Abstract
Intradermal immunization using microfabricated needles represents a potentially powerful technology, which can enhance immune responses and provide antigen sparing. Solid vaccine formulations, which can be coated onto microneedle patches suitable for simple administration, can also potentially offer improved shelf-life. However the approach is not fully compatible with many vaccine adjuvants including alum, the most common adjuvant used in the vaccine market globally. Here, we introduce a polyphosphazene immuno adjuvant as a biologically potent and synergistic constituent of microneedle-based intradermal immunization technology. Poly[di(carboxylatophenoxy)phosphazene], PCPP, functions both as a vaccine adjuvant and as a key microfabrication material. When used as part of an intradermal delivery system for hepatitis B surface antigen, PCPP demonstrates superior activity in pigs compared to intramascular administration and significant antigen sparing potential. It also accelerates the microneedle fabrication process and reduces its dependence on the use of surfactants. In this way, PCPP-coated microneedles may enable effective intradermal vaccination from an adjuvanted patch delivery system.
Keywords: polyphosphazenes, vaccine adjuvants
Skin is an attractive organ for administration of vaccines since it constitutes an anatomic barrier, defending the body against external pathogens. Due to a large population of epidermal dendritic cells, such as Langerhan cells, it has a potential to facilitate induction of more potent immune responses and provide the basis for a significant antigen sparing effect (1–6). The latter can be highly desirable during times of vaccines shortages, such as epidemic emergencies, and can also reduce the cost of vaccine manufacturing, which is especially important for expanding vaccine use in less developed areas of the world (5, 7).
Microneedle technology, which utilizes submillimeter structures to pierce the stratum corneum and deliver vaccines in the epidermis or dermis compartments, is an especially attractive pathway for intradermal delivery (8–11). Microneedles are designed to combine vaccine formulation, often as a solid coating, and metal as a means to provide the required mechanical strength. Once applied to the skin, these formulations dissolve to release vaccine antigen in the skin compartment. Solid vaccine formulations are especially attractive since they also potentially offer improved shelf life and reduced dependence on temperature-controlled supply chains (12, 13). These microneedles can also potentially be self-administered and safely disposed of.
Modern vaccine technologies rely heavily on immune-enhancing additives (i.e., immunoadjuvants) to engender the desirable protective immune responses (14). Unfortunately, many of the currently used vaccine adjuvants may not be compatible with intradermal delivery approaches. For example, alum, which is the most common adjuvant used in the vaccine market globally (14), was shown to induce serious adverse effects, such as formation of granuloma, when administered intradermally (15). Other advanced adjuvants, which contain biphasic systems, such as oil emulsions or liposomes, may not be sufficiently stable to withstand the microneedle coating and drying processes.
Polyphosphazene polyelectrolytes are one of the most remarkable classes of vaccine adjuvants due to their macromolecular nature, well-defined structure, and synthetic origin. They are representatives of a broader class of synthetic macromolecules with phosphorus-nitrogen backbone and organic side groups, which have been studied in many areas of biomedical research, such as tissue engineering and drug delivery (16). Polyphosphazene derivatives with ionic groups, however, have demonstrated excellent immunomodulating potential when tested in multiple animal models with both viral and bacterial antigens (17–21). The lead compound, poly[di(carboxylatophenoxy)phosphazene], sodium salt (PCPP) has been advanced into clinical trials (22–24) and PCPP formulated vaccines were reported to be safe and immunogenic in humans (22, 23). Most importantly, the macromolecular nature of PCPP ensures excellent film forming and microencapsulating properties (25, 26). Biodegradability of this material allows the use of high molecular weight compounds, producing highly viscous solutions, an important feature for any coating process. PCPP is a water-soluble molecule that can be formulated with proteins in aqueous solutions under mild conditions (27) and has the potential to be dissolved easily in a highly hydrated environment, such as skin. Finally, anticipated dual functionality of such a molecule, as an immunoadjuvant and film forming/microfabrication material, eliminates the need for the use of additional macromolecular excipients and thus can potentially result in a higher vaccine loading capacity.
The objective of the present study is to integrate the advantages of intradermal delivery with a potent immunoadjuvant system to afford a highly efficient immunization approach. The paper summarizes our study on the feasibility of polyphosphazene adjuvants for microneedle based intradermal immunization. It describes microfabrication of PCPP containing needles, immunoadjuvant activity of such system, and its potential for vaccine delivery.
Results
PCPP Improves Microneedle Fabrication Process.
