Abstract
The pulsatile secretion of gonadotropin-releasing hormone (GnRH) release is an intrinsic property of hypothalamic GnRH neurons and attributed to several specific mechanisms. These include the spontaneous electrical activity of GnRH neurons, calcium and cAMP signaling, a GnRH receptor autocrine regulatory component, a GnRH concentration-dependent switch in GnRH receptor (GnRH-R) coupling to specific G proteins, expression of G protein-coupled receptors (GPCRs) and steroid receptors, and homologous and heterologous interactions between cell-membrane receptors expressed in GnRH neurons. The coexistence of multiple regulatory mechanisms for pulsatile GnRH secretion provides a high degree of redundancy in maintaining this crucial component of the mammalian reproductive process. These studies provided insights into the basic cellular and molecular mechanisms involved in GnRH neuronal function.
Hypothalamic control of reproductive function
The episodic mode of GnRH secretion from the hypothalamus and of GnRH receptor (GnRH-R) activation in pituitary gonadotrophs are essential for optimal gonadotropin synthesis and secretion and ultimately for normal reproductive function [1–5]. The genesis of GnRH pulse generation in the hypothalamus is still incompletely understood. However, episodic neuropeptide secretion is an intrinsic property of the GnRH neuron and dependent on intracellular signaling and mechanism(s) leading to coordinated bursts of GnRH release [5–12]. Seminal studies by Ernst Knobil et al. in rhesus monkeys defined the importance of episodic pituitary stimulation for optimal gonadotropin secretion, as well as the relationship of GnRH release to electrical activity in the hypothalamus and the essential role of estrogen in promoting the mid-cycle LH surge [13, 14].
The neuroendocrine control of reproductive function is expressed through the episodic secretion of gonadotropic hormones from the anterior pituitary gland in response to pulsatile stimulation by GnRH, produced by a network of peptidergic neurons in the hypothalamus [11, 15–20]. The characteristic pulsatile secretion of GnRH from hypothalamic neurons is dependent on an autocrine interaction between GnRH and its receptors expressed in GnRH-producing neurons. It is noteworthy that GnRH-R expression, GnRH-dependent activation of Ca2+ signaling, and autocrine regulation of GnRH release are evident in early fetal GnRH neurons. It is probable that such activity provides a mechanism for gene expression and regulated GnRH secretion during embryonic migration [21–23].
The GnRH neuronal system
Mammalian reproduction is controlled by integrated sets of interactions between the hypothalamus, pituitary gland and gonads. Each component of the reproductive system is regulated by feedback mechanisms that coordinate the processes leading to gonadotropin secretion, gamete production and maintenance of the species [1, 24–26]. In most mammalian species, GnRH neurons are distributed in the preoptic area and adjacent sites in the rostral region of the hypothalamus, rather than concentrated in a discrete nucleus. These scattered neurons are believed to form a diffuse neural network that functions coordinately as a GnRH pulse generator [27]. The generation of pulsatile GnRH release at the median eminence is the central and essential element governing reproductive function, and depends on the coordinated activities of the 1500 or so GnRH neurons that are located in the hypothalamus [28], of which about 34% are required to control the ovarian cycle in the mouse [29]. Studies using intrahypothalamic injection of immortalized GnRH neurons (GT1–7cells) in hypogonadal mice revealed that regardless of the number of neurons injected, normal reproductive function could be restored [30].
Pulsatile GnRH secretion
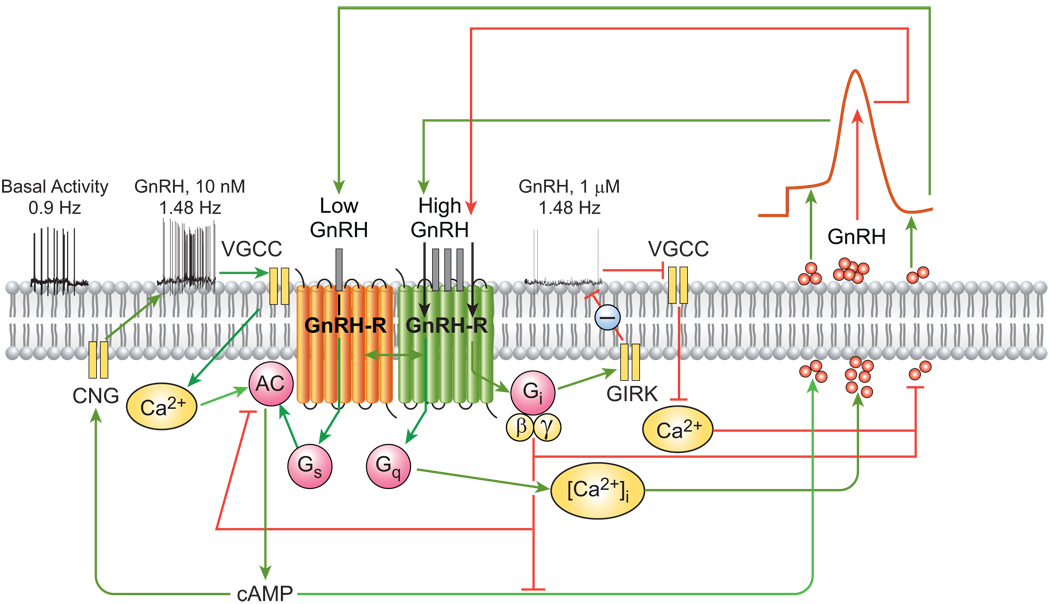
Episodic hormone secretion is a characteristic feature of the hypothalamo-pituitary-gonadal system, in which the profile of gonadotropin release from pituitary gonadotrophs reflects the pulsatile secretory activity of GnRH-producing neurons in the hypothalamus [4, 5]. Pulsatile release of GnRH is also evident in vitro during perifusion of cultured fetal hypothalamic cells, which produce GnRH for up to 2 months. Such cultures, as well as hypothalamic tissue from adult rats, express GnRH-Rs as evidenced by the presence of high-affinity GnRH binding sites and GnRH-R transcripts [11]. The ability of immortalized GnRH neurons to exhibit episodic GnRH release in the absence of other cell types indicates that intrinsic factors such as autocrine regulation of neurosecretion are important determinants of pulsatile GnRH release (Figure 1). The findings that hypothalamic GnRH neurons and GT1–7 cells express GnRH-Rs, and that agonist activation modulates pulsatile GnRH release by changing pulse frequency and amplitude [11, 12], are consistent with this proposal. The pulsatile secretion of GnRH from normal and immortalized hypothalamic GnRH neurons is highly calcium-dependent and also stimulated by cAMP [7, 31]. Upon agonist activation, the coupling of endogenous GnRH-Rs Gq/11 releases membrane-bound αq/11 subunits, activating PLC-β and increasing inositol phosphate/Ca2+ signaling. Conversely, treatment with GnRH antagonists increases membrane-associated αq/11 subunits and abolishes receptor signaling and pulsatile GnRH secretion (Figure 1). GnRH also stimulates cAMP production via Gs, but at high concentrations has a PTX-sensitive inhibitory effect due to receptor coupling to Gi.
