Abstract
Adult gains in body weight, excess adiposity, and intra-abdominal fat have each been associated with risk for type 2 diabetes mellitus (T2DM), forming the basis for preventive medicine guidelines and actuarial predictions using practical indices of weight (e.g., body mass index [BMI]) and waist circumference (WC). As obesity-related disease spreads beyond affluent western countries, application of WC thresholds to other populations has highlighted issues of their generalizability. For example, U.S. national health goals based on BMI < 25 kg/m2 and WC < 89 cm (women) and <102 cm (men) differ considerably with a recent law in Japan mandating intervention for older adults with WC exceeding 90 cm (women) and 85 cm (men). The U.S. military has also faced issues of generalizability of WC-based adiposity standards that are fair and achievable. Data from many studies indicate that WC is a reliable biomarker for T2DM risk, suggesting that, for adult men and women, action thresholds should be more stringent than current U.S. guidelines, and it would not be harmful to set worldwide targets somewhere below 90 cm for men and women, regardless of weight status. Medical technology has provided many great insights into disease, including modern imaging technologies that have differentiated fat depots that have the greatest influence on T2DM, but ultimately, an inexpensive measuring tape provides the most useful and cost-effective preventive measure for T2DM today. At some point in the future, a Star Trek-like abdominal body fat “tricorder” noninvasive assessment of tissue composition may provide an advantage over abdominal girth.
Keywords: abdominal circumference, age, anthropometry, body composition, body fat standards, body mass index, epidemiology of diabetes, ethnicity, fat topography, gender, intra-abdominal fat, percent body fat, preventive medicine, public health policy
“It is not how heavy a man is or how heavy he becomes that has maximum prognostic significance, but rather how much fat he carries and how much fat he adds. It is not unlikely that the location of the fat, as well as the gross amount, may elucidate the differential mortality rates of the different weight groups.”1
–Stanley Marion Garn, 1955
Background
The association between abdominal fat accumulation and risk for type 2 diabetes mellitus (T2DM) has been recognized at least since the start of the 20th century. By the 1930s, the Metropolitan Life Insurance Company reported a striking death rate associated with excess body weight, particularly for diabetes-associated disease; for individuals 25% or more overweight, diabetes death rates were 8 times that of a sample of average weight and 13 times that of a sample of underweights.2,3 An extra all-cause mortality of 50%, over and above the risk from excess body weight alone, was observed for overweight men with abdominal girth exceeding chest girth by two inches or more.4 Current-day refinements of these guidelines recommend that Americans should strive for a healthy weight with a body mass index (BMI) below 25 kg/m2 and waist circumferences (WCs) of less than 35 in. for women and 40 in. for men.5 International public health recommendations have also moved to the inclusion of WC.6 These guidelines emphasize the empirically observed relationship between T2DM and excess adiposity in the abdominal region.7–9 A high BMI may signify inappropriate weight gain, fat accumulation, and specific abdominal fat accumulation—but with much greater variability than WC, particularly for athletic males.10,11 Some males, in particular, are very large and still very lean.12 Excess weight, regardless of its chemical composition, is associated with other obesity-related problems such as osteoarthritis and sleep apnea, but abdominal fat accumulation has a strong and specific association with metabolic disorders, notably insulin resistance, leading to T2DM.7,8,13–16 These health risk associations have been explained on the basis of the lability of hypertrophic intra-abdominal fat cells, with release of fatty acids and cytokines into the portal flow to the liver and with subsequent effects on insulin resistance.12,17,18 Thus the health risk focus of WC is specifically on intra-abdominal or “visceral” adipose tissue (VAT) and not on other fat in the abdominal region such as subcutaneous and structural fat depots. This within-abdomen distribution of fat is important to gender differences because it affects the relationship between WC and health risks. The reliability of the relationship between VAT and the effect on insulin resistance is also of important to predict risk guidelines. This relationship may differ between populations and may change for individuals based on a variety of factors, including genetics, physical fitness, physical activity habits, and nutritional factors.19,20,21 This article considers the net variability of these two relationships (WC–VAT and VAT–insulin resistance/T2DM), with consideration of practical WC thresholds of action for T2DM risk.
