Abstract
Hemoglobin A1c (HbA1c) is widely used as an index of mean glycemia, a measure of risk for the development of diabetes complications, and a measure of the quality of diabetes care. Emerging literature suggests that, although HbA1c levels change little over time within persons without diabetes, they vary considerably among individuals, suggesting that factors other than glycemia may impact HbA1c. Racial and ethnic differences in HbA1c have been described that do not appear to be explained by differences in glycemia. It is imperative that the nonglycemic factors that affect HbA1c be more clearly defined. Even more important, it must be determined whether differences among individuals or groups correlate with susceptibility to complications or merely reflect variation in hemoglobin glycation.
Keywords: diabetes, ethnicity, hemoglobin A1c, race
Introduction
Hemoglobin is found in erythrocytes and is critical for normal oxygen delivery to tissues. It is a tetramer composed of two α (141 amino acid) and two β (146 amino acid) globin chains that bind four heme groups, each containing one iron atom. Glycated hemo-globin or hemoglobin A1 represents a post-translational modification of hemoglobin A formed by the covalent attachment of glucose or other sugars to hemoglobin. Hemoglobin A1c (HbA1c) is formed by glucose attachment to the N-terminal valine of the β globin chain.
A number of clinically important hemoglobin abnormalities have been described, including the structural hemoglobinopathies, in which mutations alter the amino acid sequence of the globin chain and alter its physiologic properties; the thalassemia syndromes, in which mutations impair globin biosynthesis and result in hypochromia and microcytosis; and the acquired hemoglobinopathies, in which hemoglobin is chemically altered. Examples of acquired hemoglobinopathies include carboxyhemoglobin, associated with cigarette smoking; carbamylhemoglobin, associated with uremia; acetylhemoglobin, associated with the consumption of large amounts of aspirin; and methemoglobin, associated with carbon monoxide poisoning.
In 1958, Allen and colleagues first described hemoglobin A1 as one of the forms of hemoglobin that could be separated by cation-exchange chromatography.1 In 1968, Rahbar recognized that HbA1c represented a glycated form of hemoglobin that was increased in diabetes,2 and in 1976, Koenig and colleagues suggested that, because HbA1c is formed slowly and nonenzymatically at a rate directly proportional to the ambient glucose concentration, it might be a useful indicator of glucose tolerance or glucose regulation in diabetes.3,4 Currently, HbA1c is widely accepted as a index of mean glycemia, a measure of risk for the development of diabetes complications, and a measure of the quality of diabetes care.
A number of different approaches have been used to measure HbA1c in clinical practice. These include ion-exchange high-performance liquid chromatography, immunoassay, boronate affinity, and enzymatic methods. In an effort to standardize these methods and to reduce the variation among them, the American Association for Clinical Chemistry established the National Glycohemoglobin Standardization Program in 1996.5 The purpose was to standardize glycohemoglobin test results to Diabetes Control and Complications Trial equivalent HbA1c values. Each year, the College of American Pathologists assesses the comparability of values within and between methods at HbA1c values.5 This initiative has resulted in substantial improvement in the comparability of assay methods and has enhanced reliability and precision. Nevertheless, it is clear that a number of factors, including structural hemoglobinopathies, thalassemia syndromes, and chemical alterations of hemoglobin, can either falsely lower HbA1c test results or raise HbA1c test results independent of glycemia.
Any condition that decreases mean erythrocyte age will falsely lower HbA1c test results regardless of the assay method used.6 Examples of such conditions include hemolytic anemia and recovery from acute blood loss. The impact of structural hemoglobinopathies and thalassemia syndromes on HbA1c test results depend on the pathologic processes involved and the assay method employed. Hemoglobin S trait, which effects approximately 8% of African Americans, hemoglobin C trait, which effects approximately 3% of African Americans, and hemoglobin E trait, which effects 10% to more than 50% of Southeast Asians in California, are all reported to effect some HbA1c assay methods.7 Elevated hemoglobin F, which is associated with thalassemia syndromes, also effects some assay methods.7 Uremia, hyperbilirubinemia, hypertriglyceridemia, chronic alcoholism, chronic ingestion of salicylates, vitamin C ingestion, and opiate addiction have all been reported to interfere with some assay methods, falsely increasing results.7 In some assays, vitamin C and vitamin E ingestion have also been reported to falsely lower HbA1c test results.7 Iron deficiency, which effects up to 20% of menstruating women8 and many pregnant women, has been reported to increase HbA1c test results by altering the structure of the hemoglobin molecule and making it easier to glycate.9 When interferences are recognized, alternative forms of testing, such as glycated serum protein testing (fructosamine or glycated albumin), may be employed to assess glycemia. Unfortunately, factors affecting the accuracy of HbA1c measurement may not be recognized clinically.
