Abstract
A central goal of cognitive neuroscience is to elucidate the neural mechanisms underlying decision-making. Recent physiological studies suggest that neurons in association areas may be involved in this process. To test this, we measured the effects of electrical microstimulation in the lateral intraparietal area (LIP) while monkeys performed a reaction-time motion discrimination task with a saccadic response. In each experiment, we identified a cluster of LIP cells with overlapping response fields (RFs) and sustained activity during memory-guided saccades. Microstimulation of this cluster caused an increase in the proportion of choices to the RF of the stimulated neurons. Choices toward the stimulated RF were faster with microstimulation, while choices in the opposite direction were slower. Microstimulation never directly evoked saccades, nor did it change reaction times in a simple saccade task. These results demonstrate that the discharge of LIP neurons is causally related to decision formation on the discrimination task.
Much progress has been made in understanding the neurobiology of decision-making by tracing the neural events that link sensory processing to a choice of action in monkeys1–4. For example, to decide whether a pattern of random dots is moving to the left or right, the brain must represent the motion information in the visual cortex, interpret this information as evidence for one or the other direction, and eventually commit to a choice, indicated by some action. Through a combination of recording, lesion, and microstimulation experiments, the activity of direction-selective neurons in area MT has been shown to represent an important source of the sensory evidence upon which such a decision about direction is based5–8. This raises the question of how that representation is converted into a categorical decision.
When performing the motion discrimination task, monkeys improve in accuracy when given more time to view the stimulus6,9. Furthermore, in a reaction-time version of the task, stronger motion leads to both faster and more accurate decisions10. These findings can be explained by a simple mechanism whereby momentary sensory evidence is accumulated over time toward a criterion level, which in turn yields a commitment to a proposition and ultimately an action.
A neural correlate of this process has been described in the macaque parietal cortex10,11. Many LIP neurons respond when visual stimuli are presented at a specific location in space or when monkeys intend to make a saccade to that same location; thus, they have combined sensory and motor RFs12,13. When monkeys indicate decisions about direction of motion with an eye movement, LIP neurons increase or decrease their firing as evidence accumulates in favor of or against the choice associated with the target in their RF10. This rise and fall in activity depends on the quality of the motion evidence from another region of the visual field, with stronger motion leading to faster and more intense changes in spike rate. Unlike neurons in area MT, which are thought to represent the moment-by-moment fluctuations in motion energy in their preferred direction14,15, neurons in LIP appear to represent the mounting evidence for or against an eye movement to the choice target in their RF. Furthermore, there is a stereotyped level of activity in these LIP neurons independent of motion strength just prior to the saccadic eye movement that indicates the choice. Thus, the decision process appears to terminate when a criterion level of activity is reached in the appropriate LIP neurons10.
These observations suggest that neurons in LIP may mediate a simple decision process by accumulating sensory evidence toward a criterion level to commit to one alternative or the other. However, a causal role for LIP neurons in decision-making has not been established. To test whether the activity of these LIP neurons influences decisions rather than merely reflecting them, we measured the effects of LIP microstimulation on decision formation while monkeys performed the direction discrimination task. We found that microstimulation influenced the monkeys’ decisions and the time taken to reach those decisions.
RESULTS
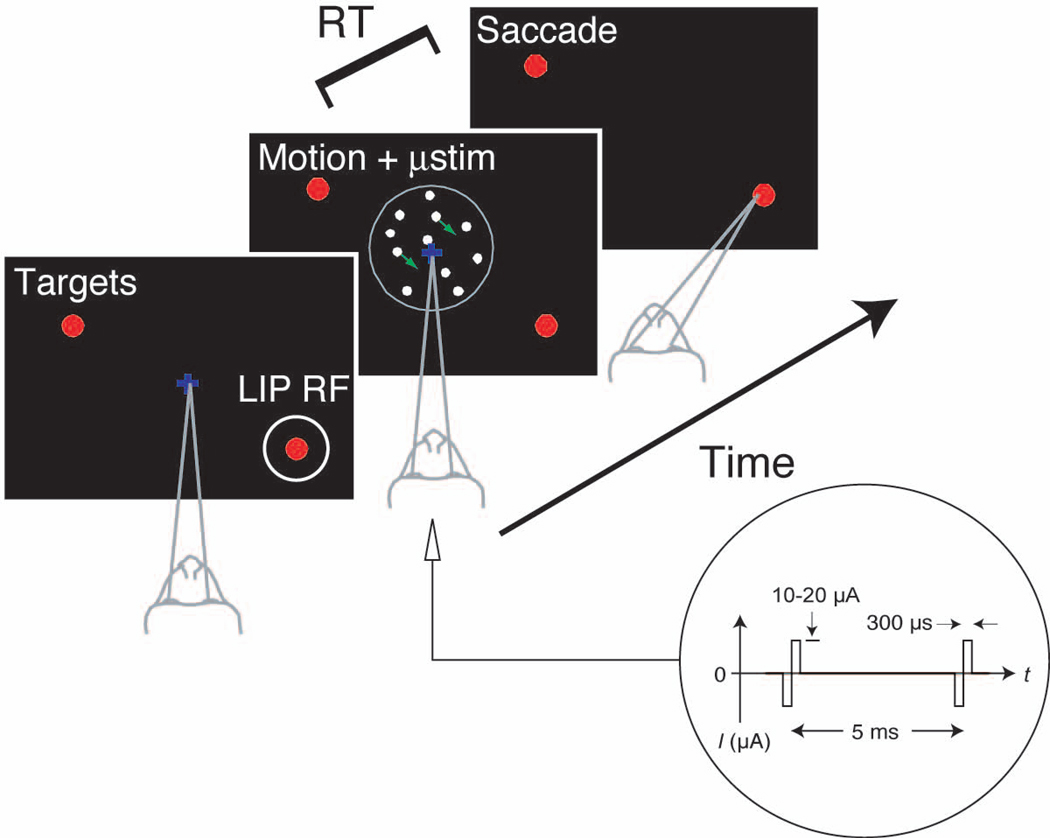
Monkeys were trained to perform a two choice direction discrimination task while viewing a random-dot motion stimulus (Fig. 1). We controlled the difficulty of the task by varying the percentage of coherently moving dots. The monkeys indicated their decision by making an eye movement to one of two choice targets any time after motion onset (see Methods). In this way, we obtained two behavioral measures: the proportion of decisions in favor of either direction of motion and the amount of time taken to decide and respond (reaction time, RT).