Waist-to-Hip Ratios Discriminate between “Apples” and “Pears”
In the 1940s, Jean Vague invigorated the concept of WC as a risk factor for metabolic disease with the expression of waist-to-hip ratio (WHR).22,23 His characterization of “apples” and “pears” has been widely tested in endocrine metabolic studies of women, where androgen and estrogen metabolism influences both the pattern of distribution as well as the metabolism of steroid hormones.24,25 Lower body obesity (LBO) or stereotypically “female” fat distribution is associated with a protective effect against metabolic disorders.26 This may be a reflection of the estrogen influence and/or the absence of a greater androgen influence on accumulation of intra-abdominal adipose tissue. Androgens also play a significant role in male and female abdominal fat accretion and distribution.27 Upper body obesity (UBO) (“male” pattern or “android” pattern) is associated with increased insulin resistance and diabetes,8 cardiovascular risk,7 and even “male-type” responses to physical exercise and weight loss.28
High WHR or UBO is most influenced in women by age and increasing weight.29,30 The prevalence of upper body fat distribution rises with increasing adiposity in healthy young military women.31 These women have male-type greater muscle mass, strength, and weight loss patterns,28,32 presenting some challenges for the military with somewhat conflicting health and strength performance standards.
For women, increasing WC is associated with greater metabolic risk through the specific association with a greater abdominal adiposity and the relative distribution of fat to other key fat depots (i.e., hips, thighs, upper arms, and breasts) appears to be relatively unimportant to metabolic disease risk. Conway and colleagues33 found that WC was the single best predictor of VAT in both UBO and LBO African American women; WHR was not a good overall predictor of VAT. Although WHR has been used in many studies, the advantage of a simple WC is rapidly overtaking artificial indices such as WHR and waist corrected for stature. Contrary to earlier perceptions, WC does not have to be adjusted for body size, as it is stature independent.34 Sagittal thickness (anterior–posterior measurement) has also been examined as a practical measure of VAT on the basis of computerized tomography scans but has not proven to be better than WC.35
Waist Circumference Prediction of Intra-Abdominal Fat—The Intra-Abdominal Space Is “Protected” in Premenopausal Women
Women compared to men can store more fat subcutaneously throughout the body before intra-abdominal fat accumulation begins, while men preferentially deposit fat in the abdomen. This was observed by Stanley Garn as “women carry more fat on and less in their smaller frames.”36 The observation was quantified by Kvist and associates37 with computerized tomography scanning and a compelling observation that intra-abdominal fat was minimal in healthy young women until approximately 30 kg of fat accumulated elsewhere on the body; for men, fat was stored intra-abdominally in a straight line relationship with increasing overall adiposity. Kvist and associates found a very high correlation between WC and total abdominal adiposity predicted at two different abdominal slices for both men and women, and specific VAT was also highly correlated.37 Other studies have repeatedly confirmed this finding with abdominal fat scans. Lemieux and coworkers38 showed that premenopausal women can accumulate more body fat than men before they reach the VAT levels found in men. Kuk and colleagues39 demonstrated similar findings with greater subcutaneous adipose tissue (SAT) and lower VAT in women compared to men and an inverse relationship in both sexes with age.
There was no sex difference in the relationship of WC to a threshold of VAT (130 cm3) in the study of French Canadians by Lemieux and associates,40 but the ratio of visceral to subcutaneous abdominal fat was doubled in men (0.59) compared to the women (0.29) in this study sample. There was an age difference in WC corresponding to the VAT thresholds (130 cm3), with lower WC for the same VAT for older men and women (i.e., WC = 100 cm, <age 40; WC = 90, >age 40). The absence of a sex difference in WC equivalents to VAT volume despite sex differences in subcutaneous fat in this study probably reflects some of the variability related to human sexual dimorphism in abdominal shape. Other differences such as sensitivity to the factors affecting insulin resistance could explain the sex differences in WC thresholds corresponding to actual disease outcome risk.
This sexual dimorphism leads to a logical and practical difference in the measurement of WC between men and women. The military-adopted sex-specific abdominal girth measurement sites, with men measured at the level of the navel (“abdominal 2”) and women measured at the thinnest point between the ribs and the suprailiac crest (“abdominal 1”).12 These measurements are made at mid-expiration, with measurements horizontal with the floor and flat against the skin (not with a spring-gauge controlled tension tape, such as the Gulick-style tapes). Most health risk studies involving both men and women have measured WC by a single standard for consistency. This WC measurement site can be any of multiple possibilities between the lowest rib margin and the top of the iliac crest; a common choice and a World Health Organization standard is to measure men and women midway between these limits.
