Abstract
The lactose permease of Escherichia coli (LacY) is a highly dynamic membrane transport protein, while the Cys154→Gly mutant is crippled conformationally. The mutant binds sugar with high affinity, but catalyzes very little translocation across the membrane. In order to further investigate the defect in the mutant, fluorescent maleimides were used to examine the accessibility/reactivity of single-Cys LacY in right-side-out membrane vesicles. As shown previously, sugar binding induces an increase in reactivity of single-Cys replacements in the tightly packed periplasmic domain of wild-type LacY, while decreased reactivity is observed on the cytoplasmic side. Thus, the predominant population of wild-type LacY in the membrane is in an inward-facing conformation in the absence of sugar, sugar binding induces opening of a hydrophilic pathway on the periplasmic side, and the sugar-binding site is alternatively accessible to either side of the membrane. In striking contrast, the accessibility/reactivity of periplasmic Cys replacements in the Cys154→Gly background is very high in the absence of sugar, and sugar binding has little or no effect. The observations indicate that an open hydrophilic pathway is present on the periplasmic side of the Cys154→Gly mutant and that this pathway is unaffected by ligand binding, a conclusion consistent with findings obtained from single-molecule fluorescence and double electron–electron resonance.
Keywords: membranes, membrane protein structure/function, transport, site-directed alkylation, cysteine scanning mutagenesis
Introduction
The lactose permease of Escherichia coli (LacY), a member of the major facilitator superfamily of membrane transport proteins, couples the stoichiometric translocation of a galactoside and an H+.1,2 As such, LacY utilizes free energy stored in an electrochemical H+ gradient (Δμ̄H+) to drive accumulation of galactosidic sugars against a concentration gradient. Conversely, in the absence of Δμ̄H+, LacYutilizes free energy released from downhill translocation of galactosides to drive uphill translocation of H+ with generation of Δμ̄H+, the polarity of which depends on the direction of the sugar gradient.
LacY has been solubilized, purified and reconstituted into proteoliopsomes in a fully functional state.3 X-ray crystal structures of LacY with a native Cys at position 154 replaced with Gly (Cys154→Gly, C154G), which is conformationally restricted,4–9 has been solved in an inward-facing conformation,10,11 and wild-type LacY has the same global fold.12–14 The proteins contain 12 transmembrane helices organized into two pseudo-symmetrical six α-helix bundles surrounding a large interior hydrophilic cavity open to the cytoplasm, which represents the inward-facing conformation. The sugar-binding site and the residues involved in H+ translocation are near the apex of the cavity at the approximate middle of the molecule and distributed such that the side chains important for sugar recognition are in the N-terminal helix bundle, while most of the side chains important for H+ translocation are in the C-terminal bundle.
Wild-type LacY is highly dynamic, and ligand binding induces widespread conformational changes.1,2,7,15,16 In contrast, although C154G LacY binds ligand with relatively high affinity, the mutant is conformationally constrained and catalyzes very little sugar translocation across the membrane.4–7,17 Isothermal titration calorimetry demonstrates that substrate binding to wild-type LacY is largely entropic, while sugar binding to C154G LacY is exclusively enthalpic.7 Thus, wild-type LacY behaves if there are multiple ligand-bound conformational states, while the C154G mutant is severely restricted. Distance measurements by both single-molecule fluorescence resonance energy transfer,8 and double electron–electron resonance data9 indicate that sugar binding induces closing of the cytoplasmic cavity with opening of a hydrophilic cleft on the periplasmic side of wild-type LacY. Remarkably, although sugar binding in the C154G mutant induces closing of the cytoplasmic cavity, the periplasmic cleft appears to be paralyzed in an open state and insensitive to sugar binding.9 In view the dramatic biophysical differences observed between the wild-type and C154G LacY, it is surprising that the X-ray crystal structures exhibit the same global fold, which raises the question of whether both crystal structures accurately reflect the structure of the proteins in the membrane.
In this study, we used site-directed alkylation with hydrophobic or hydrophilic fluorescent maleimides to examine accessibility/reactivity of strategically placed single-Cys replacements.14,18 The experiments are carried out with right-side-out (RSO) membrane vesicles containing a single mutant in LacY background devoid of the native eight Cys residues with Cys154 replaced by Val (C154V; pseudo wild-type).19 The effect of the C154G mutation is examined by using the same strategy with single-Cys mutants in which Cys154 is replaced with Gly (the C154G mutant). As shown earlier,18,20 binding of β-D-galactopyranosyl-1-thio-β-D-galactopyranoside (TDG) increases reactivity of single-Cys replacements in wild-type LacY on the periplasmic side of the sugar-binding site and decreases reactivity of those on the cytoplasmic side. In stark contrast, although TDG binding decreases labeling of single-Cys replacements on the cytoplasmic side of the C154G mutant, single-Cys replacements on the periplasmic side are highly reactive in the absence of sugar, and TDG binding has no significant effect on reactivity/accessibility. When considered with other completely independent lines of experimental support,8,9,18,21 it seems conclusive that sugar binding induces opening of a hydrophilic cleft on the periplasmic side of wild-type LacY, while introduction of the C154G mutation results in a conformation in which the periplasmic cleft is open abnormally and does not respond to sugar binding.
