Abstract
Mouse models take advantage of genetic manipulations that can be achieved in this species. There are currently two accepted mouse models of subarachnoid hemorrhage (SAH) and cerebral vasospasm (CVs). Both are technically demanding and labor intensive. In this study, we report a reproducible and technically feasible method to induce SAH, and subsequently CVs, in mice. We tested this model in multiple strains of mice that are commonly used for genetic manipulation.
Methods
SAH was induced in C57BL/6NCr, FVB, 129S1, BalbC and SJL mice, weighing 28–32 g, by an intracisternal vessel transection technique. Animals were perfused with India ink at 24 h postprocedure and vessel diameters were quantified. Brain slices were obtained for hematoxylin–eosin staining (H&E) to look for vascular changes consistent with CVs.
Results
There was no mortality during or after the procedure. Four of the five mouse strains showed significant CVs at 24 h postprocedure characterized by decreased vessel diameter of the middle cerebral artery close to the Circle of Willis. Histologically, the vessel wall displayed significant corrugation and thickening, consistent with CVs.
Conclusion
A novel mouse model to induce SAH is described and tested in several mouse strains. Four of the five strains used in this study developed CVs after the induction of SAH. The procedure is brief, straightforward, reproducible with low mortality, and applicable to commonly used background strains for genetically engineered mice.
Keywords: Subarachnoid hemorrhage, Vasospasm, Mouse models
1. Introduction
Cerebral vasospasm (CVs) after subarachnoid hemorrhage (SAH) is the most serious complication in patients who survive the first 24 h after SAH. The syndrome of CVs includes the progressive narrowing of the cerebral arteries with both a reversible vasoconstriction and static vascular wall thickening. Although there has been an extensive study of CVs for over 30 years, its etiology is still unclear.
There are two published mouse models of CVs after SAH. One model employs an endovascular perforation of the middle cerebral artery to produce the hemorrhage (Mesis et al., 2006; Parra et al., 2002). The second model is accomplished by the injection of femoral arterial blood into the occipital cisternal space (Lin et al., 2003). Both models produce blood vessel changes consistent with CV.
Murine models of human diseases have become important tools to investigate the pathophysiology of complicated biological systems. The ability to isolate a single gene product and investigate the effect of nullifying or augmenting its function has offered many new insights into disease processes. A significant problem to efficiently evaluating gene products in mouse models is the variability of responses in genetically inbred strains of mice. Because gene modifications can be made in multiple mouse strains, models of disease in mice either have to be present in the strain with the alteration or the genetic alteration must be bred into the mouse strain in which the model is used. This can be a significant impediment to rapid evaluation of complicated systems by evaluating multiple gene products.
In this study, we present a modification of the Lin mouse model of SAH (Lin et al., 2003). This procedure has the advantage of requiring surgery at only one site. In addition, we tested our model in five of the most commonly used strains of mice for genetic alteration. We find morphometric and histologic similarity with the other procedures and human CV.
2. Materials and methods
All experimental procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee (IACUC). Male C57BL/6NCr (n = 29), FVB (n = 18), BalbC (n = 20), SJL (n = 20) and 129S1 (n = 15) mice (Charles River, CA) weighing 28–32 g, 10–12 weeks of age were used for this study. Mice were anesthetized using chloral hydrate (350 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and received buprenorphine 0.1 mg/kg i.p. for analgesia postoperatively.
2.1. Induction of SAH
This model of SAH was created using a modification of the model reported by Lin and colleagues (Acalovschi et al., 2003). The aim of the new procedure was to simplify the technique by removing the need for femoral catheterization. During the surgical procedure, the body temperature was maintained at 37 °C using a heating pad. Briefly, under general anesthesia, animals were placed in prone position. A mechanical support was placed under the clavicles to position the head downward at a 30° angle. Using a surgical microscope (Zeiss, Germany), the posterior cervical muscles were dissected through a suboccipital midline skin incision, and retracted laterally. The transparent atlanto-occipital membrane was exposed, and an underlying intracisternal vein was consistently visualized. The membrane was penetrated by a 30 G needle. The vein was transected using a pair of microjeweler's forceps and allowed to bleed with the head downward until tenting of the membrane was observed (Fig. 1). The two edges of the pierced membrane were approximated using forceps to prevent blood leak. Once the membrane tenting was observed, the forceps were removed (no leak was observed in any animal once the forceps were removed). The muscle layers were approximated and the skin was sutured. Animals were kept with the head down for 10 min allowing the blood to disseminate down through the subarachnoid space. Animals were then transferred to an incubator until they were fully recovered from anesthesia and then to their home cages.
