Abstract
Impairment in executive cognition (EC) is now recognized as relatively common among older persons with mild cognitive impairment (MCI), and may be predictive of the development of dementia. However, both MCI and executive functioning are broad and heterogeneous constructs. The present study sought to determine whether impairments in specific domains of EC are associated with specific subtypes of MCI. 124 MCI patients were divided into four subgroups (amnestic versus nonamnestic, and single- versus multiple-domain) based on their performance of widely-used neuropsychological screening tests. These patients and 68 normal elderly were administered 18 clinical and experimental tests of executive function. Principal components analysis suggested two highly reliable EC components, planning/problem-solving and working memory, and a less reliable third component, judgment. Planning/problem-solving and working memory, but not judgment, were impaired among the MCI patients. This was true even among those with Apure amnestic@ MCI, the least impaired group overall. Multiple-domain MCI patients had more severe impairments in planning/problem-solving and working memory than single-domain patients, leading to the supposition that they, not pure amnestic MCIs, are at highest risk of imminent dementia.
Keywords: executive function, mild cognitive impairment, dementia, principal components analysis, flexibility, working memory, planning
The status of mild cognitive impairment (MCI) as an important clinical entity remains debated. Expert opinion ranges from it being early Alzheimer=s disease (AD) in virtually all cases (Morris et al., 2001) to it being a diagnostic nonentity (Milwain, 2000; Gauthier & Touchon, 2005; Whitehouse, 2007). Most opinions fall somewhere between these two extremes, and view MCI as a heterogeneous cognitive state that sometimes heralds the onset of progressive dementia (Chertkow et al., 2007). Much recent research has focused on determining the characteristics of patients with MCI that predict the progression to AD or another dementia.
It is now widely recognized that several subtypes of MCI can be identified, and the prognoses for them may differ (Lopez et al., 2003; Winblad et al., 2004). The most studied variety is pure amnestic MCI, an isolated and otherwise-unexplained memory impairment in an older person (Ganguli et al., 2004; Petersen, 2004). Although estimates vary, patients with this condition appear to “convert to” (i.e., develop) AD at a rate of 6% –15% per year (Daly et al., 2000; Fisk et al., 2003; Grundman et al., 1996). Patients with isolated impairments in other cognitive domains (e.g., language, spatial cognition) have also been identified, as have patients with mild impairments in multiple cognitive domains who remain functionally intact and don=t meet criteria for dementia. The prognoses for the various subtypes of MCI remain unknown (Chertkow et al., 2007).
The present study investigates the status of executive functioning in four subtypes of MCI. We conceptualize executive functions as Miyake et al. (2000) does: “general purpose control mechanisms that modulate the operation of various cognitive subprocesses and thereby regulate the dynamics of human cognition” (p.50). Although the identification and operation of these control processes has been the subject of much experimentation and discussion, a general model of executive cognition (EC) has yet to be validated or universally accepted (Burgess, 1997; Miyake et al., 2000). The present study is seen as a step toward that goal in that it attempts to identify, empirically, the latent structure underlying a large number of executive function tests.
There are many reasons to believe that decline in some aspects of EC is a strong risk factor for the imminent development of dementia. First, Baddeley and colleagues demonstrated 20 years ago that selective impairment of the central executive component of working memory is a prominent feature of Alzheimer’s-type dementia (Baddeley et al., 1986, 1991). Second, onset of executive dysfunction typically follows onset of episodic memory impairment in AD, and precedes impairment of language or spatial cognition (Lafleche & Albert, 1995; Binetti et al., 1996; Bondi et al., 2002). Third, many of the cognitive tests that are most helpful for predicting which nondemented elderly will subsequently develop dementia have substantial executive control requirements (Bondi et al., 1994; Elias et al., 2000; Jacobs et al., 1995; Albert et al., 2001, 2007; Rapp & Reischies, 2005). Finally, even among patients with pure amnestic MCI, impairments in executive function can be found (Crowell et al., 2002; Griffith et al., 2003; Kramer et al., 2006; Royall et al., 2004). These observations have led to the hypothesis that only when executive functioning becomes impaired should an MCI patient be considered to have prodromal AD (Albert et al., 2001; Royall et al., 2002).
Not only is the development of executive impairment potentially predictive of the development of dementia, it also appears to be uniquely associated with functional impairment in the elderly. One study reported that just two executive function tasks (Wisconsin Card Sorting Test [WCST] and Trail Making Test [TMT] part B) accounted for more than 50% of the variance in functional abilities of normal elderly (Bell-McGinty et al., 2002). Among community-dwelling elders, executive function tests have predictive value above and beyond demographic and health variables, overall cognitive integrity (e.g., Mini-Mental State Exam [MMSE] score) and other specific cognitive functions (language, spatial skills, and memory) for both self-reported and empirically measured everyday functioning (Cahn-Weiner et al., 2000; Grigsby et al., 1998; Lewis & Miller, 2007; Royall et al., 1998, 2004). Thus, subtle changes in EC can have a major impact on the lives of elderly persons.
Questions remain as to 1) whether impairments in specific executive domains are associated with specific subtypes of MCI, and 2) whether these impairments have particular prognostic value. The present study addresses the first of these questions by studying normal elderly subjects, patients with amnestic MCI (both single- and multiple-domain), and patients with nonamnestic MCI (both single- and multiple-domain) with an extensive set of clinical tests and experimental tasks of executive control. We selected 18 tests representing six conceptually distinct domains of EC: 1) spontaneous flexibility and generativity, 2) inhibition of prepotent responses, 3) planning and sequencing, 4) concept/rule learning and set shifting, 5) decision-making and judgment, and 6) working memory and resource-sharing. The cognitive test data were reduced using principal components analysis and the profile of each of the four MCI subgroups on the derived components was compared to each other and to normal elderly.
METHODS
Participants
One hundred, twenty-four persons with MCI and 68 cognitively normal older adults participated in this study. Most participants (81%) were recruited from the Johns Hopkins Alzheimer=s Disease Research Center (ADRC) and other research studies. They responded to direct-mail and posted announcements, newspaper ads, and solicitations of research volunteers at community lectures. A small number of subjects (19%) were referred from University clinics and physicians in the community from whom they sought evaluation of memory or other cognitive complaints. A health conditions checklist was used to gather information about major physical and psychological disorders. Volunteers were excluded from study participation if they had any history of psychosis, CNS disorder, or active systemic illness (e.g., cancer). Persons with histories of depression were not excluded, as depression is both very common in MCI and may be an important predictor of incident dementia (Jorm, 2001; Lyketsos et al., 2002; Mondrego & Ferrández, 2004; Visser, 2000) or a very early manifestation (Chen et al., 1999).
Every participant was required to have a family member or close friend available to be interviewed for a Clinical Dementia Rating (CDR) (Hughes et al., 1982). Only those with overall CDR scores of 0 or 0.5 were eligible. In addition, every participant was required to score in the normal range (i.e., at or above the 20th percentile for age and education) on the MMSE (Bravo & Hébert, 1997).
