Abstract
Background
We estimate safe screening intervals for sight-threatening diabetic retinopathy (STDR).
Methods
A 6-year retrospective follow-up study to review screening results of two cohorts of patients with diabetes mellitus (DM) was conducted; a cohort free of diabetic retinopathy (DR) and a cohort with mild nonproliferative diabetic retinopathy (NPDR) at baseline. Patients had been screened by means of a nonmydriatic retinal camera. Baseline age, sex, and diabetes characteristics were also collected. Statistical analysis was based on life-table method of risk estimation.
Results
A total of 286 patients with DM free of DR and 144 patients with mild NPDR at baseline were included in the study. For patients free of DR, the probability of remaining free of STDR was 97% (95% confidence interval [CI] 94–99%) at the end of the fourth year. In this cohort of patients, those with type 2 DM were more likely to progress to STDR than those who had type 1 DM (p < .01). For patients with mild NPDR, the probability of remaining free of STDR dropped to 94% (95% CI 88–97%) at the end of the second year, and it was still 100% at the end of the second year for those with a glycated hemoglobin level ≤7.5% at baseline (p < .05).
Conclusions
Screening at a 3–4 year interval for diabetes patients free of DR is safe because of their low risk of developing STDR. Patients with mild NPDR require screening at a 1 year interval, or at a 2 year interval with good metabolic control.
Keywords: complications, diabetes mellitus, diabetic retinopathy, follow-up studies, mass screening, risk factors, sight-threatening diabetic retinopathy
Introduction
In 1976, the Diabetic Retinopathy Study Research Group showed that laser photocoagulation could prevent visual loss in patients with diabetes mellitus (DM) and diabetic retinopathy (DR); laser treatment was beneficial in reducing the risk of further visual loss, but it was not beneficial in reversing already diminished visual acuity (VA).1 However, patients with diabetes are not receiving this preventive treatment early enough, because some patients with sight-threatening diabetic retinopathy (STDR) may not have had symptoms that prompted an eye examination.2–4 Besides, the incidence and prevalence of blindness is lower in populations where screening for DR has been established compared to populations without screening.5 Therefore, a screening schedule for retinopathy in patients with DM seems to be appropriate.
Several clinical practice recommendations for initial and subsequent screening for retinopathy in diabetes patients have been developed in United States and Europe. The American Diabetes Association recommended that patients with type 2 DM (T2DM) should have an initial eye examination shortly after the diagnosis of diabetes, and patients with type 1 DM (T1DM) should have an initial exam within 3–5 years after the onset of the disease. Afterwards, subsequent eye examinations for both T1DM and T2DM patients should be repeated annually.6 In Europe, the Retinopathy Working Party recommended that eyes of diabetes patients should be examined at diagnosis of DM, at least every 2 years thereafter, and at least annually once retinopathy appears.7 However, as pointed out by Younis et al., these recommendations were based on expert consensus opinion rather than direct evidence.8 To our knowledge, only two publications from the Liverpool Diabetic Eye Study estimated from direct evidence of a systematic screening program how frequently patients with T1DM and T2DM should be screened for STDR.8,9 Younis and colleagues estimated that screening patients with T2DM with no retinopathy (n = 3743) every 5 years provided a 95% probability of remaining free of STDR.8 The Liverpool findings were consistent with those previously reported by Vijan and associates, which concluded that annual screening for patients with T2DM with no DR was not cost-effective and that increasing the interval between screenings should be considered.10 However, long intervals between screening visits may lead to a fall in patient compliance, and therefore screening every 3 years was finally suggested instead. Although they also estimated that screening patients with T1DM with no DR (n = 305) every 5 years provided a 95% probability of remaining free of STDR, screening every 2–3 years was also considered to be more appropriate.9 Younis and colleagues found that patients with T2DM with the longest duration of diabetes were more likely to progress to STDR.8 They also reported that longer duration of diabetes was an independent risk factor for the development of STDR in patients with T1DM (i.e., Relative Risk = 1.068).9
The purpose of the current study is to estimate safe screening intervals for the presence of STDR in patients with DM who are free of DR or who already have mild nonproliferative diabetic retinopathy (NPDR).
