Abstract
Overactive WNT/beta-catenin signaling has been found in many forms of cancer in human patients. Mouse models with mutations in different components of the WNT/beta-catenin signaling pathway have been generated to mimic tumorigenesis in humans. Mice with mutations that result in overactive WNT/beta-catenin signaling developed tumors in some tissues, such as digestive tract, skin, and ovary, but they failed to develop tumors in other tissues, such as mammary gland, liver, kidney, and primordial germ cells. To investigate whether overactive beta-catenin signaling is capable of inducing Sertoli cell tumorigenesis in testes, we generated Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor male mice that express a constitutively active form of beta-catenin specifically in Sertoli cells. No tumors were observed at 4 mo of age, but 70% of the mutant males developed Sertoli cell tumors at 8 mo of age. At 1 yr of age, more than 90% of the mutant males developed tumors. No instances of extratesticular spread of the tumors were found in the mutant mice. These studies show a causal link between overactive WNT/beta-catenin signaling and Sertoli cell tumor development and provide a novel mouse model for the study of Sertoli cell tumor biology.
Keywords: Beta-catenin, Sertoli cell tumor, Sertoli cells, testis
Overactive β-catenin signaling causes Sertoli cell tumor development in the mouse, providing a novel mouse model for the study of Sertoli cell tumor biology.
INTRODUCTION
Sex cord-stromal tumors represent approximately 4% of all testicular tumors and occur over a wide age range in humans [1]. Four major groups of sex cord-stromal tumors exist: Sertoli cell tumors (SCTs), Leydig cell tumors, granulosa cell tumors, and unclassified tumors [2]. Typical tumor types of these different groups have distinct histopathological features. For example, SCTs have differentiated tubule formation, whereas Leydig cell tumors have abundant eosinophilic cytoplasm and granulosa cell tumors have follicle differentiation. Although much is known about the molecular pathogenesis of sex cord-stromal tumors, the etiology of SCTs and other sex cord-stromal tumors remains unclear.
The WNT/β-catenin signaling pathway is known to play important roles in multiple developmental processes and tumorigenesis. In the canonical WNT/β-catenin signaling pathway, β-catenin is involved in the transduction of extracellular WNT signals to the nucleus. In the absence of WNTs, cytoplasmic β-catenin is phosphorylated by glycogen synthase kinase-3β (GSK3B) and casein kinase 1 (CSNK1) bound to the adenomatosis polyposis coli (APC) complex and is targeted for degradation through the ubiquitination pathway. In the presence of WNTs, the WNT pathway is activated to inhibit GSK3B, resulting in the stabilization and cytoplasmic accumulation of β-catenin. The stabilized β-catenin translocates into the nucleus and regulates the transcription of downstream target genes [3–5]. In humans, approximately 90% of colorectal cancers are associated with activation of the WNT/β-catenin signaling pathway, including inactivating mutations in the tumor suppressor gene APC or activating mutations in CTNNB1 [6]. Activating mutations of the WNT/β-catenin signaling pathway also are described less frequently in a large number of other tumor types, such as melanoma [7, 8], ovarian carcinoma [9, 10], hepatoblastoma [11, 12], and Wilms tumor [13].
Many mouse models with mutations in the WNT/β-catenin signaling pathway have been generated to dissect the roles of individual components in cancer development. These mouse models also provide strong evidence supporting a causative role of misregulated WNT/β-catenin signaling in tumor development. For example, different inactivating mutations in Apc and constitutively active mutations in Ctnnb1 caused intestinal adenomas in mice [14–16]. Mice expressing a stabilized form of β-catenin in epidermis or ovarian granulosa cells developed pilomatricomas and ovarian granulosa tumors, respectively [17, 18]. However, overactivation of the WNT/β-catenin signaling pathway failed to cause tumor formation in many other tissues in mice, such as secretory epithelia, liver, kidney, and primordial germ cells [19–22].
β-Catenin is expressed in Sertoli cells starting from Embryonic Day (E) 15.5, mainly on the Sertoli cell membrane (undetectable in the nucleus). We recently investigated the role of β-catenin signaling in Sertoli cells during testis development by both loss-of-function and gain-of-function studies [23]. Conditional knockout of β-catenin in Sertoli cells did not affect testis formation, but stabilization of β-catenin in Sertoli cells caused severe phenotypes, including testicular cord disruption, germ cell depletion, and inhibition of Müllerian duct regression. These results suggested that inhibition of β-catenin signaling is essential for Sertoli cell and testicular cord maintenance and germ cell survival during testis development. To investigate whether overactive β-catenin signaling is capable of inducing SCT development, we generated Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor male mice that express a stabilized form of β-catenin specifically in Sertoli cells and examined the testes of mice at various ages for tumor formation. We found that mutant males developed either unilateral or bilateral SCTs after 8 mo of age. These studies demonstrate that overactive WNT/β-catenin signaling in Sertoli cells causes Sertoli cell tumorigenesis in mice, and they provide a novel mouse model for the study of SCT biology.
