Abstract
Personalized medicine may be considered an extension of traditional approaches to understanding and treating disease, but with greater precision. Physicians may now use a patient's genetic variation or expression profile as well as protein and metabolic markers to guide the selection of certain drugs or treatments. In many cases, the information provided by molecular markers predicts susceptibility to conditions. The added precision introduces the possibility of a more preventive, effective approach to clinical care and reductions in the duration and cost of clinical trials. Here, we make the case, through real-world examples, that personalized medicine is delivering significant value to individuals, to industry, and to the health care system overall and that it will continue to grow in importance if we can lift the barriers that impede its adoption and build incentives to encourage its practice.
Keywords: evidence-based medicine, molecular diagnostics, personalized medicine, pharmacogenomics, preventive medicine, regulation
There is a tectonic shift taking place in medicine. For the average patient, it is a subtle, perhaps imperceptible movement, but ultimately, it will affect the entire landscape of our health care system. The explosive growth in our knowledge of genetics and the molecular origins of disease is making its way to doctors' offices, patient bedsides, and medicine cabinets of ordinary people. Physicians can guide treatments by using genetic, mRNA, protein and metabolic markers in a way that they never have before. Since mapping the human genome in 2003, the pace of discovery, product development, and clinical adoption of what we have come to know as “personalized medicine” has accelerated.
Personalized medicine, according to the President's Council of Advisors on Science and Technology, “refers to the tailoring of medical treatment to the individual characteristics of each patient. It does not literally mean the creation of drugs or medical devices that are unique to a patient, but rather the ability to classify individuals into subpopulations that differ in their susceptibility to a particular disease or their response to a specific treatment. Preventive or therapeutic interventions can then be concentrated on those who will benefit, sparing expense and side effects for those who will not.”1
Physicians have always used observable evidence to make a diagnosis or prescribe a treatment tailored to each individual. But personalized medicine provides new tools to physicians that are more precise to probe not just the visually obvious, such as a tumor on a mammogram or the appearance of cells under a microscope, but the very molecular makeup of each patient. A profile of a patient's genetic variation can guide the selection of drugs or treatment protocols that minimize harmful side effects or ensure a more successful outcome. It can also indicate susceptibility to certain diseases before they become manifest, allowing the physician and patient to set out a plan for monitoring and prevention. The ability to profile the activity of genes, proteins, and metabolites is redefining how we classify diseases and select treatments, allowing physicians to go beyond the “one size fits all” model of medicine to make the most effective clinical decisions for each patient.
As evidence of the benefits of personalized medicine has grown, an infrastructure of laws, policy, education and clinical practice has been building around personalized medicine to support its use:
Medical institutions across the country have announced their commitment to putting personalized medicine into practice through dedicated centers or statewide initiatives.
Personalized medicine approaches are becoming “best practice” in hospitals in order to ensure that patients with serious conditions such as cancer are given the optimum therapy from the start.
The regulatory system is integrating genetic and molecular testing into the labels of pharmaceutical products, ensuring that a drug is administered in a way that minimizes the risk of adverse effects and improves the chances of effective treatment.2
Nearly every major pharmaceutical development project is incorporating information on genetic variation and its effects on the safety and effectiveness of the candidate drug.
Genomics-based medical education programs are being launched at several of the nation's leading medical schools to train the next generation of care providers.
The U.S. Department of Health and Human Services, the President's Council of Advisors on Science and Technology, and the Personalized Medicine Coalition have defined far-reaching policy recommendations for personalized medicine, and legislation supporting personalized medicine has been introduced into the U.S. Senate and House of Representatives.
Our health care system is facing many challenges, including escalating costs, declining value, and a seeming inability to institute reforms against a backwash of misaligned incentives. Personalized medicine is by no means the full prescription for reform, but it does offer a structural model for efficient health care. In its essence, it is preventive, coordinated, and evidence-based. It relies on a network of electronic health records that link clinical and molecular information to help patient and physician make optimal treatment decisions. It is proactive and participatory, engaging the patient in lifestyle choices and active health maintenance to compensate for susceptibilities written into their genome.