Methods
A 6-year retrospective follow-up study was conducted in the Department of Endocrinology of Cruces's Hospital (Vizcaya, Northern Spain). Funduscopic results of eyes from patients with DM referred by local general practitioners at primary care level were reviewed between 1998 and 2004. Patients who had retinopathy data available at the time they were first screened (baseline) and at least one subsequent screening visit were included in the study. Patients with laser treatment in at least one eye at baseline were excluded. Each screening visit had been planned to evaluate the presence and stage of retinopathy by means of a 45° nonmydriatic retinal camera with an instant film Polaroid® device (Canon® CR4-45NM). One fundus photograph centered on the macula had been taken of each eye.11 Age, sex, glycated hemoglobin level, type of DM, diabetes duration, and treatment of DM had been recorded at baseline into a questionnaire, as well as VA that had been measured for each eye separately using a Snellen chart at 6 m. Depending on age at diabetes onset, participants had been categorized as having younger onset (<30 years of age) or older onset (<30 years). Younger-onset participants were considered to have T1DM if they were also receiving insulin. Older-onset individuals who required insulin during the first postdiagnosis year were also considered to have T1DM. Type 2 DM was considered in patients with an older onset in the absence of insulin dependence during the first year after diagnosis. The duration of DM was defined as the interval between the first diagnosis of the disease by health personnel and the first screening exam for DR in the present study. The study was conducted in accordance with the 1983 Declaration of Helsinki.12
For the purpose of this study, stored Polaroid photographs were graded by the same retina specialist and classified as gradable or nongradable. Gradable images were labeled according to the following international scale that identifies five levels of DR:13 (1) no apparent retinopathy, (2) mild NPDR, (3) moderate NPDR, (4) severe NPDR, and (5) proliferative diabetic retinopathy (PDR). Gradable photographs were also classified according to two major levels of STDR: absent or present. Sight-threatening diabetic retinopathy was defined as DR requiring referral to an ophthalmologist according to the following screening threshold: moderate NPDR or more severe retinopathy and/or suspected macular edema (hard exudates within one disk diameter of the fovea associated with a VA less than 6/9 in the absence of another cause of reduced vision). Levels of disease for each patient are presented for the worst eye.
Data collection was done using a specially designed questionnaire and was carried out by a physician who was not involved in the ophthalmic care of these patients. Data from the questionnaire were entered into a portable computer IBM® ThinkPad 600 using a Microsoft Access® database program. The mean and the standard deviation of the data were given to describe continuous variables. Chi-square tests were used to compare frequencies among subgroups of patients. Nonparametric methods were used where appropriate.14 The life-table method was used to estimate the risk of reaching the outcome of interest by follow-up time intervals, and log-rank tests were performed to test for equivalence of groups.15 The screening interval that could be safely adopted for patients with DM for early detection of STDR (safe screening interval) was defined as the interval for an estimated probability of at least 95% of remaining free of STDR. Younis and colleagues (i.e., the Liverpool Diabetic Eye Study) made their recommendations “to give a minimum of 95% certainty of remaining free of STDR” in their published papers.8,9 Thus to gain comparability of our results, we used the same criteria. Calculation of probabilities was done using Stata® 8.0 for Windows (StataCorp, College Station, TX), and statistical significance was considered when p values were less than 0.05.
Results
The results in this report were based on analysis of 430 patients with DM with complete data collected after reviewing 1856 consecutive screening visits. A total of 286 diabetes patients free of DR and 144 patients with mild NPDR at baseline were included in the study. Table 1 describes patients' characteristics at baseline according to DR status (cohort free of DR and cohort with mild NPDR). The percentage of patients that had T2DM was significantly larger in the cohort with mild NPDR than in the cohort free of DR. The percentage of patients that were treated with insulin was over 90% in both cohorts. As expected, diabetes duration and level of glycated hemoglobin were also significantly larger in the cohort with mild NPDR at baseline. No patient was censored due to laser treatment after baseline. Six patients (two free of DR and four with mild NPDR at baseline) required laser treatment in one of their eyes afterward. Five of them reached the outcome of interest (STDR) after laser treatment.
Table 1.