Each participant was administered the following screening tests to determine group assignment: Logical Memory subtest (story A) of Wechsler Memory Scale-Revised (WMS-R; Wechsler, 1987), a 30-item version of the Boston Naming Test (Goodglass & Kaplan, 1983; Brandt et al., 1989), word list generation (for the letters FAS and the semantic categories animals and vegetables) (Rascovsky et al., 2007; Salmon et al.,1999), and clock drawing to request (Rouleau et al., 1992). These specific tests were chosen for their brevity and their widespread use in the neuropsychological evaluation of geriatric cognitive disorders (Attix & Welsh-Bohmer, 2006). Tests of EC were not included in this screening/subtyping battery because they constitute the outcome variables of interest. In addition, the Activities of Daily Living – Prevention Instrument (ADL-PI) developed by the Alzheimer’s Disease Cooperative Study (Galasko et al., 2006) was completed by each participant’s “study partner” to supplement the CDR’s assessment of functional capacity in everyday life.
MCI groups
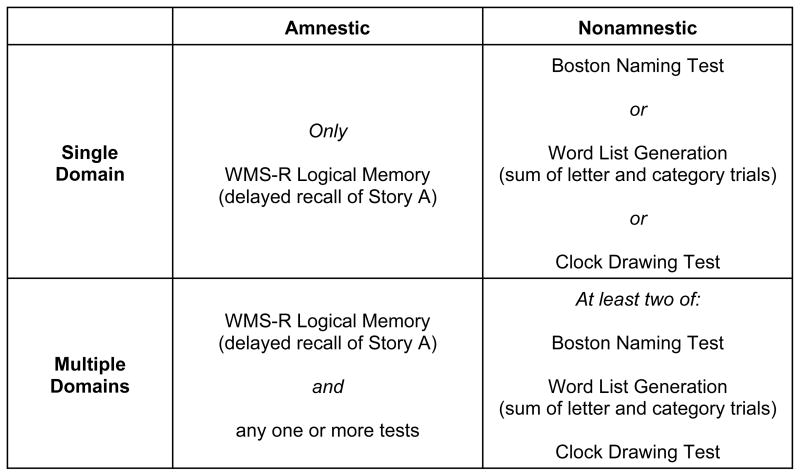
Participants were diagnosed with MCI according to the Petersen (2004) criteria. Specifically, each participant or his/her study partner reported excessive decline in one or more cognitive domain and obtained an overall CDR score of 0.5, indicating questionable dementia. In addition, participants were required to perform at or below 1.5 standard deviations below the mean for age and education (i.e., 6.7th percentile), according to published norms, on one or more of the screening tests. Applying the criteria described in Figure 1 allowed the MCI sample to be divided into four groups: amnestic single domain (AS) (N=36); amnestic multiple domain (AM) (N=45); nonamnestic single domain (NAS) (N=26), and nonamnestic multiple domain (NAM) (N=17).
Figure 1.
Operational criteria for four groups of participants with mild cognitive impairment. Subjects in each group performed at or below 1.5 SD below age-and education norms on the test(s) indicated.
Normal Control group
All participants in the normal control (NC) group were free of significant cognitive complaints. The absence of cognitive decline was confirmed by interview with the study partner; all normal control subjects obtained an overall CDR sore of 0. In addition, they all scored at or above 1.5 standard deviations below the mean for age and education on all four screening tests.
Procedures
Three clinical tests or experimental tasks were selected to evaluate each of the six EC domains proposed. Although our selection of tasks is based on previous literature, the very large number of executive tests and tasks available (Burgess et al., 1998; Miyake et al., 2000; Lezak et al., 2004) makes the ones selected somewhat arbitrary. It is also appreciated that our description of tasks as representative of particular domains is rationally based, rather than empirically based. However, this categorization was only preliminary, serving to guide test selection. An empirical categorization of tasks was achieved in the present study, using principal components analysis.
The tasks chosen to represent each domain are shown in Table 1. More detailed descriptions of the tasks, and the metrics derived from them, may be found in the on-line Appendix.
Table 1.
Performance of five subject groups on 18 executive function tests (means and SDs), with effect sizes and p-values based on one-way ANOVA.
| Proposed Domain | Test | Normal Control (CDR=0) | Amnestic MCI (CDR=.5) | Nonamnestic MCI (CDR=.5) | η2 | p | ||
|---|---|---|---|---|---|---|---|---|
| Single Domain | Multiple Domain | Single Domain | Multiple Domain | |||||
| Spontaneous Flexibility and Generativity | Alternate Uses Test (raw score) | 15.12 (5.65) | 11.86 (5.34) | 7.49 (5.83) | 11.27 (4.90) | 7.82 (5.60) | .244 | <.001 |
| Random Number Generation (written trial RNG + oral trial RNG) | 0.60 (0.09) | 0.65 (0.24) | 0.68 (0.21) | 0.73 (0.31) | 0.71 (0.25) | .052 | .041 | |
| Tinker Toy Test (raw score) | 9.19 (1.80) | 8.69 (1.98) | 7.31 (2.47) | 8.65 (1.79) | 7.25 (1.88) | .139 | <.001 | |
| Inhibition of Prepotent Responses | D-KEFS Stroop Test (inhibition trial scaled score) | 10.70 (2.23) | 10.26 (2.88) | 9.73 (3.94 | 9.38 (3.44) | 10.18 (3.01) | .024 | .349 |
| Hayling Test (total scaled score) | 5.22 (1.45) | 4.67 (1.55) | 3.89 (1.64) | 4.50 (1.68) | 3.71 (1.69) | .120 | <.001 | |
| Completions & Corrections Test total correct) | 11.06 (1.26) | 9.75 (1.95) | 9.18 (2.34) | 9.65 (1.81) | 9.18 (2.04) | .164 | <.001 | |
| Planning and Sequencing | Porteus Maze Test (test age) | 15.60 (1.74) | 15.07 (1.62) | 12.92 (3.50) | 13.83 (2.32) | 11.00 (3.86) | .254 | <.001 |
| D-KEFS Tower Test (total achievement scaled score) | 11.81 (2.59) | 10.17 (2.43) | 9.00 (3.04) | 9.77 (2.37) | 8.53 (3.26) | .175 | <.001 | |
| Tic-Tac-Toe (total score) | −4.57 (2.44) | −4.69 (1.85) | −4.76 (2.59) | −5.08 (1.65) | −5.47 (1.70) | .014 | .603 | |
| Concept/Rule Learning and Set Shifting | D-KEFS Sorting Test (confirmed sorts scaled score) | 13.16 (1.85) | 11.94 (2.51) | 10.67 (2.73) | 10.77 (2.41) | 10.12 (2.64) | .201 | <.001 |
| Brixton Test (scaled score) | 5.26 (2.12) | 4.64 (2.22) | 3.80 (2.06) | 4.73 (2.01) | 2.76 (1.79) | .124 | <.001 | |
| Verbal Concept Attainment Test (raw score) | 19.50 (2.47) | 17.33 (3.14) | 14.78 (4.03) | 16.96 (3.28) | 15.41 (3.20) | .263 | <.001 | |
| Decision-Making and Judgment | Stanford Binet Absurdities Test (raw score) | 29.47 (2.89) | 29.31 (2.05) | 25.93 (4.41) | 27.08 (2.94) | 23.88 (3.87) | .258 | <.001 |
| Iowa Gambling Test (advantageous selections on block 1 minus block 5) | 3.95 (6.45) | 4.36 (6.85) | 2.60 (5.52) | 1.77 (6.53) | 2.47 (6.38) | .022 | .403 | |
| Experimental Judgment Test (mean percent deviation) | 7.75 (4.79) | 10.25 (6.75) | 10.61 (5.58) | 6.68 (3.04) | 7.50 (4.43) | .081 | .004 | |
| Working Memory and Resource-Sharing | Trail Making Test (time on Part B minus time on Part A) | 39.15 (20.57) | 67.36 (14.31) | 78.64 (63.71) | 74.72 (43.21) | 94.53 (50.02) | .173 | <.001 |
| Brief Test of Attention (total correct) | 15.31 (3.28) | 14.31 (3.29) | 12.53 (4.23) | 14.12 (3.58) | 10.71 (3.46) | .143 | <.001 | |
| TEA Telephone Search While Counting (dual task decrement score) | 1.35 (1.81) | 1.48 (1.83) | 4.16 (5.36) | 1.58 (2.34) | 4.442 (5.48) | .130 | <.001 | |
The Johns Hopkins University Institutional Review Board fully reviewed and approved the study protocol. All participants and their study partners gave written informed consent to participate.