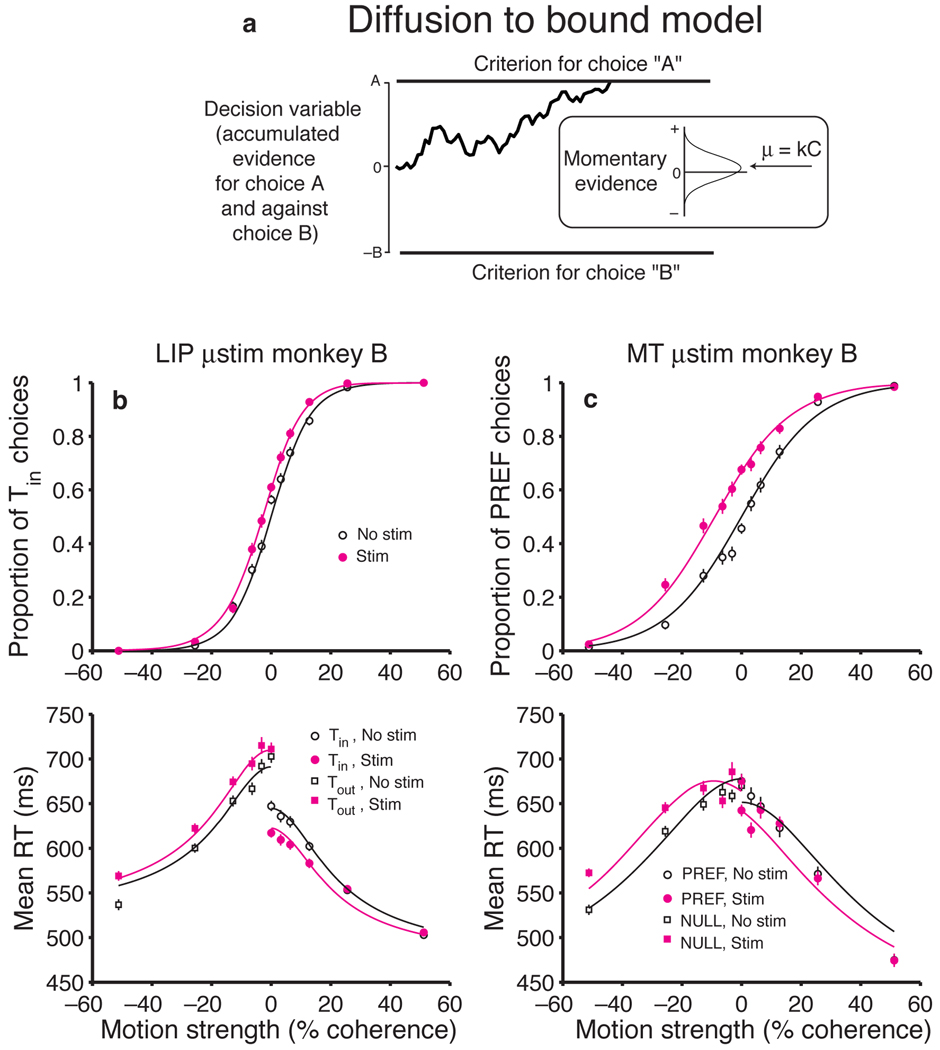
Fig. 1.
Experimental Design. A recording/stimulating microelectrode was advanced into the ventral portion of area LIP to identify a cluster of neurons with similar RFs. The monkey performed a direction discrimination task with several levels of task difficulty randomly interleaved. The monkey could respond at any time after onset of the random dot motion and it indicated its decision with a saccadic eye movement. One of the two choice targets was placed in the RF of the LIP neurons. We applied microstimulation as shown from the onset of the motion stimulus until the initiation of the saccade on a random half of the trials.
For each experiment, we identified a cluster of LIP cells with overlapping RFs. The neurons near the tip of the electrode were required to satisfy the following criteria: multineuron activity was modulated during a simple delayed eye movement task; the activity was selective for saccades to targets placed in a restricted region of the visual field (the multineuron RF); this elevation in activity persisted through a 500–1000 ms delay period separating the onset of a target and the instruction to make an eye movement to its remembered location. Further, we required that these properties (RF location and selectivity) remained similar over a range of electrode positions extending at least ±100 µm along the path of the electrode. 24 LIP sites satisfied these criteria (12 from each monkey).
The discrimination task was arranged so that one of the choice targets (Tin) was in the RF of the neurons near the electrode tip (Fig. 1). The other target (Tout) and the random dot motion were always outside the RF of the neurons nearest the electrode tip. On a random half of the trials, we applied microstimulation during motion viewing (see Methods).
LIP microstimulation affects choice and reaction time
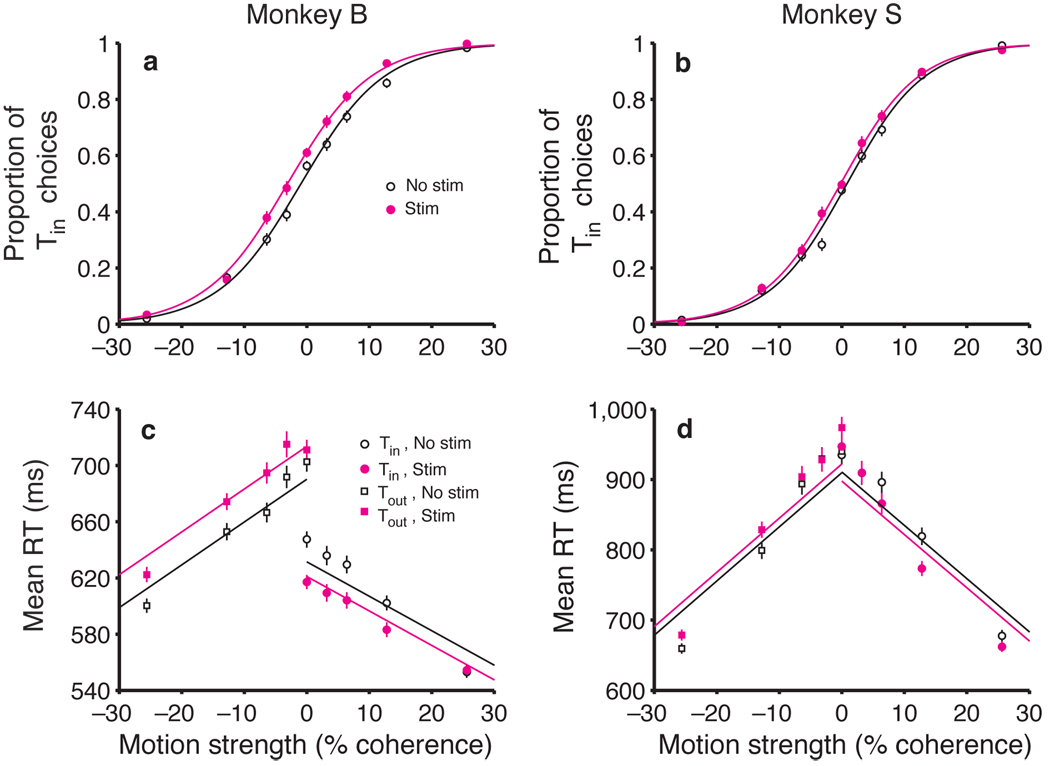
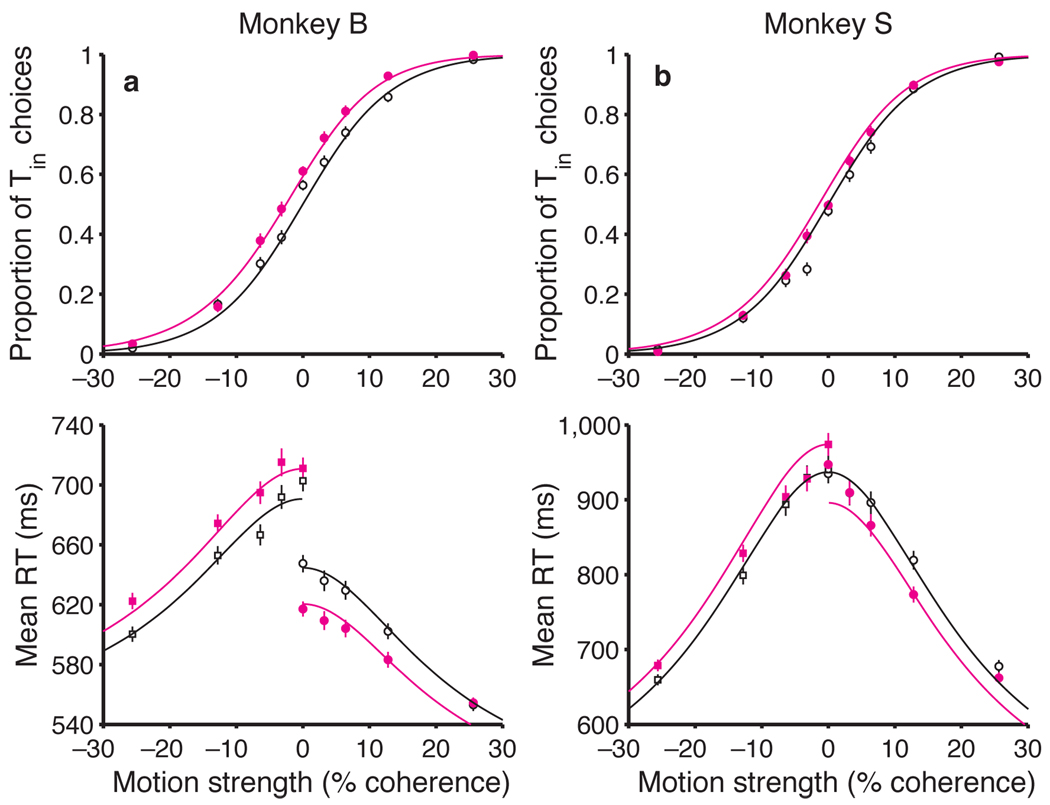
Microstimulation biased the monkeys to choose the direction of motion associated with the Tin choice target (Fig. 2a,b). On both stimulation and non-stimulation trials, stronger motion toward Tin led to more Tin choices, and stronger motion toward Tout led to more Tout choices, as previously shown10. Microstimulation caused a small change in these choice frequencies. This was most apparent at the weaker motion strengths (the middle of the plot), where stimulation caused an increase in Tin choices at nearly every point for both monkeys. It is convenient to quantify this effect by calculating the change in motion strength that would be required to produce the observed shift in probability (see Methods). For monkey B, LIP microstimulation biased decisions in favor of Tin by an amount equivalent to adding 2.13% coherent motion toward Tin (95% CI: 1.42 to 2.85% coh, P < 0.001). For monkey S, the effect of microstimulation was weaker (equivalent to 1.00% coh toward Tin), but it was still significant (CI: 0.32 to 1.68% coh, P < 0.005).
Fig. 2.
Microstimulation in LIP affects both decisions and reaction times. (a, b) Effect of motion strength and LIP microstimulation on monkeys’ choices. The probability of a Tin choice is plotted as a function of motion strength. Positive and negative motion strengths correspond to motion toward Tin and Tout, respectively. The sigmoid curves are fit using equation 1, which characterizes the microstimulation effect as a horizontal shift of the psychometric function. Data are pooled from 12 stimulation sites in monkey B and 12 sites in monkey S. (c, d) Effect of motion strength and LIP microstimulation on reaction time. Average RTs (± SEM) are plotted as a function of motion strength for all correct trials. The lines are fit using equation 2 (Methods). Data are not shown for the highest motion strength (± 51.2% coh) because the effects are similar to those seen at the next highest motion strength (± 25.6% coh).