MATERIALS AND METHODS
Mice
Tg(AMH-cre)1Flor mice and Ctnnb1tm1Mmt/tm1Mmt mice were maintained on a C57BL/6 × 129/SvEv mixed genetic background. Male Tg(AMH-cre)1Flor mice were mated with female Ctnnb1tm1Mmt/tm1Mmt mice to produce offspring with the genotype of Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor or Ctnnb1tm1Mmt/+;+/+. Genotyping was performed by polymerase chain reaction as described elsewhere [15, 24]. All experimental procedures using mice were approved by the University of Texas M.D. Anderson Cancer Center Animal Care and Use Committee.
Tissue Collection and Histological Analysis
Testes were dissected from mutant and control males at various time points. For paraffin sectioning, tissues were fixed in 4% paraformaldehyde overnight, embedded in paraffin, and sectioned at a thickness of 4 μm. After dewaxing, the sections were stained with hematoxylin and eosin for histological analyses. For frozen sectioning, samples were directly embedded in OCT medium on dry ice and stored at −80°C before sectioning. Cryosections were cut at a thickness of 10 μm.
Immunohistochemistry/Fluorescence
Immunohistochemical analysis was carried out using the Vectastain ABC (avidin-biotin-peroxidase) kit (Vector Laboratories) as recommended by the manufacturer. Endogenous peroxidase activity was destroyed using 3% hydrogen peroxide for 20 min. Antigen recovery was performed by boiling samples in 0.01 M sodium citrate buffer (pH 6.0) for 20 min. Sections were incubated with 5% bovine serum albumin in PBS for 30 min at room temperature and then incubated for 1 h at room temperature with either anti-β-catenin antibody (C 2206; Sigma), anti-3β-hydroxysteroid dehydrogenase (anti-3β-HSD) antibody (sc-30821; Santa Cruz), anti-cyclin D1 antibody (#2978; Cell Signaling), anti-MYC antibody (#9402; Cell Signaling), or anti-phosphorylated histone H3 (anti-PH3) antibody (#06-570; Upstate) at dilutions of 1:2000, 1:200, 1:25, 1:100, and 1:200, respectively. After three washes with PBS, the sections were incubated with biotinylated secondary antibody (BA-1000; Vector Laboratories) at a dilution of 1:250 for 45 min at room temperature. After incubation with avidin-biotin-peroxidase complex for 45 min, the sections were washed with PBS. The color was developed with 3-amino-9-ethylcarbazole substrate. Samples were counterstained with Harris hematoxylin. Wilms tumor 1 (WT1) immunofluorescence was performed on cryosections using an anti-WT1 primary antibody (sx-192; Santa Cruz) at a dilution of 1:200. PH3 and β-catenin double immunofluorescence was performed on paraffin sections by mixing anti-PH3 antibody and anti-β-catenin antibody (sc-1496; Santa Cruz) at dilutions of 1:200 and 1:50, respectively. All secondary antibodies were Alexa Fluor-conjugated antibodies from Molecular Probes and used at a dilution of 1:400. Sections were finally mounted using Vectashield mounting medium with 4′,6′-diamidino-2-phenylindole (H1200; Vector Laboratories).