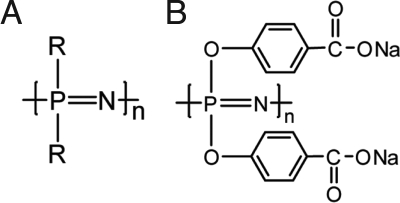
Polyphosphazenes are synthetic macromolecules with a phosphorus-nitrogen backbone and organic side groups (R) (Scheme 1A). A subclass of this family of polymers containing ionic or ionizable moieties, and polyphosphazene polyelectrolytes, are generally water-soluble polymers, which possess potent immunostimulating properties (17, 18). PCPP (Scheme 1B), the most advanced representative of the group, with a history of use in clinical trials, was selected as a target of our investigation for testing as a component of intradermal microneedle technology.
Scheme 1.
Chemical structures of polyphosphazenes (A) and poly[di(carboxylatophenoxy)phosphazene], sodium salt (PCPP) (B).
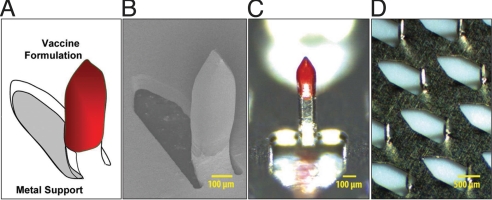
Microneedles used in our study (Fig. 1) can be described as a two-component system, containing a solid phase polymeric formulation with an antigen (functional “shell”) and titanium metal as a mechanical support (an inert “core”). Microneedles are integrated into rectangular arrays (1 × 1 cm), each containing 50 600-μm long needles. Such technical design forces the technology to rely heavily on the ability of the microfabrication polymer to form a film, which binds the antigen to the metal surface, and then dissolve upon application of the array to the skin to release the antigen.
Fig. 1.
Schematic presentation of a polyphosphazene coated microneedle (A), scanning electron microscopy image (magnification of 90×) of a coated microneedle (B), optical microscopy images of a coated microneedle (C) and an array of microneedles (D).
In general, selection of the film forming polymer for microneedle technology is influenced by a number of considerations. Candidates should have excellent viscosity enhancing characteristics, should be readily soluble in aqueous solutions, provide antigen stabilizing properties, and ideally have a history of use in humans. PCPP seems to address the requirements listed above and can also act as a film forming agent. Such a feature can be extremely important in maximizing microneedle loading, since the amount of the material that can be deposited on microneedles without compromising their dissolution and penetration characteristics is limited.
To date, sodium salt of carboxymethylcellulose (CMC) in combination with a surfactant has been one of the most frequent choices of microfabrication polymer for use with microneedle technology (28). However, higher concentration of surfactants in the formulation can be sometimes undesirable due to potential toxic effects associated with their ability to solubilize membranes or destabilizing effect on the protein (13, 29, 30). Thus, minimizing surfactant dependence in the microfabrication process can be an attractive goal for formulation development.
To investigate the performance of surfactant-free PCPP solution in the microneedle coating process, its film forming properties were compared with those of CMC, which was also used without a surfactant. The focus of such a comparative study was set on the rate of antigen loading. Rapid construction of the coating maximizes process efficiency and minimizes overall drying time. Since the latter can potentially have an adverse effect on sensitive antigens, it is highly desirable for a film-forming polymer to minimize the number of coating/drying cycles.
Microneedle coating was performed using micro dip-coating technology with individual wells for each microneedle and a 3-D positioning system (31, 32). This method was used because of its simplicity, ability to coat complex shapes, and minimal evaporation of the formulation solution. Aqueous formulations were used in the process and the coatings were dried at ambient temperature. The performance of both polymers, PCPP and CMC, was evaluated based on their ability to facilitate the deposition of the model antigen, BSA, on the microneedles. BSA was selected for these experiments due to a significant knowledge on physico-chemical behavior of BSA in formulations with PCPP and because it has commonly been used as a model antigen in previous studies (27).
The amount of the protein was detected by dissolving the coating in phosphate buffered saline (PBS) and analyzing the solute by HPLC. PCPP, in the coating formulation, was used at a concentration of 0.5% (wt/vol), while the concentration of CMC was slightly higher, 0.8% (wt/vol), to attain the same viscosity enhancing characteristics. Both polymer solutions had a viscosity of 5.1 cps in 0.1× PBS solutions at ambient temperature. Interestingly, the rate of antigen deposition was dramatically higher for PCPP solutions (Fig. 2, white columns) as compared to this CMC formulation (Fig. 2, black columns). Moreover, the increase of CMC concentration to 1.5% (wt/vol) still did not bring the rate of coating formation (Fig. 2, columns with diagonal pattern) to the levels achieved with PCPP at a much lower concentration. The advantage of PCPP formulations can be illustrated with the fact that the loading of 0.45 μg BSA per microneedle can be achieved with only four coating cycles using PCPP containing formulation, whereas the formulation based on CMC required 35 cycles to accomplish the same results. However, it is important to emphasize that the addition of surfactant to a CMC formulation can also provide efficient microneedle coating (31, 32).