Figure 1.
Proposed mechanism of autocrine control of pulsatile GnRH secretion. (i) Firing of Ca2+-dependent action potentials in GnRH neurons promotes Ca2+ influx, activation of Ca2+-dependent adenylyl cyclase (AC), increased cAMP production, and elevated GnRH secretion. (ii) GnRH-induced stimulation of the endogenous GnRH-R expressed in GnRH neurons activates three G proteins, as indicated by the time- and dose-dependent release of their specific α-subunits from the plasma membrane. This is associated with increased inositol phosphate production and [Ca2+]i mobilization, as well as prominent increases in GnRH peak amplitude. Agonist activation of GnRH-Rs in GnRH neurons regulates AC activity in a biphasic manner, such that cAMP production is stimulated at nanomolar agonist levels and decreased by micromolar concentrations. (iii) Neuronal GnRH-Rs also interact with Gi proteins. The autocrine switch from Gs to Gi at high local GnRH concentrations interrupts the rise in neurosecretion and is followed by a fall to baseline and subsequent reactivation of secretion via the resurgent Ca2+/cAMP signaling pathways. VSCC, voltage-sensitive calcium channels; AC, adenylyl cyclase; CNG, cyclic nucleotide gated channels; GIRK, G protein-activated inwardly rectifying potassium channels. Green and red lines indicate stimulatory and inhibitory actions, respectively.
The ability of the agonist-activated GnRH-R to couple to both Gs and Gi proteins was also demonstrated by the efficacy of GnRH in reducing membrane-associated G protein αs and αi3 levels, and in increasing or decreasing cAMP production at appropriate agonist concentrations (Figure 1). Membrane-associated αi3 is increased by both GnRH antagonist and pertussis toxin treatment, with concomitant loss of pulsatile GnRH secretion. Treatment with cholera toxin selectively activates and releases plasma membrane αs and increases cAMP production similar to that induced by low nanomolar GnRH concentrations [12]. Treatment with cholera toxin and 8-bromo-cAMP amplifies episodic GnRH pulses but does not affect their frequency [12]. These findings suggest that an agonist concentration-dependent switch in coupling of GnRH-R between specific G proteins modulates neuronal Ca2+ signaling via Gs-cAMP stimulatory and Gi-cAMP inhibitory mechanisms.
Activation of Gi also inhibits GnRH neuronal function and episodic secretion, probably in part by regulating membrane ion currents (Figure 1). This autocrine mechanism could serve as a timer to determine the frequency of pulsatile GnRH release by regulating Ca2+- and cAMP-dependent signaling and GnRH neuronal firing. These findings suggest that an agonist concentration-dependent switch in coupling of GnRH-R between specific G proteins modulates neuronal Ca2+ signaling via Gs-cAMP-stimulatory and Gi-cAMP-inhibitory mechanisms [12, 32, 33]. Activation of Gi could also inhibit GnRH neuronal function and episodic secretion by regulating membrane ion currents, probably through activating G protein-regulated inwardly rectifying potassium channels (Figure 1) [22, 34].
Autocrine regulation of pulsatile neurosecretion in GnRH neurons
The ontogeny and function of this autoregulatory process was investigated in GnRH neurons derived from the olfactory placode of the fetal rat. Laser-captured fetal rat hypothalamic GnRH neurons and olfactory placode-derived GnRH neurons identified by differential interference contrast microscopy co-express mRNAs encoding GnRH and its receptor. Placode-derived GnRH neurons exhibit spontaneous electrical activity that is increased by GnRH agonist treatment. This response, as well as basal neuronal firing, is abolished by GnRH antagonist treatment. GnRH stimulation elicits biphasic [Ca2+]i responses, and both basal and GnRH-stimulated levels are reduced by antagonist treatment. Perifused cultures release GnRH in a pulsatile manner that is highly dependent on extracellular Ca2+. The amplitude of GnRH pulses is increased by GnRH agonist stimulation and diminished during GnRH antagonist treatment. These findings demonstrate that GnRH-R expression, GnRH-dependent activation of Ca2+ signaling, and autocrine regulation of GnRH release are characteristics of early fetal GnRH neurons and could provide a mechanism for gene expression and regulated GnRH secretion during embryonic migration [22, 23] (Figure 1).
Integrated actions of the hypothalamic and pituitary GnRH signaling systems
In addition to the central GnRH system, there is evidence for the peripheral production of GnRH in ovarian granulosa [35] and testicular cells [36], the human placenta [37], the immune system [38], Rathkes pouch [39], and the pituitary gland [40].
