Abstract
Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, a rate-limiting step of the mevalonate pathway. The pleiotropic effects of statins may be due to inhibition of cholesterol synthesis, as well as decreased availability of several biologically important intermediate components of the mevalonate pathway, including two substrates for isoprenylation (farnesyl pyrophosphate [FPP] and geranylgeranyl pyrophosphate [GGPP]). Recently, we demonstrated statin-induced inhibition of ovarian theca-interstitial cell proliferation in vitro, as well as reduction of testosterone levels in women with polycystic ovary syndrome (PCOS). This study evaluates the relative contribution of inhibition of isoprenylation and/or cholesterol availability to the modulation of theca-interstitial proliferation. Rat theca-interstitial cells were cultured in chemically defined media with or without simvastatin, FPP, GGPP, squalene, and/or two membrane-permeable forms of cholesterol (25-hydroxycholesterol and 22-hydroxycholesterol). Simvastatin inhibited DNA synthesis and the count of viable cells. The effects of simvastatin were partly abrogated by FPP and GGPP but not by squalene or cholesterol. Inhibition of farnesyl transferase and geranylgeranyl transferase reduced cell proliferation. The present findings indicate that simvastatin inhibits proliferation of theca-interstitial cells, at least in part, by reduction of isoprenylation. These observations provide likely mechanisms explaining clinically observed improvement of ovarian hyperandrogenism in women with PCOS.
Keywords: interstitial cells, isoprenylation, ovary, proliferation, statin, theca cells
Isoprenylation may mediate statin-induced inhibition of theca-interstitial proliferation in ovarian theca-interstitial cells.
INTRODUCTION
Ovarian mesenchyma (theca-interstitial tissues) are a major source of androgens. Normal ovarian function, including folliculogenesis and steroidogenesis, requires effective mechanisms regulating theca-interstitial growth and function. Under pathological conditions such as in polycystic ovary syndrome (PCOS), ovaries are significantly enlarged, and they are characterized on histological evaluation by prominent theca-interstitial hyperplasia [1]. Potential contributors to excessive growth of theca-interstitial tissues in PCOS include insulin, insulin-like growth factor 1 (IGF1), and moderate oxidative stress. Indeed, PCOS is associated with hyperinsulinemia, elevated free IGF1, and increased oxidative stress [2–8]. Our previous in vitro studies [9–11] have demonstrated that insulin, IGF1, and moderate oxidative stress (such as that induced by hypoxanthine-xanthine oxidase) stimulate proliferation of rat theca-interstitial cells. In contrast, mevastatin induced a concentration-dependent reduction of theca-interstitial proliferation [12, 13]. Furthermore, we have evaluated the effects of another statin, simvastatin, on the endocrine profile of women with PCOS. In a randomized clinical trial, treatment with simvastatin resulted in a significant reduction of total and free testosterone [14, 15]. In a subsequent randomized trial, other investigators found that another statin, atorvastatin, also markedly reduced androgen levels in women with PCOS [16].
Statins are thought to act primarily by competitive inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, an early step of the mevalonate pathway; hence, the effects of statins may be related to decreased availability of several biologically important products of this pathway, including substrates of isoprenylation (farnesyl pyrophosphate [FPP] and geranylgeranyl pyrophosphate [GGPP]), as well as reduction of the availability of cholesterol [17–19]. This study was designed to evaluate potential contributions of these compounds to the observed inhibition of theca-interstitial proliferation by a statin.