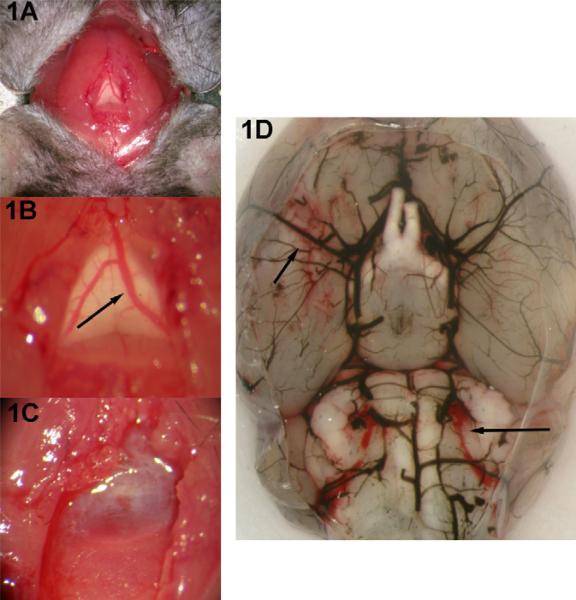
Fig. 1.
Procedure for mouse model of SAH. (A) Initial exposure consists of midline suboccipital incision and lateral retraction of strap muscles. (B) A closer view of the underlying transparent atlanto-occipital membrane with the visible subarachnoid vein (arrow). (C) After transection of the vein, blood quickly fills the subarachnoid space. The muscles and skin are closed on one layer and the animals are recovered. (D) After 24 h, there is evidence of blood in the basilar cisterns (arrows). The blood vessels are casted with India ink.
To determine if our model was similar to that of Lin et al. (2003), we compared our model to the published model with small modifications. Briefly, under general anesthesia, C57BL/6NC mice were placed in prone position with head down. Using a surgical microscope (Zeiss, Germany), the transparent atlanto-occipital membrane was exposed. Concurrently, a syngeneic sibling mouse was used as a donor for arterial blood by transcutaneous left ventricular cardiac puncture into a non-heparinized syringe. The atlanto-occipital membrane of the recipient mouse was penetrated by a 30 G needle attached to the blood-filled syringe. 50 μl of blood was injected slowly with the head angled downward. The muscle layers were approximated and the skin was sutured. Animals were kept with the head down for 10 min allowing the blood to disseminate down through the subarachnoid space. Animals were then transferred to an incubator until they were fully recovered from anesthesia.
An identical procedure was followed in the sham animals, with the exception of the vein transection or blood infusion. Instead, a 30 G needle was inserted through the atlanto-occipital membrane, and 50 μl of normal saline was slowly injected.
Neurological symptoms were evaluated by direct observation of spontaneous activity after the recovery from anesthesia. The evaluation was recorded immediately upon recovery and at 24 h postoperatively as either normal activity, weakness on gait without obtundation, or obtundation (with or without weakness).
2.2. Evaluation of CVs
After 24 h, animals were re-anesthetized with pentobarbital (6 mg/kg i.p.), and transcardially perfused with 20 ml saline, followed by 20 ml of 4% paraformaldehyde, and subsequently 10 ml of 5% India ink in gelatin. Animals were then decapitated and the brains were carefully removed preserving the vasculature. The Circle of Willis and the brain stem vasculature were examined under the surgical microscope and relevant pictures were captured using a microscope-mounted camera (Leica, DM4000M, Wetzler, Germany). Image analysis was conducted with Adobe Photoshop 9.0 (San Jose, CA).