Results
TMRM labeling of single-Cys LacY
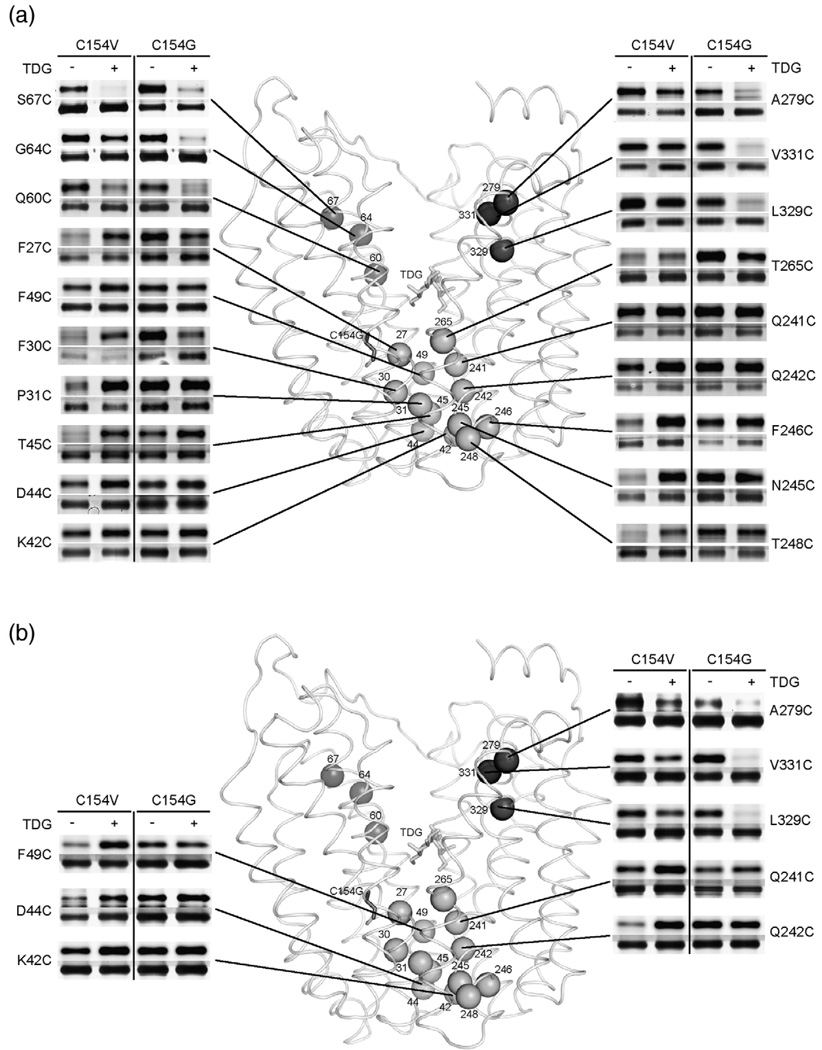
TMRM (Fig. 1a, Table 1) labeling of 19 single-Cys replacements in the C154V (pseudo wild-type) or C154G (mutant) background was carried out at 0 °C for 30 min in the absence or in the presence of TDG (Fig. 2a). In the pseudo wild-type background, Q60C, G64C and S67C (helix II) on the cytoplasmic side of the sugar-binding site exhibit decreased TMRM labeling in the presence of TDG at 30 min. With C154V/A279C (helix VIII), C154V/L329C and C154V/V331C (helix X) LacY, labeling at 0 °C for only 20 s is needed to reveal a decrease in activity upon TDG binding; otherwise the effect is obscured by saturation of the signal (Fig. 2b). In the C154G background, each single-Cys exhibits a marked decrease in labeling in the presence of TDG at 0 °C at either 30 min or 20 s (Fig. 2).
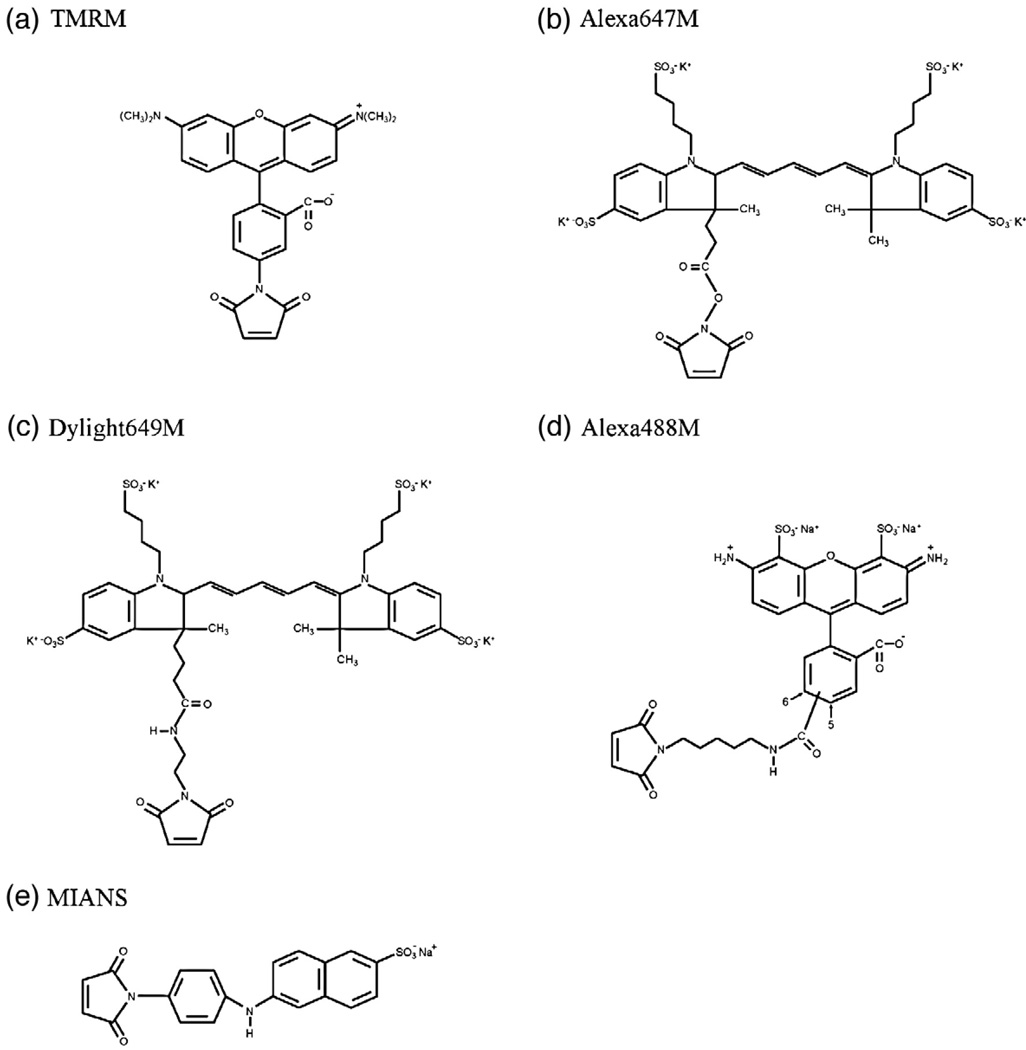
Fig. 1.
Fluorescent alkylating reagents. The structure of Alexa647M is simulated.
Table 1.
Labeling reagents
| Alkylation reagent |
Hydrophobicity | Head group width (Å) |
Arm length (Å) |
|---|---|---|---|
| TMRM | Hydrophobic | ~14 | ~9 |
| Alexa647M | Hydrophilic | ~21 | ~9 |
| Dylight649M | Hydrophilic | ~21 | ~12 |
| Alexa488M | Hydrophilic | ~14 | ~16 |
| MIANS | Hydrophilic | ~5 | ~12 |
Fig. 2.
Effect of TDG on TMRM reactivity of single-Cys replacements in the pseudo wild-type (C154V) or C154G LacY background. Single-Cys replacements in C154Vor C154G LacY were labeled with 20 µM TMRM for 30 min (a) or 20 s (b) at 0 °C in the absence or in the presence of TDG. Positions of Cys replacements are superimposed on the backbone of C154G LacY [Protein Data Bank ID code 