RESULTS
β-Catenin Stabilization in Sertoli Cells Causes Testicular Tumor Development
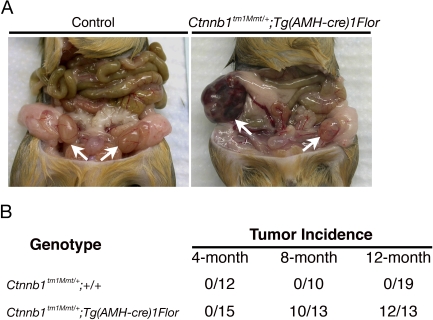
To test whether overactive β-catenin signaling results in tumorigenesis in Sertoli cells, we crossed the Ctnnb1tm1Mmt/+ mouse line to the Sertoli cell-specific Tg(AMH-cre)1Flor mouse line to generate Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor male mice. Exon 3 of Ctnnb1, which encodes several phosphorylation sites required for β-catenin degradation, is flanked by loxP sites in the Ctnnb1tm1Mmt/+ mouse line [15]. Expression of Cre in Sertoli cells removes exon 3 of Ctnnb1 and, therefore, leads to the accumulation of β-catenin protein in Sertoli cells of the Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor male mice. The accumulation of β-catenin protein in Sertoli cells started from E13.5 in Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor testes, and mutant males developed small testes at 7 wk of age because of disruption of testicular cords and loss of germ cells. Only a few degenerated tubules were found in mutant testes [23]. To investigate whether accumulation of β-catenin protein in Sertoli cells is capable of inducing SCT development in the remaining degenerated tubules, we aged the Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor male mice and examined the testes at various ages for tumor formation. We did not detect any tumor formation in 4-mo-old mutant males. Of the 15 mutant males generated, none developed tumors as determined by gross examination when killed at 4 mo of age. However, more than 70% of the mutant males developed testicular tumors at 8 mo of age (n = 10 of 13). The incidence increased over time; more than 90% of the mutant males developed testicular tumors at 1 yr of age (n = 12 of 13). The testicular tumors that developed in the β-catenin conditionally stabilized mutants were either unilateral or bilateral. At 8 mo of age, among the 10 mutant males that developed tumors, four had unilateral tumors, and six had bilateral tumors. At 12 mo of age, among the 12 mutant males that developed tumors, two had unilateral tumors, and 10 had bilateral tumors. Although some of the tumors were large in size and seemed to increase over time, detailed necropsies failed to detect any instance of extratesticular spread of the tumors in these mutant mice. Control male mice with the genotype of Ctnnb1tm1Mmt/+;+/+ developed no testicular tumors at 4-, 8-, or 12-mo of age (n = 12, 10, and 19, respectively) (Fig. 1).
FIG. 1.
Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor male mice develop testicular tumors. A) Normal testes of control males at 8 mo of age (arrows; left) and testicular tumor developed in the Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor male mice at 8 mo of age (arrows; right). B) Incidence of the testicular tumor development in Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor male mice. No tumors were found in mutant mice at 4 mo of age, but more than 70% of the mutant males (n = 10 of 13) developed tumors at 8 mo of age. The incidence increased to more than 90% at 1 yr of age (n = 12 of 13).
Tumor Cells in β-Catenin Stabilized Mutant Mice Are of Sertoli Cell Origin
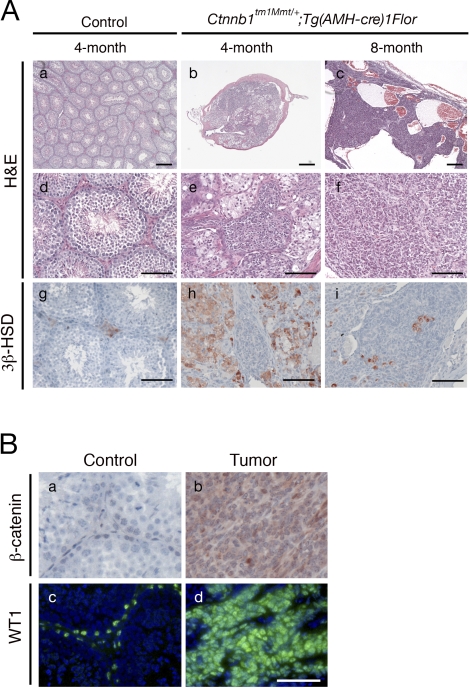
We performed histological analysis on the testes of control and β-catenin conditionally stabilized mutant mice at different ages. In the testes of 4-mo-old control mice, well-organized seminiferous tubules contained all the different stages of germ cells (spermatogonia, spermatocytes, spermatids, and spermatozoa), suggesting that spermatogenesis occurred normally (Fig. 2, Aa and Ad). However, the testes of Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice at 4 mo of age were much smaller compared to those of control mice. Unlike control testes, mutant testes lacked testicular cord structure and contained only a few degenerated tubules. Spermatogenesis was completely absent in these remaining tubules. Clumps of Sertoli cells remained in the remnants of tubules (Fig. 2, Ab and Ae). The size of mutant testes increased significantly at 8 mo of age because of tumor formation. Histological examination revealed that the tumor mainly consisted solid nests of disorganized tumor cells and fibroblast-like stroma cells. Multiple blood-filled cysts lined with tumor cells were seen in all the tumors (Fig. 2, Ac and Af).
FIG. 2.
Tumor progression in Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor male mice. Aa–Af) Normal histology in testes of 4-mo-old control males (low magnification, Aa; high magnification, Ad), and progressive development of testicular tumors in Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor males of different ages (low magnification, Ab and Ac; high magnification, Ae and Af), shown by hematoxylin and eosin staining. Ag–Ai) Immunostaining with Leydig cell marker 3β-HSD identified Leydig cells in testes of Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor and control mice at different ages. B) β-Catenin (red) and WT1 (green) immunostaining on control testes and tumors. Tumor cells were positive for β-catenin and WT1 staining, indicating their Sertoli cell origin (Bb and Bd, respectively). Bar = 250 μm (Aa–Ac) and 100 μm (Ad–Bd).