Anthropometric Associations with Body Mass Index Thresholds (25 kg/m2)
Body weight expressed as BMI is the most commonly obtained anthropometric or body fat measurement in health risk epidemiology and thus provides the key basis against which other measurements of interest such as WC or adiposity can be roughly calibrated. The corresponding values for potentially equivalent or superior thresholds of risk can then be further tested to determine if WC or adiposity provide greater predictive value than body weight.
Many studies have contributed to the change in obesity-related disease threshold with BMI, and national and international review committees have frequently revisited the question, converging on BMI >25 and >30 kg/m2 as “overweight” (or some term describing greater than healthy weight) and “obese,” respectively.41 The U.S. Army used BMI 25 kg/m2 as a basis for upper limits of military body fat standards and also considered the thresholds of WC recommended by the National Heart, Lung, and Blood Institute (NHLBI); this is discussed in further detail in the next section of this article.
Zhu and coworkers42 considered the WC values that corresponded to similar cardiovascular risk factor predictions provided by BMI 25 and 30 in white subjects, ages 20–90, in the National Health and Nutrition Examination Survey (NHANES) III sample (Table 1). A BMI 25 corresponded to 90 and 83 cm, for men and women, respectively, based on lowest probability of having one out of four measured risk factors (reference BMI for the odds ratio comparisons were set at the 25th percentile for BMI and WC). For BMI 30, the corresponding health risks were predicted at WC 100 and 93 cm for men and women, respectively. Waist circumference had better sensitivity than BMI in both men and women.42 Using the same technique in a later analysis, Zhu and colleagues compared ethnic groups in the NHANESIII data, finding higher WC thresholds for white men compared to black men and Mexican Americans; women had smaller differences between ethnic groups.43 Simplified threshold recommendations that combined values across ethic groups for BMI 25 equivalents were 89 and 83 cm for men and women, respectively (Table 1). Other studies have also suggested that some Asian populations (Chinese and South Asian) have more VAT relative to Europeans. The Multicultural Community Health Assessment Trial study44 of a large group matched by BMI and sex found that VAT was underestimated by BMI in the non-European groups (although above some levels of obesity, Asian cohorts had more VAT than Europeans).
Table 1.
Waist Circumferences (cm) Corresponding to Body Mass Index (25 kg/m2), Based on Similar Risk Factor Odds Ratios
| Race/ethnicity | |||
|---|---|---|---|
| African American | Mexican American | Non-Hispanic white | |
| Men42 | — | — | 90 |
| Men43a | 86.4 | 88.7 | 91.3 |
| Women42 | — | — | 83 |
| Women43b | 83.5 | 83.1 | 83.4 |
Simplified overall recommendations, regardless of race/ethnicity: 89 cm.
Simplified overall recommendations, regardless of race/ethnicity: 83 cm.
Also using the NHANESIII data, Janssen and associates15 examined odds ratios for metabolic syndrome by NHLBI criteria for BMI (25 and 30 kg/m2) and WC (102 and 88 cm for men and women, respectively). There were dramatic progressive increases in odd ratios for men and women for BMI 25 and 30 kg/m2 compared to BMI <25 kg/m2, but these differences were completely abolished after adjustment for WC. In other words, normal-weight individuals with the same WC as obese individuals had similar risks for metabolic disease, further suggesting that WC is the single physical measurement needed for predicting obesity-related metabolic disease. The usefulness of BMI in previous epidemiological studies of T2DM and related metabolic diseases may derive primarily from its association with WC as a less precise surrogate of VAT.
Waist circumference equivalents of BMI only provide starting points from which to conduct validation studies, as the overall adiposity as well as location of abdominal fat (and intramuscular fat) varies within subpopulations. Matsuzawa and colleagues demonstrated this variability in the dramatic example of Sumo wrestlers compared to obese patients.11 A group of young wrestlers (average age 18 years; BMI 36 kg/m2) were compared to obese patients of similar BMI, sorted by visceral- and subcutaneous-predominant abdominal fat distributions. The wrestlers and the patients with subcutaneous fat distribution had mean values of fasting plasma glucose, cholesterol, and triglycerides in the normal range, while the visceral fat obese patients were substantially elevated.11 Only 1 of 15 Sumo wrestlers studied by abdominal computed axial tomography scan had high visceral fat levels.11 Matsuzawa and colleagues also noted that many of the obese subjects actually had low visceral fat content and high subcutaneous abdominal fat stores. They suggested that the physique of Japanese subjects might be different than that of the white European samples on which some of the commonly used health risk thresholds have been based.11 Clearly, physical activity habits have important effects on body composition and alter the relationship between BMI and WC.15 This is observed in the fit and active U.S. military population when compared to nationally representative samples.