1PV7; www.pdb.org]. LacY is viewed perpendicular to the membrane with the N-terminal helix bundle on the left and the C-terminal bundle on the right. Purified proteins labeled with TMRM were subjected to SDS-PAGE, and then TMRM-labeled (upper panels) and silver-stained (lower panels) bands corresponding to LacY were imaged. Dark gray spheres, cytoplasmic positions: 60, 67, 70 (helix II), 279 (helix VIII), 329 and 331 (helix X); gray spheres, periplasmic positions: 27, 30, 31 (helix I), 42, 44, 45, 49 (helix II), 241, 242, 245, 246, 248 (helix VII), and 265 (helix VIII). Stick models are shown for TDG at the apex of the inward-facing cavity (light gray sticks) and C154G at the periplasmic side of the N-terminal bundle (gray sticks).
On the periplasmic side, TDG clearly increases TMRM labeling in incubation for 30 min at 0 °C with the following single-Cys replacements in pseudo wild-type LacY: F27C, F30C and P31C (helix I); T45C (helix II); N245C, F246C and T248C (helix VII); T265C (helix VIII) (Fig. 2a).With F49C, D44C, K42C, Q242C and Q241C, although little effect of TDG is observed at 30 min, labeling for 20 s reveals increased reactivity/accessibility in the presence of the lactose homologue. In marked contrast, in the C154G background, TMRM strongly labels each single-Cys in the absence of TDG, and addition of the sugar has little or no effect (Fig. 2). The results suggest that the periplasmic side of wild-type LacY is closed in the absence of sugar and TDG binding causes opening of a pathway on this side of the molecule. In contrast, the C154G mutation appears to cause constitutive opening of the periplasmic pathway in such a manner that sugar binding has no effect.