To determine whether tumors originated from Sertoli cells with stabilized β-catenin, we performed β-catenin immunostaining on the tumor samples. Only weak β-catenin immunoreactivity was found in Sertoli cells of the control testes, but cells in the tumor mass were strongly positive for β-catenin staining (Fig. 2, Ba and Bb). Because β-catenin was specifically stabilized in Sertoli cells, the strong staining of β-catenin in tumor cells indicates that the testicular tumors in the Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice are of Sertoli cell origin. We also performed immunohistochemical analysis on tumor samples for a Sertoli cell marker, WT1. Tumor cells were strongly positive for WT1, further confirming their Sertoli cell origin (Fig. 2, Bc and Bd).
Sertoli cells are thought to be important for the development and function of Leydig cells. They secrete either stimulatory or inhibitory factors to regulate the production of testosterone in Leydig cells [25]. To investigate whether Leydig cells were affected by the stabilization of β-catenin in Sertoli cells, we performed immunohistochemical analysis on mutant testes at different ages using the Leydig cell marker, 3β-HSD. At 4 mo of age, we observed clumps of Leydig cells surrounding the degenerated tubules in mutant testes as indicated by 3β-HSD immunostaining (Fig. 2Ah). With the onset of Sertoli cell tumorigenesis, tumor cells proliferated and became the dominant cell type in the mutant testes at 8 mo of age. Leydig cells were more isolated but still present inside or peripheral to the tumor mass (Fig. 2Ai).
Tumor Onset Coincides with Increased Cell Proliferation
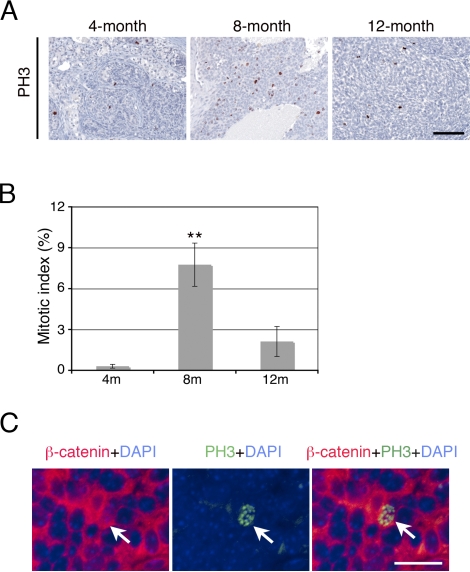
Because we observed tumor formation and increased testis size in the Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice after 8 mo of age, the proliferation status of the cells in the testes was assessed by PH3 immunohistochemistry in mutant mice of different ages (Fig. 3). We found a limited number of cells within the disrupted cords to be proliferating at 4 mo of age (<1% of cells were positive for PH3 staining). However, the number of proliferating cells increased significantly at 8 mo of age, when approximately 8% of cells were positive for PH3 staining, which is consistent with the tumor onset time. Tumor cell proliferation was reduced at 12 mo of age, when approximately 2% of the cells were shown to be positive for PH3 staining. To investigate whether those proliferating cells at 8 mo of age were tumor cells, we performed double-immunofluorescent staining of β-catenin and PH3 and found that the proliferating cells were positive for β-catenin staining (Fig. 3C).
FIG. 3.
Increased cell proliferation in tumor cells of Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice at 8 mo of age. A) Immunostaining with the proliferation marker PH3 identified mitotic cells in testes of Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice at different ages. The number of mitotic cells increased significantly at 8 mo of age, which is consistent with the tumor onset time. B) Quantitative analysis showed that approximately 8% of cells were positive for PH3 staining at 8 mo of age, as compared with less than 1% and approximately 2% at 4 mo of age and 1 yr of age, respectively. **P < 0.01. C) Double-immunofluorescent staining of β-catenin and PH3 on tumors at 8 mo of age. Left, β-catenin (red) was highly accumulated in tumor cells; middle, one PH3-positive (green) proliferating cell; right, merge showing the proliferating cell was positive for β-catenin staining (arrow). Bar = 100 μm.