Although CV was noted throughout the Circle of Willis and in the basilar artery when it was apparent, the intra-animal variability of the basilar artery made quantitative comparison difficult. Therefore, the diameter of the MCA segment was measured at the point 1 mm from the posterior wall of the carotid artery into the MCA, and the values from SAH and sham animals were compared. In addition, to assess the incidence of CVs, we arbitrarily assigned any vessel diameter that was more than one standard deviation lower than the sham mean as CVs.
2.3. Histopathological study
We evaluated the morphology of the blood vessels in CVs by histology in C57BL/6NCr mice. After perfusion with paraformaldehyde, the mice were decapitated, and the heads were incubated in 2% formic acid in 4% paraformaldehyde for 24 h to decalcify the skull. The entire skull was then sectioned at 10 μm thick slices. Sections were stained with hematoxylin and eosin.
2.4. Statistics
All statistical analysis was performed using Graphpad Instat 3.05 and Graphpad Prism 5 (Graphpad Software Inc., San Diego, CA). Differences in the means of arterial diameters were compared with unpaired Student's t-test. Because diameters greater than the mean are not physiologically relevant, only the lower tail was considered and concordantly one-tailed t-tests were used for vessel diameter comparisons. A value of p < 0.05 was considered statistically significant for all comparisons.
To evaluate the incidence of CVs, we defined CVs any vessel diameter that was more than one standard deviation lower than the sham mean. The animals were grouped into four categories using the following variables: presence or absence of CV and control or SAH treated animals. Differences were evaluated by Fisher's exact test. A value of p < 0.05 was considered statistically significant.
3. Results
Intraoperative and early postoperative temperatures (37 ± 0.01) and body weights (30 ± 1 g) did not differ between the groups. The procedure was straightforward and well tolerated by the animals. All animals recovered from surgery and returned to their baseline activity with no motor weakness on spontaneous gait. Gross pathological examination of the meninges and external surface of the brain revealed diffuse blood prominently over the brain stem and the area of the Circle of Willis (Fig. 1). There was no indication that the procedure caused any significant damage to the brain stem.
Our morphometric study of the arterial tree with India ink revealed significant narrowing in proximal arteries and segmental paucity of ink in the distal vasculature (Fig. 2A and C). Histological examination with H&E staining showed normal arterial wall features in control animals. Animals with SAH demonstrated significantly corrugated and thickened wall structure with decreased diameter on cross-section (Fig. 2B and D).
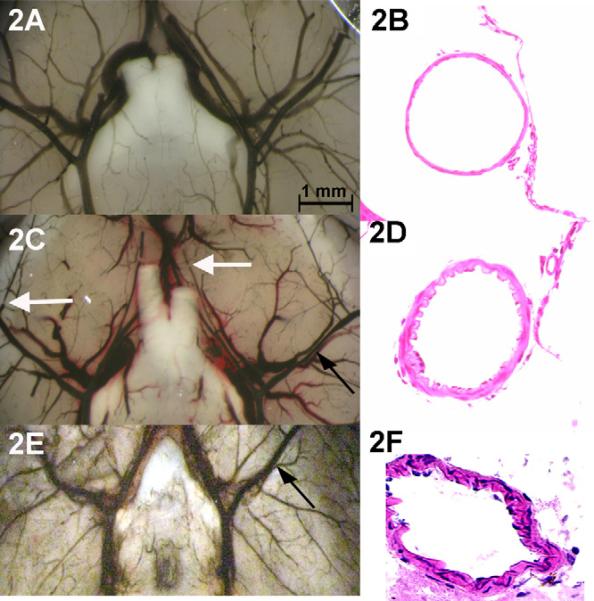
Fig. 2.
Evidence of CVs in an animal with SAH (A) compared to control (D). Note the segmental narrowing of the MCA (closed arrows) and the abrupt interruption of dye in medium vessels (open arrows). Vessels are casted with India ink. H&E preparation of meninges in control (B) shows normal blood vessel wall morphology. In SAH (E) the arterial smooth muscle layer of the MCA artery is thickened and the wall shows rogations consistent to the histology seen in human CVs. When compared to a model of arterial blood injection into the subarachnoid space, the degree of vasospasm by angiography was similar (C) and the arterial thickening was similar although more prominent in the smaller pial vessels than in the MCA (F).