Labeling with hydrophilic alkylation reagents
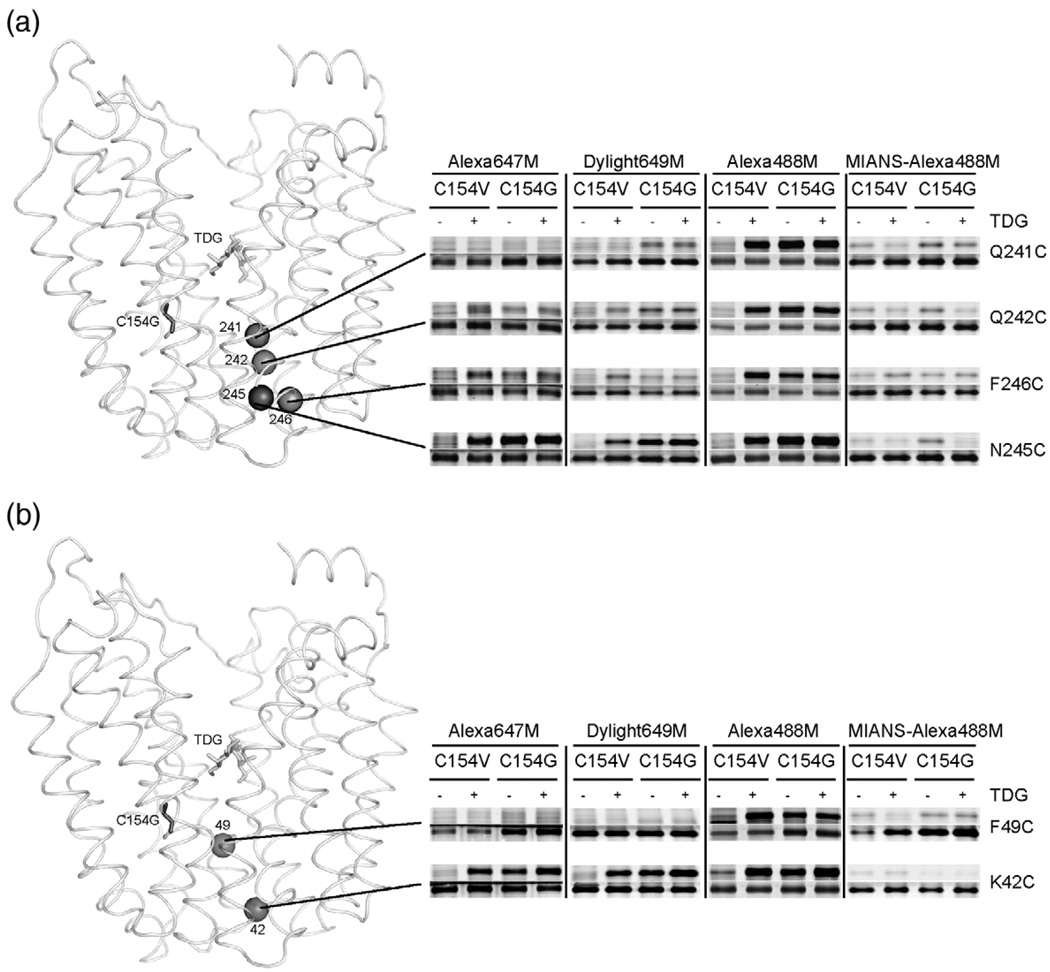
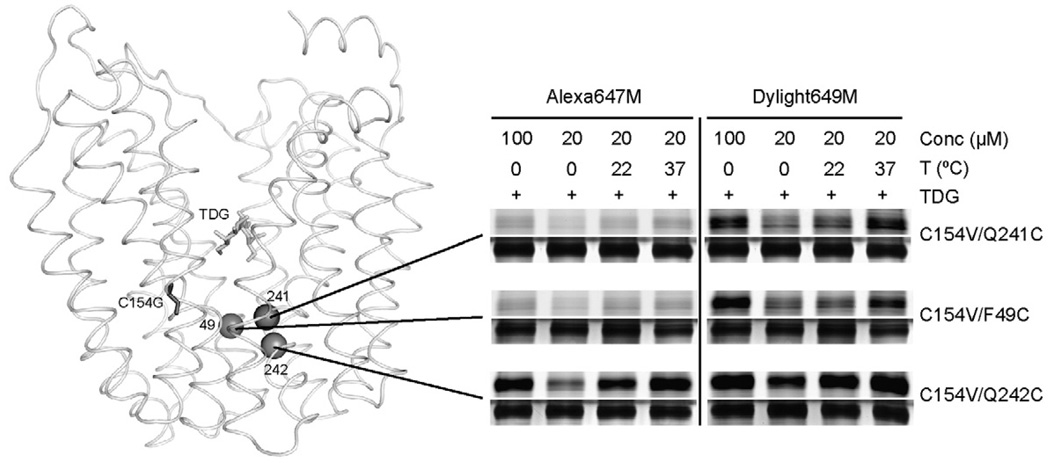
In order to examine the physical nature of the periplasmic pathway, hydrophilic alkylating agents differing in distance between the maleimide and a bulky, hydrophilic head group were tested for reactivity with selected single-Cys. Alexa647M, with distance of ~ 9 Å between the maleimide and the largest, hydrophilic moiety (Fig. 1b; Table 1), does not label Q241C, Q242C, N245C or F246C (helix VII) in pseudo wild-type LacY in the absence of TDG (Fig. 3a). However, in the presence of TDG, Alexa647M labels C154V/N245C, C154V/F246C and C154V/Q242C LacY progressively less strongly, while C154V/Q241C LacY is only labeled faintly, if at all, in the presence of TDG (Fig. 3a). In contrast, in the C154G background, Alexa647M labels the four single-Cys replacements with the same relative intensities in the absence of TDG, and little or no change is observed in the presence of the sugar (Fig. 3a). A higher concentration of Alexa647M (100 µM) or increased temperatures (22 °C and 37 °C) significantly increases Alexa647M labeling of C154V/Q242C LacY in the presence of TDG, but labeling of C154V/Q241C LacY is still not observed in the presence of TDG under any condition tested (Fig. 4). Similar results are observed for single-Cys replacements in helix II (Fig. 3b). Alexa647M does not label C154V/K42C LacY in the absence of TDG, but accessibility/reactivity is increased markedly upon TDG binding. Alexa647M labels C154G/K42C LacY strongly in the absence or presence of TDG. Essentially no labeling is observed with C154V/F49C or C154G/F49C LacY under any condition tested (Fig. 3b and Fig. 4).