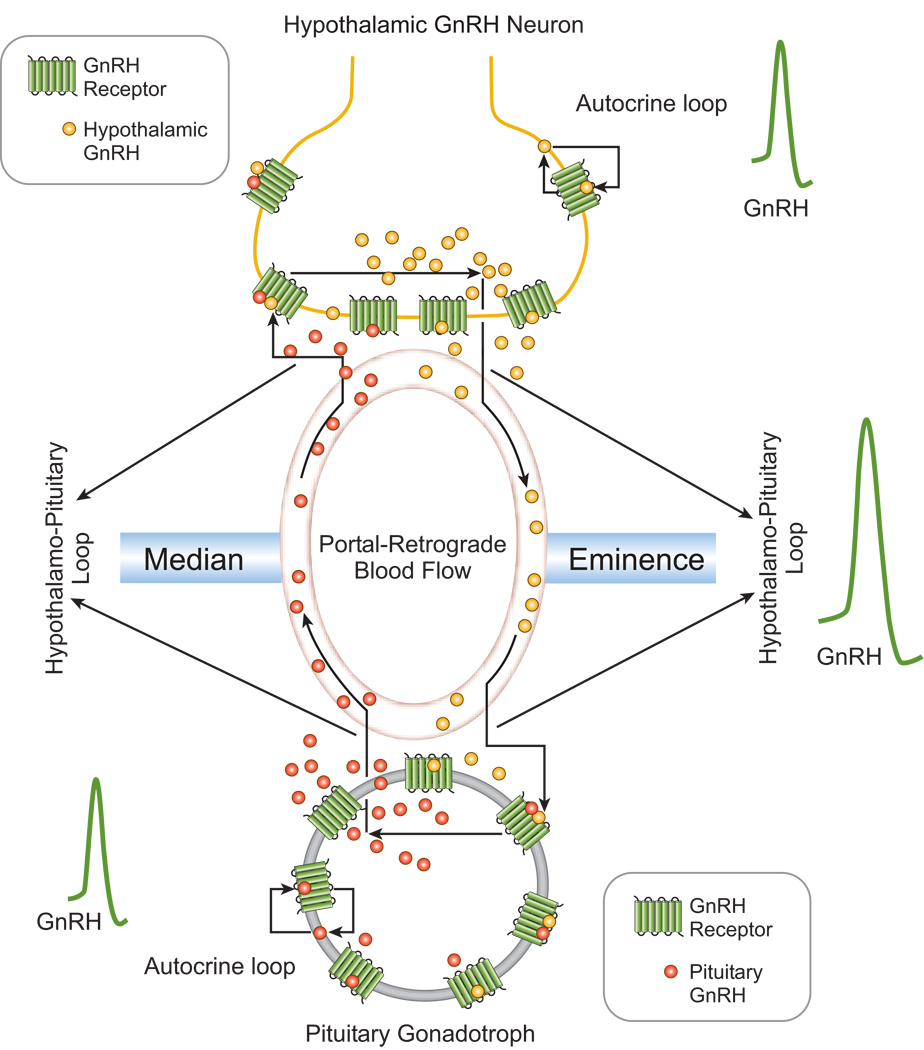
Both rat hypothalamic GnRH neurons and pituitary gonadotrophs express GnRH and its specific receptors that mediate the actions of GnRH agonist and antagonist analogs on GnRH secretion and luteinizing hormone (LH) secretion. In cultured hypothalamic cells, co-expression of GnRH and its receptors in individual GnRH neurons was demonstrated by double immunostaining with specific antisera and expression of mRNAs for both GnRH and the GnRH-R [11]. Identified pituitary gonadotrophs also exhibited positive immunostaining for LH, GnRH and the GnRH-R. Furthermore, rat anterior pituitary cells and αT3-1 gonadotrophs release GnRH and LH and LHα albeit in lesser amounts than hypothalamic cells and GT1–7 neurons. Temporal analysis of the pulsatile production of GnRH and LH in perifused pituitary cells revealed that release of GnRH preceded that of LH [41]. Furthermore, exposure of perifused pituitary cells to pulsatile GnRH agonist treatment increased LH and GnRH release. In contrast, treatment with a GnRH antagonist analog abolished spontaneous [Ca2+]i oscillations in pituitary gonadotrophs, decreased both basal and agonist-stimulated LH release, and converted the pulsatile pattern of endogenous GnRH release to a monotonic non-pulsatile profile [23]. Supportive evidence for GnRH-R participation in GnRH release from pituitary and αT3-1 cells was provided by the stimulatory action of the potent GnRH-R agonist, [D-Ala6]Ag. Discrete pulses of [D-Ala6]Ag, followed by successive washing periods, increased GnRH as well as LH release. This pattern of GnRH and LH release indicates that GnRH-R activation stimulates hormone release, consistent with the rapid [Ca2+]i increase and the absence of long-lasting refractoriness of GnRH-Rs [42, 43]. However, basal pulsatile LH release was abolished by the GnRH-R antagonist, SB-75, while agonist-stimulated GnRH release was switched to a non-oscillatory secretory profile. The operation of such an autocrine process, and its action as a positive feedback mechanism, could be a fundamental factor for amplifying basal LH release from cultured pituitary cells in vitro (Figure 2). The presence of both GnRH and LH receptors in pituitary cells and αT3-1 gonadotrophs [44, 45] as well as hypothalamic and GT1–7 neurons [10, 11] would permit the convergence of Gq- and Gi-mediated signaling pathways, and thus provide for the modulation of GnRH and LH release. These rapid changes, and the suppression of GnRH biosynthesis after long-term exposure to hCG [45], are consistent with the operation of a short-loop feedback mechanism at the hypothalamo-pituitary level that accounts for the inhibition of GnRH release after the preovulatory LH surge and during pregnancy [46].
Figure 2.