Microstimulation also biased the monkeys’ RTs (Fig. 2c,d). On both stimulation and non-stimulation trials, stronger motion led to faster RTs for both Tin and Tout choices, compared to the more difficult conditions, as has been previously shown10. The first effect of microstimulation on decision time was a reduction in RT for Tin choices (Fig. 2c,d, solid circles). For monkey B, this effect was most evident at the weaker motion strengths, and it fell to nonsignificant levels with stronger motion. For monkey S, the effect was smaller and most evident at intermediate motion strengths. Again, we quantified these effects by calculating the change in motion strength that would be required to cause the same change in RT, on average (see Methods). For monkey B, stimulation reduced Tin RTs by an amount equivalent to adding 4.26% coherence motion toward Tin (CI: 2.04 to 6.49% coh, P < 0.005). The effects for monkey S were equivalent to adding 1.71% coh (CI: 0.39 to 3.04% coh, P < 0.05). Although the fits exhibit systematic error, they describe the general trend reasonably well (R2 = 0.96 for both monkeys). This crude description of the trend will allow us to compare the effects of stimulation on choice and RT (see below).
The second effect of microstimulation on decision time was an increase in RT for Tout choices (Fig. 2c,d, solid squares). In monkey B, this was a strong effect, apparent at all motion strengths. Microstimulation increased RTs for Tout choices by an amount equivalent to adding 7.70% coh motion toward Tin —that is, in the direction away from Tout (CI: 5.28 to 10.13% coh, P < 0.001). The effects for monkey S were smaller but consistent across the range of motion strengths. The changes mimicked the addition of 1.60% coh toward Tin (CI: 0.33 to 2.87% coh, P < 0.05). Like the fits to the Tin RTs, the fits to the Tout RTs exhibit systematic error, but again, the general trend is described reasonably well (R2 = 0.98 and 0.95 for monkeys B and S, respectively). When expressed in terms of motion strength, there is a hint that the size of the microsimulation effects on choice and RT may be different. We will attempt to resolve this discrepancy in a moment.
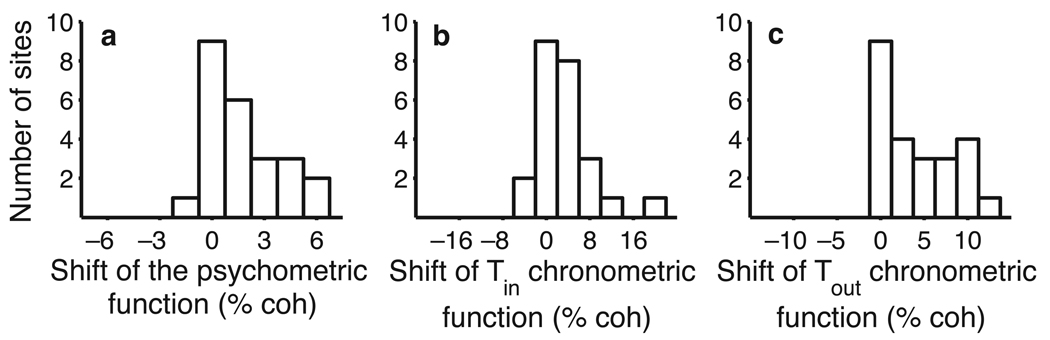
Overall, the effects of LIP microstimulation were modest but consistent across the 24 sites (Fig. 3). In 17/24 sites, microstimulation increased the probability of Tin choices, and there was only one site in which microstimulation caused a substantial increase in Tout choices. Moreover, stimulation caused the monkeys to decrease their RTs when making decisions in favor of Tin in 16/24 sites and to increase their RTs for Tout choices in 23/24 sites. These effects were only weakly correlated with each other: no pairwise correlation was statistically significant in either animal individually (P > 0.2; Fisher z).
Fig. 3.
Effects of LIP microstimulation are evident during individual experiments. (a) Effect of microstimulation on choices at different electrode sites for two monkeys. The equivalent motion strength, calculated using equation 1, is the horizontal shift in the psychometric function with microstimulation at each site. (b) Effect of microstimulation on Tin RTs at different electrode sites for two monkeys. The equivalent motion strength is calculated using equation 2a. (c) Effect of microstimulation on Tout RTs at different electrode sites for two monkeys. The equivalent motion strength is calculated using equation 2b.