Statistical Analysis
The baseline demographic and clinical characteristics of the five groups (four MCI and one normal control) were compared with one-way ANOVA, with planned comparisons between: 1) NC versus all MCI, 2) NC versus AS, 3) AS + AM versus NAS + NAM, and 4) AS + NAS versus AM + NAM. Given the large number of comparisons, α for both the omnibus ANOVAs and the planned comparisons was set to .01; this was viewed as a compromise between risking type-I and type-II statistical error. One-way ANOVAs were also performed on each of the 18 executive function measures
To derive composite scores summarizing executive test performance, an exploratory principal components analysis (PCA) of the 18 EC variables was performed. The number of components retained was determined by examination of the scree function and by factor analysis fitted by the maximum likelihood method and using the Akaike Information Criterion (AIC) (Akaike, 1974). Since the distributions of many of the EC variables were highly skewed, the 18 variables were first transformed to probit scores using the percentile method [in which the ith sorted value is assigned the percentile z-score of (i/(n+1)) ×100, for each i=1, …, n] (Rosner & Glynn, 2007). These analyses were restricted to data from the MCI participants only, because large differences between the MCI and normal groups were found in descriptive analyses. The normal control group performed near test ceiling on several measures, producing clearly bimodal distributions for many of the tests. We restricted the PCA analysis to MCI subjects because our primary aim was to discover covariance among tests in MCI, rather than to identify scores that distinguish MCIs from controls. The derived components were subjected to orthogonal rotation using the varimax method and were standardized to have variance of 1 in order to maximize their interpretability. In light of our sample size and composition, we relied primarily on PCA rather than factor analysis to minimize reliance on model assumptions.
Component scores were computed for each participant using the principal components coefficients derived from analysis of the MCI subjects. Cronbach’s alpha was used to estimate the reliabilities of the derived component scores. The mean component scores of the five groups were compared using analysis of covariance, with the same four planned comparisons described earlier. Given the total of 5 tests per component score (the omnibus comparison plus 4 planned comparisons), α was set to .01 (.05 divided by 5 = .01). In light of the descriptive nature of our study, this was seen as a reasonable compromise between risking type-I and type-II statistical errors.
RESULTS
Table 2 shows demographic and clinical characteristics for the five groups of participants. The groups were well matched for education, but they differed in age, with the NCs being slightly younger than the MCI groups (contrast = 3.55, t=3.11, df=187, p=.002). For reasons that are not clear, the sex distribution of the groups differed (χ2 =17.43 df = 4, p=.002); men predominated among the amnestic MCIs, while women predominated among the cognitively normal subjects. As expected, the NC group also had a lower mean total score on the CDR sum-of-boxes score (contrast = 1.20, t=13.40, df=186, p<.001) and on the ADL-PI (contrast = 3.00, t=5.62, df=167, p<.001) than the MCI groups. They also had a higher mean MMSE score than the MCI groups (contrast = 1.11, t=7.08, df=187, p<.001). Also not surprisingly, the two single-domain groups (AS and NAS) were less impaired overall than the two multiple-domain groups (AM and NAM). This contrast was significant for the CDR sum-of-boxes (contrast = 0.37, t=2.69, df=186, p=.001), MMSE (contrast = 0.56, t=2.65, df=187, p=.009) and ADL-PI (contrast = 2.12, t=3.25, df=167, p=.002). Geriatric Depression Scale score was higher among MCIs than among normal elderly (contrast = 1.15, t=3.28, df=180, p=.001). None of the contrasts comparing amnestic to nonamnestic MCI patients (pooling over single- and multiple-domain subtypes) on demographic and clinical characteristics was significant.
Table 2.
Demographic and clinical characteristics of study participants. Means (± SE), except as noted. For continuously distributed variables, effect sizes are eta-squared and p-values are based on one-way ANOVA. For frequency counts (sex) and percentages (prevalence of health conditions), effect sizes are Cramer’s V and p-values are based on Pearson’s chi-squared tests.
| Normal Control (CDR=0) | Amnestic MCI (CDR=.5) | Nonamnestic MCI (CDR=.5) | η2 or V | p | |||
|---|---|---|---|---|---|---|---|
| Single Domain | Multiple Domain | Single Domain | Multiple Domain | ||||
| N | 68 | 36 | 45 | 26 | 17 | ||
| Age, years | 72.41 (SD=7.25) | 75.08 (SD=5.69) | 78.33 (SD=7.66) | 74.81 (SD=8.62) | 75.59 (SD=7.69) | .087 | .002 |
| Education, highest grade completed | 15.93 (SD=2.49) | 15.92 (SD=2.17) | 15.93 (SD=2.50) | 15.42 (SD=2.73) | 15.18 (SD=2.16) | .011 | .714 |
| Sex, male:female | 27:41 | 26:10 | 28:17 | 14:12 | 4:13 | .301 | .002 |
| Clinical Dementia Rating, sum of boxes | 0.03 (0.01) | 0.89 (0.09) | 1.46 (0.12) | 1.21 (0.15) | 1.41 (0.18) | .528 | <.001 |
| Mini-Mental State Exam, score | 29.26 (0.11) | 28.33 (0.19) | 28.11 (0.18) | 28.54 (0.27) | 27.65 (.27) | .208 | <.001 |
| Activities of Daily Living - Prevention Instrument, score | 0.73 (0.18) | 3.17 (0.60) | 3.80 (0.63) | 2.17 (0.58) | 6.71 (1.80) | .195 | <.001 |
| Geriatric Depression Scale, score | 1.24 (0.26) | 2.12 (0.39) | 2.76 (0.39) | 1.96 (0.35) | 2.74 (0.52) | .075 | .007 |
| Prevalence of Specific Health Conditions (percent of subjects): | |||||||
| Hypertension | 44.8 | 47.1 | 54.6 | 34.8 | 47.1 | .119 | .647 |
| Hypercholesterolemia | 38.8 | 40.0 | 33.3 | 55.6 | 46.7 | .137 | .587 |
| Peripheral Vascular Disease | 5.8 | 3.6 | 2.6 | 5.9 | 0 | .098 | .838 |
| TIA | 0 | 0 | 2.8 | 5.3 | 6.7 | .174 | .321 |
| Seizure | 0 | 0 | 2.7 | 0 | 0 | .143 | .541 |
| Minor Head Injury | 3.5 | 3.1 | 11.9 | 8.7 | 0 | .174 | .270 |
| Cancer | 8.5 | 6.3 | 7.1 | 0 | 0 | .132 | .572 |
| Diabetes | 13.1 | 6.3 | 16.3 | 16.0 | 5.9 | .193 | .608 |
| Kidney Disease | 1.6 | 0 | 2.4 | 0 | 0 | .094 | .817 |
| Gastrointestinal Disease | 18.0 | 11.1 | 13.5 | 33.3 | 26.7 | .183 | .296 |
| Depression | 4.8 | 2.9 | 33.3 | 15.4 | 11.8 | .349 | <.001 |
| Anxiety | 1.6 | 2.9 | 10.0 | 0 | 0 | .205 | .111 |
Generally speaking, the groups were comparable in their medical histories (see Table 2). The only exception was in self-reported history of depressive disorder (χ2= 22.30, df = 4, p<.001, Cramer’s V = .349). Whereas 4.8% of the NC subjects and 2.9% of the AS patients described histories of depressive disorder, these figures rose to 11.8% in the NAM group, 15.4% in the NAS group, and 33.3% in the AM group.