Overall body composition equivalency to BMI 25 has also been examined. These comparisons have been based on observed body composition relative to body size and not compared on the basis of equivalent health risks. For the military, this was the initial basis that anchored the body fat standards, working in reverse from established weight-for-height tables that derive originally from the 1912 actuarial tables; this will be further discussed in the next section. In a large multinational study, percent body fat was accurately assessed using four-compartment model estimations and compared across three ethnic groups for correspondence to BMI 2545 (Table 2). Asian men and women tended to have higher percent body fat for the same BMI, as did white and African Americans, and relative body fat increased in all groups by age. A separate study of Asian (predominantly Chinese immigrants) and white samples resulted in similar findings46 (Table 2). Overall predicted body fat equivalents of BMI 25 can be practically summarized as 21–24% for men and 33–36% for women, but with wide variation by groups and individuals.
Table 2.
Percent Body Fat Corresponding to Body Mass Index (25 kg/m2), Based on Four-Compartment Model Estimations of Body Composition
Waist Circumference Calibrated to Percent Body Fat
Of all the anthropometric circumferences, skin folds, and thicknesses or breadths that have been studied, WC is the single best predictor of overall adiposity in men and women. Comparing BMI, WC, and WC/height ratio to dual energy x-ray absorptiometry-derived percent body fat from the recent NHANES data, Flegal and coworkers47 reported that none of these measures were accurate for individual percent body fat predictions but that, within sex and age groups, all of these measures corresponded well to overall percent body fat. Waist circumference was superior to BMI for men, but BMI worked well for women compared to WC alone. Height-adjusted WC was slightly better than WC alone. From original work for the U.S. Marine Corps in the late 1970s,48,49 the Department of Defense has used a combination of circumferences and stature to estimate a percent body fat, which is WC-based and reasonably accurate for individuals.12,50
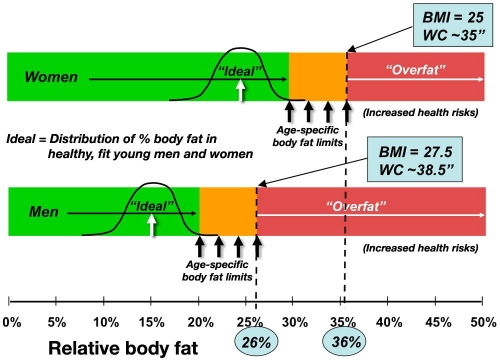
The U.S. Department of Defense uses a “calibrated” WC as the basis of their body fat standards, with downward adjustment of the relative body fat for increasing height and neck circumference and with the addition of a hip circumference for women, to the main measurement of WC.50 The predictions correspond to an upper limit for all military services of 26% and 36% body fat, roughly equating to 98 cm (38.5 in.) and 89 cm (35.0 in.) WC for the typical service member and 27.5 and 25.0 kg/m2 BMI upper limits for men and women, respectively (Table 3). The lower limits are set to fit young observed averages plus a statistical window, while these upper limits are anchored expressly to health limits. The army applies these upper limits as the threshold for action for service members over age 40, with more stringent standards applied to younger age categories12 (Figure 1).
Table 3.
Summary of Corresponding Values between Army Percent Body Fat Thresholds for Action, Waist Circumference, and Body Mass Index12
| Men | |
|---|---|
| <21 years | Upper limit was set at 20% body fat, based on mean fitness values
|
| >39 years | Upper limit was set at 26% body fat, based on thresholds of health risk
|
| Women | |
|---|---|
| <21 years | Upper limit was set at 30% body fat, based on mean fitness values
|
| >39 years | Upper limit was set at 36% body fat, based on thresholds of health risk
|
Figure 1.
Army body fat standards for men and women and the corresponding BMI and WC for health-related upper limits of the oldest soldiers.