Fig. 2.
BSA loading per microneedle as a function of a number of coating cycles using formulations containing 0.8% (wt/vol) of CMC (black columns), 1.5% (wt/vol) of CMC (columns with diagonal pattern), and 0.5% of PCPP (white columns) (all formulations contained 5% (wt/vol) BSA; 0.1× PBS; pH 7.4; ambient temperature). Data represent the mean values of duplicates, and error bars indicate SD in each series.
Doses of Antigen and PCPP on Microneedles Can Be Controlled Volumetrically.
One of the main challenges of solid phase microneedle technology is the difficulty of direct analysis without destroying the formulation. Thus, the establishment of accurate dosing approaches is one of the main objectives of technology development. Although, it is common to assume direct correlation between the amount of antigen on the microneedles and the number of coating cycles (dips) the microneedle was subjected to, this can be somewhat ambiguous. The effect of evaporation processes leading to changes in concentration and viscosity of the formulation can result in the unequal deposition of the antigen with every subsequent coating. The frequency of microwell replenishment, intervals between dips, and humidity of the environment may have more profound effect on the antigen loading than the actual numbers of coating cycles.
To minimize these variable effects, an approach based on measuring the actual volume of formulation supplied to the microneedles in the coating process has been developed. This volumetric method takes into account the actual consumption of the formulation in the micro dip-coating process, rather than the number of cycles. Essentially, the microneedle is repeatedly immersed in the coating formulation until the formulation in the microwell of the coating apparatus is fully consumed. Then the microwell is refilled and the process is repeated, if necessary.
The correlation between the loading of the protein and PCPP on the microneedle as detected by HPLC analysis, and the amount of antigen and polymer supplied volumetrically to be consumed in the micro dip-coating process is shown in Fig. S1. Results for both FITC-BSA (Fig. S1A) and PCPP (Fig. S1B) demonstrate high accuracy of the approach and linear dependence of the dose on the amounts of supplied materials (slope practically equals unity).
A critical advantage of the volumetric dosing approach is that it can dramatically reduce the possibility of drug or vaccine overdosing or underdosing. This is because a potentially uneven loading in each coating cycle, associated with evaporation and increase in the solution viscosity, does not result in a change of overall loading of the microneedle.
PCPP Coated Microneedles Enable Fast Release of Protein in Vitro and in Vivo.
Microneedles coated with polyphosphazene formulations were evaluated for their ability to deliver biologically active compounds into the skin. In vitro and in vivo studies were conducted in which microneedles were applied to the skin to investigate their capability to penetrate the stratum corneum, as well as dissolution (erosion) of the coating, and release of a model protein.
Arrays of microneedles were assembled in patches containing an adhesive layer to maintain close contact between the skin and the array. FITC-BSA was used as a model protein. Polyphosphazene coating also contained Red-40 dye to help with visualization of the coating and penetration sites. The area of application on the skin was shaved and cleaned. The patches containing microneedle arrays were manually put on the skin and pressure was applied using a thumb on the center of the patch for 1 min to facilitate microneedle insertion. The patches were then allowed to remain in place, undisturbed, for an additional 14 min. They were then removed from the skin, and the microneedle arrays were analyzed for the content of protein and polymer by dissolving the residual coating and analyzing the solution using HPLC.
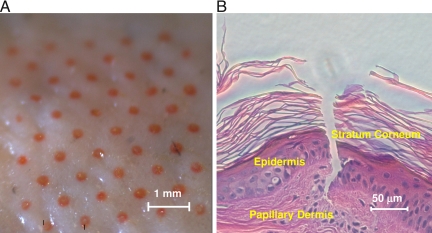
Porcine cadaver skin was used for in vitro experiments. After removal of the patch, the application site was wiped with a wet paper towel and examined under the microscope. The presence of 50 red dots, each corresponding to a single microneedle on the array, indicated apparent formation of channels in the stratum corneum and deposition of the dye (Fig. 3A). Histological evaluation of porcine cadaver skin section revealed the formation of a channel in the stratum corneum (Fig. 3B). Both, visual examination of the coating (Fig. S2) and its quantitative analysis before and after the insertion, demonstrated practically complete dissolution of the formulation in the skin accompanied with release of the protein and PCPP [(90.0 ± 5.6)% (wt/wt) and (92.4 ± 8.9)% (wt/wt), correspondingly].
Fig. 3.