The hypothalamo-pituitary GnRH system. Rat hypothalamic GnRH neurons and anterior pituitary cells express both GnRH and specific GnRH receptors that mediate the actions of GnRH agonist and antagonist analogs on intracellular signaling, GnRH secretion, and gonadotropin secretion. Identified pituitary gonadotrophs also exhibited positive immunostaining for GnRH and GnRH receptors, and mRNA transcripts for GnRH were found in pituitary cells as well as hypothalamic neurons. Temporal analysis of the pulsatile production of GnRH and LH in perifused pituitary cells revealed that release of GnRH preceded the episodes of LH secretion. Furthermore, perifused pituitary cells exposed to pulsatile GnRH agonist treatment exhibited increased release of both LH and GnRH. In contrast, treatment with a GnRH antagonist abolished spontaneous [Ca2+]i oscillations in pituitary gonadotrophs, decreased both basal and agonist-stimulated LH release, and converted the pulsatile pattern of endogenous GnRH release to a monotonic non-pulsatile profile. In vivo, the two GnRH systems are connected by the hypothalamo-pituitary portal vessels and potentially by retrograde neurohypophysial blood flow, leading to synchronous pulsatile or episodic discharges of GnRH with consequent increases in LH release.
In vivo, the autonomous pituitary GnRH system could communicate with the hypothalamic GnRH system through hypothalamo-pituitary portal vessels and retrograde neurohypophysial blood flow [47, 48]. Such vascular organizations, linking two autocrine-controlled GnRH systems, provide an additional regulatory mechanism (short-loop feedback) that controls LH and GnRH secretion (Figure 2). Due to their integration into the hypothalamo-pituitary-gonadal axis, the autonomous hypothalamic and pituitary GnRH systems are also amenable to positive and negative regulation by gonadal steroids. Such a functional link provides the pulsatile or episodic discharges of GnRH that trigger LH release from the pituitary gland [41].
Expression of a GPR54-Kisspeptin autoregulatory system in GnRH neurons
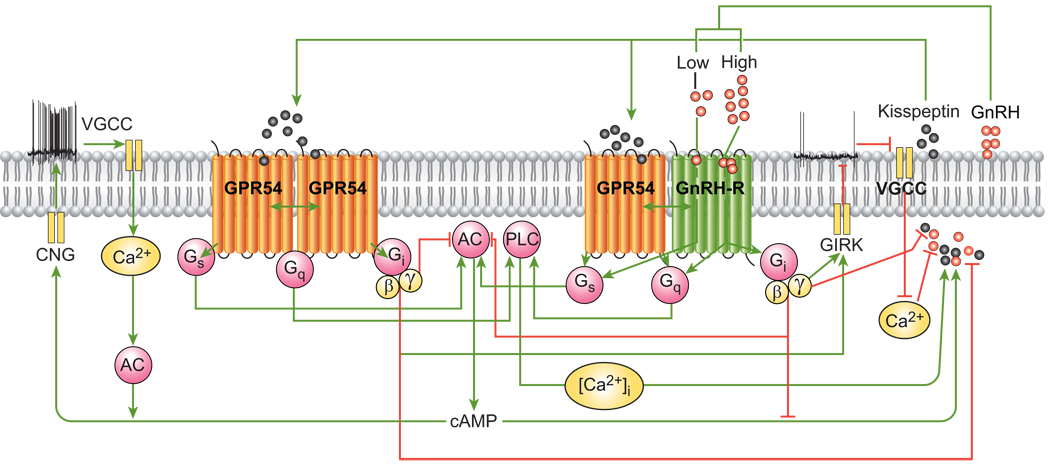
The G protein-coupled receptor 54 (GPR54) and its endogenous ligand, kisspeptin, are essential for activation and regulation of the hypothalamic-pituitary-gonadal axis. Analysis of RNA extracts from individually identified hypothalamic GnRH neurons revealed the expression of GnRH, kisspeptin-1, and GPR54. Also, constitutive and GnRH agonist-induced BRET between the Renilla luciferase-tagged GnRH receptor and GPR54 tagged with green fluorescent protein, expressed in HEK 293 cells, revealed hetero-oligomerization of the two receptors (Figure 3). Similar to other G protein-coupled receptors in which hetero-oligomer formation influences binding and/or signaling properties [49, 50], the formation of GnRH-R-GPR54 hetero-oligomers may provide for integrated cellular signaling that regulates GnRH and kisspeptin secretion from GnRH neurons (Figure 3). Whole cell patch-clamp recordings from identified GnRH neurons showed initial depolarizing effects of kisspeptin on membrane potential, followed by increased action potential firing. In perifused GT1–7 cells, kisspeptin-10 increased GnRH peak amplitude and duration. The maintenance of pulsatile GnRH release during kisspeptin treatment indicates that activation of GPR54 does not interrupt the signaling pathway that drives pulsatile GnRH release, in which activation of both Gs and Gq is necessary to initiate pulse formation, and activation of Gi causes its termination (Figure 3) [12, 33, 51]. Functional coupling of GPR54 to Gq/11 occurs in hippocampal neurons and GPR54-transfected COS-7 cells [52, 53]. Also, prediction of GPR54 coupling using Hidden Markov models [54] indicates its potential ability for Gi/o and Gs activation. The inhibition of kisspeptin secretion by GnRH suggests that its activation of the heterodimeric GnRH-R/GPR54 receptor complex favors selective coupling to the Gi/o signaling pathway. The production and secretion of kisspeptin in cultured hypothalamic neurons and GT1–7 cells are significantly reduced by treatment with GnRH (Figure 3). The expression of kisspeptin and GPR54 mRNAs in identified hypothalamic GnRH neurons, as well as kisspeptin secretion, indicate that kisspeptins may act as paracrine and/or autocrine regulators of the GnRH neuron. Stimulation of GnRH secretion by kisspeptin and the opposing effects of GnRH on kisspeptin secretion, suggest that GnRH receptor/GnRH and GPR54/kisspeptin autoregulatory systems are integrated by negative feedback to control GnRH and kisspeptin secretion from GnRH neurons [55].
Figure 3.
Homo- and hetero-oligomerization of G protein-coupled receptors expressed in GnRH neurons. The expression of kisspeptin and GPR54 mRNAs in identified hypothalamic GnRH neurons and the constitutive and agonist-induced hetero-oligomerization of GnRH-R and GPR54 indicate that both receptors are involved in the regulating GnRH neuronal function. Green and red lines indicate stimulatory and inhibitory actions, respectively.