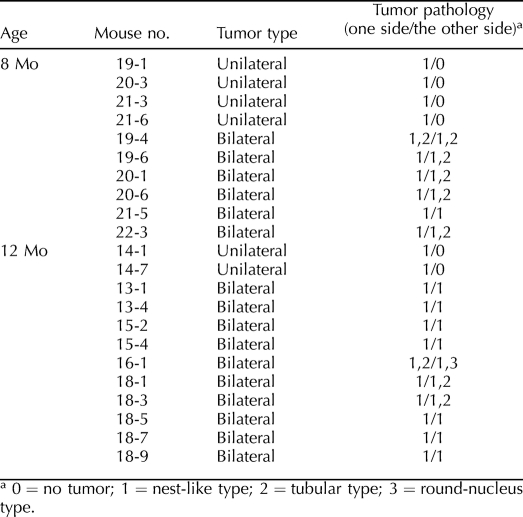
Other Histologic Subtypes of SCTs in the Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor Tumor Model
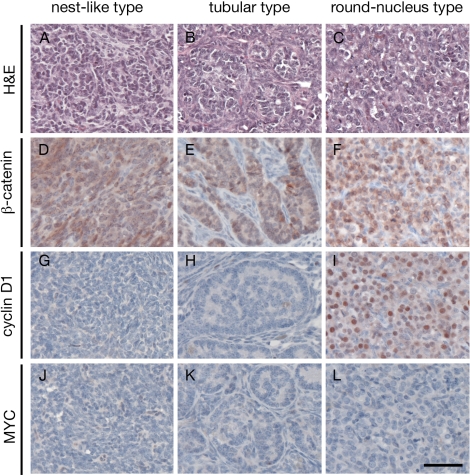
The tumor cells in the Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor testes generally were polygonal, with little to moderate amounts of eosinophilic cytoplasm and oval regular nuclei. Tumor cells formed a nest-like pattern with no obvious structure. Fibroblast-like stromal cells often were found to be mingled with the tumor cells. The nest-like type of pathology was found in all of the tumors (n = 22, with 16 bilateral and 6 unilateral) (Fig. 4A and Table 1). However, areas of tubular differentiation also were found in some of the bilateral tumors (n = 8 of 16). Tumor cells formed cord-like structures having a clear boundary with surrounding tissues (Fig. 4B). In one 12-mo-old mouse that developed bilateral tumors (mouse 16-1), a clump of tumor cells with moderate amounts of eosinophilic cytoplasm but very round nuclei were found focally, whereas the rest of the tumor was of the nest-like type in one side of the testes (Fig. 4C). It should be noted that the tubular or round-nucleus type of pathology was always found with the nest-like type inside the tumors.
FIG. 4.
Microscopic features of testicular tumors in β-catenin stabilized mutants. A–C) Three types of tumor morphologies developed in mutant mice: tumor cells forming a nest-like pattern as the dominant type, tumor cells forming a tubular pattern, and tumor cells with very round nuclei. D–F) Immunohistochemical analysis of β-catenin on tumor samples. β-Catenin staining were observed in all three types of tumor cells. G–I) Immunohistochemical analysis of cyclin D1 on tumor samples. In tumors with a nest-like pattern and tubular pattern, very few tumor cells were positive for cyclin D1 staining. However, in the round-nucleus tumor type, almost all tumor cells were positive for cyclin D1 staining. J–L) Immunohistochemical analysis of MYC showed that tumor cells were negative for MYC staining in all three types of tumor cells. Bar = 50 μm.
TABLE 1.
Histological analysis of the SCTs in Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice.
To confirm cells within the tubular and round-nucleus types of pathology also are of Sertoli cell origin, we performed immunohistochemical analysis of β-catenin on tumors with mixed tumor pathology. Similar to the nest-like type of cells, the presence of β-catenin staining also was found in the tubular and round-nucleus types of tumor cells, indicating that they are all from Sertoli cells with stabilized β-catenin (Fig. 4, D–F). Cyclin D1 (CCND1) and MYC are two well-known downstream target genes of the β-catenin signaling pathway that are involved in the colorectal tumorigenesis [26, 27]. To determine whether cyclin D1 played a role during the development of SCTs in the Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice, we performed immunohistochemical analysis of cyclin D1 on the tumor samples. In tumor cells with a nest-like or tubular pattern, very few cells were positive for cyclin D1 staining. However, the round-nucleus type of tumor cells was strongly positive for cyclin D1 staining, suggesting that the round-nucleus tumor might be a different subtype of SCT (Fig. 4, G–I). We also performed immunohistochemistry of MYC on the tumor samples and found that tumor cells were negative for MYC staining in all three types of tumor cells, suggesting that MYC is not involved in the SCT tumorigenesis in the Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice (Fig. 4, J–L).