Baseline Characteristics of Patients Included in the Study by Diabetic Retinopathy Status at Baseline
| Grade of DR | |||
|---|---|---|---|
| No DR (n = 286) | Mild NPDR (n = 144) | p value | |
| Sex (% female) | 48.9 | 50.0 | .837 |
| Type of DM (%) | |||
| Type 1 | 79.0 | 64.9 | <.01 |
| Type 2 | 21.0 | 35.1 | – |
| Insulin treatment (%) | 91.2 | 92.6 | .520 |
| Age (years)a | 52.2 ± 8.6 | 53.8 ± 9.2 | .083 |
| Diabetes duration (years)a | 10.1 ± 7.5 | 14.9 ± 8.3 | <.001 |
| Glycated hemoglobin level (%) | 7.9 ± 1.5 | 8.4 ± 1.5 | <.001 |
Mean ± standard deviation.
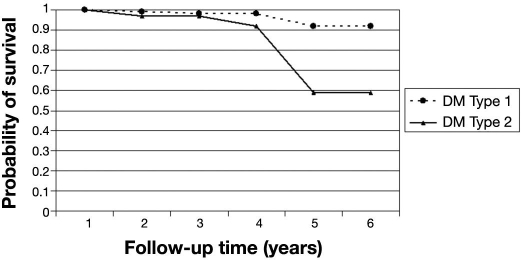
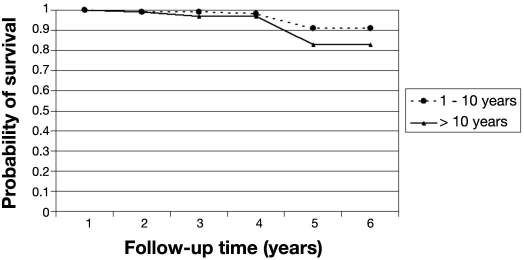
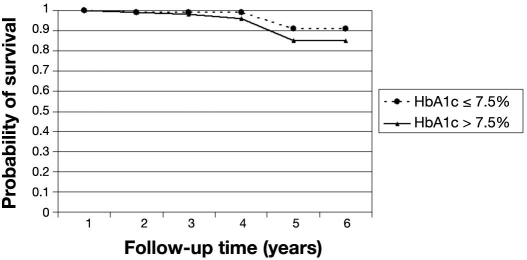
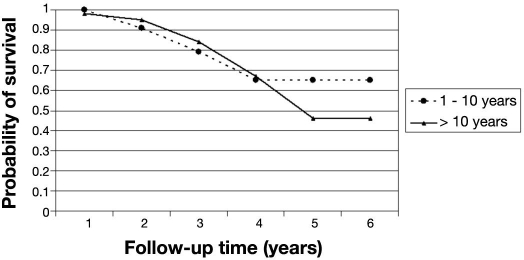
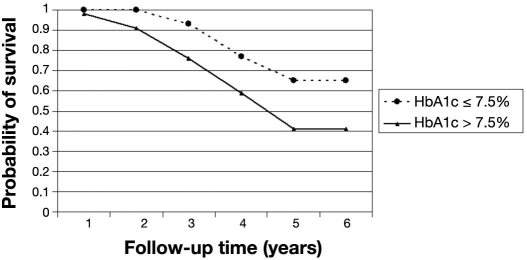
The probability of remaining free of STDR for patients free of DR at baseline was 97% (95% CI 94–99%) at the end of the fourth year (Table 2). After 4 years, the probability of STDR-free survival dropped to 87%. In this cohort of patients, those with T2DM were more likely to progress to STDR than those who had T1DM (p < .01). Patients who had T2DM had a probability of STDR-free survival of 92% (95% CI 70–98%) after the fourth year (Figure 1). The probability of STDR-free survival was not statistically significant (p = .247) between patients who had had diabetes for longer than 10 years compared to patients with shorter diabetes duration at baseline (Figure 2). Similar results were also found for patients with glycated hemoglobin level over 7.5% at baseline (p = .317) compared to those with lower levels (Figure 3).
Table 2.