Role of plasma membrane estrogen receptors in GnRH signaling and secretion
Estradiol (E2) acts as a potent feedback molecule between the ovary and hypothalamic GnRH neurons, exerting both positive and negative regulatory actions on GnRH synthesis and secretion. The regulation of GnRH neuronal function by estrogen receptors ERα and ERβ has been a controversial topic over the last decade due to the plethora of conflicting evidence about the expression and functions of these receptors in GnRH neurons. Early studies by Shivers at al. [56] found no evidence of estradiol concentration in the nuclei of LHRH-immunoreactive neurons. However, Kelly et al. [57] observed estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Conversely, Langub et al. [58] found that most LHRH neurons in the guinea-pig were not directly estrogen-responsive. Likewise, Herbison et al. [59] did not find ERs in the preoptic nucleus containing LHRH in male and female rats. Subsequently, Roy et al. [60] reported the expression of both ERα and ERβ in immortalized GnRH (GT1–7) neuronal cells.
The first evidence of ER expression in rat GnRH neurons using immunoprecipitation and double-label immunohistochemistry with an ERα selective antibody was reported by Butler et al. in 1999 [61]. Shortly after, Skynner et al. [62] used single-cell RT-PCR to show ERα expression in more than 50% of prepubertal GnRH neurons. However, the original positive findings by Skynner et al. [62] were retracted after subsequent experiments by the authors did not detect ERα expression in mouse GnRH neurons [63, 64]. In our study [65], single-cell RT-PCR revealed expression of both ERα and ERβ in cultured fetal and adult rat hypothalamic GnRH neurons. Both ERα and ERβ or individual ERs were expressed in 94% of cultured fetal GnRH neurons. In adult female rats at diestrous, 68% of GnRH neurons expressed ERs, followed by 54% in estrous and 19% in proestrous. Expression of individual ERs was found in 24% of adult male GnRH neurons. ERα has marked Gi-mediated inhibitory effects on spontaneous AP firing, cAMP production, and pulsatile GnRH secretion, indicating its capacity for negative regulation of GnRH neuronal function (Figure 4). In addition to the direct negative feedback action of E2 on ERα in rat hypothalamic GnRH neurons and the mouse pituitary [66], an alternative indirect stimulatory action of E2 was observed in the mouse where GnRH neurons do not express ERα [67]. In adult rat GnRH neurons, increased E2 concentrations and ERβ agonists increase the rate of AP firing, GnRH secretion, and cAMP production, consistent with ERβ-dependent positive regulation of GnRH secretion (Figure 4). Consonant with the coupling of ERα to pertussis toxin (PTX)-sensitive Gi/o proteins, E2 also activates G protein-activated inwardly rectifying potassium (GIRK) channels, decreasing membrane excitability and slowing the firing of spontaneous APs in hypothalamic GnRH neurons (Figure 4). These findings demonstrate that the dual actions of E2 on GnRH neuronal membrane excitability, cAMP production, and GnRH secretion are mediated by the dose-dependent activation of ERα and ERβ receptors expressed in hypothalamic GnRH neurons [65].
Figure 4.
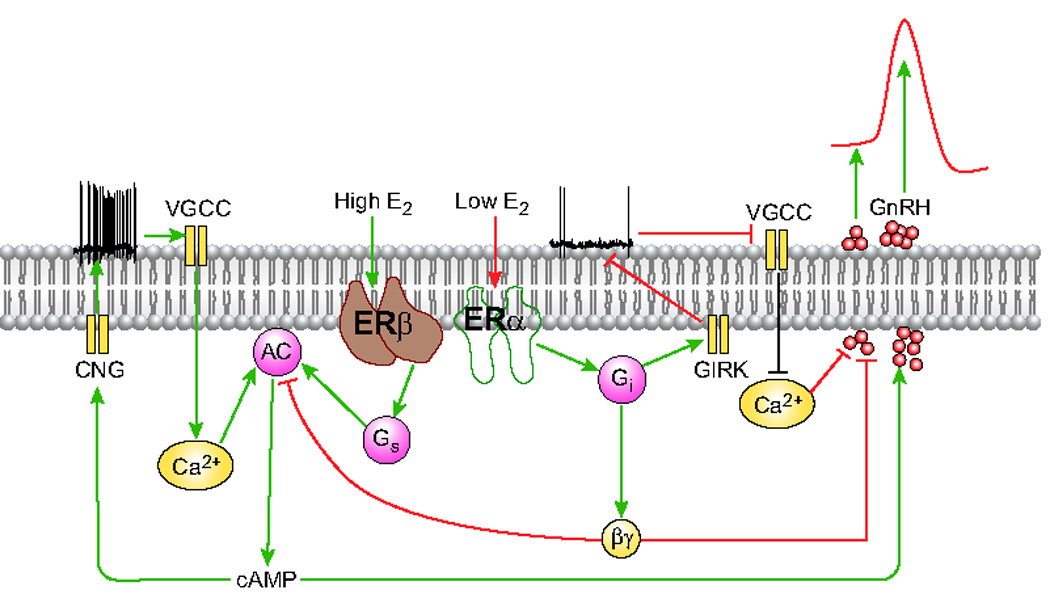
Expression of ERs in hypothalamic GnRH neurons. Estrogen receptor alpha (ERα) and beta (ERβ) are expressed in cultured hypothalamic GnRH neurons. (i) In cultured hypothalamic cells and immortalized GT1–7 neurons, low (picomolar) E2 concentrations and an ERα agonist suppressed spontaneous action potential (AP) firing, decreased cAMP production, and inhibited pulsatile GnRH release. The marked inhibitory effects of Gi-coupled ERα on spontaneous AP firing, cAMP production, and pulsatile GnRH secretion, indicate its capacity for negative regulation of GnRH neuronal function. (ii) In contrast, higher (nanomolar) E2 concentrations and an ERβ agonist increased the rate of AP firing, cAMP production, and GnRH secretion, consistent with positive regulation of GnRH secretion. In accord with the coupling of ER to PTX-sensitive Gi/o proteins, E2 also activates G protein-activated inwardly rectifying potassium (GIRK) channels, decreasing membrane excitability and slowing the firing of spontaneous APs in hypothalamic GnRH neurons. Green and red lines indicate stimulatory and inhibitory actions, respectively.