Comparison of our venous transection model with the arterial injection model of Lin and colleagues, we found similar blood staining of the ventral brain matter, angiographic vasospasm and histological evidence vasculopathy by H&E staining (Fig. 2E and F). These data suggest that the model we propose is similar in the character of the SAH, degree of vessel narrowing after SAH and histological characteristics.
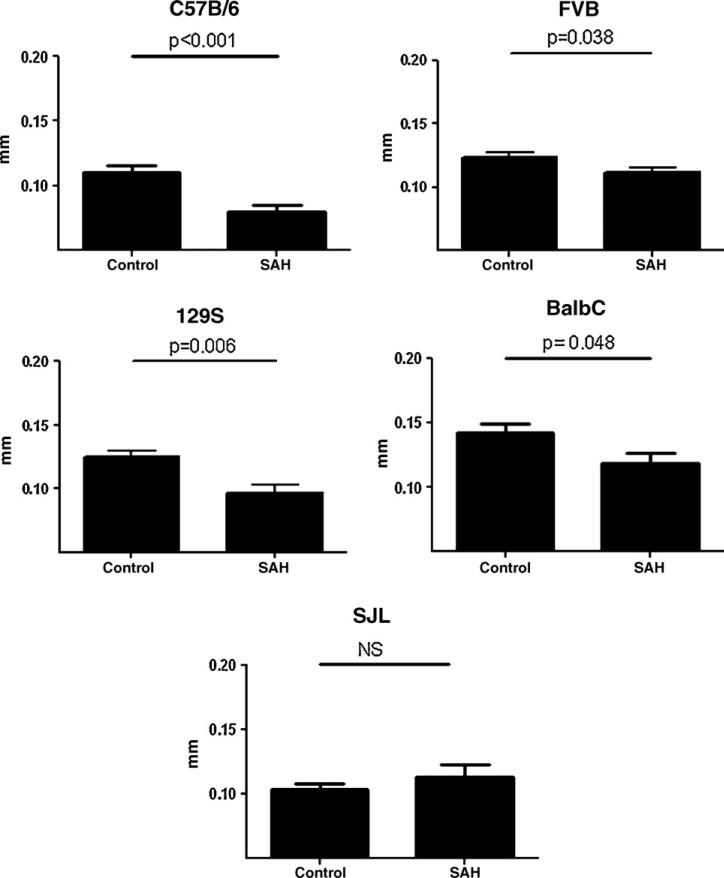
The mean vessel caliber in SAH animals was significantly decreased in C57BL/6NCr, FVB, BalbC, and 129S1 mice compared to controls. SJL mice did not have significantly smaller diameter vessels in SAH than control (Fig. 3). When we evaluated individual animals using an arbitrary cut point for CVs of vessel diameter more than 1 SD smaller than the mean control diameter, C57BL/6NCr, FVB, BalbC, and 129S1 mice with SAH showed significantly more CV than control. Again, SJL mice with SAH did not have more CV than control.
Fig. 3.
Measurement of MCA diameter in five strains of mice (C57B/6J, 129, FVB, BalbC, and SJL). All strains showed decreased mean diameters compared to control suggesting CVs except the SJL strain. Comparisons were made with one-tailed Student's t-test with a p < 0.05 considered significant.
Taken together, these data strongly support the effectiveness of this model for the study of CVs for most of the commonly used for genetic manipulation. Interestingly, SJL mice did not exhibit CVs with SAH.
4. Discussion
In this study, we present a reproducible method to induce SAH in mice that causes significant CVs. The procedure is technically straightforward, well tolerated by the animals and obviates the need for femoral catheterization. CVs was visible in proximal and distal branches of the Circle of Willis and displayed typical histological changes characteristic of CVs.
The major advantage of mouse models of SAH and CVs is the availability of genetically engineered animals that allows mechanistic evaluation of specific gene products. Mice are available commercially with transgenic, knock-out, or knock-in genotypes. In addition, there are a large number of antibodies and reagents available for the study of mice especially in the areas of inflammation and cellular energetics.