Originally, different sets of “generalized” predictive equations were developed for the army, navy, marines, and air force on the premise that the populations differed in ethnic representation typical of each of the military services; this was a fundamental flaw in logic, calling into question the generalizability of any of these predictions if they did not work across populations and provide reasonable estimations at the individual level. This was resolved with universal adoption of the equations originally developed by the navy after further study and validation across groups and against a four-compartment model of body composition.50 The greatest difference in the service-specific equations were for women, with each military service emphasizing different circumferential measurements (combinations of waist, hips, thighs, forearms, and wrists), and each equation provided very different percent body fat results for the same woman. The army equation selected from a family of regression equations drawn from a sample of female soldiers included body weight along with height and four circumferences. This proved to be a good equation for female body fat, which was relatively sensitive to changes in body composition,51,52 even though it was too complex and did not target the abdominal circumference, which is most relevant to military concerns.12 The advantage of including body weight in female body fat estimation is that, at least in large samples of female soldiers, lean mass does not increase dramatically with increasing body size, but body fat has an excellent correlation. This is in contrast to male soldiers, where lean mass, more than relative body fat, increases with body size; for men, WC is a clear and separate discriminator of adiposity compared to body size. Experience with military body fat standards has been reviewed previously in detail.12,32
Waist Circumference Prediction of Type 2 Diabetes
In the Treatment Outcomes Prospective Study of 1969, thousands of older (age 40–59) white women in the United States and Canada were examined for health consequences associated with increasing WHR. Split into upper and lower tertiles, the upper tertile of WHR has a three-fold relative risk for diabetes after adjustment for age and other body measurements, and this was the most important disease association.9,29
Lundgren and associates8 found that BMI and WHR were significant predictors of diabetes in a Swedish population 12 years later. Elevated serum glucose, however, was predicted only by increases in BMI and increases in WHR, emphasizing the importance of inappropriate weight gain over body size.53
The Health Professionals Follow-Up Study (51,529 U.S. male health professionals) demonstrated that both overall and abdominal adiposity strongly and independently predict risk of T2DM.15 Recommended cutoff for WC should be lower than the 102 cm for men, and 84% of T2DM cases were >94 cm, while a threshold value of 101 cm included 56% of cases. The authors recommended a lower WC cutoff at 95 cm rather than the current NHLBI 102 cm for men, based on actual T2DM outcome associations.15
This year, Gallagher reported significantly greater VAT, higher intermuscular adipose tissue (IMAT), and lower SAT in white compared to African American T2DM cases, suggesting the possibility that VAT and IMAT depots affect insulin resistance differently in different populations.54
Diabetes affects body composition; thus confirmed disease produces different relationships than predicting risk of disease in advance of actual onset of clinical symptoms. Gallagher and colleagues55 compared patients with T2DM to healthy controls and found that, for men and women, VAT and IMAT were higher and SAT was lower in patients with T2DM. Thus accumulating visceral fat may be a risk factor and/or a cause or accelerator of the metabolic derangements associated with T2DM.
Han and associates34 studied a group of overweight British white women and demonstrated that weight loss was associated with reduction in WC, and the largest stable reductions in WC after 6 months produced improvements in at least one of several risk factors (e.g., serum cholesterol, low-density lipoprotein cholesterol, and diastolic blood pressure). This is suggestive evidence that some amount of weight loss for individuals within action zones set by WC thresholds can be beneficial to health risk outcomes.
Application of Waist Circumference Thresholds to Public Health Intervention
As the problem of obesity-related disease spreads from affluent western countries to the rest of the world, the generalizability of WC thresholds across populations is put to the greatest test. The U.S. military has a relatively small proportion of Asian Pacific ethnic members and has never tested body composition measurement and thresholds of action in a significant cohort or subpopulation of Asian Americans at military bases in Hawaii or elsewhere. Within this group, there are clearly differences at least as large as the variability seen just within Europeans,56 ranging from lower WC thresholds proposed for Japanese men and women57 compared to higher WC thresholds proposed for Maori men and women.58
In a bold campaign to stem the rise in diabetes and other obesity-related diseases, the Japanese government imposed a new law in 2009 requiring measurement of WC of adults between the ages of 40 and 74 during annual checkups. Individuals who exceed 85 cm (men) and 90 cm (women) based on thresholds established in 2005 by the International Diabetes Federation59 must lose weight within 3 months or receive dietary guidance and additional intervention after an additional 6 months of unsuccessful progress. Companies and local governments will receive financial penalties for failure to meet WC targets. This national scale enforcement of obesity prevention measures is very similar to the U.S. military enforcement of obesity prevention directed by President Carter in 1980 that continues today. Unfortunately, no careful assessment has been made of the effectiveness compared to a similar group without these policies in place.
The approach is controversial not only because a specific threshold for action was mandated, where other national programs have typically only set population goals, but also because of the application of the International Diabetes Federation recommendations that include WC thresholds that are higher for men than women—only for Japanese of all the Pacific Rim populations considered.