Probability of Remaining Free of STDR Based on the Classification at Baseline (No DR or Mild NPDR)
| Time from baseline (years) | Number entering time interval | Number of patients reaching STDR | Probability of remaining free of STDR (95% CI) |
|---|---|---|---|
| No DR (n = 286) | |||
| 1 | 286 | 0 | 1.00 |
| 2 | 274 | 2 | 0.99 (0.96–0.99) |
| 3 | 224 | 1 | 0.98 (0.95–0.99) |
| 4 | 154 | 1 | 0.97 (0.94–0.99) |
| 5 | 82 | 6 | 0.87 (0.76–0.93) |
| 6 | 23 | 0 | 0.87 (0.76–0.93) |
| Mild NPDR (n = 144) | |||
| 1 | 144 | 1 | 0.99 (0.95–1.00) |
| 2 | 134 | 6 | 0.94 (0.88–0.97) |
| 3 | 92 | 10 | 0.81 (0.72–0.88) |
| 4 | 50 | 8 | 0.65 (0.52–0.76) |
| 5 | 24 | 4 | 0.49 (0.33–0.65) |
| 6 | 5 | 0 | 0.49 (0.33–0.65) |
Figure 1.
Probability of STDR-free survival by type of DM. Cohort of diabetes patients free of retinopathy at baseline (n = 286).
Figure 2.
Probability of STDR-free survival by diabetes duration. Cohort of diabetes patients free of retinopathy at baseline (n = 286).
Figure 3.
Probability of STDR-free survival by glycated hemoglobin level. Cohort of diabetes patients free of retinopathy at baseline (n = 286). HbA1c, glycated hemoglobin level.
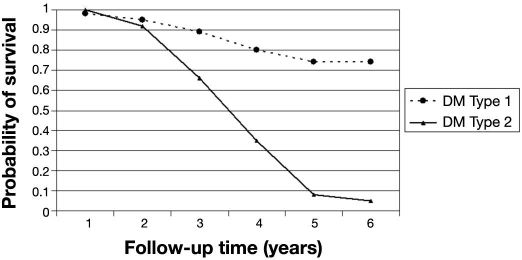
For patients with mild NPDR at baseline, the probability of remaining free of STDR was 99% (95% CI 95–100%) at the end of the first year of follow-up and dropped to 94% (95% CI 88–97%) at the end of the second year (Table 2). Figure 4 shows the relationship between probability of STDR-free survival and type of diabetes in this cohort of patients. Patients who had T2DM had a probability of STDR-free survival of 92% (95% CI 78–97%) at the end of the second year compared to the probability of 95% (95% CI 87–98%) in those who had T1DM (p < .001). The probability of STDR-free survival was not significantly different (p = .945) between those patients who had had DM for longer that 10 years compared to those patients with shorter diabetes duration at baseline (Figure 5). Different results were found for patients with glycated hemoglobin level over 7.5% at baseline compared to those with lower levels (Figure 6), because the probability of remaining free of STDR was still 100% at the end of the second year for those with a glycated hemoglobin level less or equal to 7.5% at baseline (p < .05).
Figure 4.
Probability of STDR-free survival by type of DM. Cohort of diabetes patients with mild NPDR at baseline (n = 144).
Figure 5.
Probability of STDR-free survival by diabetes duration. Cohort of diabetes patients with mild NPDR at baseline (n = 144).
Figure 6.
Probability of STDR-free survival by glycated hemoglobin level. Cohort of diabetes patients with mild NPDR at baseline (n = 144). HbA1c, glycated hemoglobin level.
The screening interval for at least a 95% estimated probability of remaining free of STDR for no baseline retinopathy was 4 years for those who had T1DM and 3 years for those who had T2DM. For patients with mild NPDR at baseline, the screening interval for at least a 95% estimated probability of STDR-free survival was 1 year, but it rose to 2 years in those who had T1DM or in those with a level of glycated hemoglobin less or equal to 7.5%.