Optical microscope image of porcine cadaver skin surface after application of microneedle containing patch (A) and histological section of porcine cadaver skin after insertion of coated microneedle (B) (in vitro; array coating: 7.2 μg FITC-BSA, 42.5 μg PCPP, 1.3 μg Red 40; application time 15 min).
In vivo experiments were also conducted in 3- to 4-week-old Land Race Cross pigs. The application sites were clipped of all hair and then shaved to further ensure a smooth surface. The results were in agreement with in vitro findings and showed practically complete dissolution of the polymer coating and release of the protein and PCPP [(87.3 ± 6.9)% (wt/wt) and (90.7 ± 3.9)% (wt/wt), correspondingly] in a short period (15 min).
In vitro and in vivo release studies were also conducted to evaluate the stability of biological material released from the microfabricated needles. Horseradish Peroxidase and recombinant Hepatitis B surface antigen (HBsAg) were tested and the results indicated that both agents survived the microfabrication process well (see SI Text for more information on antigen and protein stability).
PCPP Demonstrates Superior Activity as an Intradermal Immunoadjuvant.
The ultimate test for the PCPP microneedle approach is in vivo assessment of its performance in intradermal immunization studies. Pigs were used as an animal model because of the similarity of their skin anatomy to human skin (33) and recombinant HBsAg was used as an immunogen. Microneedle arrays were applied as part of an adhesive patch by pressing them firmly into the skin for 1 min and leaving them attached afterward so that the total application time amounted to 30 min.
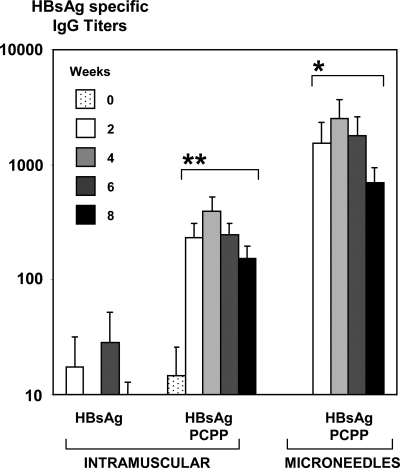
Single dose immunization with microneedles containing HBsAg-PCPP formulation as a coating induced IgG titers that were approximately 10 times higher than the same formulation administered intramuscularly (Fig. 4). The immune responses induced, using polyphosphazene microneedles, were up to three orders of magnitude higher than those obtained for nonadjuvanted antigen administered intramuscularly. In the case of microneedles they were also characterized by a rapid onset in antibody responses.
Fig. 4.
Serum IgG specific HBsAg titers after single dose immunization of pigs intramuscularly with HBsAg, HBsAg formulated with PCPP, and intradermally using microneedles coated with HBsAg and PCPP formulation (seven animals per group, 20 μg of HBsAg, PCPP formulations contained 66 μg of the polymer). *, Significantly higher than in both intramuscular groups. P < 0.05 (ANOVA). **, Significantly higher than in a nonadjuvanted intramuscular group. P < 0.05 (ANOVA).
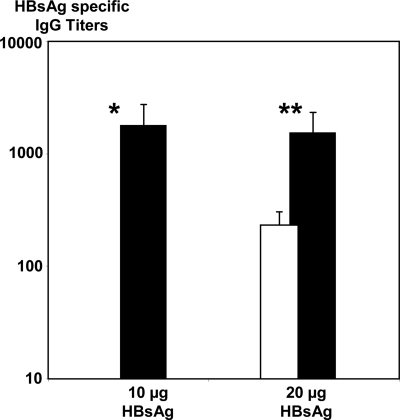
Intradermal immunization using microneedles with PCPP coating also showed a notable dose sparing effect. Ten micrograms of HBsAg administered intradermaly using microneedles induced 10-fold higher immune responses than 20 μg of the same formulation injected intramuscularly (Fig. 5). In our experiments we also did not observe a significant difference between 10 and 20 μg of the antigen when administered intradermally using PCPP.
Fig. 5.
Serum IgG specific HBsAg titers after single dose immunization of pigs with 20 μg of HBsAg formulated with PCPP intramuscularly (open column), 10 and 20 μg of HBsAg formulated with PCPP intradermally (black columns) (seven animals per group, week 2 data, intradermal immunization was performed using microneedles, all formulations contained 66 μg of PCPP). *, Significantly higher than in an intramuscular group (open column). P < 0.05 (ANOVA). **, Significantly higher than in an intramuscular group (open column). P < 0.05 (ANOVA).