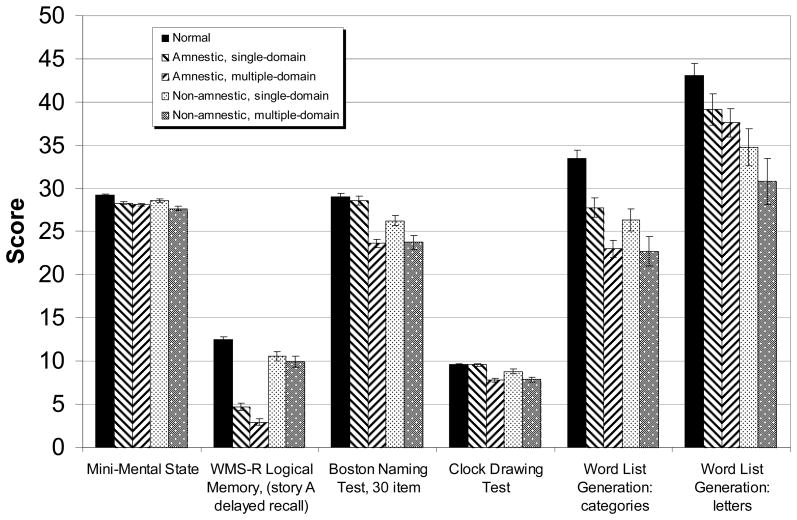
Performance on the neuropsychological screening battery that was used to determine group assignment is shown in Figure 2. The groups differed in expected ways, with statistically significant differences among the 5 groups (p<.001) on all tasks. Effect sizes (η2) ranged from .136 on word list generation to letter cues to .682 on delayed recall of the Logical Memory passages. These differences are to be expected, of course, as the groups were constituted based on subjects’ performances on these tests.
Figure 2.
Performance of five subject groups on neuropsychological screening battery. Age- and education-adjusted means ± standard errors. Note that although the data are drawn on one set of axes, the possible range of scores for the tests differ.
PCA of the 18 executive function variables yielded six components with eigenvalues greater than 1.0, together accounting for 63% of the variance. However, inspection of the scree function and the factor analysis results suggested that models with three components fully accounted for the shared covariance among the measures. Thus, we opted for a three-component solution, which accounted for 44% of the variance.
Fifteen of the 18 tests loaded highly (≥.50) on one of the three rotated components. Tests from four of our six putative domains load highly on the first component (see Table 3), which may be a relatively general factor. We have labeled it “planning/problem-solving” to capture its contributions from tests requiring strategy formation and application as well as those requiring creativity and the production of novelty. Tests requiring multiple tracking, divided attention, and inhibitory control load significantly on component two, which is labeled here “working memory.” Finally, the Iowa Gambling Task and the Experimental Judgment Test load highly and specifically on the third component, which we are labeling “judgment.”
Table 3.
Results of principal components analysis of executive function tasks (correlations between z-transformed test scores and components). Varimax rotated components. For clarity of presentation, only correlations ≥ 0.50 are shown.
| Proposed Domain | Test | Component | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| 18% of variance Planning/Problem-Solving | 17% of variance Working Memory | 8% of variance Judgment | ||
| Spontaneous Flexibility and Generativity | Alternate Uses Test (raw score) | 0.504 | ||
| Random Number Generation (written trial RNG + oral trial RNG) | −0.594 | |||
| Tinker Toy Test (raw score) | 0.766 | |||
| Inhibition of Prepotent Responses | D-KEFS Stroop Test (inhibition trial scaled score) | 0.655 | ||
| Hayling Test (total scaled score) | ||||
| Completions & Corrections Test (total correct) | 0.639 | |||
| Planning and Sequencing | Porteus Maze Test (test age) | 0.617 | ||
| D-KEFS Tower Test (total achievement scaled score) | 0.661 | |||
| Tic-Tac-Toe (total score) | ||||
| Concept/Rule Learning and Set Shifting | D-KEFS Sorting Test (confirmed sorts scaled score) | 0.507 | ||
| Brixton Test (scaled score) | 0.706 | |||
| Verbal Concept Attainment Test (raw score) | ||||
| Decision-Making and Judgment | Stanford Binet Absurdities Test (raw score) | 0.751 | ||
| Iowa Gambling Test (advantageous selections on block 1 minus block 5) | 0.655 | |||
| Experimental Judgment Test (mean percent deviation) | 0.726 | |||
| Working Memory and Resource-Sharing | Trail Making Test (time on Part B minus time on Part A) | −0.693 | ||
| Brief Test of Attention (total correct) | 0.662 | |||
| TEA Telephone Search While Counting (dual task decrement score) | −0.548 | |||
Within the MCI group, Cronbach’s alpha coefficients were 0.73, 0.72, and 0.34 for components one, two and three, respectively. For the entire sample, the reliabilities were 0.76, 0.76, and 0.22. Thus, the planning/problem-solving and working memory components had reasonably high internal consistency reliability. While the reliability of the judgment component was low, we choose to report it because of its clear interpretability. However, we recognize that ability to detect meaningful associations with the judgment factor are limited by the measure’s low reliability.