Fig. 3.
Labeling of periplasmic single-Cys replacements by hydrophilic fluorescent reagents. Single-Cys replacements at positions 241, 242, 245 and 246 (helix VII) (a), 42 and 49 (helix II) (b) in the C154V or C154G background were labeled with Alexa647M, Dylight649M and Alexa488M. MIANS labeling was performed by inhibiting Alexa488M labeling, as described in Materials and Methods, and shown in the last columns (MIANS-Alexa488M). Fluorescent reagent-labeled (upper panels) and silver-stained (lower panels) bands corresponding to LacY in SDS-PAGE gels were imaged.
Fig. 4.
Higher concentrations of reagent and increased temperature enhance labeling of periplasmic single-Cys replacements in C154V LacY in the presence of TDG. Alexa647M or Dylight649M labeling of C154V/F49C, C154V/Q241C and C154V/Q242C LacY in the presence of TDG was performed for 30 min at 0 °C with 100 µM alkylation reagents, or at 0 °C, 22 °C or 37 °C with 20 µM alkylation reagents. Fluorescent reagent-labeled (upper panels) and silver-stained (lower panels) bands corresponding to LacY in SDS-PAGE gels were imaged.
Dylight649M (Fig. 1c; Table 1), with a distance of ~ 12 Å between the maleimide and the same bulky, hydrophilic moiety as Alexa647M, does not label Q241C, Q242C, F246C or N245C in the pseudo wild-type background in the absence of TDG (Fig. 3a). However, the sugar significantly increases labeling of C154V/N245C, C154V/N246C and C154V/Q242C LacY with decreasing intensity, respectively. Furthermore, a higher concentration of the reagent or increased temperature enhances Dylight649M labeling of C154V/Q241C LacY in the presence of TDG (Fig. 4). In the C154G background, Dylight649M labels each mutant comparably in the absence or in the presence of TDG (Fig. 3a). Similar results are observed for single-Cys in the helix II (Fig. 3b). Dylight649Mdoes not label C154V/K42C LacY in the absence of sugar, but TDG binding strongly increases labeling. Dylight649M labels C154G/K42C LacY strongly in the absence of sugar, and perhaps a slight increase is observed in the presence of TDG. With F49C LacY, no significant labeling is observed regard-less of TDG and/or the C154G mutation. However, intense labeling of C154V/F49C LacY is observed with TDG at 100 µM Dylight649M, and labeling is increased progressively at 0 °C, 22 °C and 37 °C, respectively, at 20 µM Dylight649M (Fig. 4).
Alexa488M, with a distance of ~ 16 Å between the maleimide and a smaller hydrophilic moiety relatively similar to TMRM in size (Fig. 1d; Table 1), does not react with the single-Cys replacements in the C154V background in helix VII (N245C, F246C, Q242C and Q241C) or helix II (K42C and F49C) in the absence of sugar, but TDG clearly increases reactivity/accessibility of each mutant (Fig. 3). In the C154G background, Alexa488M labels all positions about equally well in the absence and in the presence of TDG (Fig. 3).
2-(4′-Maleimidylanilino) naphthalene-6-sulfonic acid, sodium salt (MIANS) with a distance of ~ 12 Å between themaleimide and the hydrophilicmoiety (Fig. 1e; Table 1), has excitation and emission spectra that prohibit direct measurement of Cys reactivity in the available instrument. Therefore, the ability of MIANS to block Alexa488M labeling was determined. In both the pseudo wild-type and C154G backgrounds, MIANS blocks Alexa488M labeling of Q241C, Q242C, N245C and F246C LacY (helix VII; Fig. 3a), as well as K42C and F49C LacY (helix II; Fig. 3b). Therefore, it is apparent that MIANS can enter the periplasmic pathway and reach positions as deep as 241 (helix VII) and 49 (helix II).
Discussion
TMRM labeling of single-Cys replacements
In this study, a simple but sensitive alkylation method using fluorescent reagents was utilized to examine the effect of ligand binding and/or the C154G mutation on the reactivity of single-Cys replacements. 18 On the cytoplasmic side of the sugar-binding site, TMRM reactivity of single-Cys replacements, Q60C, G64C, L70C, A279C, L329C or V331C in both the pseudo wild-type and C154G LacY backgrounds is decreased in the presence of TDG (Fig. 2). In contrast, binding of TDG leads to opening of a pathway on the periplasmic side of the sugar-binding site, as reflected by a clear increase in accessibility/reactivity of single-Cys replacements in this region. Furthermore, on the periplasmic side of the sugar-binding site, the C154G mutation clearly causes all the periplasmic positions tested to become highly accessible/reactive, and TDG binding has little or no effect. Thus, in the C154G mutant, an open periplasmic cleft appears to be present in the absence of sugar, which is not significantly altered by TDG binding. The observations are consistent with other independent lines of evidence,8,9 indicating that sugar binding leads to closing of the cytoplasmic cavity in wild-type LacY in a manner that is relatively unaffected by the C154G mutation, while the mutation affects mobility directly on the periplasmic side of the molecule.