MATERIALS AND METHODS
Animals
Female Sprague-Dawley rats were obtained at age 25 days from Charles River Laboratories (Wilmington, MA) and housed with 12L:12D in an air-conditioned environment. All animals received standard rat chow and water ad libitum. At ages 28, 29, and 30 days, the rats were injected with 17β-estradiol (1 mg/0.3 ml of sesame oil s.c.) to stimulate ovarian development and growth of antral follicles. Approximately 24 h after the last injection (at age 31 days), the animals were anesthetized using ketamine and xylazine (i.p.) and euthanized during intracardiac perfusion using 0.9% saline. All treatments and procedures were carried out in accord with accepted standards of humane animal care as outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
Cell Culture and Reagents
Collection and purification of ovarian theca-interstitial cells were performed as previously described [9, 20]. Briefly, ovaries were dissected, and theca-interstitial cells were purified using discontinuous Percoll gradient centrifugation. The cells were counted, and viability was routinely in the 85%–95% range. Theca-interstitial cells were incubated in 96-well plates (35 000 cells/well). The cultures were carried out for 48 h at 37°C in an atmosphere of 5% CO2 in humidified air in serum-free McCoy 5A medium (supplemented with antibiotics, 0.1% bovine serum albumin, and 2 mM l-glutamine). The cells were incubated without (control) or with simvastatin (10 μM), FPP (1–30 nM), GGPP (1–30 nM), farnesyltransferase inhibitor 277 (FTI) (1–10 μM), geranyltransferase inhibitor 298 (GGTI) (1–10 μM), squalene (3–30 μM), 25-hydroxycholesterol (3–30 μM), and 22-hydroxycholesterol (3–30 μM). All of the chemicals were purchased form Sigma Chemical Co. (St. Louis, MO).
Proliferation Assays
Theca-interstitial cells were incubated for 48 h in 96-well culture plates with or without individual additives. DNA synthesis was quantified using a thymidine incorporation assay. Radiolabeled [3H] thymidine (1 μCi/well) was added to the cells during the last 24 h of culture. At the end of the culture period, the cells were harvested using a multiwell cell harvester (PHD Harvester, model 290; Cambridge Technology, Inc., Watertown, MA). Radioactivity was measured in a liquid scintillation counter (SL 4000; Intertechnique, Fairfield, NJ). Each treatment was carried out in at least eight replicates.
The total viable cell number was estimated using a 4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenil)-2H-tetrazolium (MTS) assay (CellTiter 96 AQueous One; Promega, Madison, WI). This assay involves conversion of MTS to colored formazan by mitochondrial dehydrogenase within living cells; the maximal absorbance of the formazan released to the culture medium is 490 nm [21]. The MTS assay is an indirect measure of the number of viable cells. The actual absorbance observed during each experiment depends not only on the number of cells but also on the duration of incubation of reagents with cells, the temperature, and the state of the cells (freshly collected vs. cells attached to the plate). Consequently, a direct comparison of the actual cell number at the beginning and at the end of any individual experiment is not reliable. Hence, in this study, the absorbance in the treatment cultures was expressed relative to the absorbance detected in the control cultures (% of control). Based on experiments in which the known cell number (directly counted under the microscope) was evaluated also by MTS, we estimate that the number of cells per well in the control culture typically doubles to quadruples after 48 h of culture.
Statistical Analysis
Baseline data are presented as the mean ± SEM. Statistical analysis was performed using JMP 7.0 (SAS Institute, Cary, NC). Normality of distribution was evaluated using Shapiro-Wilk test. When required, data were logarithmically transformed [22]. Statistical analysis was performed using ANOVA, followed by post hoc pairwise comparisons using Bonferroni correction.
RESULTS
Inhibition of the mevalonate pathway by statins leads to decreased production of two substrates for isoprenylation: FPP, which is required for farnesylation, and GGPP, which is required for geranylgeranylation. To test whether statin-induced reduction of DNA synthesis is related to decreased isoprenylation, theca-interstitial cells were cultured without (control) or with simvastatin (10 μM) and/or varying concentrations of FPP and GGPP.