Our results suggest that microstimulation of selected clusters of LIP neurons affects the decision process that underlies the monkey’s choices and RTs, but a potential concern is that we have merely affected the motor response. It is therefore important to test whether the effects of LIP microstimulation are confined to the decision-making task or whether they are also apparent in a comparable motor task. For this reason, we randomly interleaved control trials in which monkeys made delayed saccades at unpredictable times to single targets, without having to make a decision about motion direction (see Methods). These control trials mimicked the waiting times and eye movement responses seen in the direction-decision task, but they removed the spatial uncertainty associated with choosing a target. On these trials, microstimulation never evoked a saccade. The monkeys made eye movements only after the delay period ended and the FP was dimmed.
These control trials also allowed us to determine whether any portion of the RT effects seen on the motion discrimination trials could be attributed to changes of a purely motor nature. This, however, was not the case because the latencies of the motor responses in the control trials were not affected by microstimulation (Tin: RT increased 1.4 ± 1.6 ms, P = 0.4; Tout: RT increased 0.7 ± 1.5 ms, P = 0.6; mean ± SEM, n = 19 sites, 2,550 trials; mean RT: 221 ms). Thus, microstimulation appears to exert its effect on choice and RT by affecting the decision process, rather than generic aspects of eye movement preparation and execution.
Comparison of LIP and MT microstimulation
At face value, microstimulation of neurons in LIP appears to affect decisions about motion in a manner that is qualitatively similar to stimulation of direction-selective neurons in area MT during the same task16. However, there are several important differences. Perhaps the most obvious is reflected in the design of the experiments. For the MT experiments, the hypothesis concerned neurons with receptive fields overlapping the motion stimulus. In contrast, the hypothesis of this study concerns LIP neurons with RFs overlapping the target of an eye movement. Thus, the two experiments required stimulation of neurons representing different regions of the visual field. Of course, this limits any conclusions that can be drawn about these two groups of neurons.
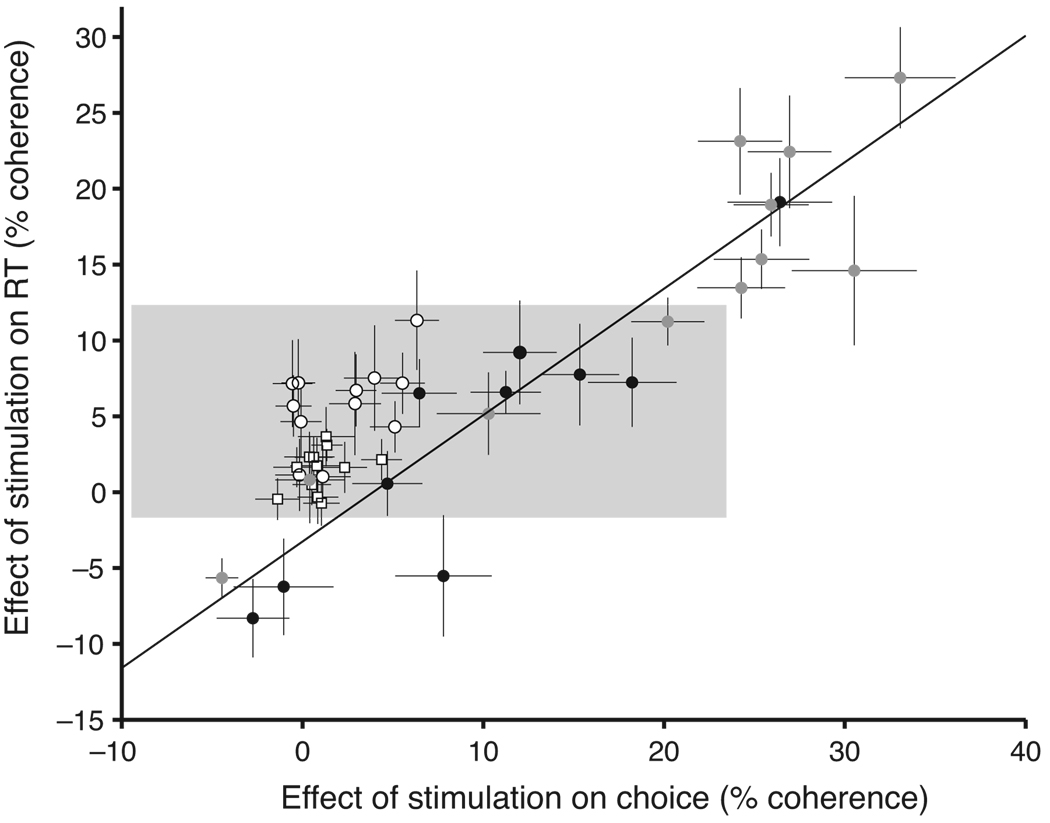
More detailed observations are consistent with the idea that the neurons we stimulated in LIP and MT lie at different points along a causal chain connecting stimulus representation to decision and action. Indeed, examination of the size and pattern of the stimulation effects in LIP reveals important differences from the effects of MT microstimulation using the same RT direction discrimination task16. The most obvious difference is that LIP stimulation led to relatively small changes in RT and choice, especially the latter (Fig. 4). We will explain why the effects are small in a moment. First, we wish to draw attention to a more telling distinction alluded to earlier: LIP stimulation has a greater average effect on RT than on choice, in contrast to the effects of MT stimulation. Even for sites restricted to have comparable changes in RT, MT stimulation had much stronger effects on choice than LIP stimulation (11.06 ± 0.72% coh vs. 1.62 ± 0.25% coh, P < 0.001, t test; Fig. 4, shaded region). This suggests that stimulation in MT and LIP affect the decision process in qualitatively different ways. Stimulation in MT seems to affect the decision process by mimicking a change in motion strength. On the other hand, our attempt to characterize LIP stimulation by an equivalent change in motion strength leads to a logical inconsistency.
Fig. 4.
Microstimulation in MT and LIP have different effects on decisions and RTs. Each point represents the effect of microstimulation on choices and RTs in one experiment. This is characterized by the equivalent motion strength that would have been needed to produce the observed choice bias or RT shift (equations 1 and 2; α2 and α5 constrained to be equal). Open symbols show LIP stimulation sites (○, monkey B;□, monkey S), and filled symbols show MT stimulation sites (black, monkey B; gray, monkey N). Error bars show the standard error of the parameter estimates (see Methods). The line is fit to the MT sites (weighted type II regression50). All LIP sites lie above this line, indicating that LIP stimulation has larger effects than MT stimulation on RT for a given effect on choices. One MT data point from monkey N lies outside the boundaries of this figure at (62, 60). The gray box highlights all sites in LIP and those in MT with similarly sized RT effects.
Why should LIP microstimulation affect RT more than it affects choice? Up to now, we have tried to describe the effects of microstimulation on choice and RT using descriptive methods. To gain insight, it is helpful to consider these results in the context of a model for decision-making. This exercise provides an explanation for both the difference in the magnitude of the stimulation effects in MT and LIP and the larger effect of LIP stimulation on RT than on choice.
A simple diffusion model provides a plausible account for the stimulation effects in both areas (Fig. 5). The model draws on an analogy between decision-making and the noisy accumulation of evidence to a decision criterion, formalized as a diffusion to bound10,17,18. Models of this variety provide a useful framework to understand a wide range of decision processes17,19–21. By invoking a single mechanism to explain which choice is made and when the process terminates, the diffusion-to-bound model provides a unified explanation for choices and RTs. It gives a particularly compelling account of the choice and RT functions for the motion discrimination task in monkeys and humans22. We can thus use this framework to gain insight into the underlying computations of neurons in MT and LIP by determining how the model best accounts for the effects of microstimulation in each area.