The performance of PCPP microneedle delivery system was also benchmarked against a traditional intradermal delivery of nonadjuvanted HBsAg via injection using a hypodermic syringe. This approach also eliminates the potential effect of CMC, required to fabricate nonadjuvanted microneedles. The results indicated up to a 100-fold increase in antibody titers for the polyphosphazene microneedle delivery system over intradermal injection of nonadjuvanted HBsAg formulation (Fig. S3).
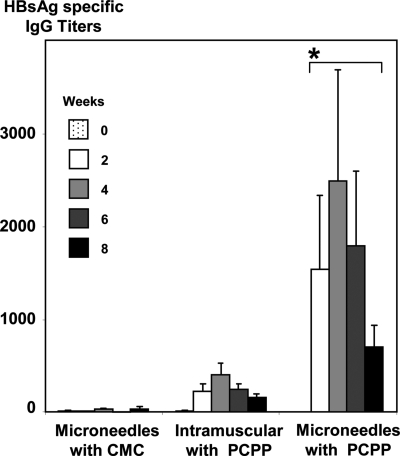
To assess the role of the PCPP adjuvant in the context of microneedle delivery, we compared microneedles coated with an inert CMC formulation to microneedles coated with PCPP. Comparison of the intradermal adjuvant effect of PCPP to its intramuscular adjuvant effect and the results of intradermal delivery using microneedles, in which PCPP was replaced with an inert coating material, CMC, suggests a synergy between intradermal and PCPP adjuvant approaches (Fig. 6).
Fig. 6.
Serum IgG specific HBsAg titers after single dose immunization of pigs with HBsAg intradermally using microneedles with CMC coatings, intramuscularly with PCPP containing formulation, and intradermally using microneedles with PCPP coatings (seven animals per group, 20 μg of HBsAg in all formulations). *, Significantly higher than in both other groups. P < 0.05 (ANOVA).
Discussion
Remarkable progress in the development of microscale devices for transdermal macromolecular delivery (34, 35) sets the stage for broader clinical application of intradermal vaccination (see SI Text for more on intradermal immunization). Although the intradermal immunization approach appears to be promising, its technical realization faces significant challenges, such as special training of personnel who would be needed to administer vaccinations through the intradermal route effectively (36). Microneedles and microinjection devices can provide a convenient alternative potentially offering ease of application and distribution, possibility of self-administration and pain-free delivery (9).
Recent clinical and preclinical studies on intradermal administration of seasonal influenza vaccine using a prefilled microinjection system suggested significant antigen sparing effect and provided consistent and reliable immunization results (1, 2, 37). Although there are clear indications that intradermal immunization can potentially offer significant improvements over intramuscular vaccination (9), it appears that the majority of studies have been conducted using nonadjuvanted vaccine formulations, such as influenza formulations above. Since the reliance of commercial vaccines on adjuvants at present time is enormous, it is important to understand if intradermal delivery systems can potentially eliminate the need for immunostimulants by comparing adjuvanted intramuscular formulations and their nonadjuvanted intradermal counterparts. It is also critical to elucidate the level of compatibility of modern adjuvant systems with intradermal approach and to investigate if they can provide the same advantages as they offer for intramuscular delivery of vaccines.
Recent studies explored intradermal delivery of adjuvanted formulations using coated microneedle arrays in hairless guinea pig model (38). Administration of patches with microprojections coated with dry formulation containing ovalbumin and an adjuvant, glucosaminyl muramyl dipeptide (GMDP), demonstrated augmented immune responses compared to a nonadjuvanted intradermal formulation. However, the effect of the adjuvant for intradermal formulations appeared to be much less pronounced when compared to intramuscular injections (38). Other studies on intradermal immunization using a simple patch demonstrated the induction of significant systemic immune responses in the presence of bacterial adjuvants, such as heat-labile enterotoxin (39–41).
Our results demonstrate that a macromolecular adjuvant, PCPP, exhibits potent immunoadjuvant activity when delivered intradermally and also enables efficient incorporation of vaccine antigens into microfabricated delivery devices. This molecular adjuvant is not only compatible with solid state microneedle technology, but can potentially eliminate the need for inert “engineering” polymers, such as CMC, whose sole role is to serve as a microfabrication material. Since both immunostimulating and “engineering” functionalities can now be integrated in a single compound (PCPP) the approach creates opportunities for maximizing antigen doses or achieving faster dissolution profiles using the same amount of the formulation.
Protein-loading experiments indicate that, as a microfabrication component, PCPP outperforms CMC under the conditions studied. It is important to emphasize that the performance of CMC formulations can be dramatically improved through the addition of surfactant (31, 32), so that the coating construction and protein loading can be accelerated to the levels of PCPP formulation. However, the results suggest that PCPP can potentially provide surfactant-free or low surfactant coating solutions allowing more flexibility in formulation development, which is especially important when high contents of surfactant are undesirable (13, 42).