Conclusions
Medical technology has provided many great insights into disease, including modern imaging technologies that have differentiated fat depots that have the greatest influence on T2DM. Ultimately, a 50-cent measuring tape provides the most useful and cost-effective preventive measure for T2DM today. At some point in the future, a Star Trek-like abdominal body fat “tricorder” noninvasive assessment of adiposity and perhaps biochemical composition will provide a significant advantage over abdominal girth. The effects of physical fitness and physical activity habits on T2DM risk60 also need to be better understood, and benefits of energy flux and exercise-associated changes need to be distinguished from effects on abdominal fat reduction. Despite concerns about the specific WC thresholds for action, continuing reports from new studies of specific populations suggest that the action levels for WC and T2DM risk should be more stringent than NHLBI guidelines for men (102 cm) and women (89 cm), and it would not be harmful to set worldwide “gut reduction” targets somewhere below 90 cm for both sexes. The calculation of an adjusted WC as used by the U.S. military actually provide the finer adjustment for body sizes that may accommodate some of the differences seen across populations, but this remains to be demonstrated.
Abbreviations
- BMI
body mass index
- IMAT
intermuscular adipose tissue
- LBO
lower body obesity
- NHANES
National Health and Nutrition Examination Survey
- NHLBI
National Heart, Lung, and Blood Institute
- SAT
subcutaneous adipose tissue
- T2DM
type 2 diabetes mellitus
- UBO
upper body obesity
- VAT
visceral adipose tissue
- WC
waist circumference
- WHR
waist-to-hip ratio
References
- 1.Garn SM, Harper RV. Fat accumulation and weight gain in the adult male. Hum Biol. 1955;27(1):39–49. [PubMed] [Google Scholar]
- 2.Marks HH. Body weight: facts from life insurance records. Hum Biol. 1956;28(2):217–231. [PubMed] [Google Scholar]
- 3.Metropolitan Life Insurance Company. Body weight and longevity. Vol. 3. Statistical Bulletin of the Metropolitan Life Insurance Company; 1922. pp. 3–4. [Google Scholar]
- 4.Metropolitan Life Insurance Company. Girth and death. Vol. 18. Statistical Bulletin of the Metropolitan Life Insurance Company; 1937. pp. 2–5. [Google Scholar]
- 5.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 6.Seidell JC, Kahn HS, Williamson DF, Lissner L, Valdez R. Report from a Centers for Disease Control and Prevention Workshop on use of adult anthropometry for public health and primary health care. Am J Clin Nutr. 2001;73(1):123–126. doi: 10.1093/ajcn/73.1.123. [DOI] [PubMed] [Google Scholar]
- 7.Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J. (Clin Res Ed) 1984;288(6428):1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundgren H, Bengtsson C, Blohme G, Lapidus L, Sjöström L. Adiposity and adipose tissue distribution in relation to incidence of diabetes in women: results from a prospective population study in Gothenburg, Sweden. Int J Obes. 1989;13(4):413–423. [PubMed] [Google Scholar]
- 9.Hartz AJ, Rupley DC, Rimm AA. The association of girth measurements with disease in 32,856 women. Am J Epidemiol. 1984;119(1):71–80. doi: 10.1093/oxfordjournals.aje.a113727. [DOI] [PubMed] [Google Scholar]
- 10.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzawa Y, Fujioka S, Tokunaga K, Tarui S. Classification of obesity with respect to obesity. Proc Soc Exp Biol Med. 1992;200(2):197–201. doi: 10.3181/00379727-200-43417. [DOI] [PubMed] [Google Scholar]
- 12.Friedl KE. Can you be large and not obese? The distinction between body weight, body fat, and abdominal fat in occupational standards. Diabetes Technol Ther. 2004;6(5):732–749. doi: 10.1089/dia.2004.6.732. [DOI] [PubMed] [Google Scholar]
- 13.Björntorp P. Obesity and adipose tissue distribution as risk factors for the development of disease. A review. Infusionstherapie. 1990;17(1):24–27. doi: 10.1159/000222436. [DOI] [PubMed] [Google Scholar]
- 14.Seidell J, Björntorp P, Sjöström L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39(9):897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81(3):555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Katzmarzyk PT, Ross R, Leon AS, Skinner JS, Rao DC, Wilmore JH, Rankinen T, Bouchard C. Fitness alters the associations of BMI and waist circumference with total and abdominal fat. Obes Res. 2004;12(3):525–537. doi: 10.1038/oby.2004.60. [DOI] [PubMed] [Google Scholar]