Fig. 5.
Microstimulation of LIP and MT affect the decision process at different points. (a) Diffusion-to-bound model of the decision process. Momentary evidence in favor of the Tin direction and against the Tout direction is accumulated as a function of time. The accumulation is termed the decision variable. The process terminates with a Tin or Tout choice when the decision variable reaches the upper or lower bound, respectively, at +A or −B. The momentary evidence is distributed as a unit-variance Gaussian whose mean, μ, is proportional to motion strength. On a single trial, the decision variable follows a random “diffusion” path, like the one shown. Both decision time and the proportion of Tin choices are governed by A, B, and μ. (b) Diffusion model fit to psychometric and chronometric functions for monkey B with and without LIP microstimulation. Data are the same as in Figs. 2a and c. LIP stimulation mainly affects the decision process by adding a constant to the decision variable. The faster RTs associated with Tin choices are explained by less nondecision time for leftward choices, which was always the direction of Tin for this monkey. (A = 13.4, k = 0.0056, Tout nondecision time = 511 ms, Tin nondecision time = 465 ms). (c) Diffusion model fit to psychometric and chronometric functions for monkey B with and without MT microstimulation. Data are from a previously published experiment using this monkey16. Although the average effects on RT were similar for MT and LIP stimulation, MT stimulation caused a stronger choice bias: 13.1% more choices in favor of the preferred direction of the stimulated neurons compared to 3.5% more Tin choices with LIP stimulation. MT stimulation mainly affects the decision process by adding to the momentary evidence. Less nondecision time for leftward choices was also frequently present in individual MT experiments, but it is not apparent in the averages because the preferred direction of individual MT sites corresponded to different saccade directions. (A = 16.7, k = 0.0023, Null nondecision time = 398 ms, Pref nondecision time = 372 ms).
In principle, microstimulation could affect choices and RTs by adding to the momentary evidence like additional random dot motion, or it could add to the accumulated evidence, or both. Adding to the momentary evidence would affect the rate of change of the decision variable (i.e., the drift rate of the diffusion process); adding to the accumulated evidence would offset the decision variable, bringing it closer to one of the bounds that terminate the decision process. Within this framework, these are the only options worthy of serious consideration because they are the only ones that could cause the combined choice and RT effects that were observed. For example, microstimulation could not have simply added more noise to the process, because that would decrease the RT for both choices and lead to poorer accuracy without biasing choices in one direction.
We therefore solved for the diffusion-to-bound model that best explained the effect of microstimulation of MT and LIP in monkey B by assuming that stimulation added to the momentary evidence or the decision variable or both (see Methods). These fits are remarkably good in both cases (Fig. 5b,c; R2 > 0.97 in both cases). According to the model, MT stimulation was accounted for best as a change in the momentary evidence equivalent to 9.3% coh (CI: 7.8 to 10.8% coh). This value matches the average of the effects displayed in Fig. 4, which were estimated using a more descriptive method. The model fits also indicate that MT stimulation did not affect the accumulated evidence: the estimated offset of the decision variable was negligible and in the opposite direction as the stimulation effects (mean: −1.2% of the bound, CI: −5.3 to 2.9%). This suggests that the comparable effects on RT and choice for MT stimulation are due to the changes in the momentary evidence, as if microstimulation added to the spike rate of neurons that represent motion in their preferred direction16.
In contrast, LIP stimulation was accounted for best as a change in the decision variable. The model fits indicate that LIP stimulation caused an offset of the decision variable by 16.9% of the excursion to the bound (CI: 11.4 to 22.3%). LIP stimulation did not appear to change the momentary evidence (mean: 0.49% coh, CI: −0.51 to 1.50% coh). This suggests that the larger effects on RT than on choice for LIP stimulation are explained by an offset of the decision variable, as if microstimulation added to the spike rate of neurons that accumulate evidence in favor of an eye movement to the choice target in their RF. The same analysis on the data from monkey S showed that the effect of LIP microstimulation was also captured by an offset in the decision variable (mean: 4.7% of the bound, CI: 0.6 to 8.8%). The change in the momentary evidence was negligible and in the opposite direction as the stimulation effects (mean: −0.29% coh, CI: −1.11 to 0.53% coh). We cannot make a direct comparison between LIP and MT stimulation in this monkey because we have not stimulated in MT.
The diffusion-to-bound framework also explains the paradoxical observation that although LIP lies further than MT along the causal chain from sensation to decision to action, LIP microstimulation has weaker effects on decisions. According to this framework, motion-selective neurons in MT provide the momentary evidence that is accumulated into the decision variable represented by neurons in LIP. Thus, a small change in the firing rates of these MT neurons is integrated as a function of time; the cumulative effect on the decision variable is substantial. In contrast, a change in the firing rates of the LIP neurons brings the decision variable closer to the bound, but the effect is not cumulative. The following exercise allows us to pursue this intuition more quantitatively.
Size of the stimulation effects
We can use the diffusion-to-bound framework to make a rough prediction for the effects of LIP stimulation on choice and RT using as a starting point the measured effects of MT stimulation. While this exercise is certainly speculative, it helps to make the above insight more concrete. We will focus, initially, on monkey B because MT stimulation data and recordings from LIP have been obtained from this monkey during the reaction time task. For this monkey, microstimulation of area MT produced a change in momentary evidence equivalent to ~10% coherent motion in the preferred direction of the stimulated neurons (see Methods)16. Based on recordings from MT of other monkeys, a change in motion strength of 10% coherence results in a change in firing rate of 5 sp/s for the average MT neuron15. Thus, absent detailed knowledge about how microstimulation affects the neurons in MT, a reasonable first approximation is to say that it is equivalent to a change in spike rate of ~5 sp/s. Suppose then that microstimulation were to affect LIP similarly to MT.
What are the predicted consequences of adding 5 sp/s to the LIP neurons? A previous study measured the firing rate of LIP neurons from monkey B during the RT direction discrimination task10. The firing rate at the first moment when motion affects the discharge is 42 sp/s, and it increases to 69 sp/s, on average, at the end of the decision process terminating in a Tin choice (see their Fig. 7, ~200 ms after onset of random dot motion and 70 ms before onset of the saccade10). The upper bound is thus 27 sp/s above the starting point. If microstimulation adds 5 sp/s to the average firing rate of LIP neurons with the Tin target in their RF, it would be equivalent to a change in the starting point of the accumulation by 18.5% of the overall excursion. This value predicts psychometric and chronometric functions similar to the ones observed in monkey B (Fig. 6a).
Fig. 6.
Predicted magnitude of LIP microstimulation effects on choice and RT. Predicted psychometric and chronometric functions for the two monkeys. Microstimulation was assumed to change the spike rates of LIP neurons that represent the decision variable by 5 sp/s. The size of the change was estimated by analyzing MT microstimulation experiments in monkey B. It is the change in firing rates of MT neurons that would be required to produce the observed effects on choice and RT, equivalent to a change in motion strength of 10% coh. See text for details. The data points are the same as Fig. 2. Dashed and solid lines are predictions for non-stimulated and stimulated conditions, respectively. (a) Predictions for monkey B. Solid curve shows the diffusion model fit to the non-stimulation trials (A = 13.4, k = 0.0056, Tout nondecision time = 511 ms, Tin nondecision time = 465 ms). Dashed curve shows predicted change in the choices and RTs if the decision variable were offset by 2.48 units (18.5%) toward A. (b) Predictions for monkey S. Same conventions as in a (model fit to non-stimulation trials: A = 23.6, k = 0.0032, Tout nondecision time = 385 ms, Tin nondecision time = 385 ms). The prediction is based on the same offset of 2.48 units toward A, which constitutes a smaller fraction of the bound height for this monkey (10.5%).