Two techniques, endovascular rupture and blood injection into the cistern, are reported for the induction of CVs in mice (Lin et al., 2003; Parra et al., 2002). Endovascular puncture is performed through insertion of a monofilament nylon suture into the carotid artery terminus. The suture is then advanced further to perforate the artery. The procedure is successful in eliciting CVs, although it is associated with mortality up to 29%, with an unpredictable pattern and amount of bleeding (Kamii et al., 1999).
Blood injection into the atlanto-occipital membrane is a relatively less lethal procedure in mice. In this method, autologous blood is withdrawn from a cannulated femoral artery or through cardiac puncture in a non-heparinized syringe and is injected into the atlanto-occipital membrane. This model elicits CVs reliably with a mortality of 3%.
In our hands, the most cumbersome aspect of the procedure is the femoral catheterization prior to injection. This requires the animal to be supine for the catheter placement and prone for the blood injection. We chose to do a cardiac puncture of a syngeneic sibling but this requires a second animal as a donor. In addition, the amount of blood injected into the brain in this model must be precise. Lin et al. (2003) noted in their work that volumes below 50 μl did not reliably elicit CVs while those above were associated with increased mortality. Our approach simplifies the procedure.
It is worth noting that the vessel transected in our model is a vein and not an artery. Although it is known that venous bleeding is less likely to cause CVs in human SAH, a very early model has used venous blood in susceptible animals (Bagley, 1928). The trend after this very early model focused on arterial blood to mimic the human condition. In the present study, the venous blood distributing into the basal cisterns was reliably associated with CVs. An added advantage to the vein transaction is that the bleeding does not occur at arterial or injection pressure. This, we believe limits the mortality. In fact we found no animal deaths in our study.
Important questions arise about the validity of our venous subarachnoid hemorrhage model considering the body of research that suggests that oxyhemoglobin contributes to the mechanism of CVs. Because our model is similar to the arterial injection model, we believe that it represents CVs as well as other models. It is true that there is more oxyhemoglobin in arterial blood than venous blood. Mouse blood has SvO2 of 0.05, which in a susceptible species may constitute enough oxyhemoglobin to catalyze the reaction toward vasospasm (Sasaki et al., 2006). In a very susceptible animal species, lower oxygen tension may be sufficient to develop vasospasm.
An important question for any animal model of human disease is how well it correlates with the human disease. Our model has very similar characteristics to those of the other published mouse models of CVs. As our data shows, the histological and morphometric characteristics are typical of human CVs. There are differences that limit the correlation with human disease. First, human CVs typically occur only in a fraction of patients (15–65% of patients depending on the method used to determine CVs) with aneurismal SAH and in less than 5% of patients with traumatic SAH (which is thought to be commonly due to venous bleeding). In our mouse model, CVs is present in 57–88% of animals after experimental hemorrhage in susceptible strains (again, consistent with the other published models). Second, human CVs typically occur from the third day after hemorrhage to the 14th day. In mice, CVs reliably occurs in the first 16–24 h. Finally, all animal models of CVs lack an aneurysm rupture which limits studies of endothelial dysfunction in aneurysms which may contribute to the development of CVs.
An important consideration in mouse models of disease is variable susceptibility in different strains of mice. This becomes more important when genetic mutant mice are considered due to the different strain backgrounds of these mutants and the difficulty of directly comparing experimental results across different strains. If a model could be used in multiple common strains, back-breeding into different strains, which can be time-consuming and costly, could be avoided. We could not find any comparative studies of CVs induced in different strains of mice. In the present study, we tested our method in five different strains whose backgrounds are commonly used in the generation of mutant mice. In all strains except SJL mice, the rate and the amount of bleeding were relatively predictable with consistent development of CVs.