The American Diabetes Association, the European Association for the Study of Diabetes, and the International Diabetes Federation sponsored a study called the A1c-Derived Average Glucose (ADAG) Study to define the relationship between HbA1c and mean blood glucose.10 An international group of investigators from ten clinical centers recruited 268 type 1 and 159 type 2 diabetes patients and 80 nondiabetes control patients. Volunteers with conditions that might interfere with measurement of HbA1c, including anemia, reticulocytosis, blood loss and/or transfusions, erythropoietin treatment, hemoglobinopathies, chronic liver or renal disease, and high-dose vitamin C treatment, were excluded. Hemoglobin A1c was measured centrally at baseline and every month for 3 months. In addition, continuous glucose monitoring and 7-point self-monitoring profiles were performed for 2 days every month and 7-point daily self-monitoring of blood glucose was performed 3 days per week. At the end of the study, the mathematical relationship between HbA1c and average glucose was explored. Linear regression analysis between the HbA1c and average glucose values provided good correlation (R2 = 0.84), allowing calculation of an estimated average glucose for HbA1c values. Table 1 illustrates this relationship. Although the overall relationship was strong, there were mismatches between peripheral blood measurements and HbA1c on an individual subject basis that could lead to erroneous conclusions. (See the study by Chalew and associates in this issue of Journal of Diabetes Science and Technology.)
Table 1.
Estimated Average Glucose
| HbA1c (%) | mg/dla |
|---|---|
| 5 | 97 (76–120) |
| 6 | 126 (100–152) |
| 7 | 154 (123–185) |
| 8 | 183 (147–217) |
| 9 | 212 (170–249) |
| 10 | 240 (193–282) |
| 11 | 269 (217–314) |
| 12 | 298 (240–347) |
Linear regression estimated average glucose (mg/dl) = 28.7 × HbA1c – 46,7. Data in parentheses are 95% confidence intervals.
Despite recognition of known assay interferences, initiatives to enhance assay standardization, and studies to define the relationship between mean blood glucose and HbA1c, emerging literature suggests that factors other than glycemia may contribute to the variance in HbA1c across individuals. The seminal observation was that, although HbA1c levels change little over time within persons without diabetes, they vary considerably among individuals.11–13 Among individuals without diabetes, only approximately one-third of the variance in HbA1c levels is explained on the basis of measures of glycemia.11 This observation suggests that other factors must operate to produce variation in HbA1c levels. Some factors proposed to be associated with variation in HbA1c independent of glycemia include female sex,14 sex hormones,15 visceral fat distribution,16 and genetics.17,18
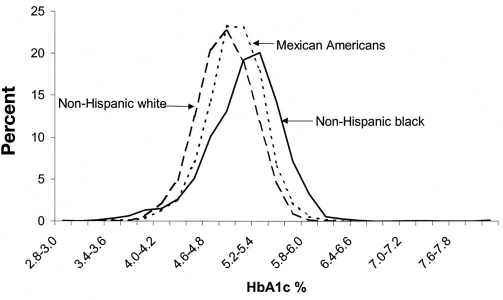
Racial and ethnic differences in HbA1c have been described in nondiabetes populations that do not appear to be explained by differences in glycemia. Saaddine and coworkers described HbA1c levels among young participants without diabetes in the National Health and Nutrition Examination Survey-3 (NHANES-3) before and after adjusting for age, sex, education, and weight.19 They found different HbA1c distributions among non-Hispanic white, Mexican American, and non-Hispanic black populations with shifts of the distributions for both Mexican Americans and non-Hispanic blacks to higher levels than those for whites (Figure 1). Eberhardt and colleagues analyzed data from a community-based sample of 3175 adults without diabetes in the South Carolina Cardiovascular Disease Prevention Project.20 After adjusting for age and body mass index (BMI), HbA1c remained 0.3% and 0.4% higher in black men and women with no reported diabetes compared to white men and women with no reported diabetes (p < .05). Additional studies comparing HbA1c levels across racial and ethnic groups with type 2 diabetes have generally demonstrated higher HbA1c levels among African Americans, Hispanics, and Asian/Pacific Islanders compared to non-Hispanic whites.21 Studies that have compared HbA1c levels across racial and ethnic groups within organized systems of health care and have carefully adjusted for processes of care have demonstrated persistent though attenuated differences in HbA1c.22–28
Figure 1.