Conclusion
The neuroendocrine control of reproductive function is expressed through the episodic secretion of gonadotropic hormones from the anterior pituitary gland in response to pulsatile stimulation by GnRH released from a network of hypothalamic peptidergic neurons. The characteristic pulsatile secretion of GnRH from hypothalamic neurons is dependent on an autocrine interaction between GnRH and its receptors expressed in GnRH-producing neurons, which provides the mechanism for episodic GnRH secretion.
Given the primacy of the hypothalamic pulse generator in pituitary regulation, the production of GnRH by pituitary gonadotrophs raises the question of its physiological role. In vivo the hypothalamus and pituitary gland are organized as a morpho-physiological unit and are connected by the hypothalamo-pituitary portal system. This raises the possibility that the coordinated actions of two individual systems contribute to the synchronous pulsatile or episodic discharge of GnRH that triggers LH release from the pituitary gland.
The expression of kisspeptin and GPR54 mRNAs in identified hypothalamic GnRH neurons, and the constitutive and agonist-induced hetero-oligomerization of the GnRH-R and GPR54, indicate that both receptors are involved in the regulation of hypothalamic GnRH neurons. Moreover, stimulation of GnRH secretion by kisspeptin, and inhibition of kisspeptin secretion by GnRH, indicate that GnRH-receptor-GnRH and GPR54-kisspeptin autoregulatory systems are integrated by negative feedback action to control GnRH and kisspeptin secretion from cultured hypothalamic GnRH neurons.
The regulation of GnRH neuronal function by ERα and ERβ has been a controversial topic over the last decade, due to conflicting findings on the expression and functions of these receptors in GnRH neurons. The expression of both ERα and ERβ in identified rat GnRH neurons suggests that E2 might directly modulate GnRH neuronal function via these receptors. Selective activation of ERβ increases the rate of AP firing, GnRH secretion, and cAMP production, consistent with ERβ-dependent positive regulation of GnRH secretion. In contrast, selective activation of ERα activates G protein-activated inwardly rectifying potassium (GIRK) channels, thus decreasing membrane excitability and GnRH secretion. These findings demonstrate that the dual actions of E2 on GnRH neuronal membrane excitability, cAMP production, and GnRH secretion are mediated by the dose-dependent activation of ERα and/or ERβ receptors expressed in hypothalamic GnRH neurons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Catt KJ, et al. GnRH receptors and actions in the control of reproductive function. J Steroid Biochem. 1985;23:677–689. doi: 10.1016/s0022-4731(85)80003-0. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen DD. Episodic gonadotropin-releasing hormone release from the rat isolated median eminence in vitro. Neuroendocrinology. 1993;58:511–518. doi: 10.1159/000126584. [DOI] [PubMed] [Google Scholar]
- 3.Krsmanovic LZ, et al. Pulsatile gonadotropin-releasing hormone release and its regulation. Trends Endocrinol Metab. 1996;7:56–59. doi: 10.1016/1043-2760(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 4.Terasawa E. Cellular mechanism of pulsatile LHRH release. Gen Comp Endocrinol. 1998;112:283–295. doi: 10.1006/gcen.1998.7155. [DOI] [PubMed] [Google Scholar]
- 5.Moenter SM, et al. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 6.Krsmanovic LZ, et al. Receptors and neurosecretory actions of endothelin in hypothalamic neurons. Proc Natl Acad Sci U S A. 1991;88:11124–11128. doi: 10.1073/pnas.88.24.11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krsmanovic LZ, et al. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci U S A. 1992;89:8462–8466. doi: 10.1073/pnas.89.18.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner RI, Martinez de la Escalera G. Pulsatile release of gonadotrophin releasing hormone (GnRH) is an intrinsic property of GT1 GnRH neuronal cell lines. Hum Reprod. 1993;8 Suppl 2:13–17. doi: 10.1093/humrep/8.suppl_2.13. [DOI] [PubMed] [Google Scholar]
- 9.Wetsel WC, et al. Regulation of gonadotropin-releasing hormone by protein kinase-A and -C in immortalized hypothalamic neurons. Endocrinology. 1993;132:2360–2370. doi: 10.1210/endo.132.6.8504741. [DOI] [PubMed] [Google Scholar]
- 10.Krsmanovic LZ, et al. Expression of gonadotropin-releasing hormone receptors and autocrine regulation of neuropeptide release in immortalized hypothalamic neurons. Proc Natl Acad Sci U S A. 1993;90:3908–3912. doi: 10.1073/pnas.90.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krsmanovic LZ, et al. Autocrine regulation of gonadotropin-releasing hormone secretion in cultured hypothalamic neurons. Endocrinology. 1999;140:1423–1431. doi: 10.1210/endo.140.3.6588. [DOI] [PubMed] [Google Scholar]
- 12.Krsmanovic LZ, et al. An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc Natl Acad Sci U S A. 2003;100:2969–2974. doi: 10.1073/pnas.0535708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knobil E. The hypothalamic gonadotrophic hormone releasing hormone (GnRH) pulse generator in the rhesus monkey and its neuroendocrine control. Hum Reprod. 1988;3:29–31. doi: 10.1093/oxfordjournals.humrep.a136647. [DOI] [PubMed] [Google Scholar]
- 14.Ordog T, Knobil E. Estradiol and the inhibition of hypothalamic gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Proc Natl Acad Sci U S A. 1995;92:5813–5816. doi: 10.1073/pnas.92.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wray S. Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol. 2002;23:292–316. doi: 10.1016/s0091-3022(02)00001-8. [DOI] [PubMed] [Google Scholar]