Such superior performance of polyphosphazene excipient in coating experiments can be potentially explained in light of previous studies on the protein complex forming properties of PCPP (27). Noncovalent interactions between polyphosphazene and BSA can lead to a dramatic rise in the viscosity of the formulation solution and formation of a physical network with protein acting as a cross-linker. The microheterogeneous nature of such complexes can potentially provide amphiphilic characteristics needed for surface wetting in the coating process, thus further reducing the need for an additional surfactant. Both of these factors can contribute to improved film forming properties and superior coating performance of PCPP.
Intradermal administration of PCPP containing formulations demonstrates its potency to dramatically increase antibody responses compared to intramuscular and nonadjuvanted intradermal formulations. It also provides significant antigen sparing ability. Although PCPP has already been reported as a potent adjuvant for intramuscularly administered vaccine formulations, such effect appears to be inferior to the performance of the same adjuvant administered intradermally.
These results appear especially significant for the field of intradermal immunization as the anatomy of pig skin presents a reasonable model for a human skin (33). Pigs, like most other mammals and unlike humans and some primates, are not susceptible to natural or experimental Hepatitis B virus infection (43), which eliminates the possibility of survival studies. However, the importance of our findings stems from the fact that antibodies to HBsAg (anti-HBs) are neutralizing and immunocompetent persons who achieve anti-HBs concentrations >10 mIU/mL after preexposure vaccination have virtually complete protection against both acute disease and chronic infection (44, 45). Anti-HB is the only easily measurable correlate of vaccine-induced protection and thus is used as serological endpoint in clinical trials to define seroprotection (44, 46).
The synergistic effect observed in our immunization studies requires further mechanistic studies focusing on the specifics of immunological pathways associated with delivery to skin and the role of PCPP (see SI Text for more on the mechanism of action of PCPP). One possible factor contributing to the synergy between intradermal delivery and adjuvant effect of PCPP may, however, lie in the formulation area. It has been previously reported that PCPP-antigen complexation is an important step in the overall mechanism and that adjuvant activity can be linked to the biophysical and physico-chemical properties of such complex (27). Microneedle delivery of PCPP-antigen formulation reported here is an example of solid state delivery of PCPP, which requires its in vivo dissolution. Although, the dissolution process leading to the delivery of the formulation in the skin appears to be fast, the viscosity of the formulation and concentration of the complex can be dramatically different, which can affect its presentation to immunocompetent cells. The superior performance of PCPP as an intradermal adjuvant dictates further investigation of both physico-chemical and immunological components of its mechanism of action.
Materials and Methods
Full details are available in SI Materials and Methods.
Materials.
Poly[di(carboxylatophenoxy)phosphazene] (PCPP) (Aldrich) was purified by multiple precipitations using sodium chloride (please see SI Materials and Methods). Hepatitis B Surface Antigen, recombinant (HBsAg) (Fitzgerald Industries International, Inc.) was used as received. Titanium microneedle arrays, each containing 50 microneedles of approximately 600 μm in length, were similar to previously reported stainless steel microneedles (31, 32) with the exception that they were produced of titanium sheets by chemical etching using hydrofluoric acid.
Coating Procedure.
The coating formulation was fed to a 50-microwell reservoir, using a Genie Plus syringe pump (Kent Scientific). A microneedle array was secured on an array holder and then attached to an X-Y-Z micropositioning system using alignment pins and holders. Using the micropositioning system, the coating procedure was performed by submerging the microneedles into the wells in the coating reservoir and then immediately removing them, followed by a drying step in which the arrays were purged with anhydrous nitrogen gas. The needles were dipped until the wells were depleted of formulation, at which time they were replenished. A stereo zoom microscope (STZ-45-BS-FR) with a digital camera (Caltex Scientific) was used to monitor the process.
In Vivo Immunization Experiments.
In vivo immunization experiments were conducted in Land Race Cross pigs, which were divided into groups, each containing seven animals. The pigs were 3–4 weeks old, at the start of the study, and weighed 5–8 kg each. The application sites for intradermal administration were clipped of all hair and then shaved to further ensure a smooth surface. The sites were then washed with water and allowed to air dry. The patches containing microneedle arrays were manually put on the skin and pressure was applied on the center of the patch for 1 min to facilitate microneedle insertion. The patch was allowed to remain in place, undisturbed, for an additional 29 min before removal. Each animal immunized via intramuscular route, received a 1-mL injection of liquid formulation in the neck, right behind the ears. Intradermal injection using hypodermic syringe was carried out using four 50-μL injections of liquid formulation in four different spots on the ear, for a total of 200 μL of 100 μg/mL HBsAg solution (i.e., 20-μg dose). All subjects were anesthetized during the immunization with a combination of Xylazine and Ketamine. The blood samples were collected before being immunized (0 weeks) and then at 2, 4, 6, and 8 weeks after being immunized. The application site was monitored through periodic visual examinations. Mildly red skin marks, apparently corresponding to microneedle insertion sites, were noticed immediately after application of the patch, which disappeared shortly thereafter. No severe adverse reactions were observed at any time during the experiment.