Clinical Applications
Ultimately, the success of personalized medicine will rise or fall on its ability to demonstrate its value to the health care system, to the industries that develop its products, and to patients. Many claims have been attached to the “promise” of personalized medicine. They include the ability to
Shift Emphasis in Medicine from Reaction to Prevention
Personalized medicine introduces the ability to use molecular markers that signal the risk of disease or its presence before clinical signs and symptoms appear. This information underlies a health care strategy focused on prevention and early intervention rather than reaction to advanced stages of disease. Such a strategy can delay disease onset or minimize symptom severity.
One example of this approach is a test being used to look for BRCA1 and BRCA2 genetic variants indicating a hereditary propensity for breast and ovarian cancer.3 Women with BRCA1 or BRCA2 genetic risk factors have a 36% to 85% lifetime chance of developing breast cancer compared with a 13% chance among the general female population.4–6 For ovarian cancer, women with certain BRCA1 or BRCA2 gene variants have a 16% to 60% chance of disease compared with a 1.7% chance among the general population. Use of the BRCA1 and BRCA2 genetic test can be used to guide preventive measures such as increased frequency of mammography, prophylactic surgery, and chemoprevention.
Preventive approaches to common diseases such as diabetes will be guided increasingly by more complex genetic profiles made available through resources such as the HapMap or the Diabetes Genetics Initiative (a public–private collaboration that published a genome-wide map of genetic differences related to type 2 diabetes and other metabolic disorders).7,8
Select Optimal Therapy
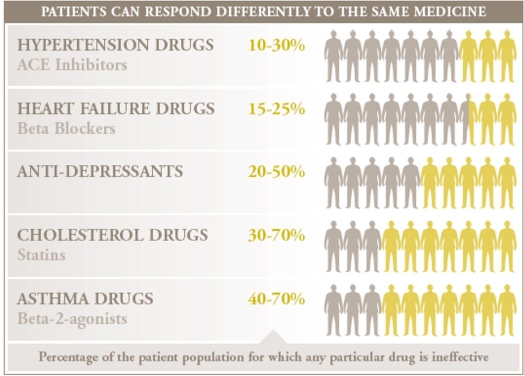
On average, a drug on the market works for only 50% of the people who take it.9 The consequences in terms of quality and cost of care are significant, leaving patients to contend with their disease and their medical bills as they switch from one drug to another until they find an effective therapy. Studies have linked differences in response to the differences in genes that code for the drug-metabolizing enzymes, drug transporters, or drug targets (Figure 1). The use of genetic and other forms of molecular screening allows the physician to select an optimal therapy the first time and avoid the frustrating and costly practice of trial-and-error prescriptions. Getting to the right drug sooner can lead to improved health for the patient or, in the case of deadly conditions, a better chance of survival.
Figure 1.
Examples of how patients respond differently to medications.
One of the most common applications of personalized medicine has been for women with breast cancer. Approximately 30% of breast cancer cases are characterized by overexpression of a cell surface protein called human epidermal growth factor receptor 2 (HER2).10,11 For these women, standard therapy is not effective, but one treatment does work—an antibody drug called Herceptin. Herceptin can actually reduce the recurrence of a tumor by 52% when used in combination with chemotherapy compared to chemotherapy alone. Molecular diagnostic tests for HER2 are used to identify the 30% of patients who will benefit from receiving the drug.
Make Drugs Safer
According to a review of several studies, approximately 5.3% of hospital admissions are associated with adverse drug reactions (ADRs).12 Many ADRs are the result of variations in genes coding for the cytochrome P450 family of enzymes.13,14 These variants may cause a drug to be metabolized either faster or slower than in the general population. As a result, some individuals may have trouble inactivating a drug and eliminating it from their body, leading, effectively, to an “overdose” as it accumulates, while others eliminate the drug before it has a chance to work. The consequences of not considering variation in these genes when dosing can be anything from unpleasant to fatal.