The same exercise also plausibly explains the smaller effects of LIP stimulation in monkey S. The fits suggest that this monkey exercised a higher diffusion bound than monkey B. If the higher bound is instantiated as a higher neural bound in LIP, then smaller shifts in the psychometric and chronometric functions are expected for the same 5 sp/s change in the decision variable. These predictions also match the data reasonably well (Fig. 6b). Given the assumptions and simplifications that went into making these predictions, the fact that they match the data as well as they do is not particularly telling. The numbers used in this exercise are far from perfect, especially for monkey S in which neither LIP recording nor MT microstimulation have been performed. Moreover, microstimulation is unlikely to exert identical changes in MT and LIP firing rates. Nonetheless, this numerical exercise serves as a benchmark. It tells us that if microstimulation were to exert comparable changes in the two areas, we would expect to observe the pattern of RT and choices seen in our data.
In summary, the diffusion-to-bound framework explains the effect of stimulation in MT as a change in the momentary evidence in favor of the direction preferred by the stimulated neurons. It explains the effect of LIP stimulation as an offset in the accumulation of momentary evidence in favor of the choice target in the RF of the stimulated neurons. In doing so, it makes sense of the comparable effects of MT stimulation on RT and choice and the relatively larger effects of LIP stimulation on RT than on choice. It also provides an intuition for why LIP stimulation causes such small effects on choice in comparison to MT stimulation. Of course, it should be noted that these comparisons are limited by the experimental design (stimulating a cluster of cells with RFs overlapping the motion stimulus for the MT experiment versus a cluster of cells with RFs overlapping one of the targets for the LIP experiment) and variability in baseline behavior between animals and across experiments.
DISCUSSION
The central finding of our study is the demonstration that LIP neurons with RFs overlapping a choice target play a causal role in decision formation for this task. This puts LIP neurons on similar footing to MT neurons, which have been shown to play a causal role in the perceptual judgment of visual motion in their receptive fields7,16,23. It also complements previous results from single-unit recording studies that suggest that many LIP neurons accumulate evidence over time from sensory areas such as MT to a criterion level in order to form a decision10. These LIP neurons most likely play this role because decisions about motion are, in our task, decisions about where to move the eyes.
Indeed, our primary conclusion is that neurons in LIP are involved in representing a decision variable about where to move the eyes. A related hypothesis is that LIP is involved in the control of spatial attention24–27, in this case to one or the other choice target, or that it represents a motor preparatory signal13,28,29. These “alternatives” may be unified into the single framework that LIP serves as a saliency map that is used to choose both where to look and where to attend. In the decision task, it is possible to refine these concepts by demonstrating a quantitative relationship between LIP neural activity and a cognitive (decision) variable10,30. The present finding takes this one step further by establishing that the activity is not just correlated with these computations but in the causal chain leading to decisions.
Previous applications of microstimulation to area LIP have led to the idea that this area plays a role in the timing of saccades but not to eye movement selection31. Indeed, attempts to influence decisions on a motion task were inconsistent (E. Seidemann & W. T. Newsome, Soc. Neurosci. Abstr. 666.11, 1996). The key difference with this study is the use of a combined choice-reaction time paradigm. In addition to providing a sensitive measure (RT), it permits application of microstimulation during the rather prolonged period of decision formation that could be identified on each trial.
We propose that neurons in LIP account for decisions about where to look and the time taken to reach those decisions through the single process of accumulation of evidence to a bound. One of the more surprising results in this study was that stimulation increased RT for Tout choices. It is possible that this slowing is a non-specific effect of stimulation on saccades as shown in FEF32,33, but we did not observe any slowing with LIP stimulation during simple delayed saccades. Thus, the slowing we observe seems to require a second potential choice-target in the RF of the stimulated neurons. According to the diffusion-to-bound model, the slowing is produced by raising the bound of the competing mechanism, or equivalently by subtracting from the accumulation of evidence. This suggests a negative interaction between the pools of neurons selective for the two different choice targets.
Several other brain structures such as the superior colliculus, the dorsolateral prefrontal cortex (Walker area 46), and the FEF are thought to represent quantities like the accumulated evidence in area LIP2,10,11,34–37, and the superior colliculus is also known to play a causal role in the selection of targets for eye movements38. We do not yet know if LIP affects the decision process through mechanisms involving these structures, and we cannot rule out the possibility that some of the observed effects are due to antidromic activation of these areas. Orthodromic activation of these areas, on the other hand, is consistent with the conclusion that LIP is in the causal chain. An obvious question is whether altering the activity of neurons in the FEF, the superior colliculus, the prefrontal cortex, and other related areas would influence the monkey’s decision process, and if so, whether those areas would affect decisions similarly to MT or LIP or in some other way.
Ultimately, we would like to know the detailed mechanisms underlying decision-making. Our results tell us that these mechanisms can be studied in LIP. In addition, previous studies have shown that LIP responses are modulated by many factors, such as attention, expectation, time, motor intention, experienced value, reward, and the prior probability of making an eye movement12,13,39–42. This suggests that the responses in LIP are based on a convergence of multiple signals relevant for making a decision. It is now clear that when these cognitive signals affect neurons in area LIP they are likely to influence behavior.
METHODS
Behavioral Task
Stimuli were presented on a computer monitor (75 Hz frame rate) using Matlab 5.2 for the Macintosh and the Psychophysics Toolbox43. Trials started with the appearance of a single dot that the monkey was required to fixate. Upon fixation, two bright red (4.5 cd/m2) choice targets appeared at an equal distance from the FP and 180 degrees apart. The random-dot motion stimulus next appeared in a 5° diameter aperture that was either centered at the FP or located 5° away from the center orthogonal to the target locations. To generate this stimulus, a set of dots was shown and then replotted 40 ms later. When replotted, a subset of dots was offset from their original location to create apparent motion while the remaining dots were relocated randomly. The dot density was 16.7 dots/deg2/sec and the displacement was chosen to give a dot speed of 6.0 deg/sec.
Both the direction and motion strength (the percentage of coherently moving dots) were chosen randomly on each trial. The monkey’s task was to determine the direction of net motion, which it indicated by making a saccade to the appropriate choice target (right target for rightward motion, etc.). The monkey could indicate its decision at any time after motion onset. The monkey received a liquid reward for all correct choices and on a random half of the trials when there was no net motion (0% coh). Rewards were administered one second after motion onset or upon choice target acquisition, whichever came later. The rules governing reward were not altered on microstimulation trials.
The monkey also performed a delayed saccade task while we searched for stimulation sites and during interleaved control trials. The monkey fixated as above, but only a single target was presented. The monkey was required to maintain fixation until we extinguished the FP after a random delay, at which time the monkey was rewarded for making a saccade to the target. The delay time was drawn from a truncated exponential distribution (mean = 600 ms for monkey B; 700 ms for monkey S). The control trials comprised one-seventh of the total trials.