- 16.Herbison AE, et al. Molecular and cellular properties of GnRH neurons revealed through transgenics in the mouse. Mol Cell Endocrinol. 2001;185:185–194. doi: 10.1016/s0303-7207(01)00618-9. [DOI] [PubMed] [Google Scholar]
- 17.Ordog T, et al. On the role of gonadotropin-releasing hormone (GnRH) in the operation of the GnRH pulse generator in the rhesus monkey. Neuroendocrinology. 1997;65:307–313. doi: 10.1159/000127189. [DOI] [PubMed] [Google Scholar]
- 18.Weiner RI. Cellular basis of the GnRH pulse generator. Nippon Sanka Fujinka Gakkai Zasshi. 1996;48:573–577. [PubMed] [Google Scholar]
- 19.Caraty A, et al. Neuroendocrine control of ovulation in sheep. Ann Endocrinol (Paris) 1995;56:539–542. [PubMed] [Google Scholar]
- 20.Halasz B, et al. Regulation of the gonadotropin-releasing hormone (GnRH) neuronal system: morphological aspects. J Steroid Biochem. 1989;33:663–668. doi: 10.1016/0022-4731(89)90475-5. [DOI] [PubMed] [Google Scholar]
- 21.Pronina T, et al. Influence of serotonin on the development and migration of gonadotropin-releasing hormone neurones in rat foetuses. J Neuroendocrinol. 2003;15:549–558. doi: 10.1046/j.1365-2826.2003.01029.x. [DOI] [PubMed] [Google Scholar]
- 22.Romanelli RG, et al. Expression and function of gonadotropin-releasing hormone (GnRH) receptor in human olfactory GnRH-secreting neurons: an autocrine GnRH loop underlies neuronal migration. J Biol Chem. 2004;279:117–126. doi: 10.1074/jbc.M307955200. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Fuentes AJ, et al. Gonadotropin-releasing hormone (GnRH) receptor expression and membrane signaling in early embryonic GnRH neurons: role in pulsatile neurosecretion. Mol Endocrinol. 2004;18:1808–1817. doi: 10.1210/me.2003-0321. [DOI] [PubMed] [Google Scholar]
- 24.Fink G. Neuroendocrine control of gonadotrophin secretion. In: Knobil E, Neill J, editors. The physiology of reproduction. Raven Press; 1988. pp. 1349–1377. [Google Scholar]
- 25.Knobil E, Krey LC. Neuroendocrine control of gonadotropin secretion in the rhesus monkey. In: Anand Kumar TC, editor. Neuroendocrine regulation of fertility. Basel, Karger; 1976. pp. 278–285. WQ 205 I6167n 1974. [Google Scholar]
- 26.Sagrillo CA, et al. Hormonal and neurotransmitter regulation of GnRH gene expression and related reproductive behaviors. Behav Genet. 1996;26:241–277. doi: 10.1007/BF02359383. [DOI] [PubMed] [Google Scholar]
- 27.Knobil E. The GnRH pulse generator. Am J Obstet Gynecol. 1990;163:1721–1727. doi: 10.1016/0002-9378(90)91435-f. [DOI] [PubMed] [Google Scholar]
- 28.Wray S. Review Article: Development of Luteinizing Hormone Releasing Hormone Neurones. J Neuroendocrinol. 2001;13:3–11. doi: 10.1046/j.1365-2826.2001.00609.x. [DOI] [PubMed] [Google Scholar]
- 29.Herbison AE, et al. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149:597–604. doi: 10.1210/en.2007-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman AJ, et al. Intrahypothalamic injection of a cell line secreting gonadotropin-releasing hormone results in cellular differentiation and reversal of hypogonadism in mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10668–10672. doi: 10.1073/pnas.89.22.10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krsmanovic LZ, et al. Regulation of Ca2+sensitive adenylyl cyclase in gonadotropin-releasing hormone neurons. Mol Endocrinol. 2001;15:429–440. doi: 10.1210/mend.15.3.0610. [DOI] [PubMed] [Google Scholar]
- 32.Li YX, Khadra A. Robust synchrony and rhythmogenesis in endocrine neurons via autocrine regulations in vitro and in vivo. Bulletin of Mathematical Biology. 2008;70:2103–2125. doi: 10.1007/s11538-008-9328-z. [DOI] [PubMed] [Google Scholar]
- 33.Khadra A, Li YX. A model for the pulsatile secretion of gonadotropin-releasing hormone from synchronized hypothalamic neurons. Biophys J. 2006;91:74–83. doi: 10.1529/biophysj.105.080630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuen T, et al. Coupling of GnRH concentration and the GnRH receptor-activated gene program. Mol Endocrinol. 2002;16:1145–1153. doi: 10.1210/mend.16.6.0853. [DOI] [PubMed] [Google Scholar]
- 35.Peng C, et al. Expression and regulation of gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acids in human granulosa-luteal cells. Endocrinology. 1994;135:1740–1746. doi: 10.1210/endo.135.5.7956897. [DOI] [PubMed] [Google Scholar]
- 36.Di Matteo L, et al. Localization of GnRH molecular forms in the brain, pituitary, and testis of the frog, Rana esculenta. J Exp Zool. 1996;274:33–40. doi: 10.1002/(SICI)1097-010X(19960101)274:1<33::AID-JEZ4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 37.Gohar J, et al. GnRH in pregnancy. Arch Gynecol Obstet. 1996;259:1–6. doi: 10.1007/BF02505301. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson JD, et al. Cyclical expression of GnRH and GnRH receptor mRNA in lymphoid organs. Neuroendocrinology. 1998;67:117–125. doi: 10.1159/000054306. [DOI] [PubMed] [Google Scholar]
- 39.Van Bael A, et al. Presence of gonadotropin-releasing hormone (GnRH) mRNA in Rathke's pouch and effect of the GnRH-antagonist ORG 30276 on lactotroph development in vitro. J Neuroendocrinol. 1998;10:437–445. doi: 10.1046/j.1365-2826.1998.00205.x. [DOI] [PubMed] [Google Scholar]
- 40.Bauer TW, et al. Studies of immunoreactive gonadotropin-releasing hormone (GnRH) in the rat anterior pituitary. J Histochem Cytochem. 1981;29:1171–1178. doi: 10.1177/29.10.6795258. [DOI] [PubMed] [Google Scholar]