- 17.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 18.Shimomura I, Funahasm T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med. 1996;2(7):800–803. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 19.Ross R, Rissanen J. Mobilization of visceral and subcutaneous adipose tissue in response to energy restriction and exercise. Am J Clin Nutr. 1994;60(5):695–703. doi: 10.1093/ajcn/60.5.695. [DOI] [PubMed] [Google Scholar]
- 20.Han TS, Richmond P, Avenell A, Lean ME. Waist circumference reduction and cardiovascular benefits during weight loss in women. Int J Obes Relat Metab Disord. 1997;21(2):127–134. doi: 10.1038/sj.ijo.0800377. [DOI] [PubMed] [Google Scholar]
- 21.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86(8):3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 22.Vague J. La differenciation sexuelle facteur determinant des formes de l'obesite. Presse Med. 1947;55(30):339–340. [PubMed] [Google Scholar]
- 23.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric-calculous disease. Am J Clin Nutr. 1956;4(1):20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 24.Evans DJ, Hoffmann RG, Kalkhoff RK, Kissebah AH. Relationship of androgenic activity to body fat topography, fat cell morphology, and metabolic aberrations in premenopausal women. J Clin Endocrinol Metab. 1983;57(2):304–310. doi: 10.1210/jcem-57-2-304. [DOI] [PubMed] [Google Scholar]
- 25.Peiris AN, Aiman EJ, Drucker WD, Kissebah AH. The relative contributions of hepatic and peripheral tissues to insulin resistance in hyperandrogenic women. J Clin Endocrinol Metab. 1989;68(4):715–720. doi: 10.1210/jcem-68-4-715. [DOI] [PubMed] [Google Scholar]
- 26.Terry RB, Stefanick ML, Haskell WL, Wood PD. Contributions of regional adipose tissue depots to plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism. 1991;40(7):733–740. doi: 10.1016/0026-0495(91)90093-c. [DOI] [PubMed] [Google Scholar]
- 27.Mårin P, Odén B, Björntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab. 1995;80(1):239–243. doi: 10.1210/jcem.80.1.7829619. [DOI] [PubMed] [Google Scholar]
- 28.Andersson B, Xu XF, Rebuffé-Scrive M, Terning K, Krotkiewski M, Björntorp P. The effects of exercise training on body composition and metabolism in men and women. Int J Obes. 1991;15(1):75–81. [PubMed] [Google Scholar]
- 29.Hartz AJ, Rupley DC, Jr, Kalkhoff RD, Rimm AA. Relationship of obesity to diabetes: influence of obesity level and body fat distribution. Prev Med. 1983;12(2):351–357. doi: 10.1016/0091-7435(83)90244-x. [DOI] [PubMed] [Google Scholar]
- 30.Lanska DJ, Lanska MJ, Hartz AJ, Rimm AA. Factors influencing anatomic location of fat tissue in 52,953 women. Int J Obes. 1985;9(1):29–38. [PubMed] [Google Scholar]
- 31.Westphal KA, Friedl KE, Sharp MA, King N, Kramer TR, Reynolds KL, Marchitelli LJ. Natick: U.S. Army Research Institute of Environmental Medicine; 1995. Health, performance, and nutritional status of U.S. Army women during basic combat training. AD-A302042. Technical Report No. T96-2. [Google Scholar]
- 32.Friedl KE. Carlson-Newberry SJ, Costello RB. Emerging technologies for nutrition research: potential for assessing military performance capability. Washington DC: National Academy Press; 1997. Military application of body composition assessment technologies; pp. 81–126. [PubMed] [Google Scholar]
- 33.Conway JM, Chanetsa FF, Wang P. Intra-abdominal adipose tissue and anthropometric surrogates in African American women with upper- and lower-body obesity. Am J Clin Nutr. 1997;66(6):1345–1351. doi: 10.1093/ajcn/66.6.1345. [DOI] [PubMed] [Google Scholar]
- 34.Han TS, Seidell JC, Currall JE, Morrison CE, Deurenberg P, Lean ME. The influences of height and age on waist circumference as an index of adiposity in adults. Int J Obes Relat Metab Disord. 1997;21(1):83–89. doi: 10.1038/sj.ijo.0800371. [DOI] [PubMed] [Google Scholar]
- 35.Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73(7):460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 36.Garn SM. Fat weight and fat placement in the female. Science. 1957;125(3257):1091–1092. doi: 10.1126/science.125.3257.1091. [DOI] [PubMed] [Google Scholar]
- 37.Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48(6):1351–1361. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 38.Lemieux S, Prud'homme D, Bouchard C, Trembley A, Després JP. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am J Clin Nutr. 1993;58(4):463–467. doi: 10.1093/ajcn/58.4.463. [DOI] [PubMed] [Google Scholar]