Hydrophilic alkylation reagents label periplasmic single-Cys replacements from outside of the membrane
None of the hydrophilic alkylation reagents used here labels any periplasmic single-Cys replacements in pseudo wild-type LacY (K42C, F49C, Q241C, Q242C, N245C or F246C) in the absence of TDG (Fig. 3), demonstrating that pseudo wild-type LacY is tightly closed on the periplasmic side so that the sugar-binding site is inaccessible from outside the membrane. TDG induces strong labeling at these positions, and Alexa488M labeling is blocked by MIANS (Fig. 3a and b), indicating that TDG binding opens a hydrophilic periplasmic pathway that renders the sugar-binding site accessible from the outside.
According to the crystal structure,10 the Cα–Cα distances are 9.4 Å between positions 241 and 245, 6.2 Å between positions 242 and 245, and 3.8 Å between positions 246 and 245. Cα 245 is ~ 2 Å from the outside opening of the molecule. Therefore, the distances to reach each position from outside the membrane are ~ 2 Å for position 245, ~ 5.8 Å for position 246, ~ 8.2 Å for position 242 and ~ 11.4 Å for position 241. Similarly, the distances to reach positions 42 and 49 are ~ 2 Å and ~ 11.9 Å, respectively, from the external medium. In the presence of TDG, Alexa647M (arm length ~ 9 Å) labels positions as deep as 242, but not 241 or 49, while Dylight649M (arm length ~ 12 Å) labels all of these positions (Fig. 3 and Fig. 4). Thus, when the periplasmic pathway is open, the maleimide group of Alexa647M or Dylight649M enters the periplasmic pathway and reacts with the Cys thiol group, while the head group of the reagents (~ 21 Å wide) is too big to enter and stays outside at the opening of the periplasmic pathway. Alexa488M strongly labels all the positions, particularly the deeper ones, such as 241 and 49 (Fig. 3), most likely because the first ring (~ 4 Å long, ~ 5 Å wide) also enters the periplasmic pathway. MIANS is a relatively small hydrophilic reagent with a head group ~ 5 Å wide (Fig. 1b, Table 1) and blocks Alexa488M labeling of all positions tested (Fig. 3). Furthermore, TMRM strongly labels Q241C and Q242C LacY in the presence of TDG at 0 °C (Fig. 2), and complete TMRM labeling of these mutants totally inhibits the transport activity of these mutants in RSO vesicles (data not shown). Therefore, it is very likely that when ligand binding causes opening of the periplasmic pathway, TMRM modifies deeply placed Cys residues at positions 241 and 242, thereby suggesting that the periplasmic pathway is larger than 14 Å in width. Taken together, the results show clearly that TDG binding induces opening of a hydrophilic periplasmic cleft in wild-type LacY that is sufficiently large to accommodate molecules such as MIANS, as well as TMRM, but not big enough for the head group of either Alexa647M or Dylight649M. Therefore, the periplasmic pathway in wild-type LacY is most likely between ~ 14 Å and ~ 21 Å in width, which is consistent with double electron–electron resonance studies and with the recent observation showing directly that the periplasmic cavity must open to ~ 17 Å for transport to occur.21
For the periplasmic single-Cys replacements in the C154G background, these hydrophilic reagents label similar positions according to the size of the reagents but, strikingly, they do so in the absence of TDG, and the presence of the sugar has little or no effect (Fig. 3). Therefore, the C154G mutant apparently occupies a conformation in which the hydrophilic cleft is paralyzed in an open position, while the cytoplasmic cavity remains capable of closing. These results for the C154G mutant are consistent with observations from both the single-molecule fluorescence resonance energy transfer,8 and double electron–electron resonance data,9 which also show that sugar binding induces no change in distance on the periplasmic side.
In summary, the data presented here indicate that the predominant population of wild-type LacY in the membrane is predominantly in an inward-facing conformation with a hydrophilic cavity facing the cytoplasm and a potential periplasmic cavity that is tightly closed. Moreover, these cavities reciprocally open and close upon sugar binding. However, the predominant population of the C154G mutant in the membrane exhibits open cytoplasmic, as well as periplasmic cavities, and although the cytoplasmic cavity closes in the presence of sugar, the periplasmic cavity is unaffected. Thus, ligand binding to the C154G mutant is uncoupled with respect to alternative closing and opening of cytoplasmic and periplasmic cavities in wild-type LacY.20 Since X-ray structures of wild-type and C154G LacY are all in the same inward-facing conformation, it is likely that the crystallization process selects a single conformer of LacY that is in the lowest free energy state.10,11,13
Materials and Methods
Materials
Alexa Fluor 488 C5-maleimide (Alexa488M, Cat. A-10254), Alexa Fluor 647 C2-maleimide (Alexa647M, Cat. A-20347), 2-(4′-maleimidylanilino) naphthalene-6-sulfonic acid, sodium salt (MIANS, Cat. M-8), and tetramethylrhodamine-5-maleimide (TMRM, Cat. T-6027) were obtained from Molecular Probes, Invitrogen Corp. (Carlsbad, CA). DyLight 649 maleimide (Dylight649M, Cat. 46615) and ImmunoPure immobilized monomeric avidin (Cat. 20228) were obtained from Pierce (Rockford, IL). All other materials were reagent grade and obtained from commercial sources.