DISCUSSION
SCT Model
In humans, most SCTs in children are benign, but up to 20% in adults are malignant [28]. The molecular mechanisms underlying Sertoli cell tumorigenesis largely remain unknown. Mouse models have been widely used as a powerful tool for the study of tumorigenesis in various tissues and for the development of potential treatments. However, very few mouse models of SCT have been established. Transgenic mice carrying SV40 T antigen driven by human Müllerian-inhibiting substance regulatory sequences developed testicular tumors composed of a cell type histologically resembling the Sertoli cell [29]. The tumor incidence was not studied in this model, however, because all transgenic lines could not be maintained as a result of reduction in fertility. Inhibin α was identified as a tumor-suppressor gene in the gonads and adrenals [30]. The inhibin α knockout male mice develop mixed gonadal stromal tumors with 100% penetrance. These tumors develop as early as 4 wk of age and cause a progressive, cachexia-like wasting syndrome; 95% of the mice die of the severe cachexia-like syndrome by 12 wk of age. In another study, double-conditional knockout of Smad1 and Smad5 in mice using Amhr2tm3(cre)Bhr/+ causes the development of metastatic Sertoli-Leydig cell tumors [31]. The tumors develop after 28 wk of age and show lymphatic and peritoneal metastases. These studies provide important insights regarding the development of mixed sex cord-stromal tumors; however, their value as animal models for human patients with SCT are limited. In the present study, we generated a novel mouse model of SCT by stabilizing β-catenin in Sertoli cells. The stabilization of β-catenin in Sertoli cells caused unilateral or bilateral SCT development with high penetrance but no extratesticular spread of the tumors, even in 1-yr-old mutants. In the SCTs that Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice developed, tumor cells predominantly formed a nest-like pattern, but they also formed tubular structures. Both of these tumor structures also are found in human patients with SCTs [32]. These features make our model a better mimic of Sertoli cell tumorigenesis in humans.
β-Catenin, both as an important effector of the canonical WNT signaling pathway and as a regulator of cell adhesion, has been demonstrated to be involved in tumorigenesis in many tissue types. However, its potential role in Sertoli cell tumorigenesis is unknown. Boyer et al. [33] crossed the same Ctnnb1tm1Mmt mouse line with Amhr2tm3(cre)Bhr/+ to stabilize β-catenin in both Leydig and Sertoli cells. Mutant mice had testicular atrophy starting at 5 wk of age, but no animals developed tumors by 8 mo of age. The relatively mild phenotype of Ctnnb1tm1Mmt/+;Amhr2 tm3(cre)Bhr/+ mice probably results from the weak Cre activity. It is not clear whether the Ctnnb1tm1Mmt/+;Amhr2 tm3(cre)Bhr/+ male mice will develop SCT later in life, because no mice were examined beyond 8 mo of age in their study. We generated Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor male mice with β-catenin specifically stabilized in Sertoli cells and found that 70% of the mutant males developed SCT at 8 mo of age. Our study shows a causal link between overactive β-catenin signaling and SCT development in mice. In the future, analyzing the β-catenin expression level or searching for β-catenin mutations in tumor samples from human patients will provide knowledge about whether overactivation of β-catenin signaling also plays a role in Sertoli cell tumorigenesis in humans.
Leydig Cells Cross Talk with Sertoli and Germ Cells
Several studies have indicated that other cell types in the testes, such as Sertoli cells and germ cells, can affect Leydig cell morphology and function [25]. Several Sertoli cell factors have either stimulatory or inhibitory effects on the production of testosterone in primary-cultured Leydig cells in vitro [25, 34, 35]. Results of several in vivo studies also have suggested that Leydig cell morphology and function are affected when spermatogenesis is disrupted [36, 37]. However, evidence has been conflicting in terms of whether Sertoli cells and germ cells are required for normal Leydig cell function. A recent study indicated that Leydig cells are viable and function normally in the absence of Sertoli cells and germ cells. In Wt1 Sertoli cell knockout mice, the testicular cords are disrupted at embryonic stages followed by the loss of germ cells. However, Leydig cells proliferated normally, and the levels of serum testosterone are within normal ranges in the adult mutant males [38]. In our study, we stabilized β-catenin protein in Sertoli cells using the Tg(AMH-cre)1Flor mouse line. The mutant testes lacked normal testicular cord structures, and spermatogenesis was completely abolished. However, before the tumor onset, Leydig cells were found in the interstitial spaces of the mutant testes. We also measured serum testosterone levels of adult mutants and found that they were not significantly different from those of the controls (data not shown). After the tumor onset, Sertoli cells proliferated rapidly and became the dominant cell type in the mutant testes after 8 mo of age. Leydig cells were isolated but still existed inside or at the periphery regions outside the tumor mass. Our results suggest that Leydig cells at adult stages can survive and function normally in the absence of normal Sertoli cells and spermatogenesis.