Hemoglobin A1c distribution by ethnicity in U.S. children and young adults ages 5–24 NHANES–3, 1988–1994.
Hemoglobin A1c has been described by race and ethnicity among patients with impaired glucose tolerance enrolled in the Diabetes Prevention Program (DPP).29 There were 3819 individuals eligible to participate in the DPP who were ≥25 years of age and had BMI ≥ 24 kg/m2 (≥22 kg/m2 for Asians). Fasting plasma glucose was between 95 and 125 mg/dl (<125 mg/dl for American Indians), and 2 h plasma glucose was between 140 and 199 mg/dl. Hemoglobin A1c levels were higher among blacks, Hispanics, American Indians, and Asian Americans compared to whites both before and after using multiple linear regression to adjust for factors that differed among groups and might affect glycemia. These included age, sex, education, marital status, adiposity (BMI and waist circumference), blood pressure, fasting and postload glucose, glucose area under the curve, β-cell function, insulin resistance, and hematocrit. Table 2 summarizes these results. In fully adjusted models, up to 8% of the variance in HbA1c was associated with race or ethnicity, a proportion similar to that explained by age and fasting glucose level.
Table 2.
Differences in HbA1c by Race and Ethnicity among Patients with Impaired Glucose Tolerance in the Diabetes Prevention Program
| White | Black | Hispanic | American Indian | Asian | |
|---|---|---|---|---|---|
| n | 2117 | 752 | 609 | 174 | 167 |
| HbA1c (%) | 5.80 ± 0.44 | 6.19 ± 0.59 | 5.89 ± 0.46 | 5.96 ± 0.46 | 5.96 ± 0.45 |
| Adjusted HbA1c (%)a | 5.78 | 6.18 | 5.93 | 6.12 | 6.00 |
Adjusted for age; sex; education; marital status; BMI; waist circumference; blood pressure; fasting, 30 min, and 120 min plasma glucose; glucose area under the curve; fasting insulin; corrected insulin response; homeostasis model assessment of insulin resistance; and hematocrit. All differences p < .0001 adjusted for multiple comparison. Results were not changed using common criteria for BMI and fasting plasma glucose.
In another analysis of patients with recent-onset, drug-na age, sex, and BMI was significantly higher among blacks and Hispanics compared to whites, despite similar fasting plasma glucose levels and similar or lower postglucose load glucose levels.30 In another study of type 2 diabetes patients failing oral antidiabetes therapies, it was found that HbA1c levels were significantly higher in African Americans, Hispanics, Asians, and other races and ethnicities compared to whites before and after adjusting for age, gender, BMI, duration of diabetes, oral medication use, mean fasting plasma glucose, mean postprandial glucose, insulin resistance, and β-cell function.31 In the ADAG Study, although there were no significant differences in the slope or intercept for the regression equations for any of the subgroup comparisons, including age, sex, type of diabetes, or smoking status, there was a suggestion (p = .07) that the regression line was different for African Americans compared to Caucasians, such that for a given mean glucose level, African Americans had a slightly higher HbA1c level.10 A study in children with type 1 diabetes found substantial variation in the relationship between mean glucose and HbA1c and questioned the advisability of transforming HbA1c into calculated mean glucose values.32
The reasons for these racial and ethnic differences in HbA1c remain to be explained. Heritable differences in intraerythrocyte 2, 3-diphosphoglycerate, which catalyzes production of HbA1c,33 and intraerythrocyte fructosemine 3-kinase, which deglycates intracellular fructosemines,34 have been proposed to account for interindividual and intergroup variation in HbA1c. Their role has not, however, been established. Indeed, since fructosemine 3-kinase mediates deglycation at amine side chains such as on lysine, it is unlikely to affect HbA1c, which involves glycation of an N-terminal valine.
Enthusiasm is growing for greater use of HbA1c for both screening and diagnosis of diabetes.35 Although HbA1c provides an integrated measure of glycemia that is less susceptible to short-term modulation than fasting glucose and is useful for tracking therapy within individuals with diabetes, it is imperative that the nonglycemic factors that affect assays be more clearly defined. Additional studies are needed to determine which factors account for intraindividual variability in HbA1c and for differences among racial and ethnic groups. Even more important, it must be determined whether differences among individuals and groups correlate with susceptibility to complications or merely variation in hemoglobin glycation.