Electrophysiology
Two rhesus monkeys were subjects in the experiments. Details of surgical, training, and recording procedures have been previously published10. Briefly, a head-holding device, scleral search coil for monitoring eye position, and recording chamber were implanted under general anesthesia. Tungsten microelectrodes (Alpha Omega) suitable for multi-unit recording and microstimulation (impedances usually ranged from 0.8 MOhm to 1.2 MOhm, never exceeding 1.8 MOhm) were advanced into LIPv44, which we targeted using MRI scans in combination with the CARET software package45.
Multiunit RFs were mapped by hand using the delayed saccade task described above. RFs were typically 9° eccentric (range 5° to 15°) and did not overlap the random dot motion stimulus. Specifically, there was never any discernable modulation when a target was placed at the location of the motion stimulus or at the Tout location. Sites for microstimulation were selected based on a consistent location of the multi-unit RF over a span of at least 200 µm, and the electrode was placed in the middle of the cluster. All stimulation sites also exhibited sustained responses during memory saccade trials. When a site was found, experiments were never stopped prematurely unless the monkey refused to continue. We preset the minimum number of discrimination trials for including data at 500, and we always stopped collecting data after 800–900 discrimination trials. We only performed one experiment at any particular stimulation site, so that each site corresponds to a unique location within LIP.
Microstimulation was controlled by a Grass S88 with two optical isolation units (Grass PSIU6). Stimulation trains consisted of 10–20 µA biphasic current pulses of 300 µs duration at a rate of 200 Hz. These levels are thought to directly activate neurons within an approximately 100 µm radius of the electrode tip46, but indirect activation of neural elements may increase this spread47. We applied microstimulation during the entire duration of the motion stimulus on a randomly chosen half of the trials. Stimulation was terminated upon initiation of the saccade. The initiation of the saccade was detected as the time when the eye exited a small window around the FP, which triggered a bit change (TTL) that controlled the stimulator. The MT stimulation study to which we compare our data used the same stimulation protocol, except the amplitude of the current pulses was 5 µA16. For the control delayed saccade trials, microstimulation started when the saccade target appeared and continued until saccade initiation. The time between target onset and the go signal was set so that the average duration of microstimulation was roughly equal in control trials to that applied during the discrimination trials.
All training, surgery and experimental procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Washington Animal Care Committee.
Data Analysis
The choice data were analyzed using logistic regression (Fig. 2 and Fig 3), whereby the probability of a Tin choice is given by
| (1) |
where C is motion strength expressed as percentage coherence using the convention that positive and negative values denote motion toward Tin and Tout, respectively. IE is 1 if stimulation accompanied the trial and 0 otherwise. The βi are free parameters that were fit using the maximum likelihood method. The size of the stimulation effect can be expressed in units of equivalent motion strength by taking the ratio β2: β1, corresponding to a horizontal shift in the sigmoid function. Standard errors of parameters were estimated from the Hessian matrix of second partial derivatives of the log likelihood.
RTs were defined as the interval between motion onset and initiation of the saccade. The RT data were analyzed by fitting lines separately to the chronometric functions for correct Tin and Tout choices (Fig. 2), as follows:
| (2a) |
| (2b) |
where C and IE are the same as in equation 1. The αi are free parameters that were fit using the maximum likelihood method. The size of the stimulation effect for Tin and Tout RTs is given in units of equivalent motion strength by α2 and α5, respectively. For all fits, only the RTs of correct choices were analyzed. Unless otherwise stated, all tests of statistical significance were t-tests based on the standard error of the fitted coefficients.
Although the stimulation currents used in our experiments were an order of magnitude smaller than what has been previously reported to elicit saccades in LIP48, we did detect subtle changes in saccade metrics with microstimulation for some experiments. None of these changes were significant overall in either monkey. To test whether these changes in saccade metrics could account for the observed effects of microstimulation, we retested the stimulation effects after incorporating additional terms for saccade duration, mean velocity, peak velocity, and distance of saccade in equations 1 and 2. In neither monkey did changes in saccade metrics explain the effect of stimulation on choice or RT.
The MT data (Fig. 4 and Fig 5) are from a previously published study16 and incorporate two additional sites acquired after publication. Unlike the previous study, our figures and analyses incorporate all MT sites, including those without significant choice effects. We include all stimulation sites from the MT study here in order to provide a fair comparison with the LIP results. The goals of the present study differs from the previous one, which was focused on stimulation-induced changes in RT in an area already known to affect choice in this paradigm7.
Diffusion Model
We also fit psychometric and chronometric functions using a modified diffusion model (also known as a random walk to bounds). The model works by accumulating momentary evidence to an upper bound (+A) or a lower bound (−B), corresponding to the two direction choices. We refer to the accumulated evidence as the decision variable in this model. Positive evidence favors choice “A” and negative evidence favors choice “B”. The momentary evidence gathered in each time step is drawn from a unit-variance Gaussian distribution with mean μ determined by a linear transform of the motion strength:
| (3) |
where C is the motion strength and k is a free parameter that scales the motion appropriately. This relationship is reasonable because the expected difference in firing rates between direction selective neurons in area MT is zero when C=0 and is known to vary linearly, on average, as a function of motion strength15. Both the momentary evidence and the decision variable of this model can be related to neural responses18. The bound reached first by the accumulated evidence determines the choice, and the decision time is determined by how long it took to reach that bound. One advantage of this model is that analytic solutions exist for both of these22,49. The probability that the decision variable hits bound “A” first is
| (4) |
In the limit as μ approaches zero, this converges to
| (5) |
The mean time to bound “A” is
| (6) |
In the limit as μ approaches zero, this converges to
| (7) |
The mean time to bound “B” can be found by exchanging A and B in the above equations. Notice that near C=0, the decision time varies linearly as a function of C and approximately quadratically as a function of the criterion height (A+B). The total RT is a combination of the decision time and a nondecision “residual” time, which accounts for sensory and motor latencies and presumably involves other processes that we do not fully understand37. The RT asymmetry in monkey B for the non-stimulation trials provides a particularly striking example. We accommodated this by allowing different nondecision times for the two choice directions.
We always assumed symmetric bounds in the absence of stimulation so that B = A. This is justified because (1) monkey S was unbiased in its choices with no stimulation, and (2) although monkey B exhibited a small bias (56% Tin choices at 0% coh; see Fig. 2), accounting for this bias with a change in the decision variable and momentary evidence for non-stimulated trials did not alter the conclusions. Note that the diffusion model is formally equivalent to a race between competing accumulators when the accumulated quantities (e.g., evidence for left vs. right) are anticorrelated.
To incorporate the effects of microstimulation into the diffusion model, we added a constant to the incoming sensory evidence (equivalent to altering the motion strength, C) or the accumulated decision variable, or both. Adding a fixed offset to the decision variable is formally equivalent to subtracting a constant from the bounds. Thus, increasing the decision variable by one unit can be implemented in the above equations by making A one unit smaller and B one unit larger. Using this method, we fit the chronometric and psychometric function in the presence and absence of microstimulation with a model that has six parameters: k, A, Tin nondecision time, Tout nondecision time, microstimulation effect on motion strength, and microstimulation effect on decision variable. Note that this involves three fewer parameters than equations 1 and 2, used to generate the descriptive fits to the data (Fig. 2). The best-fit parameters and their standard errors were found using the maximum likelihood method. The fraction of the variance reported in the text (R2) compares the fitted curves to the RT means and choice frequencies.