Discussion
Little research has been done on the screening intervals that could be safely adopted for patients with DM for early detection of STDR. Most of the published studies have examined the incidence and risk factors for progression of DR.16–30 A review of the 10-year screening for DR in Iceland between 1995 and 2005 revealed that screening every other year seems to be safe in patients without retinopathy.31 Although the present study differed from those reported by Younis and colleagues on retinopathy classification (mild NPDR and moderate NPDR in the present study would have been included in their background retinopathy definition), our results from patients free of DR at baseline might be compared to theirs.8,9 It has been recognized that long screening intervals may lead to difficulties in maintaining contact with patients.32,33 In the present study, the screening interval that could be safely adopted for early detection of STDR for patients with DM with no baseline retinopathy was estimated to be 4 years for those who had T1DM and 3 years for those who had T2DM. The slightly shorter screening intervals estimated by our study for patients with diabetes free of DR may be explained by the higher average diabetes duration of our patients at baseline (10.1 years) compared that reported by Younis and colleagues (7.8 years in T1DM and 3.0 years in T2DM).8,9
In the present study, we did not find a statistically significant association between duration of diabetes at baseline and the probability of survival free of STDR, in contrast to cross-sectional studies that have shown an increased prevalence of retinopathy with longer diabetes duration.3,34–37 For patients with mild NPDR, our study revealed a positive association between metabolic control of DM and STDR. A significantly lower probability of survival was found for patients with mild NPDR and glycated hemoglobin level over 7.5% at baseline. Therefore, diabetes patients with mild NPDR and glycated hemoglobin levels less than or equal to 7.5% may be more protected from progression to STDR. Our findings are consistent with those previously reported by the U.K. Prospective Diabetes Study that showed that intensive blood glucose control decreased the risk of microvascular complications in patients with T2DM.38 Younis and colleagues did not report on metabolic control of DM, because glycated hemoglobin was not measured routinely in retinopathy screening programs in the United Kingdom, especially in primary care.8,9
One potential limitation of this study may be that the photographic procedure used for screening had a sensitivity of 91.9% and a specificity of 89.7% (i.e., the study designed to assess its validity compared a 45° nonmydriatic retinal camera with a Polaroid instant film versus biomicroscopy with a 78D lens and reverse image ophthalmoscope as the standard method).11 Even though some deviation from the true incidence of STDR is expected (i.e., sensitivity and specificity of the screening procedure was less than 100%), the potential for bias seems to be low, because both parameters are close to 90%. Other studies assessing the validity of Polaroid photography compared to digital imaging suggested that nonmydriatic retinal photography using Polaroid film could be as effective as digital imaging.39 Besides, our definition of macular edema is different from the U.S. standard of clinically significant macular edema. We considered “suspected macular edema” (i.e., presence of hard exudates within one disk diameter of the fovea associated with a VA less than 6/9 in the absence of another cause of reduced vision). We did not consider swelling, because stereo vision of the macula (i.e., by ophthalmoscopy or stereo fundus images) would have been needed to determine its presence.
Conclusions
In summary, although the optimum frequency of screening may still remain controversial, this study provides some evidence that recommendations to patients with DM may be given on the basis of their risk. Therefore, changing the frequency of screening for diabetes patients with no retinopathy from 1 to 3–4 years may not have a detrimental effect on early detection of STDR, while possibly reducing screening costs. The ability to generalize the results from the present study to other screening situations will depend on the comparability with the populations being screened, as well as the validity of the method used to detect STDR.
Further research using prospective data from larger studies would be required to fully characterize optimal screening intervals before final recommendations can be made.