Acknowledgments
This work was supported by the Michigan Diabetes Research and Training Center, funded by DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- ADAG
A1c-derived average glucose
- BMI
body mass index
- DPP
Diabetes Prevention Program
- HbA1c
hemoglobin A1c
References
- 1.Allen DW, Schroeder WA, Balog J. Observations on the chromatographic heterogeneity of normal adult and fetal human hemogloba study of the effects of crystallization and chromatography on the heterogeneity and isoleucine content. J Am Chem Soc. 1958;80(7):1628–1634. [Google Scholar]
- 2.Rajbar S. An abnormal hemoglobin in red cells of diabetics. Clin Chem Acta. 1968;22(2):296–298. doi: 10.1016/0009-8981(68)90372-0. [DOI] [PubMed] [Google Scholar]
- 3.Koenig RJ, Peterson CM, Kilo C, Cerami A, Williamson JR. Hemoglobin A1c as an indicator of the degree of glucose intolerance in diabetes. Diabetes. 1976;25(3):230–232. doi: 10.2337/diab.25.3.230. [DOI] [PubMed] [Google Scholar]
- 4.Koenig RJ, Peterson CM, Jones RL, Saudek C, Lehrman M, Cerami A. Correlation of glucose regulation and hemoglobin A1c in diabetes mellitus. N Engl J Med. 1976;295(8):417–420. doi: 10.1056/NEJM197608192950804. [DOI] [PubMed] [Google Scholar]
- 5. National Glycohemoglobin Standardization Program http://www.ngsp.org/
- 6.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM. Tests of glycemia in diabetes. Diabetes Care. 1995;18(6):896–909. doi: 10.2337/diacare.18.6.896. [DOI] [PubMed] [Google Scholar]
- 7.National Glycohemoglobin Standardization Program. Factors that interfere with GHB (HbA1c) test results. Updated 4/08. http://www.ngsp.org/prog/factors.htm.
- 8.Cook JD, Finch CA, Smith NJ. Evaluation of the iron status of a population. Blood. 1976;48(3):449–455. [PubMed] [Google Scholar]
- 9.Coban E, Ozdogan M, Timuragaoglu A. Effect of iron deficiency anemia on the levels of hemoglobin A1c in nondiabetic patients. Acta Haematol. 2004;112(3):126–128. doi: 10.1159/000079722. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yudkin JS, Forrest RD, Jackson CA, Ryle AJ, Davie S, Gould BJ. Unexplained variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Diabetologia. 1990;33(4):208–215. doi: 10.1007/BF00404798. [DOI] [PubMed] [Google Scholar]
- 12.The DCCT Research Group. Diabetes Control and Complications Trial (DCCT): results of a feasibility study. Diabetes Care. 1987;10(1):1–19. doi: 10.2337/diacare.10.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Meigs JB, Nathan DM, Cupples LA, Wilson PW, Singer DE. Tracking of glycated hemoglobin in the original cohort of the Framingham Heart Study. J Clin Epidemiol. 1996;49(4):411–417. doi: 10.1016/0895-4356(95)00513-7. [DOI] [PubMed] [Google Scholar]
- 14.Strickland MH, Paton RC, Wales JK. Hemoglobin A1c concentrations in men and women with diabetes. Br Med J. 1984;289:733. doi: 10.1136/bmj.289.6447.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalish GM, Barrett-Connor E, Laughlin GA, Gulanski BI. Postmenopausal Estrogen/Progestin Intervention Trial. Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. J Clin Endocrinol Metab. 2003;88(4):1646–1652. doi: 10.1210/jc.2002-021375. [DOI] [PubMed] [Google Scholar]
- 16.Araneta MR, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and white women. Obes Res. 2005;13(8):1458–1465. doi: 10.1038/oby.2005.176. [DOI] [PubMed] [Google Scholar]
- 17.Snieder H, Sawtell PA, Ross L, Walker J, Spector TD, Leslie RD. HbA(1c) levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes. 2001;50(12):2858–2863. doi: 10.2337/diabetes.50.12.2858. [DOI] [PubMed] [Google Scholar]
- 18.Cohen RM, Snieder H, Lindsell CJ, Beyan H, Hawa MI, Blinko S, Edwards R, Spector TD, Leslie RD. Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care. 2006;29(8):1739–1743. doi: 10.2337/dc06-0286. [DOI] [PubMed] [Google Scholar]
- 19.Saaddine JB, Fagot-Campagna A, Rolka D, Narayan KM, Geiss L, Eberhardt M, Flegal KM. Distribution of HbA(1c) levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Study. Diabetes Care. 2002;25(8):1326–1330. doi: 10.2337/diacare.25.8.1326. [DOI] [PubMed] [Google Scholar]