It is striking that the SJL mouse strain did not develop CVs. Mice are very susceptible to CVs compared to other animal models as evidenced by the fact that single hemorrhage models work well in consistently producing CVs. In other animals, CVs generation requires two hemorrhages. It is not clear why this is the case. It is interesting that the major differences between SJL mice and other strains have to do with immunocompetence and the absence of dysferlin. Dysferlin functions in cell membrane repair and its deficiency causes a unique subtype of muscular dystrophy. SJL mice are deficient in dysferlin unlike other mouse strains tested for this report (Vafiadaki et al., 2001). Dysferlin is over-expressed in cerebrovascular endothelial cells in mouse experimental encephalitis (Hochmeister et al., 2006). It is tempting to speculate that dysferlin deficiency confers protection against CVs in SJL mice, in our SAH model. In addition, there is evidence that inflammation may be an important mediator of CVs in humans (Provencio and Vora, 2005). Differences in immune capabilities may play a role. Regardless of the cause, we feel that the SJL mouse may not be suitable for vasospasm research using our model.
The model described here inherits the advantages of currently used mouse models of CVs. Additionally; we have streamlined the procedure without losing reliability of development of CVs. Finally, we assessed the model in multiple strains of mice to inform decisions about strain choice for genetic mutants. We feel that selection of mouse strains for vasospasm research using our model is broader than previously published models.
Acknowledgements
The authors would like to thank Drs. Deren Huang and Nicholas Stephen Potter for their technical assistance in the early stages of development of the mouse model.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Acalovschi D, Wiest T, Hartmann M, Farahmi M, Mansmann U, Auffarth GU, et al. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34:1864–9. doi: 10.1161/01.STR.0000079815.38626.44. [DOI] [PubMed] [Google Scholar]
- Bagley C. Blood in the cerebrospinal fluid: resultant functional and organic alterations in the central nervous system. Part A. Experimental data. Arch Surg. 1928;17:18–38. [Google Scholar]
- Hochmeister S, Grundtner R, Bauer J, Engelhardt B, Lyck R, Gordon G, et al. Dysferlin is a new marker for leaky brain blood vessels in multiple sclerosis. J Neuropathol Exp Neurol. 2006;65:855–65. doi: 10.1097/01.jnen.0000235119.52311.16. [DOI] [PubMed] [Google Scholar]
- Kamii H, Kato I, Kinouchi H, Chan PH, Epstein CJ, Akabane A, et al. Amelioration of vasospasm after subarachnoid hemorrhage in transgenic mice overexpressing CuZn-superoxide dismutase. Stroke. 1999;30:867–71. doi: 10.1161/01.str.30.4.867. [discussion 72] [DOI] [PubMed] [Google Scholar]
- Lin C L, Calisaneller T, Ukita N, Dumont AS, Kassell NF, Lee KS. A murine model of subarachnoid hemorrhage-induced cerebral vasospasm. J Neurosci Methods. 2003;123:89–97. doi: 10.1016/s0165-0270(02)00344-8. [DOI] [PubMed] [Google Scholar]
- Mesis RG, Wang H, Lombard FW, Yates R, Vitek MP, Borel CO, et al. Dissociation between vasospasm and functional improvement in a murine model of subarachnoid hemorrhage. Neurosurg Focus. 2006;21:E4. doi: 10.3171/foc.2006.21.3.4. [DOI] [PubMed] [Google Scholar]
- Parra A, McGirt MJ, Sheng H, Laskowitz DT, Pearlstein RD, Warner DS. Mouse model of subarachnoid hemorrhage associated cerebral vasospasm: methodological analysis. Neurol Res. 2002;24:510–6. doi: 10.1179/016164102101200276. [DOI] [PubMed] [Google Scholar]
- Provencio JJ, Vora N. Subarachnoid hemorrhage and inflammation: bench to bedside and back. Seminars Neurology. 2005;25:435–44. doi: 10.1055/s-2005-923537. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Asanuma K, Johkura K, Kasuga T, Okouchi Y, Ogiwara N, et al. Ultrastructural analysis of TiO2 nanotubes with photodecomposition of water into O2 and H2 implanted in the nude mouse. Ann Anat. 2006;188:137–42. doi: 10.1016/j.aanat.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Vafiadaki E, Reis A, Keers S, Harrison R, Anderson LV, Raffelsberger T, et al. Cloning of the mouse dysferlin gene and genomic characterization of the SJL-Dysf mutation. Neuroreport. 2001;12:625–9. doi: 10.1097/00001756-200103050-00039. [DOI] [PubMed] [Google Scholar]