Abbreviations
- CI
confidence interval
- DM
diabetes mellitus
- DR
diabetic retinopathy
- NPDR
nonproliferative diabetic retinopathy
- PDR
proliferative diabetic retinopathy
- STDR
sight-threatening diabetic retinopathy
- T1DM
type 1 DM
- T2DM
Type 2 DM
- VA
visual acuity
References
- 1.Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81(4):383–396. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- 2.Witkin SR, Klein R. Ophthalmologic care for persons with diabetes. JAMA. 1984;251(19):2534–2537. [PubMed] [Google Scholar]
- 3.Soto-Pedre E, Hernaez-Ortega MC, Piniés JA. Duration of diabetes and screening coverage for retinopathy among patients with type 2 diabetes. Ophthalmic Epidemiol. 2007;14(2):76–79. doi: 10.1080/09286580600879032. [DOI] [PubMed] [Google Scholar]
- 4.Soto-Pedre E, Hernaez-Ortega MC. Screening coverage for diabetic retinopathy using a three-field digital non-mydriatic fundus camera. Prim Care Diabetes. 2008;2(3):141–146. doi: 10.1016/j.pcd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Stefánsson E, Bek T, Porta M, Larsen N, Kristinsson JK, Agardh E. Screening and prevention of diabetic blindness. Acta Ophthalmol Scand. 2000;78(4):374–385. doi: 10.1034/j.1600-0420.2000.078004374.x. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association Clinical Practice Recommendations 2001. Diabetes Care. 2001;24(Suppl 1):S1–S133. [PubMed] [Google Scholar]
- 7.Retinopathy Working Party. A protocol for screening for diabetic retinopathy in Europe. Diabet Med. 1991;8(3):263–267. [PubMed] [Google Scholar]
- 8.Younis N, Broadbent DM, Vora JP, Harding SP. Liverpool Diabetic Eye Study. Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet. 2003;361(9353):195–200. doi: 10.1016/s0140-6736(03)12267-2. [DOI] [PubMed] [Google Scholar]
- 9.Younis N, Broadbent DM, Harding SP, Vora JP. Incidence of sight threatening retinopathy in type 1 diabetes in a systematic screening programme. Diabet Med. 2003;20(9):758–765. doi: 10.1046/j.1464-5491.2003.01035.x. [DOI] [PubMed] [Google Scholar]
- 10.Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA. 2000;283(7):889–896. doi: 10.1001/jama.283.7.889. [DOI] [PubMed] [Google Scholar]
- 11.Hernáez-Ortega MC, Soto-Pedre E, Vázquez JA, Gutiérrez MA, Asúa J. Estudio de la eficiencia de una cámara de retina no-midriática en el diagnóstico de retinopatía diabética. Rev Clin Esp. 1998;198(4):194–199. [PubMed] [Google Scholar]
- 12.World Medical Association. Ferney-Voltaire: World Medical Association; 2004. Declaration of Helsinki: ethical principles for research involving human subjects (as amended in Tokyo) [Google Scholar]
- 13.Wilkinson CP, Ferris FL, III, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT. Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular oedema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 14.Snedecor GW, Cochran WG. 8th ed. Ames: Iowa State University Press; 1989. Statistical methods. [Google Scholar]
- 15.Cox DR, Oakes D. Analysis of survival data. London: Chapman & Hall; 1984. [Google Scholar]
- 16.Klein R, Meuer SM, Moss SE, Klein BE. Retinal microaneurysm counts and 10-year progression of diabetic retinopathy. Arch Ophthalmol. 1995;113(11):1386–1391. doi: 10.1001/archopht.1995.01100110046024. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105(10):1801–1815. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Krzentowski G, Albert A, Lefebvre PJ. Risk of developing retinopathy in Diabetes Control and Complications Trial type 1 diabetic patients with good or poor metabolic control. Diabetes Care. 2001;24(7):1275–1279. doi: 10.2337/diacare.24.7.1275. [DOI] [PubMed] [Google Scholar]
- 19.Porta M, Sjoelie AK, Chaturvedi N, Stevens L, Rottiers R, Veglio M, Fuller JH. EURODIAB Prospective Complications Study Group. Risk factors for progression to proliferative diabetic retinopathy in the EURODIAB Prospective Complications Study. Diabetologia. 2001;44(12):2203–2209. doi: 10.1007/s001250100030. [DOI] [PubMed] [Google Scholar]
- 20.Keen H, Lee ET, Russell D, Miki E, Bennett PH, Lu M. The appearance of retinopathy and progression to proliferative retinopathy: the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S22–S30. doi: 10.1007/pl00002935. [DOI] [PubMed] [Google Scholar]