- 41.Krsmanovic LZ, et al. Local regulation of gonadotroph function by pituitary gonadotropin-releasing hormone. Endocrinology. 2000;141:1187–1195. doi: 10.1210/endo.141.3.7392. [DOI] [PubMed] [Google Scholar]
- 42.Jinnah HA, Conn PM. GnRH-stimulated LH release from rat anterior pituitary cells in culture: refractoriness and recovery. Am J Physiol. 1985;249:E619–E625. doi: 10.1152/ajpendo.1985.249.6.E619. [DOI] [PubMed] [Google Scholar]
- 43.Mason DR, et al. Homologous down-regulation of gonadotropin-releasing hormone receptor sites and messenger ribonucleic acid transcripts in alpha T3-1 cells. Endocrinology. 1994;135:1165–1170. doi: 10.1210/endo.135.3.8070359. [DOI] [PubMed] [Google Scholar]
- 44.Lei ZM, Rao CV. Signaling and transacting factors in the transcriptional inhibition of gonadotropin releasing hormone gene by human chorionic gonadotropin in immortalized hypothalamic GT1–7 neurons. Mol Cell Endocrinol. 1995;109:151–157. doi: 10.1016/0303-7207(95)03497-u. [DOI] [PubMed] [Google Scholar]
- 45.Lei ZM, Rao CV. Novel presence of luteinizing hormone/human chorionic gonadotropin (hCG) receptors and the down-regulating action of hCG on gonadotropin-releasing hormone gene expression in immortalized hypothalamic GT1–7 neurons. Mol Endocrinol. 1994;8:1111–1121. doi: 10.1210/mend.8.8.7997235. [DOI] [PubMed] [Google Scholar]
- 46.Mores N, et al. Activation of LH receptors expressed in GnRH neurons stimulates cyclic AMP production and inhibits pulsatile neuropeptide release. Endocrinology. 1996;137:5731–5734. doi: 10.1210/endo.137.12.8940408. [DOI] [PubMed] [Google Scholar]
- 47.Porter JC, et al. Hypothalamic peptide and catecholamine secretion: roles for portal and retrograde blood flow in the pituitary stalk in the release of hypothalamic dopamine and pituitary prolactin and LH. Clin Obstet Gynaecol. 1978;5:271–282. [PubMed] [Google Scholar]
- 48.Porter JC, et al. Hypothalamic-hypophysial vasculature and its relationship to secretory cells of the hypothalamus and pituitary gland. Vitam Horm. 1983;40:145–174. [PubMed] [Google Scholar]
- 49.Rocheville M, et al. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 50.Charest PG, et al. Monitoring agonist-promoted conformational changes of beta-arrestin in living cells by intramolecular BRET. EMBO Rep. 2005;6:334–340. doi: 10.1038/sj.embor.7400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wada K, et al. Serotonin (5-HT) receptor subtypes mediate specific modes of 5-HT-induced signaling and regulation of neurosecretion in gonadotropin-releasing hormone neurons. Mol Endocrinol. 2006;20:125–135. doi: 10.1210/me.2005-0109. [DOI] [PubMed] [Google Scholar]
- 52.Stafford LJ, et al. Identification and characterization of mouse metastasis-suppressor KiSS1 and its G-protein-coupled receptor. Cancer Res. 2002;62:5399–5404. [PubMed] [Google Scholar]
- 53.Brailoiu GC, et al. KiSS-1 expression and metastin-like immunoreactivity in the rat brain. J Comp Neurol. 2005;481:314–329. doi: 10.1002/cne.20350. [DOI] [PubMed] [Google Scholar]
- 54.Sgourakis NG, et al. Prediction of the coupling specificity of GPCRs to four families of G-proteins using hidden Markov models and artificial neural networks. Bioinformatics. 2005;21:4101–4106. doi: 10.1093/bioinformatics/bti679. [DOI] [PubMed] [Google Scholar]
- 55.Quaynor S, et al. Expression of a functional g protein-coupled receptor 54-kisspeptin autoregulatory system in hypothalamic gonadotropin-releasing hormone neurons. Mol Endocrinol. 2007;21:3062–3070. doi: 10.1210/me.2007-0207. [DOI] [PubMed] [Google Scholar]
- 56.Shivers BD, et al. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304:345–347. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- 57.Kelly MJ, et al. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- 58.Langub MC, Jr, Watson RE., Jr Estrogen receptor-immunoreactive glia, endothelia, and ependyma in guinea pig preoptic area and median eminence: electron microscopy. Endocrinology. 1992;130:364–372. doi: 10.1210/endo.130.1.1727710. [DOI] [PubMed] [Google Scholar]
- 59.Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- 60.Roy D, et al. Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-alpha (ERalpha)- and ERbeta-expressing GT1–7 GnRH neurons. Endocrinology. 1999;140:5045–5053. doi: 10.1210/endo.140.11.7117. [DOI] [PubMed] [Google Scholar]
- 61.Butler JA, et al. Evidence for oestrogen receptor alpha-immunoreactivity in gonadotrophin-releasing hormone-expressing neurones. J Neuroendocrinol. 1999;11:331–335. doi: 10.1046/j.1365-2826.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 62.Skynner MJ, et al. Detection of estrogen receptor alpha and beta messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology. 1999;140:5195–5201. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- 63.Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- 64.Herbison A, et al. Lack of detection of estrogen receptor-αtranscripts in mouse gonadotropin-releasing hormone neurons. Endocrinology. 2001;42:493. [Google Scholar]
- 65.Hu L, et al. Converse regulatory functions of estrogen receptor-alpha and -beta subtypes expressed in hypothalamic gonadotropin-releasing hormone neurons. Mol Endocrinol. 2008;22:2250–2259. doi: 10.1210/me.2008-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Couse JF, et al. Characterization of the Hypothalamic-Pituitary-Gonadal Axis in Estrogen Receptor (ER) Null Mice Reveals Hypergonadism and Endocrine Sex Reversal in Females Lacking ER{alpha} But Not ER{beta} Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 67.Wintermantel TM, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]