Plasmid construction
DNA fragments encoding given single-Cys mutants were isolated from plasmids in the library of single-Cys mutations22–26 by restriction enzyme digestion and inserted into plasmid pT7-5 encoding Cys-less LacY with either the C154Vor C154G mutation and a biotin acceptor domain (BAD) from a Klebsiella pneumoniae oxaloacetate decarboxylase at the C terminus.27 All constructs were sequenced for the full length of lacY genes.
Growth of bacteria
E. coli T184 (lacY−Z−) transformed with plasmid pT7-5 encoding a given mutant were grown aerobically at 37 °C in Luria-Bertani broth containing ampicillin (100 µg/ml). Fully grown cultures were diluted tenfold and grown for 2 h. After induction with 1 mM isopropyl 1-thio-β-d-galactopyranoside for 2 h, cells were harvested and used for the preparation of RSO membrane vesicles.
Preparation of RSO membrane vesicles
RSO membrane vesicles were prepared from 0.8 l cultures of E. coli T184 expressing a specific LacY by treatment with lysozyme/EDTA and osmotic lysis.28,29 The vesicles were resuspended to a protein concentration of 10 mg/ml in 100 mM potassium phosphate (KPi, pH 7.5), 10 mM MgSO4, frozen in liquid nitrogen and stored at −80 °C.
Fluorescent maleimide labeling
Labeling with TMRM and other fluorescent alkylation reagents was performed following the protocol developed recently.18 Briefly, RSO membrane vesicles in RSO buffer (0.1 mg of total protein in 50 µl of 100 mM KPi (pH 7.5), 10 mM MgSO4) containing a single-Cys LacY were incubated with 40 µM TMRM or 20 µM Alexa488M, Alexa647M or Dylight649M in the absence or in the presence of 10 mM TDG at 0 °C for 30 min, or 20 sec when indicated. When the concentration of alkylating reagents or temperature was varied, experiments were performed by incubating RSO membrane vesicles with 100 µM Alexa647M or Dylight649M at 0 °C for 30 min, or 20 µM at 0 °C, 22 °C or 37 °C for 30 min. When inhibition of labeling was studied, experiments were performed by incubating RSO membrane vesicles with 20 µM MIANS at 0 °C for 30 min, washing the vesicles with 2 ml ice-cold RSO buffer twice, resuspending in 50 µl of RSO buffer, adding 10 mM TDG to the reaction when indicated, and incubating the vesicles with 20 µM Alexa488M at 0 °C for 30 min. Dithiothreitol was added to 10 mM in all cases to stop the reaction. The membranes were then solubilized in 2% n-dodecyl β-d-maltopyranoside and biotinylated LacY was purified with immobilized monomeric avidin Sepharose chromatography. Purified proteins (10 µl out of a total of 50 µl) were subjected to SDS-PAGE (16% (w/v) poly-acrylamide gel). The wet gels were imaged directly on an Amersham Typhoon™ 9410 Workstation (λex = 532 nm and λem = 580 nm for TMRM; λex = 633 nm and λem = 670 nm for Alexa647M and Dylight649M; λex = 488 nm and λem = 526 nm for Alexa488M). The gels were then stained with silver to quantify the proteins.
Acknowledgements
The authors thank Natalia Ermolova for initiating the project, and for advice and discussion.We thank Junichi Sugihara for help with growth of bacteria and all the members of the laboratory for critically reading the manuscript. This work was supported by NIH grantsDK051131,DK069463, GM073210 and GM074929, and NSF grant 0450970 to H.R.K.