- 21.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 22.Lövestam-Adrian M, Agardh CD, Torffvit O, Agardh E. Diabetic retinopathy, visual acuity, and medical risk indicators: a continuous 10-year follow-up study in type 1 diabetic patients under routine care. J Diabetes Complications. 2001;15(6):287–294. doi: 10.1016/s1056-8727(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 23.Henricsson M, Nyström L, Blohmé G, Ostman J, Kullberg C, Svensson M, Schölin A, Arnqvist HJ, Björk E, Bolinder J, Eriksson JW, Sundkvist G. The incidence of retinopathy 10 years after diagnosis in young adult people with diabetes: results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS) Diabetes Care. 2003;26(2):349–354. doi: 10.2337/diacare.26.2.349. [DOI] [PubMed] [Google Scholar]
- 24.Looker HC, Krakoff J, Knowler WC, Bennett PH, Klein R, Hanson RL. Longitudinal studies of incidence and progression of diabetic retinopathy assessed by retinal photography in Pima Indians. Diabetes Care. 2003;26(2):320–326. doi: 10.2337/diacare.26.2.320. [DOI] [PubMed] [Google Scholar]
- 25.van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Polak BC. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol. 2003;121(2):245–251. doi: 10.1001/archopht.121.2.245. [DOI] [PubMed] [Google Scholar]
- 26.Sloan FA, Brown DS, Carlisle ES, Ostermann J, Lee PP. Estimates of incidence rates with longitudinal claims data. Arch Ophthalmol. 2003;121(10):1462–1468. doi: 10.1001/archopht.121.10.1462. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM. U.K. Prospective Diabetes Study Group. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122(11):1631–1640. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- 28.Tapp RJ, Zimmet PZ, Harper CA, McCarty DJ, Chitson P, Tonkin AM, Söderberg S, Taylor HR, Alberti KG, Tuomilehto J, Shaw JE. Six year incidence and progression of diabetic retinopathy: results from the Mauritius diabetes complication study. Diabetes Res Clin Pract. 2006;73(3):298–303. doi: 10.1016/j.diabres.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Roy MS, Affouf M. Six-year progression of retinopathy and associated risk factors in African American patients with type 1 diabetes mellitus: the New Jersey 725. Arch Ophthalmol. 2006;124(9):1297–1306. doi: 10.1001/archopht.124.9.1297. [DOI] [PubMed] [Google Scholar]
- 30.Tam VH, Lam EP, Chu BC, Tse KK, Fung LM. Incidence and progression of diabetic retinopathy in Hong Kong Chinese with type 2 diabetes mellitus. J Diabetes Complications. 2009;23(3):185–193. doi: 10.1016/j.jdiacomp.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Ólafsdóttir E, Stefánsson E. Biennal eye screening in patients with diabetes without retinopathy: 10-year experience. Br J Ophthalmol. 2007;91(12):1599–1601. doi: 10.1136/bjo.2007.123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fong DS, Gottlieb J, Ferris FL, III, Klein R. Understanding the value of diabetic retinopathy screening. Arch Ophthalmol. 2001;119(5):758–760. doi: 10.1001/archopht.119.5.758. [DOI] [PubMed] [Google Scholar]
- 33.Klein R. Screening interval for retinopathy in type 2 diabetes. Lancet. 2003;361(9353):190–191. doi: 10.1016/S0140-6736(03)12317-3. [DOI] [PubMed] [Google Scholar]
- 34.National Diabetes Data Group. Diabetes in America. 2nd ed. Bethesda: National Institutes of Health; 1995. [Google Scholar]
- 35.McKay R, McCarty CA, Taylor HR. Diabetic retinopathy in Victoria, Australia: the Visual Impairment Project. Br J Ophthalmol. 2000;84(8):865–870. doi: 10.1136/bjo.84.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tapp RJ, Shaw JE, Harper CA, de Courten MP, Balkau B, McCarty DJ, Taylor HR, Welborn TA, Zimmet PZ. AusDiab Study Group. The prevalence of and factors associated with diabetic retinopathy in the Australian Population. Diabetes Care. 2003;26(6):1731–1737. doi: 10.2337/diacare.26.6.1731. [DOI] [PubMed] [Google Scholar]
- 37.Varma R, Torres M, Peña F, Klein R, Azen SP. Los Angeles Latino Eye Study Group. Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111(7):1298–1306. doi: 10.1016/j.ophtha.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 38.U.K. Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 39.Phiri R, Keeffe JE, Harper CA, Taylor HR. Comparative study of the Polaroid and digital non-mydriatic cameras in the detection of referrable diabetic retinopathy in Australia. Diabet Med. 2006;23(8):867–872. doi: 10.1111/j.1464-5491.2006.01824.x. [DOI] [PubMed] [Google Scholar]