Administration of the drug, warfarin, used to prevent blood clots, is complicated by genetic variations in a drug metabolizing enzyme (CYP2C9) and a vitamin K metabolizing enzyme (VKORC1). Dosing is typically adjusted for the individual patient through multiple rounds of trial and error throughout the first year of treatment, during which the patient may be at risk of excessive bleeding or further blood clots. The need to get warfarin dosing right the first time to avoid adverse effects led the Food and Drug Administration to recommend genotyping for all patients receiving warfarin.15
Increase Patient Compliance to Treatment
Patient noncompliance to treatment leads to adverse health effects and increased costs. When personalized therapies prove more effective or present fewer side effects, it can be assumed that patients will more likely comply with their treatments. The impact could be greatest for the treatment of diseases such as asthma and diabetes, in which noncompliance commonly exacerbates the condition. At least one study supports this point.16 Inherited forms of hypercholesterolemia (high cholesterol) can increase the risk of myocardial infarction before the age of 40 by more than 50-fold in men and 125-fold in women. Conventional monitoring of cholesterol levels can catch the condition early, but genetic testing offers additional benefits. In addition to detecting the condition before there are observable signs of disease, knowledge of a genetic predisposition for hypercholesterolemia provides patients with a powerful incentive to make lifestyle changes and to treat their condition seriously. Patients with a genetic diagnosis have shown more than 86% adherence to their treatment program after 2 years compared to 38% prior to testing.
Reduce Time, Cost, and Failure Rate of Clinical Trials
Developing a new drug is a costly and lengthy process. Theoretically, the use of pharmacogenomic data, or information about how patients' genes affect their drug responses, could reduce the time and cost of drug development. Using genetic tests, researchers could preselect patients for studies, using those most likely to respond or least likely to suffer side effects. “Enriching” the clinical trial pool, as this approach is called, could reduce the size, time, and expense of clinical trials. Moreover, use of pharmacogenomics early in the drug development process could reduce product failures by focusing resources on drug candidates most likely to be safe and effective.
Rescue Drugs Failing Clinical Trials
Herceptin for breast cancer was a failed project before it became a resounding success and a poster drug for personalized medicine. Phase III trials in 1997 showed the drug to be ineffective in the overall population, but a careful review of the trial results revealed that women who tested positive for HER2 overexpression had a significantly better response. In 1998, the Food and Drug Administration was presented with clinical data suggesting that the HER2-positive group, defined by a diagnostic test, would benefit from the drug, and they approved the drug/diagnostic combination.17
In Europe, regulators rejected Amgen's colon cancer drug, Vectibix, for market approval because it did not demonstrate a benefit to the broad population of patients tested. The company took a closer look at the data from the clinical trial and discovered that Vectibix worked better in patients whose tumors lacked the KRAS gene mutation. The drug was later approved for those patients only.18
Reduce the Cost of Health Care
The cost of health care in the United States is on an unsustainable upward climb. Incorporating personalized medicine into the fabric of the health care system can help resolve many embedded inefficiencies, such as trial-and-error dosing, hospitalization of patients who have severe reactions to a drug, late diagnoses, and reactive treatment.
Economists have estimated that the use of a genetic test to properly dose the blood thinner, warfarin, could prevent 17,000 strokes and 85,000 “serious bleeding events” each year and avoid as much as 43,000 visits to the emergency room. If the 2 million people that start taking warfarin each year were tested at a cost of $125 to $500 per patient, the overall cost savings to the health care system would be $1.1 billion annually.19
An economic analysis of the OncoType Dx gene expression test looked at the real costs of treating women with breast cancer in a 2-million-member health plan.20 If half of the 773 eligible patients received the test, then the savings in terms of adjuvant chemotherapy, supportive care, and management of adverse events would be approximately $1930 per patient tested (based on a 34% reduction in chemotherapy use).