All experiments were carried out according to the Guide to the Care and Use of Experimental Animals, provided by the Canadian Council on Animal Care. Experimental protocols were approved by the University of Saskatchewan Animal Care Committee.
Supplementary Material
Acknowledgments.
We thank Drs. Rox Anderson and William Farinelli for help with histology studies.
Footnotes
Conflict of interest statement: A.K.A., D.P.D., H.A.G., H.H.K. and A.M. are employees of Apogee Technology, Inc. M.R.P. is a member of the scientific advisory board of Apogee Technology, Inc. and is an inventor on patents involving microneedle technology.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908842106/DCSupplemental.
References
- 1.Holland D, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: A randomized controlled trial. J Infect Dis. 2008;198:650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 2.Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007;14:375–381. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belshe RB, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 4.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme P, et al. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 7.Weeratna R, Comanita L, Davis HL. CPG ODN allows lower dose of antigen against hepatitis B surface antigen in BALB//c mice. Immunol Cell Biol. 2003;81:59–62. doi: 10.1046/j.1440-1711.2003.01135.x. [DOI] [PubMed] [Google Scholar]
- 8.Mikszta JA, et al. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nature Medicine. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 9.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-Based Vaccines. In: Compans RW, Orenstein WA, editors. Curr. Top. Microbiol Immunol Vol. 333: Vaccines for Pandemic Influenza. Warren, MI: Springer; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widera G, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 11.McAllister DV, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc Natl Acad Sci USA. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rexroad J, Wiethoff CM, Jones LS, Middaugh CR. Lyophilization and the thermostability of vaccines. Cell Preserv Techno. 2002;1:91–104. [Google Scholar]
- 13.Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203:1–60. doi: 10.1016/s0378-5173(00)00423-3. [DOI] [PubMed] [Google Scholar]
- 14.Singh M. Vaccine Adjuvants and Delivery Systems. Hoboken, NJ: Wiley-Interscience; 2006. p. 449. [Google Scholar]
- 15.Vogelbruch M, et al. Aluminium-induced granulomas after inaccurate intradermal hyposensitization injections of aluminium-adsorbed depot preparations. Allergy. 2000;55:883–887. [PubMed] [Google Scholar]
- 16.Nukavarapu SP, Kumbar SG, Allcock HR, Laurencin CT. Biodegradable Polyphosphazene Scaffolds for Tissue Engineering. In: Andrianov AK, editor. Polyphosphazenes for Biomedical Applications. NJ: Wiley-Interscience Hoboken; 2009. pp. 119–138. [Google Scholar]
- 17.Andrianov AK. Water-soluble polyphosphazenes for biomedical applications. J Inorg Organomet Polym Mater. 2006;16:397–406. [Google Scholar]
- 18.Andrianov AK. Polyphosphazenes as vaccine adjuvants. In: Singh M, editor. Vaccine Adjuvants and Delivery Systems. Hoboken, NJ: John Wiley & Sons; 2007. pp. 355–378. [Google Scholar]
- 19.Andrianov AK, Marin A, Chen J. Synthesis, properties, and biological activity of Poly[di(sodium carboxylatoethylphenoxy)phosphazene] Biomacromolecules. 2006;7:394–399. doi: 10.1021/bm050790a. [DOI] [PubMed] [Google Scholar]
- 20.Payne LG, et al. Poly[di(carboxylatophenoxy)phosphazene] (PCPP) is a potent immunoadjuvant for an influenza vaccine. Vaccine. 1998;16:92–98. doi: 10.1016/s0264-410x(97)00149-7. [DOI] [PubMed] [Google Scholar]
- 21.Mutwiri G, et al. Poly[di(sodium carboxylatoethylphenoxy)phosphazene] (PCEP) is a potent enhancer of mixed Th1/Th2 immune responses in mice immunized with influenza virus antigens. Vaccine. 2007;25:1204–1213. doi: 10.1016/j.vaccine.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Bouveret Le Cam NN, Ronco J, Francon A, Blondeau C, Fanget B. Adjuvants for influenza vaccine. Res Immunol. 1998;149:19–23. [Google Scholar]
- 23.Kim JH, et al. A phase I, open label, dose ranging trial of the pasteur merieux connaught (PMC) oligomeric HIV-1 Gp160mn/LAI-2 vaccine in HIV seronegative adults. (Abstracts of the 37th Annual Meeting of the Infectious Diseases Society of America); 1999. p. 1028. [Google Scholar]