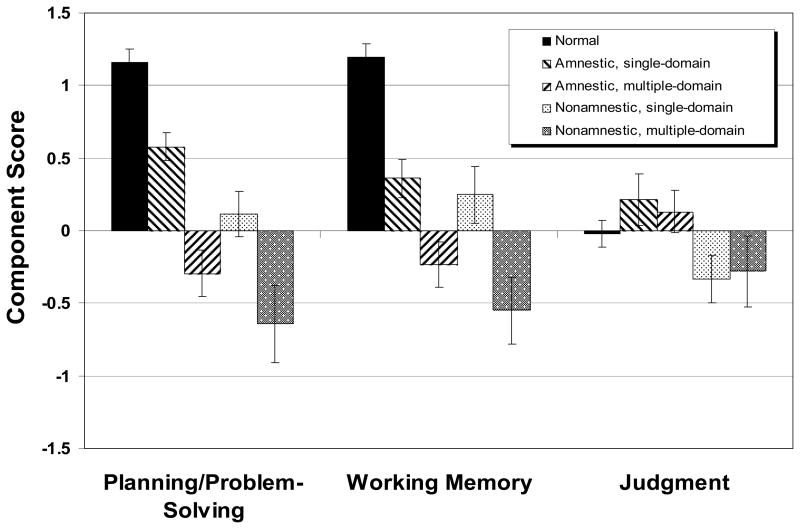
The mean score on each of the executive function components for each group of participants is shown in Figure 3. A regression model, with age, sex, and MMSE scores as covariates, was computed on each of the three components. For component 1, there was a significant effect of group (F=15.38, df=4,184, p<.0001, adjusted R2=.451). The results of the four planned contrasts appear in Table 4. The MCI patients, as a whole, performed less well than the normal control subjects. Even the AS group, the least impaired MCI subgroup overall, was severely impaired in planning/problem-solving compared to normal subjects. The difference between amnestic and nonamnestic MCI patients in this EC domain was not statistically significant, but the multiple-domain patients performed less well than single-domain patients.
Figure 3.
Scores of normal control subjects and four groups of MCI participants on three executive functioning summary scores derived from principal components analysis. The component scores were derived from data of MCI subjects only. Means ± standard errors.
Table 4.
Results of ANCOVA on three executive function components and significance level and effect sizes (ES), in SD units, for planned contrasts.
| Component 1: Planning/Problem-Solving | Component 2: Working Memory | Component 3: Judgment | |
|---|---|---|---|
| Overall model, with age, sex, and MMSE as covariates | F(4,184)=15.38 p<.001 Adjusted R2=.451 |
F(4,184)=10.61 p<.001 Adjusted R2=.493 |
F(4,184)=1.42 p=.230 Adjusted R2=.044 |
| Contrasts: | |||
| Normal v. all MCI | ES=.955, p<.001 | ES=.816, p<.001 | N/A |
| Normal v. Amnestic Single MCI | ES=.464, p=.009 | ES=.486, p=.005 | N/A |
| Amnestic v. Nonamnestic MCI | ES=.278, p=.079 | ES=.276, p=.073 | N/A |
| Single v. Multiple-Domain | ES=.550, p=.001 | ES=.480, p=.002 | N/A |
For component 2, working memory, the five groups differed significantly (F=10.61, df=4,184, p<.0001), with the normal subjects again out-performing the MCI patients as a group. As in Component 1, the AS group differed from the normal group, and single-domain patients outperformed multiple-domain patients, but the amnestics did not outperform the nonamnestics.
For component 3, judgment, the five groups did not differ significantly (F=1.42, df=4,184, p=.230). Therefore, no planned comparisons were undertaken.
DISCUSSION
There are four major findings of this study. First, using a broad array of clinical tests and experimental tasks, we found moderate support for the existence of two highly reliable domains of executive functioning -- planning/problem-solving and working memory -- among elderly persons with MCI, and a less reliable third domain, judgment. Second, we found planning/problem-solving and working memory, but not judgment, to be selectively impaired in MCI compared to cognitively normal elderly. Third, even patients with “pure” amnestic MCI, the least impaired subgroup overall, displayed major impairments in these two executive domains. Finally, multiple-domain MCI patients (i.e., those with deficits in at least two domains [of episodic memory, language and spatial cognition]) have more significant planning/problem-solving and working memory deficits than single-domain patients.
Much of the previous research on the latent structure of executive cognition, including the elegant work of Miyake and colleagues on inhibitory control (Friedman & Miyake, 2004), used data from young normal subjects performing experimental paradigms. Few previous studies have used latent structure methods to determine the components of executive functions in the elderly. Fisk & Sharp (2004) studied normal subjects ranging from age 20–81 and found evidence for Miyake’s three factors (updating, shifting, and inhibition), plus a fourth factor, access, which reflected efficiency in accessing long-term memory. Lamar et al. (2002) performed a PCA of data from a large collection of cognitive tests administered to 417 nondemented elderly in the Baltimore Longitudinal Study on Aging. Tests of executive functioning featured prominently in their battery. Two components of their four-component solution were interpreted as primarily executive. One, labeled “sustained attention and mental tracking,” had particularly high loadings for both parts A and B of the TMT. The other, “brief attention and mental manipulation,” had high loadings on forward and backward digit span. Both these factors appear most highly related to the working memory component found in the present study. Rodríguez-Aranda & Sundet (2006) reported evidence for four executive factors (cognitive flexibility, speed of processing, word production, and loss of set) in their neurocognitive test data from 101 normal older adults. However, they included only four tests in their analysis, and multiple measures from each test, resulting in possibly spurious results.
Based on a principal components analysis of data from AD patients, Bondi et al. (2002) reported that the WCST and part B of the TMT loaded on a common executive factor. The WCST has characteristics in common with both the D-KEFS Sorting Test and the Brixton Test used in the present study, which loaded on planning/problem-solving and working memory, respectively. The TMT also loaded on working memory. Bondi and colleagues found that the Stroop Color-Word Test covaried with tests requiring rapid visual processing and visuomotor sequencing rather than executive cognition per se. It is not unreasonable to assume that the more advanced neuropathology of AD alters the relationship among cognitive mechanisms, thereby contributing to differences in the structure of executive control found by Bondi and colleagues and the present study.
Our principal components solution accounted for a modest 43% of the variance among measures. While this figure is somewhat lower than that obtained in some other studies (e.g., 86% in Lamar et al., 2002), we were intentionally conservative in our interpretation of the PCA results and our selection of a solution. Deciding the number of components to be retained in a PCA is a controversial issue, and several methods have been proposed. Perhaps the most commonly employed method, the Kaiser criterion, involves retaining all components with eigenvalues ≥ 1.0. We opted against this method as overly liberal (i.e., indicating a Astructure@ where the evidence is weak). There must be eigenvalues ≥ 1 in any correlation-based principal components analysis (given that the mean eigenvalue is always 1 in such analyses), even when all items are truly independent of each another. We also opted against a formal factor analysis to determine the number of components because of our modest sample size. In contrast, the scree criterion we employed estimates the number of systematic dimensions of shared covariation, and the AIC criterion does not rely on thresholds (as formal tests do) which may not be valid with small samples. Thus, we regarded the scree and AIC criteria as most suitable to determining the number of dimensions in this study. We find the convergence between them reassuring, and we believe our selection is appropriately reproducible while allowing for meaningful test structure to be detected.