Acknowledgments
We thank A. Huk, M. Mazurek, and W. Newsome for helpful discussion on all aspects of this project. We also thank S. Allred, A. Churchland, R. Kiani, J. Palmer, and T. Yang for comments on this manuscript and useful suggestions, and M. Mihali, J. McNulty, and V. K. Skypeck for technical assistance. This study was supported by the Howard Hughes Medical Institute (HHMI) and the National Eye Institute. T.H. is also supported by a HHMI predoctoral fellowship.
References
- 1.de Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- 2.Schall J, Thompson K. Neural selection and control of visually guided eye movements. Annu Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- 3.Glimcher PW. The neurobiology of visual-saccadic decision making. Annu Rev Neurosci. 2003;26:133–179. doi: 10.1146/annurev.neuro.26.010302.081134. [DOI] [PubMed] [Google Scholar]
- 4.Romo R, Salinas E. Flutter discrimination: neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 5.Pasternak T, Merigan WH. Motion perception following lesions of the superior temporal sulcus in the monkey. Cereb Cortex. 1994;4:247–259. doi: 10.1093/cercor/4.3.247. [DOI] [PubMed] [Google Scholar]
- 6.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salzman CD, Murasugi CM, Britten KH, Newsome WT. Microstimulation in visual area MT: Effects on direction discrimination performance. J. Neurosci. 1992;12:2331–2355. doi: 10.1523/JNEUROSCI.12-06-02331.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newsome WT, Paré EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J. Neurosci. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold JI, Shadlen MN. The influence of behavioral context on the representation of a perceptual decision in developing oculomotor commands. J Neurosci. 2003;23:632–651. doi: 10.1523/JNEUROSCI.23-02-00632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci. 2002;22:9475–9489. doi: 10.1523/JNEUROSCI.22-21-09475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- 12.Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu. Rev. Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- 13.Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosi. 2000;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- 14.Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- 15.Britten KH, Shadlen MN, Newsome WT, Movshon JA. Responses of neurons in macaque MT to stochastic motion signals. Vis. Neurosci. 1993;10:1157–1169. doi: 10.1017/s0952523800010269. [DOI] [PubMed] [Google Scholar]
- 16.Ditterich J, Mazurek M, Shadlen MN. Microstimulation of visual cortex affects the speed of perceptual decisions. Nat Neurosci. 2003;6:891–898. doi: 10.1038/nn1094. [DOI] [PubMed] [Google Scholar]
- 17.Ratcliff R, Rouder JN. Modeling response times for two-choice decisions. Psychological Science. 1998;9:347–356. [Google Scholar]
- 18.Mazurek ME, Roitman JD, Ditterich J, Shadlen MN. A role for neural integrators in perceptual decision making. Cereb Cortex. 2003;13:1257–1269. doi: 10.1093/cercor/bhg097. [DOI] [PubMed] [Google Scholar]
- 19.Holmes P, et al. Optimal decisions: from neural spikes, through stochastic differential equations, to behavior. IEICE Transactions on Fundamentals of Electronics, Communications and Computer Science. 2005;88:2496–2503. [Google Scholar]
- 20.Ratcliff R, Smith P. A comparison of sequential sampling models for two-choice reaction time. Psychol Rev. 2004;111:333–367. doi: 10.1037/0033-295X.111.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Link SW. The wave theory of difference and similarity. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 22.Palmer J, Huk AC, Shadlen MN. The effect of stimulus strength on the speed and accuracy of a perceptual decision. J Vis. 2005;5:376–404. doi: 10.1167/5.5.1. [DOI] [PubMed] [Google Scholar]
- 23.Bisley JW, Zaksas D, Pasternak T. Microstimulation of cortical area MT affects performance on a visual working memory task. J Neurophysiol. 2001;85:187–196. doi: 10.1152/jn.2001.85.1.187. [DOI] [PubMed] [Google Scholar]
- 24.Gottlieb J, Goldberg ME. Activity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nat Neurosci. 1999;2:906–912. doi: 10.1038/13209. [DOI] [PubMed] [Google Scholar]
- 25.Wardak C, Olivier E, Duhamel JR. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- 26.Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- 28.Platt ML, Glimcher PW. Responses of intraparietal neurons to saccadic targets and visual distractors. J Neurophysiol. 1997;78:1574–1589. doi: 10.1152/jn.1997.78.3.1574. [DOI] [PubMed] [Google Scholar]
- 29.Pare M, Wurtz RH. Progression in neuronal processing for saccadic eye movements from parietal cortex area lip to superior colliculus. J Neurophysiol. 2001;85:2545–2562. doi: 10.1152/jn.2001.85.6.2545. [DOI] [PubMed] [Google Scholar]
- 30.Gold JI, Shadlen MN. Neural computations that underlie decisions about sensory stimuli. Trends Cogn Sci. 2001;5:10–16. doi: 10.1016/s1364-6613(00)01567-9. [DOI] [PubMed] [Google Scholar]
- 31.Schiller PH, Tehovnik EJ. Look and see: how the brain moves your eyes about. Prog Brain Res. 2001;134:127–142. doi: 10.1016/s0079-6123(01)34010-4. [DOI] [PubMed] [Google Scholar]
- 32.Burman DD, Bruce CJ. Suppression of task-related saccades by electrical stimulation in the primate's frontal eye field. J Neurophysiol. 1997;77:2252–2267. doi: 10.1152/jn.1997.77.5.2252. [DOI] [PubMed] [Google Scholar]
- 33.Opris I, Barborica A, Ferrera VP. Effects of electrical microstimulation in monkey frontal eye field on saccades to remembered targets. Vision Res. 2005;45:3414–3429. doi: 10.1016/j.visres.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Romo R, Hernandez A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron. 2004;41:165–173. doi: 10.1016/s0896-6273(03)00817-1. [DOI] [PubMed] [Google Scholar]
- 35.Kim J-N, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- 36.Horwitz GD, Newsome WT. Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J Neurophysiol. 2001;86:2543–2558. doi: 10.1152/jn.2001.86.5.2543. [DOI] [PubMed] [Google Scholar]
- 37.Huk AC, Shadlen MN. Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making. J Neurosci. 2005;25:10420–10436. doi: 10.1523/JNEUROSCI.4684-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43:575–583. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 39.Eskandar EN, Assad JA. Dissociation of visual, motor and predictive signals in parietal cortex during visual guidance. Nat Neurosci. 1999;2:88–93. doi: 10.1038/4594. [DOI] [PubMed] [Google Scholar]
- 40.Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38:317–327. doi: 10.1016/s0896-6273(03)00185-5. [DOI] [PubMed] [Google Scholar]
- 41.Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304:1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- 42.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 43.Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:443–446. [PubMed] [Google Scholar]
- 44.Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Van Essen DC, et al. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tehovnik EJ. Electrical stimulation of neural tissue to evoke behavioral responses. J. Neurosci. Methods. 1996;65:1–17. doi: 10.1016/0165-0270(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 47.Butovas S, Schwarz C. Spatiotemporal effects of microstimulation in rat neocortex: a parametric study using multielectrode recordings. J Neurophysiol. 2003;90:3024–3039. doi: 10.1152/jn.00245.2003. [DOI] [PubMed] [Google Scholar]
- 48.Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. J. Neurophysiol. 1998;80:1713–1735. doi: 10.1152/jn.1998.80.4.1713. [DOI] [PubMed] [Google Scholar]
- 49.Smith P. A note on the distribution of response times for a random walk with gaussian increments. Journal of Mathematical Psychology. 1990;34:445–459. [Google Scholar]
- 50.Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical Recipes in C. Cambridge: Cambridge University Press; 1998. p. 735. [Google Scholar]