Finally, a study in January 2009 has found that $600 million could be saved annually if the anti-EGFR therapies, Vectibix and Erbitux, were limited to those patients with metastatic colorectal cancer whose KRAS gene is not mutated, because those are the only patients who benefit from the drugs.21
While many fear that new, sophisticated tests to guide smart medical decisions will add unnecessary costs to an already overburdened system, personalized medicine—prescribing the right treatment to the right patient at the right time to increase efficacy and reduce side effects—can lead to both improved clinical results and reduced costs. Along with increased access, these should be the goals of intelligent health care reform.
Abbreviations
- ADR
adverse drug reaction
- HER2
human epidermal growth factor receptor 2
References
- 1.President's Council of Advisors on Science and Technology. Priorities for personalized medicine. 2008. Sep, http://www.ostp.gov/galleries/PCAST/pcast_report_v2.pdf. Accessed June 19, 2009.
- 2.U.S. Food and Drug Administration. Table of valid biomarkers in the context of approved drug labels. http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm. Accessed June 19, 2009.
- 3.Nelson HD, Huffman LH, Fu R, Harris EL. U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143(5):362–379. doi: 10.7326/0003-4819-143-5-200509060-00012. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. BRCA1 and BRCA2: cancer risk and genetic testing. http://www.cancer.gov/cancertopics/factsheet/risk/brca. Accessed May 27, 2009.
- 5.Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- 6.Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Eisner MP, Horner MJ, Howlader N, Hayat M, Hankey BF, Edwards BK. Bethesda: National Cancer Institute; 2006. SEER cancer statistics review, 1975–2003. [Google Scholar]
- 7.Zhang W, Ratain MJ, Dolan ME. The HapMap resource is providing new insights into ourselves and its application to pharmacogenomics. Bioinform Biol Insights. 2008;2:15–23. doi: 10.4137/bbi.s455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broad Institute. New genomic tool for diabetes. 2007. Feb 12, http://www.broad.mit.edu/news/159. Accessed May 27, 2009.
- 9.Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med. 2001;7(5):201–204. doi: 10.1016/s1471-4914(01)01986-4. [DOI] [PubMed] [Google Scholar]
- 10.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Eng J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 11.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Eng J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 12.Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42(7):1017–1025. doi: 10.1345/aph.1L037. [DOI] [PubMed] [Google Scholar]
- 13.Phillips KA, Veenstra DL, Oren E, Lee JK, Sadee W. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA. 2001;286(18):2270–2279. doi: 10.1001/jama.286.18.2270. [DOI] [PubMed] [Google Scholar]
- 14.Special report: genotyping for cytochrome P450 polymorphisms to determine drug-metabolizer status. Technol Eval Cent Asses Program Exec Summ. 2004;19(9):1–2. [PubMed] [Google Scholar]
- 15.U.S. Food and Drug Administration. FDA approves updated warfarin (Coumadin) prescribing information. 2007. Aug 16, http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108967.htm. Accessed June 19, 2009.
- 16.Umans-Eckenhausen MA, Defesche JC, van Dam MJ, Kastelein JJ. Long-term compliance with lipid-lowering medication after genetic screening for familial hypercholesterolemia. Arch Intern Med. 2003;163(1):65–68. doi: 10.1001/archinte.163.1.65. [DOI] [PubMed] [Google Scholar]
- 17.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G, Slamon DJ. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 18.Looking forward, looking back. Nat Biotechnol. 2008;26(5):475. doi: 10.1038/nbt0508-475. [DOI] [PubMed] [Google Scholar]
- 19.McWilliam A, Lutter R, Nardinelli C. Health care savings from personalizing medicine using genetic testing: the case of warfarin. 2006. http://aei-brookings.org/admin/authorpdfs/redirect-safely.php?fname=..pdffiles/WP06-23_topost.pdf. Accessed May 27, 2009.
- 20.OncotypeDX. Has the economic validity of the Oncotype DX® assay been investigated? http://www.oncotypedx.com/ManagedCareOrgs/EconomicValidity.aspx?Sid=25. Accessed May 27, 2009.
- 21.Shankaran V. California: San Francisco; Conference presentation at the Gastrointestinal Cancers Symposium January 2009. http://www.medscape.com/viewarticle/586946. Accessed May 27, 2009. Requires free registration to access. [Google Scholar]