While several previous investigations have reported impairments in executive cognition among MCI patients, most included very small samples and did not consider the heterogeneity of MCI (Winblad et al., 2004). Crowell et al. (2002) reported that 25 MCI patients -- defined using Petersen=s (2000) criteria but requiring that memory be below only −1 SD, and not further subtyped -- performed less well than 22 normal elderly on part B of the TMT, part B minus part A (the same metric used in the present study), and backward digit span. The MCI patients performed normally on tests of language, constructional praxis, and psychomotor speed. These authors concluded that executive dysfunction is frequently a second deficit in patients who present with “selective” memory impairment, and recommend that future studies sample a wider range of “both traditional and nontraditional executive measures.” Kramer et al. (2006) identified 22 MCI patients with isolated memory impairments based on stringent CDR criteria (not a combination of clinical and psychometric criteria, as in the present study). They found that these patients performed more poorly than normal elderly on a modification of the TMT, the Stroop Test, and word-list generation (animal fluency). They concluded that exclusively amnestic MCI patients are probably extremely rare, and that concomitant executive dysfunction is common. They recommended comprehensive cognitive assessments for all MCI patients, even when patients and families report only memory decline. Albert et al. (2007) found that scores on an executive functioning factor were lower at baseline in MCI patients who subsequently converted to dementia than in normal subjects or in MCI patients who declined but didn’t convert. Rate of change in executive cognition over longitudinal assessments was greatest among those MCIs who subsequently converted.
A major finding of the present study is that the impairment of executive cognition in MCI is not global; only certain empirically-defined domains are affected. This is consistent with previous observations that some specific executive tests are performed normally and others are impaired in MCI. For example, Traykov et al. (2007) found their 20 MCI patients to be impaired on the Stroop Test and a modified WCST, but normal on the TMT, the Bells Test (a visual search task) and the WAIS Digit Symbol subtest. The authors conclude that response inhibition, switching, and cognitive flexibility are selectively impaired, while sustained and divided attention are intact. Zhang et al. (2007) reported that the TMT, Porteus Maze Test, and verbal fluency tests -- which they described as measures of planning -- were impaired among 32 MCI patients, whereas no-go accuracy, Stroop task performance, and negative priming -- described as measures of inhibition -- were not. In the present study, the Porteus Maze Test contributed to a planning/problem-solving factor, and the TMT and Stroop loaded together on a working memory factor, both of which were impaired among MCI patients. Differences in assignment of tasks to domains (done empirically in our study and conceptually in the Traykov and Zhang studies) may be a primary reason for the apparent discrepancies.
No previous study of EC in either normal or cognitively-impaired elderly has identified a specific factor related to judgment. Judgment, especially as it involves risk-taking and decision-making, appears to rely on neural circuitry (orbitofrontal cortex and its striatal, thalamic, and limbic connections) that is distinguishable from that supporting planning/problem-solving and working memory (primarily dorsolateral prefrontal cortex) (Rogers et al., 1998, 1999). Our finding that judgment is selectively spared in MCI may suggest that orbitofrontal cortex is largely unaffected in these patients, although the low reliability of our judgment component score dictates extreme caution in its interpretation.
Few previous studies of executive cognition in MCI have considered differences among MCI subtypes. In the present study, all four MCI subtypes, even the group with “pure” memory impairment, displayed deficits in planning/problem-solving and working memory, and multiple-domain patients were more severely impaired than single-domain patients. In contrast, neither the 10 amnestic nor the 28 “multiple cognitive deficits” MCI patients in the Cardiovascular Health Study were found to have impairments on a composite executive function measure (Lopez et al., 2006). The specifics of the sample characteristics and cognitive tests employed are likely responsible for the differences among studies.
The present study found differences in the lifetime prevalence of major depression among our groups, with the highest rate (33.3%) in the amnestic multiple-domain group. Other investigators have also found an association of depression with MCI (Jorm, 2000; Lopez et al., 2003), although differences in prevalence by MCI subtype has not, to our knowledge, been previously reported. There is a large and complex literature on neuropsychological deficits associated with late-life depression (Steffans et al., 2006), and several studies suggest that executive function deficits predominate (Lockwood et al., 2000; Butters et al., 2004). However, we do not believe the executive functioning deficits of our patients can be accounted for entirely by their histories of affective disorder, since our MCI group with the most severe executive impairment (NAM) had an only modestly elevated lifetime prevalence of depression (11.8%). Additional studies are clearly needed to resolve this issue.
Needless to say, the findings of the present study are not definitive and require replication. First, our MCI sample was recruited largely from other research studies and from memory disorder clinics rather than from population screening. Although this undoubtedly results in some selection bias, we suspect that our sample is quite comparable to most MCI samples described in the clinical literature. Second, the specific criteria we used for diagnosing MCI and classifying patients into subtypes may be questioned. Although our screening battery was composed of frequently-used, standardized neuropsychological tests, it was admittedly very brief, and “single-domain” deficits were identified by failure of single tests. In addition, we relied on the report of knowledgeable informants for the assessment of functional capacity rather than direct assessment of participants (as in Cahn-Weiner et al., 2000, 2002). However, we contend that our requirement of a cognitive test failure (score < 7th percentile) in the setting of a normal MMSE score and the report of borderline functioning by a knowledgeable informant (i.e., CDR=0.5) conforms to the current standard for the diagnosis of MCI (Winblad et al., 2004). And although our MCI groups differed from each other and from the normal control group on both the CDR sum-of-boxes score and ADL-PI score, with a particularly high ADL-PI score in the NAM group, their functional deficits were not of sufficient magnitude to interfere significantly with daily life or to merit the diagnosis of dementia.
Among the other limitations of this study was that we did not allow MCI to be defined by a selective impairment in executive control. This was done to avoid conflating the independent and the dependent variables. However, it does complicate the meaning of “single-domain” (memory, language, or spatial cognition) MCI, as defined in this study. Another limitation of the study is its modest sample size for multivariate statistical approaches. Given the large number of executive function measures we employed (18), basing a principal components analysis on data from 124 subjects may result in a somewhat unstable structure. Because of this, we chose a relatively conservative approach to analysis.
A major implication of this study is that whether patients have impairment in memory or some other cognitive domain (language or spatial cognition) is less important in predicting their executive functioning (and, hence, their vulnerability to everyday functional impairment) than whether they are impaired in only one or more than one domain. It is our prediction that patients with multiple-domain MCI are at higher risk for the development of dementia, or to develop it sooner, than patients with pure amnestic MCI. This is supported by the findings of several recent studies (Alexopoulos et al., 2006; Rasquin et al., 2005; Tabert et al., 2006), but not all (Fischer et al., 2007; Yaffe et al., 2006). Continued follow-up of the participants in this study will allow us to test this prediction.