- 24.Thongcharoen P, et al. A phase 1/2 comparative vaccine trial of the safety and immunogenicity of a CRF01_AE (Subtype E) candidate vaccine: ALVAC-HIV (vCP1521) prime with oligomeric gp160 (92TH023/LAI-DID) or bivalent gp120 (CM235/SF2) boost. JAIDS J Acq Imm Defic Synd. 2007;46:48. doi: 10.1097/QAI.0b013e3181354bd7. [DOI] [PubMed] [Google Scholar]
- 25.Andrianov AK, Chen J. Polyphosphazene microspheres: Preparation by ionic complexation of phosphazene polyacids with spermine. J Appl Polym Sci. 2006;101:414–419. [Google Scholar]
- 26.Andrianov AK, Chen J, Payne LG. Preparation of hydrogel microspheres by coacervation of aqueous polyphosphazene solutions. Biomaterials. 1998;19:109–115. doi: 10.1016/s0142-9612(97)00227-5. [DOI] [PubMed] [Google Scholar]
- 27.Andrianov AK, Marin A, Roberts BE. Polyphosphazene polyelectrolytes: A link between the formation of noncovalent complexes with antigenic proteins and immunostimulating activity. Biomacromolecules. 2005;6:1375–1379. doi: 10.1021/bm049329t. [DOI] [PubMed] [Google Scholar]
- 28.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Delivery Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Heinig K, Vogt C. Determination of Triton X-100 in influenza vaccine by high-performance liquid chromatography and capillary electrophoresis. Anal Bioanal Chem. 1997;359:202–206. [Google Scholar]
- 30.Yang YW, Wu CA, Morrow WJW. Cell death induced by vaccine adjuvants containing surfactants. Vaccine. 2004;22:1524–1536. doi: 10.1016/j.vaccine.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 31.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Controlled Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24:1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]
- 33.Monteiro-Riviere NA. Comparative anatomy, physiology, and biochemistry of mammalian skin. In: Hobson D, editor. Dermal and Ocular Toxicology: Fundamentals and Methods. Boca Raton, FL: CRC Press; 1991. pp. 3–71. [Google Scholar]
- 34.Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int J Pharm. 2008;364:227–236. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogura M, Paliwal S, Mitragotri S. Low-frequency sonophoresis: Current status and future prospects. Adv Drug Delivery Rev. 2008;60:1218–1223. doi: 10.1016/j.addr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 36.La Montagne JR, Fauci AS. Intradermal influenza vaccination—Can less be more? N Engl J Med. 2004;351:2330–2332. doi: 10.1056/NEJMe048314. [DOI] [PubMed] [Google Scholar]
- 37.Laurent PE, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–8842. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Matriano JA, et al. Macroflux microprojection array patch technology: A new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- 39.Frech SA, et al. Improved immune responses to influenza vaccination in the elderly using an immunostimulant patch. Vaccine. 2005;23:946–950. doi: 10.1016/j.vaccine.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 40.Frerichs DM, et al. Controlled, single-step, stratum corneum disruption as a pretreatment for immunization via a patch. Vaccine. 2008;26:2782–2787. doi: 10.1016/j.vaccine.2008.02.070. [DOI] [PubMed] [Google Scholar]
- 41.Glenn GM, Kenney RT, Hammond SA, Ellingsworth LR. Transcutaneous immunization and immunostimulant strategies. Immuno Allergy Clin N Amer. 2003;23:787–813. doi: 10.1016/s0889-8561(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 42.Katakam M, Bell LN, Banga AK. Effect of surfactants on the physical stability of recombinant human growth hormone. J Pharm Sci. 1995;84:713–716. doi: 10.1002/jps.2600840609. [DOI] [PubMed] [Google Scholar]
- 43.Glebe D. Recent advances in hepatitis B virus research: A German point of view. 2007;13:8–13. doi: 10.3748/wjg.v13.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mast EE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. MMWR Morb Mortal Wkly Rep. 2005;54:1. [PubMed] [Google Scholar]
- 45.Chen DS. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50:805–816. doi: 10.1016/j.jhep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Sangaré L, Manhart L, Zehrung D, Wang CC. Intradermal hepatitis B vaccination: A systematic review and meta-analysis. Vaccine. 2009;27:1777–1786. doi: 10.1016/j.vaccine.2009.01.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.