Abbreviations used
- LacY
lactose permease
- C154V
functional LacY devoid of native Cys residues and containing Val in place of Cys154
- C154G
LacY devoid of native Cys residues and containing Gly in place of Cys154
- RSO
right-side-out
- TDG
β-d-galactopyranosyl 1-thio-β-d-galactopyranoside
- TMRM
tetramethylrhodamine-5-maleimide
- Alexa488M
Alexa Fluo 488 C5-maleimide
- Alexa647M
Alexa Fluo 647 C2-maleimide
- Dylight649M
DyLight 649 maleimide
- MIANS
2-(4′-maleimidylanilino) naphthalene-6-sulfonic acid, sodium salt
References
- 1.Kaback HR. Structure and mechanism of the lactose permease. C R Biol. 2005;328:557–567. doi: 10.1016/j.crvi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Guan L, Kaback HR. Lessons from lactose permease. Annu. Rev. Biophys. Biomol. Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- 4.Menick DR, Sarkar HK, Poonian MS, Kaback HR. Cys154 is important for lac permease activity in Escherichia coli. Biochem. Biophys. Res. Commun. 1985;132:162–170. doi: 10.1016/0006-291x(85)91002-2. [DOI] [PubMed] [Google Scholar]
- 5.Smirnova IN, Kaback HR. A mutation in the lactose permease of Escherichia coli that decreases conformational flexibility and increases protein stability. Biochemistry. 2003;42:3025–3031. doi: 10.1021/bi027329c. [DOI] [PubMed] [Google Scholar]
- 6.Ermolova NV, Smirnova IN, Kasho VN, Kaback HR. Interhelical packing modulates conformational flexibility in the lactose permease of Escherichia coli. Biochemistry. 2005;44:7669–7677. doi: 10.1021/bi0502801. [DOI] [PubMed] [Google Scholar]
- 7.Nie Y, Smirnova I, Kasho V, Kaback HR. Energetics of ligand-induced conformational flexibility in the lactose permease of Escherichia coli. J. Biol. Chem. 2006;281:35779–35784. doi: 10.1074/jbc.M607232200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majumdar DS, Smirnova I, Kasho V, Nir E, Kong X, Weiss S, Kaback HR. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc. Natl Acad. Sci. USA. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smirnova I, Kasho V, Choe JY, Altenbach C, Hubbell WL, Kaback HR. Sugar binding induces an outward facing conformation of LacY. Proc. Natl Acad. Sci. USA. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 11.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H+ symport in LacY. EMBO J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan L, Smirnova IN, Verner G, Nagamoni S, Kaback HR. Manipulating phospholipids for crystallization of a membrane transport protein. Proc. Natl Acad. Sci. USA. 2006;103:1723–1726. doi: 10.1073/pnas.0510922103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc. Natl. Acad. Sci. USA. 2007;104:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan L, Kaback HR. Site-directed alkylation of cysteine to test solvent accessibility of membrane proteins. Nature Protoc. 2007;2:2012–2017. doi: 10.1038/nprot.2007.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.le Coutre J, Kaback HR, Patel CK, Heginbotham L, Miller C. Fourier transform infrared spectroscopy reveals a rigid alpha-helical assembly for the tetrameric Streptomyces lividans K+ channel. Proc. Natl Acad. Sci. USA. 1998;95:6114–6117. doi: 10.1073/pnas.95.11.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaback HR, Sahin-Toth M, Weinglass AB. The kamikaze approach to membrane transport. Nature Rev. Mol. Cell Biol. 2001;2:610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 17.van Iwaarden PR, Driessen AJ, Lolkema JS, Kaback HR, Konings WN. Exchange, efflux, and substrate binding by cysteine mutants of the lactose permease of Escherichia coli. Biochemistry. 1993;32:5419–5424. doi: 10.1021/bi00071a017. [DOI] [PubMed] [Google Scholar]
- 18.Nie Y, Ermolova N, Kaback HR. Site-directed alkylation of LacY: effect of the proton electrochemical gradient. J. Mol. Biol. 2007;374:356–364. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Iwaarden PR, Pastore JC, Konings WN, Kaback HR. Construction of a functional lactose permease devoid of cysteine residues. Biochemistry. 1991;30:9595–9600. doi: 10.1021/bi00104a005. [DOI] [PubMed] [Google Scholar]
- 20.Kaback HR, Dunten R, Frillingos S, Venkatesan P, Kwaw I, Zhang W, Ermolova N. Site-directed alkylation and the alternating access model for LacY. Proc. Natl Acad. Sci. USA. 2006;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc. Natl Acad. Sci. USA. 2008;105:3774–3778. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahin-Tóth M, Persson B, Schwieger J, Cohan M, Kaback HR. Cysteine scanning mutagenesis of the N-terminal 32 amino acid residues in the lactose permease of Escherichia coli. Protein Sci. 1994;3:240–247. doi: 10.1002/pro.5560030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frillingos S, Sahin-Tóth M, Persson B, Kaback HR. Cysteine-scanning mutagenesis of putative helix VII in the lactose permease of Escherichia coli. Biochemistry. 1994;33:8074–8081. doi: 10.1021/bi00192a012. [DOI] [PubMed] [Google Scholar]
- 24.Sahin-Tóth M, Frillingos S, Bibi E, Gonzalez A, Kaback HR. The role of transmembrane domain III in the lactose permease of Escherichia coli. Protein Sci. 1994;3:2302–2310. doi: 10.1002/pro.5560031215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frillingos S, Sun J, Gonzalez A, Kaback HR. Cysteine-scanning mutagenesis of helix II and flanking hydrophilic domains in the lactose permease of Escherichia coli. Biochemistry. 1997;36:269–273. doi: 10.1021/bi9618629. [DOI] [PubMed] [Google Scholar]
- 26.Frillingos S, Ujwal ML, Sun J, Kaback HR. The role of helix VIII in the lactose permease of Escherichia coli. I Cys-scanning mutagenesis. Protein Sci. 1997;6:431–437. doi: 10.1002/pro.5560060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Consler TG, Persson BL, Jung H, Zen KH, Jung K, Prive GG, et al. Properties and purification of an active biotinylated lactose permease from Escherichia coli. Proc. Natl Acad. Sci. USA. 1993;90:6934–6938. doi: 10.1073/pnas.90.15.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Short SA, Kaback HR, Kohn LD. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J. Biol. Chem. 1975;250:4291–4296. [PubMed] [Google Scholar]
- 29.Kaback HR. Bacterial membranes. Methods Enzymol. 1971;22:99–120. [Google Scholar]