Supplementary Material
Acknowledgments
The authors thank Laura Wulff, Ph.D., Chiadi Onyike, M.D., and the staff and participants of the Johns Hopkins Alzheimer=s Disease Research Center. Arnold Bakker, M.S. and Egberdina J. van der Hulst assisted in data collection. This study was supported by grant AG-005146 from the National Institute on Aging.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/neu.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;6:716–723. [Google Scholar]
- Albert M, Blacker D, Moss MB, et al. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. Journal of the International Neuropsychological Society. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Alexopoulos P, Grimmer T, Perneczky R, et al. Progression to dementia in clinical subtypes of mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2006;22:27–34. doi: 10.1159/000093101. [DOI] [PubMed] [Google Scholar]
- Attix DK, Welsh-Bohmer KA, editors. Geriatric neuropsychology: assessment and intervention. New York: Guilford Press; 2006. [DOI] [PubMed] [Google Scholar]
- Axelrod BN, Millis SR. Preliminary standardization of the Cognitive Estimation Test. Assessment. 1994;1:269–274. [Google Scholar]
- Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer’s disease. Brain. 1991;114:2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Logie R, Bressi S, Della Sala S, Spinnler H. Dementia and working memory. Quarterly Journal of Experimental Psychology. 1986;38A:603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. Journal of Neuroscience. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-McGinty S, Podell K, Franzen M, et al. Standard measures of executive function in predicting instrumental activities of daily living in older adults. International Journal of Geriatric Psychiatry. 2002;17:828–834. doi: 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- Binetti G, Magni E, Padovani A, et al. Executive dysfunction in early Alzheimer=s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1996;60:91–93. doi: 10.1136/jnnp.60.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Monsch AU, Galasko D, et al. Preclinical cognitive markers of dementia of the Alzheimer type. Neuropsychology. 1994;8:374–384. [Google Scholar]
- Bondi MW, Serody AB, Chan AS, et al. Cognitive and neuropathologic correlates of Stroop Color-Word Test performance in Alzheimer=s disease. Neuropsychology. 2002;16:335–343. doi: 10.1037//0894-4105.16.3.335. [DOI] [PubMed] [Google Scholar]
- Bornstein RA. A factor analytic study of the Verbal Concept Attainment Test. Journal of Clinical Neuropsychology. 1982;4:43–50. doi: 10.1080/01688638208401115. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Leason M. Effects of localized lesions on the Verbal Attainment Test. Journal of Clinical and Experimental Neuropsychology. 1985;7:421–429. doi: 10.1080/01688638508401274. [DOI] [PubMed] [Google Scholar]
- Brandt J, Mellits ED, Rovner B, et al. Relation of age at onset and duration of illness to cognitive functioning in Alzheimer=s disease. Neuropsychiatry, Neuropsychology and Behavioral Neurology. 1989;2:93–101. [Google Scholar]
- Brandt J. Tic-Tac-Toe as a simple test of planning and problem-solving for use in dementia research. 2008 Manuscript in preparation. [Google Scholar]
- Bravo G, Hébert R. Age- and education-specific reference values for the Mini-Mental and modified Mini-Mental State Examinations derived from a non-demented elderly population. International Journal of Geriatric Psychiatry. 1997;12:1008–1018. doi: 10.1002/(sici)1099-1166(199710)12:10<1008::aid-gps676>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Brugger P, Monsch AU, Salmon DP, Butters N. Random number generation in dementia of the Alzheimer type: A test of frontal executive functions. Neuropsychologia. 1996;34:97–103. doi: 10.1016/0028-3932(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. The Hayling and Brixton Tests Manual. Bury St. Edmunds, England: Thames Valley Test Company Limited; 1997. [Google Scholar]
- Burgess PW. Theory and methodology in executive function research. In: Rabbitt P, editor. Methodology of frontal and executive function. East Sussex: Psychology Press; 1997. pp. 81–116. [Google Scholar]
- Burgess PW, Alderman N, Evans J, et al. The ecological validity of tests of executive function. Journal of the International Neuropsychological Society. 1998;4:547–558. doi: 10.1017/s1355617798466037. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61:587–596. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner D, Malloy PF, Boyle PA, et al. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. The Clinical Neuropsychologist. 2000;14:187–195. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Applied Neuropsychology. 2002;9:187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- Chen P, Ganguli M, Mulsant BH, DeKosky ST. The temporal relationship between depressive symptoms and dementia. Archives of General Psychiatry. 1999;56:261–266. doi: 10.1001/archpsyc.56.3.261. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Nasreddine Z, Joanette Y, et al. Mild cognitive impairment and cognitive impairment, no dementia: Part A, concept and diagnosis. Alzheimer=s & Dementia. 2007;3:266–282. doi: 10.1016/j.jalz.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Crowell TA, Luis CA, Vanderploeg RD, et al. Memory patterns and executive functioning in mild cognitive impairment and Alzheimer=s disease. Aging, Neuropsychology, and Cognition. 2002;9:288–297. [Google Scholar]
- Crowley K, Siegler RS. Flexible strategy use in young children=s tic-tac-toe. Cognitive Science. 1993;17:531–561. [Google Scholar]
- Daly E, Zaitchick D, Copeland M, et al. Predicting Aconversion@ to AD using standardized clinical information. Archives of Neurology. 2000;57:675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System examiner=s manual. San Antonio: Psychological Corporation; 2001. [Google Scholar]
- Elias MF, Beiser A, Wolfe PA, et al. The preclinical phase of Alzheimer disease. Archives of Neurology. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Evans FJ. Monitoring attention deployment by random number generation: An index to measure subjective randomness. Bulletin of the Psychonomic Society. 1978;12:35–38. [Google Scholar]
- Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- Fisk JD, Merry HR, Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology. 2003;61:1179–1184. doi: 10.1212/01.wnl.0000089238.07771.c7. [DOI] [PubMed] [Google Scholar]
- Fisk JE, Sharp CA. Age-related impairment in executive functioning: updating, inhibition, shifting, and access. Journal of Clinical and Experimental Neuropsychology. 2004;26:874–890. doi: 10.1080/13803390490510680. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: A latent variable analysis. Journal of Experimental Psychology: General. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Galasko D, Bennett DA, Sano M, et al. ADCS Prevention Instrument Project: Assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer’s Disease and Associated Disorders. 2006;20:S152–169. doi: 10.1097/01.wad.0000213873.25053.2b. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Touchon J. Mild cognitive impairment is not a clinical entity and should not be treated. Archives of Neurology. 2005;62:1164–1166. doi: 10.1001/archneur.62.7.1164. [DOI] [PubMed] [Google Scholar]
- Gillespie DC, Evans RI, Gardener EA, Bowen A. Performance of older adults on tests of cognitive estimation. Journal of Clinical and Experimental Neuropsychology. 2002;24:286–293. doi: 10.1076/jcen.24.3.286.988. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Griffith HR, Belue K, Sicola A, et al. Impaired financial abilities in mild cognitive impairment. Neurology. 2003;60:449–457. doi: 10.1212/wnl.60.3.449. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Kaye K, Baxter J, et al. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. Journal of the American Geriatrics Society. 1998;46:590–596. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Morris JC. ADCS Cooperative Study: rate of dementia of the Alzheimer=s type (DAT) in subjects with mild cognitive impairment. Neurology. 1996;46:A403. [Google Scholar]
- Guilford JP, Christensen PR, Merryfield PR, Wilson RC. Alternate uses, Form B, Form C; manual of instructions and interpretations. Orange, California: Sheridan Psychological Services Inc; 1978. [Google Scholar]
- Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Sano M, Dooneief G, et al. Psychological detection and characterization of preclinical Alzheimer=s disease. Neurology. 1995;5:957–962. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- Jahanshshi M, Dirnberger G, Fuller R, et al. The role of the dorsolateral prefrontal cortex in random number generation: a study with positron emission tomography. Neuroimage. 2000;12:713–725. doi: 10.1006/nimg.2000.0647. [DOI] [PubMed] [Google Scholar]
- Jorm AF. Is depression a risk factor for dementia or cognitive decline? Gerontology. 2000;46:219–227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- Jorm AF. History of depression as a risk factor for dementia: an updated review. Australian and New Zealand Journal of Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Nelson A, Johnson JK, et al. Multiple cognitive deficits in amnestic mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2006;22:306–311. doi: 10.1159/000095303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss E, Paterson MB, Mack JL, et al. Reliability and validity of the Tinker Toy Test in evaluating individuals with Alzheimer=s disease. The Clinical Neuropsychologist. 1998;12:325–329. [Google Scholar]
- Lafleche G, Albert MS. Executive function deficits in mild Alzheimer=s disease. Neuropsychology. 1995;9:313–330. [Google Scholar]
- Lamar ML, Zonderman AB, Resnick S. Contribution of specific cognitive processes to executive functioning in an aging population. Neuropsychology. 2002;16:156–165. doi: 10.1037//0894-4105.16.2.156. [DOI] [PubMed] [Google Scholar]
- Lewis MS, Miller LS. Executive control functioning and functional ability in older adults. The Clinical Neuropsychologist. 2007;21:274–285. doi: 10.1080/13854040500519752. [DOI] [PubMed] [Google Scholar]
- Lezak MD. The problem of assessing executive functions. International Journal of Psychology. 1982;17:281–297. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- Lockwood KA, Alexopoulos GS, Kakuma T, Van Gorp WG. Subtypes of cognitive impairment in depressed older adults. American Journal of Geriatric Psychiatry. 2000;8:201–208. [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Jagust WJ, et al. Neuropsychological characteristics of mild cognitive impairment subgroups. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77:159–165. doi: 10.1136/jnnp.2004.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Cognition Study, part 1. Archives of Neurology. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Cognition Study, part 2. Archives of Neurology. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. Journal of the American Medical Association. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Manning KJ, Brandt J. ACompletions and Corrections@ as a test of executive control. Presented at the 34th Annual Meeting of the International Neuropsychological Society; Boston, Massachusetts. 2006. Feb, [Google Scholar]
- Milwain E. Mild cognitive impairment: further caution [Letter] The Lancet. 2000;355:1018. doi: 10.1016/S0140-6736(05)74764-4. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mondrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases risk of developing dementia of Alzheimer type. Archives of Neurology. 2004;61:1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer=s disease. Neurologica. 2000;15:93–101. [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Challenges of epidemiological studies of mild cognitive impairment. Alzheimer=s Disease and Associated Disorders. 2004;18:1–2. doi: 10.1097/00002093-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Porteus SD. Fifty year=s application. New York: Psychological Corporation; 1965. Porteus Maze Test. [Google Scholar]
- Rapp MA, Reischies FM. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE) American Journal of Geriatric Psychiatry. 2005;13:134–141. doi: 10.1176/appi.ajgp.13.2.134. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, et al. Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2007;21:20–30. doi: 10.1037/0894-4105.21.1.20. [DOI] [PubMed] [Google Scholar]
- Rasquin SMC, Lodder J, Visser PJ, et al. Predictive accuracy of MCI subtypes for Alzheimer=s disease and vascular dementia in subjects with mild cognitive impairment: A 2-year follow-up study. Dementia and Geriatric Cognitive Disorders. 2005;19:113–119. doi: 10.1159/000082662. [DOI] [PubMed] [Google Scholar]
- Reitan RM. The validity of the Trail-Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, Nimmo-Smith I. The Test of Everyday Attention (TEA) Manual. Bury St. Edmunds, England: Thames Valley Test Company; 1994. [Google Scholar]
- Rodríguez-Aranda C, Sundet K. The frontal hypothesis of cognitive aging: factor structure and age effects on four frontal tests among healthy individuals. Journal of Genetic Psychology. 2006;167:269–287. doi: 10.3200/GNTP.167.3.269-287. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience. 1999;20:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Sahakian BJ, Hodges JR, et al. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain. 1998;121:815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- Rosner B, Glynn RJ. Interval estimation for rank correlation coefficients based on the probit transformation with extension to measurement error correction of correlated ranked data. Statistics in Medicine. 2007;26:633–646. doi: 10.1002/sim.2547. [DOI] [PubMed] [Google Scholar]
- Rouleau I, Salmon DP, Butters N, et al. Quantitative and qualitative analyses of clock drawings in Alzheimer=s and Huntington=s disease. Brain and Cognition. 1992;18:70–87. doi: 10.1016/0278-2626(92)90112-y. [DOI] [PubMed] [Google Scholar]
- Royall DR, Cabello M, Polk MJ. Executive dyscontrol: An important factor affecting the level of care received by older retirees. Journal of the American Geriatric Society. 1998;46:1519–1524. doi: 10.1111/j.1532-5415.1998.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Cummings JL, et al. Executive control function: A review of its promise and challenges for clinical research. Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- Royall DR, Chiodo LK, Polk MJ. Misclassification is likely in the assessment of mild cognitive impairment. Neuroepidemiology. 2004;23:185–191. doi: 10.1159/000078504. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: The Freedom House Study. Journal of the American Geriatrics Society. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Heindel WC, Lange KL. Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer’s disease: Implications for the integrity of semantic memory. Journal of the International Neuropsychological Society. 1999;5:692–703. doi: 10.1017/s1355617799577126. [DOI] [PubMed] [Google Scholar]
- Schretlen D, Brandt J, Bobholz JH. Development and psychometric properties of the Brief Test of Attention. The Clinical Neuropsychologist. 1996;10:80–89. [Google Scholar]
- Steffens DC, Otey E, Georgeopoulos GS, Butters MA, Cuthbert B, Ganguli M, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Archives of General Psychiatry. 63:130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Archives of General Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. The Stanford-Binet Intelligence Scale: Fourth Edition, Guide for Administration and Scoring. Chicago, IL: Riverside Publishing Company; 1986. [Google Scholar]
- Towse JN, Neil D. Analyzing human random generation behavior: A review of methods used and a computer program for describing performance. Behavior Research 1998 [Google Scholar]
- Traykov L, Raoux N, Latour F, et al. Executive functions deficit in mild cognitive impairment. Cognitive and Behavioral Neurology. 2007;20:219–224. doi: 10.1097/WNN.0b013e31815e6254. [DOI] [PubMed] [Google Scholar]
- Visser P, Verhey F. Distinction between preclinical Alzheimer=s disease and depression. Journal of the American Geriatrics Society. 2000;48:479–484. doi: 10.1111/j.1532-5415.2000.tb04992.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Whitehouse PJ. Mild cognitive impairment – a confused concept? Nature Clinical Practice Neurology. 1007;3:62–63. doi: 10.1038/ncpneuro0403. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment -- beyond controversies, toward a consensus. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Petersen RC, Lindquist K, et al. Subtype of mild cognitive impairment and progression to dementia and death. Dementia and Geriatric Cognitive Disorders. 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Han B, Verhaeghen P, Nilsson LG. Executive functioning in older adults with mild cognitive impairment: MCI has effects on planning, but not on inhibition. Aging, Neuropsychology, and Cognition. 2007;14:557–570. doi: 10.1080/13825580600788118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.