- 39.Kuk JL, Lee S, Heymsfield SB, Ross R. Waist circumference and abdominal adipose tissue distribution: influence of age and sex. Am J Clin Nutr. 2005;81(6):1330–1334. doi: 10.1093/ajcn/81.6.1330. [DOI] [PubMed] [Google Scholar]
- 40.Lemieux S, Prud'homme D, Bouchard C, Temblay A, Després JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64(5):685–693. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 41.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074–1081. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 42.Zhu SK, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76(4):743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 43.Zhu S, Heymsfield SB, Toyoshima H, Wang Z, Pietrobelli A, Heshka S. Race-ethnicity-specific waist circumference cutoffs for identifying cardiovascular disease risk factors. Am J Clin Nutr. 2005;81(2):409–415. doi: 10.1093/ajcn.81.2.409. [DOI] [PubMed] [Google Scholar]
- 44.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs acc ording to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT) Am J Clin Nutr. 2007;86(2):353–359. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 45.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN., Jr Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr. 1994;60(1):23–28. doi: 10.1093/ajcn/60.1.23. [DOI] [PubMed] [Google Scholar]
- 47.Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, Harris TB, Everhart JE, Schenker N. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89(2):500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright HF, Wilmore JH. Estimation of relative body fat and lean body weight in a United States Marine Corps population. Aerosp Med. 1974;45(3):301–306. [PubMed] [Google Scholar]
- 49.Wright HF, Dotson CO, Davis PO. A simple technique for measurement of percent body fat in man. US Navy Med. 1981;72(5):23–27. [PubMed] [Google Scholar]
- 50.Hodgdon JA, Friedl KE. Technical Document 99-2B. San Diego: Naval Health Research Center; 1999. Development of the DoD body composition estimation equations. [Google Scholar]
- 51.Vogel JA, Kirkpatrick JW, Fitzgerald PI, Hodgdon JA, Harman EA. Natick: U.S. Army Research Institute of Environmental Medicine; 1984. Derivation of anthropometry based body fat equations for the Army's weight control program. ADA197706. [Google Scholar]
- 52.Friedl KE, Westphal KA, Marchitelli LJ, Patton JF, Chumlea WC, Guo SS. Evaluation of anthropometric equations to assess body-composition changes in young women. Am J Clin Nutr. 2001;73(2):268–275. doi: 10.1093/ajcn/73.2.268. [DOI] [PubMed] [Google Scholar]
- 53.Hubbard VS. Defining overweight and obesity: what are the issues? Am J Clin Nutr. 2000;72(5):1067–1068. doi: 10.1093/ajcn/72.5.1067. [DOI] [PubMed] [Google Scholar]
- 54.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB. Aidpose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81(4):903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, Pi-Sunyer FX, Heshka S. MRI Ancillary Study Group of the Look AHEAD Research Group. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89(3):807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cigolini M, Seidell JC, Charzewska J, Ellsinger BM, DiBiase G, Björntorp P, Hautvast JG, Contaldo F, Szostak V, Scuro LA. Fasting serum insulin in relation to fat distribution, serum lipid profile, and blood pressure in European women: the European Fat Distribution Study. Metabolism. 1991;40(8):781–787. doi: 10.1016/0026-0495(91)90003-f. [DOI] [PubMed] [Google Scholar]
- 57.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Minimum waist and visceral fat values for identifying Japanese Americans at risk for the metabolic syndrome. Diabetes Care. 2007;30(1):120–127. doi: 10.2337/dc06-0739. [DOI] [PubMed] [Google Scholar]
- 58.Rush EC, Crook N, Simmons D. Br J Nutr. 2009. Optimal waist cutpoint for screening for dysglycaemia and metabolic risk: evidence from a Maori cohort. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 59.Asian-Pacific Type 2 Diabetes Policy Group. Type 2 diabetes, practical targets and treatments. 4th ed. Melbourne: International Diabetes Institute; 2005. p. 58. [Google Scholar]
- 60.Blair SN, Kohl KW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]