- 20.Eberhardt MS, Lackland DT, Wheeler FC, German RR, Teutsch SM. Is race related to glycemic control? An assessment of glycosylated hemoglobin in two South Carolina communities. J Clin Epidemiol. 1994;47(10):1181–1189. doi: 10.1016/0895-4356(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 21.Kirk JK, Bell RA, Bertoni AG, Arcury TA, Quandt SA, Goff DC, Jr, Narayan KM. Ethnic disparities: control of glycemia, blood pressure, and LDL cholesterol among US adults with type 2 diabetes. Ann Pharmacother. 2005;39(9):1489–1501. doi: 10.1345/aph.1E685. [DOI] [PubMed] [Google Scholar]
- 22.Summerson JH, Konen JC, Dignan MB. Race-related differences in metabolic control among adults with diabetes. South Med J. 1992;85(10):953–956. doi: 10.1097/00007611-199210000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Wisdom K, Fryzek JP, Havstad SL, Anderson RM, Dreiling MC, Tilley BC. Comparison of laboratory test frequency and test results between African-Americans and Caucasians with diabetes: opportunity for improvement. Findings from a large urban health maintenance organization. Diabetes Care. 1997;20(6):971–977. doi: 10.2337/diacare.20.6.971. [DOI] [PubMed] [Google Scholar]
- 24.Gary TL, McGuire M, McCauley J, Brancati FL. Racial comparisons of health care and glycemic control for African American and white diabetic adults in an urban managed care organization. Dis Manag. 2004;7(1):25–34. doi: 10.1089/109350704322918970. [DOI] [PubMed] [Google Scholar]
- 25.Brown AF, Gerzoff RB, Karter AJ, Gregg E, Safford M, Waitzfelder B, Beckles GL, Brusuelas R, Mangione CM, the TRIAD Study Group Health behaviors and quality of care among Latinos with diabetes in managed care. Am J Public Health. 2003;93(10):1694–1698. doi: 10.2105/ajph.93.10.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown AF, Gregg EW, Stevens MR, Karter AJ, Weinberger M, Safford MM, Gary TL, Caputo DA, Waitzfelder B, Kim C, Beckles GL. Race, ethnicity, socioeconomic position, and quality of care for adults with diabetes enrolled in manage care: the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care. 2005;28(12):2864–2870. doi: 10.2337/diacare.28.12.2864. [DOI] [PubMed] [Google Scholar]
- 27.Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the Health and Retirement Study. Arch Intern Med. 2007;167(17):1853–1860. doi: 10.1001/archinte.167.17.1853. [DOI] [PubMed] [Google Scholar]
- 28.Adams AS, Trinacty CM, Zhang F, Kleinman K, Grant RW, Meigs JB, Soumerai SB, Ross-Degnan D. Medication adherence and racial differences in A1C control. Diabetes Care. 2008;31(5):916–921. doi: 10.2337/dc07-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E. Diabetes Prevention Program Research Group. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453–2457. doi: 10.2337/dc06-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viberti G, Lachin J, Holman R, Zinman B, Haffner S, Kravitz B, Heise MA, Jones NP, O'Neill MC, Freed MI, Kahn SE, Herman WH ADOPT Study Group. A Diabetes Outcome Progression Trial (ADOPT): baseline characteristics of type 2 diabetic patients in North America and Europe. Diabet Med. 2006;23(12):1289–1294. doi: 10.1111/j.1464-5491.2006.02022.x. [DOI] [PubMed] [Google Scholar]
- 31.Herman WH, Dungan KM, Wolffenbuttel BH, Buse JB, Fahrbach JL, Jiang H, Martin S. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(5):1689–1694. doi: 10.1210/jc.2008-1940. [DOI] [PubMed] [Google Scholar]
- 32.Diabetes Research in Children Netword (DirecNet) Study Group, Wilson DM, Kollman. Relationship of A1C to glucose concentrations in children with type 1 diabetes: assessments by high-frequency glucose determinations by sensors. Diabetes Care. 2008;31(3):381–385. doi: 10.2337/dc07-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould BJ, Davie SJ, Yudkin JS. Investigation of the mechanism underlying the variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Clin Chim Acta. 1997;260(1):49–64. doi: 10.1016/s0009-8981(96)06508-4. [DOI] [PubMed] [Google Scholar]
- 34.Delpierre G, Collard F, Fortpied J, Van Schaftingen E. Fructosamine 3-kinase is involved in an intracellular deglycation pathway in human erythrocytes. Biochem J. 2002;365(Pt 3):801–808. doi: 10.1042/BJ20020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008;93(7):2447–2453. doi: 10.1210/jc.2007-2174. [DOI] [PubMed] [Google Scholar]