Model of SCT Tumorigenesis in Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor Mice
Stabilization of β-catenin can cause either cell-cycle arrest followed by apoptosis [22, 39, 40] or enhanced cell proliferation [15, 41–43], depending on tissue types. However, stabilization of β-catenin in embryonic Sertoli cells did not lead to either apoptosis or overproliferation. β-Catenin was highly accumulated in Sertoli cells of Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice after E14.5. However, Sertoli cells proliferated normally and did not undergo apoptosis, but they lost Sertoli cell markers SOX9 and AMH and failed to support spermatogenesis [23]. In the present study, we found increased cell proliferation in Sertoli cells with stabilized β-catenin at 8 mo of age. It does not appear that the increased cell proliferation is directly caused by β-catenin stabilization, because β-catenin was stabilized at embryonic stages. We hypothesize that stabilization of β-catenin might create a “first hit” in Sertoli cells, which makes Sertoli cells dysfunctional. They lose expression of some Sertoli cell markers, such as SOX9 and AMH, and fail to support spermatogenesis. However, they are not tumor cells at this stage, and they do not proliferate actively. Over time, a “second hit,” such as other somatic mutations, might transform these cells to tumor cells, and they start to expand very quickly by active proliferation.
It is important to note that in both human patients with SCT and our SCT mouse model, SCTs contain more than one type of pathology. We observed a nest-like type of tumor cells as the dominant form in Ctnnb1tm1Mmt/+;Tg(AMH-cre)1Flor mice, and we observed a tubular type or round-nucleus type of tumor cells less frequently. It is possible that all three types of tumor cells are primary tumor cells and that the difference in pathology results from different secondary hits in Sertoli cells with stabilized β-catenin. It also is possible that the tubular and round-nucleus type of tumor cells (especially the round-nucleus type) are secondary to nest-like tumor cells, because they were found less frequently and always mixed with nest-like type of tumor in the testes.
Acknowledgments
We thank Ying Wang for helpful discussions.
Footnotes
Supported by National Institutes of Health (NIH) grant HD30284 and the Ben F. Love Endowment to R.R.B. Veterinary resources were supported by the NIH Cancer Center Support Grant CA16672.
REFERENCES
- Ulbright TM, Amin MB, Young RH. Atlas of Tumor Pathology, 3rd series, fascicle 25. Washington, DC:: Armed Forces Institute of Pathology;; 1999. Tumors of the testis, adnexa, spermatic cord, and scrotum. [Google Scholar]
- Henley JD, Young RH, Ulbright TM.Malignant Sertoli cell tumors of the testis: a study of 13 examples of a neoplasm frequently misinterpreted as seminoma. Am J Surg Pathol 2002; 26: 541–550.. [DOI] [PubMed] [Google Scholar]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R.Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997; 16: 3797–3804.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ.Beta-catenin signaling and cancer. Bioessays 1999; 21: 1021–1030.. [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P.Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 2000; 287: 1606–1609.. [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H.Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003; 1653: 1–24.. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P.Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science 1997; 275: 1790–1792.. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Caca K, Hu G, Harrison FB, Fearon ER.Frequent nuclear/cytoplasmic localization of beta-catenin without exon 3 mutations in malignant melanoma. Am J Pathol 1999; 154: 325–329.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios J, Gamallo C.Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res 1998; 58: 1344–1347.. [PubMed] [Google Scholar]
- Wu R, Zhai Y, Fearon ER, Cho KR.Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res 2001; 61: 8247–8255.. [PubMed] [Google Scholar]
- Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T.Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res 1999; 59: 269–273.. [PubMed] [Google Scholar]
- Oda H, Imai Y, Nakatsuru Y, Hata J, Ishikawa T.Somatic mutations of the APC gene in sporadic hepatoblastomas. Cancer Res 1996; 56: 3320–3323.. [PubMed] [Google Scholar]
- Koesters R, Ridder R, Kopp-Schneider A, Betts D, Adams V, Niggli F, Briner J, von Knebel Doeberitz M.Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms' tumors. Cancer Res 1999; 59: 3880–3882.. [PubMed] [Google Scholar]
- Moser AR, Pitot HC, Dove WF.A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 1990; 247: 322–324.. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM.Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 1999; 18: 5931–5942.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnolo B, Berrebi D, Saadi-Keddoucci S, Porteu A, Pichard AL, Peuchmaur M, Vandewalle A, Kahn A, Perret C.Intestinal dysplasia and adenoma in transgenic mice after overexpression of an activated beta-catenin. Cancer Res 1999; 59: 3875–3879.. [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E.De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998; 95: 605–614.. [DOI] [PubMed] [Google Scholar]
- Boerboom D, Paquet M, Hsieh M, Liu J, Jamin SP, Behringer RR, Sirois J, Taketo MM, Richards JS.Misregulated Wnt/beta-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res 2005; 65: 9206–9215.. [DOI] [PubMed] [Google Scholar]
- Gounari F, Signoretti S, Bronson R, Klein L, Sellers WR, Kum J, Siermann A, Taketo MM, von Boehmer H, Khazaie K.Stabilization of beta-catenin induces lesions reminiscent of prostatic intraepithelial neoplasia, but terminal squamous transdifferentiation of other secretory epithelia. Oncogene 2002; 21: 4099–4107.. [DOI] [PubMed] [Google Scholar]
- Harada N, Miyoshi H, Murai N, Oshima H, Tamai Y, Oshima M, Taketo MM.Lack of tumorigenesis in the mouse liver after adenovirus-mediated expression of a dominant stable mutant of beta-catenin. Cancer Res 2002; 62: 1971–1977.. [PubMed] [Google Scholar]
- Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C.Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 2001; 20: 5972–5981.. [DOI] [PubMed] [Google Scholar]
- Kimura T, Nakamura T, Murayama K, Umehara H, Yamano N, Watanabe S, Taketo MM, Nakano T.The stabilization of beta-catenin leads to impaired primordial germ cell development via aberrant cell cycle progression. Dev Biol 2006; 300: 545–553.. [DOI] [PubMed] [Google Scholar]
- Chang H, Gao F, Guillou F, Taketo MM, Huff V, Behringer RR.Wt1 negatively regulates beta-catenin signaling during testis development. Development 2008; 135: 1875–1885.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lécureuil C, Fontaine I, Crepieux P, Guillou F.Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis 2002; 33: 114–118.. [DOI] [PubMed] [Google Scholar]
- Fujisawa M.Cell-to-cell cross talk in the testis. Urol Res 2001; 29: 144–151.. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW.Identification of c-MYC as a target of the APC pathway. Science 1998; 281: 1509–1512.. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F.Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999; 398: 422–426.. [DOI] [PubMed] [Google Scholar]
- Mostofi FK.Epidemiology and pathology of tumors of human testis. Recent Results Cancer Res 1977; 60: 176–195.. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Behringer RR, Cate RL, Harwood KA, Idzerda RL, Brinster RL, Palmiter RD.Directed expression of an oncogene to Sertoli cells in transgenic mice using mullerian-inhibiting substance regulatory sequences. Mol Endocrinol 1992; 6: 1403–1411.. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A.Alpha-inhibin is a tumor-suppressor gene with gonadal specificity in mice. Nature 1992; 360: 313–319.. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, Gutierrez C, Wang D, Martin JF, Jamin SP, Behringer RR, Robertson EJ, Matzuk MM.Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol 2008; 28: 248–257.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RH, Koelliker DD, Scully RE.Sertoli cell tumors of the testis, not otherwise specified: a clinicopathologic analysis of 60 cases. Am J Surg Pathol 1998; 22: 709–721.. [DOI] [PubMed] [Google Scholar]
- Boyer A, Hermo L, Paquet M, Robaire B, Boerboom D.Seminiferous tubule degeneration and infertility in mice with sustained activation of WNT/CTNNB1 signaling in Sertoli cells. Biol Reprod 2008; 79: 475–485.. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Dahl KD, Vaughan J, Tucker E, Rivier J, Bardin CW, Vale W.Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. Proc Natl Acad Sci U S A 1987; 84: 5082–5086.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Haskell J, Vinson N, Terracio L.Characterization of insulin and insulin-like growth factor I receptors of purified Leydig cells and their role in steroidogenesis in primary culture: a comparative study. Endocrinology 1986; 119: 1641–1647.. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Rich KA, De Kretser DM.Alterations of the fine structure and androgen secretion of interstitial cells in the experimentally cryptorchid rat testis. Biol Reprod 1979; 20: 409–422.. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Maddocks S, Kerr JB.Cell-cell interactions in the control of spermatogenesis as studied using Leydig cell destruction and testosterone replacement. Am J Anat 1990; 188: 3–20.. [DOI] [PubMed] [Google Scholar]
- Gao F, Maiti S, Alam N, Zhang Z, Deng JM, Behringer RR, Lécureuil C, Guillou F, Huff V.The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc Natl Acad Sci U S A 2006; 103: 11987–11992.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Pang KM, Evans M, Hay ED.Overexpression of beta-catenin induces apoptosis independent of its transactivation function with LEF-1 or the involvement of major G1 cell cycle regulators. Mol Biol Cell 2000; 11: 3509–3523.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmeda D, Castel S, Vilaró S, Cano A.Beta-catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol Biol Cell 2003; 14: 2844–2860.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B.Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev 2004; 18: 1072–1087.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T, Taketo MM, Cardiff RD, Miyoshi K, Wagner KU, Robinson GW, Hennighausen L.Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene 2003; 22: 3875–3887.. [DOI] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hülsken J, Müller T, Walther I, Taketo MM, Crenshaw EB, III, Birchmeier W, Birchmeier C.Beta-catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol 2003; 258: 406–418.. [DOI] [PubMed] [Google Scholar]