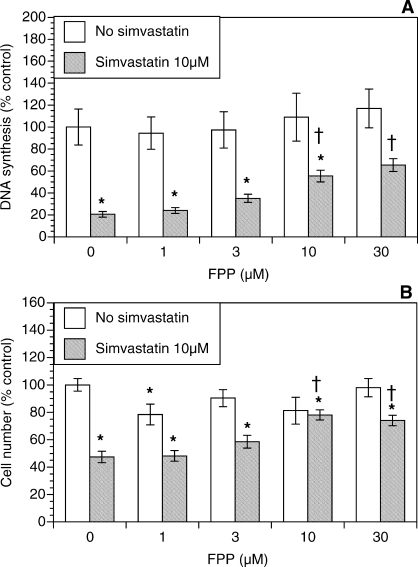
As shown in Figure 1A, simvastatin alone induced profound inhibition of DNA synthesis by 79% (P < 0.001). In contrast, FPP alone had no significant effect on DNA synthesis. However, in the presence of simvastatin, the addition of FPP resulted in a concentration-dependent restoration of DNA synthesis. A statistically significant restoration of DNA synthesis was observed starting at 10 μM FPP; at the highest concentration of 30 μM, FPP significantly increased thymidine incorporation 3.1-fold above the level in the presence of simvastatin alone (P < 0.001).
FIG. 1.
Effect of FPP (1–30 μM) on proliferation of ovarian theca-interstitial cells in the absence and presence of simvastatin (10 μM). Cells were cultured for 48 h in chemically defined media. Proliferation was evaluated by determination of DNA synthesis by thymidine incorporation (A) and by estimation of the number of viable cells using MTS assay (B). Each bar represents the mean ± SEM (N = 8). *Denotes means significantly different from control in the absence of FPP (P < 0.05). †Denotes means significantly different from simvastatin alone (P < 0.05 [applies only to comparison among cultures containing simvastatin]).
To determine whether these effects were also reflected by changes in the number of viable theca-interstitial cells, we also performed the MTS assay. Figure 1B shows the effects of simvastatin and FPP on the cell number. Simvastatin alone significantly reduced the cell number by 52% (P < 0.01). In contrast, FPP partly reversed this inhibition; the initial and maximal effect was observed at 10 μM FPP, with an increase in the cell number 62% above the cell number observed in the presence of simvastatin alone (P < 0.001).
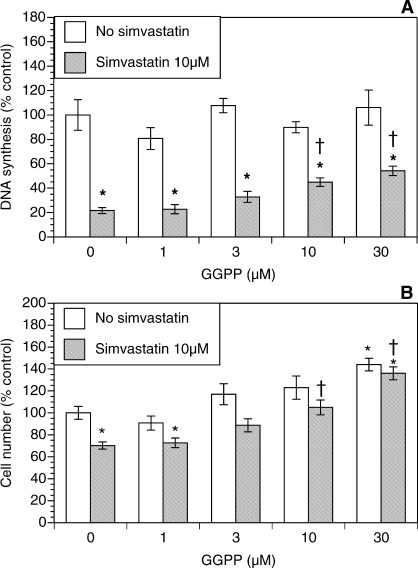
Figure 2 shows the role of GGPP in amelioration of the simvastatin-induced effects. The GGPP alone had no significant effect on DNA synthesis, while the total number of viable cells increased by 44% (P < 0.01) at the highest concentration of GGPP (Fig. 2B). The addition of GGPP to simvastatin-treated cultures resulted in a concentration-dependent restoration of DNA synthesis. A statistically significant increase in DNA synthesis was initially observed at 10 μM GGPP; at the highest concentration of GGPP (30 μM), DNA synthesis was 2.5-fold greater (P < 0.001) than that in the presence of simvastatin alone. In a similar fashion, simvastatin-induced inhibition of the number of viable cells was partly reversed by GGPP. A significant 50% increase in the cell number (P < 0.01) was initially observed at 10 μM GGPP; at the highest concentration of GGPP (30 μM), the cell number increased by 94% (P < 0.001) above the level detected in the presence of simvastatin alone.
FIG. 2.
Effect of GGPP (1–30 μM) on proliferation of ovarian theca-interstitial cells in the absence and presence of simvastatin (10 μM). The cells were cultured as described for Figure 1. A) Effects on DNA synthesis. B) Effects on the number of viable cells. Each bar represents the mean ± SEM (N = 8). *Denotes means significantly different from control in the absence of GGPP (P < 0.05). †Denotes means significantly different from simvastatin alone (P < 0.05 [applies only to comparison among cultures containing simvastatin]).
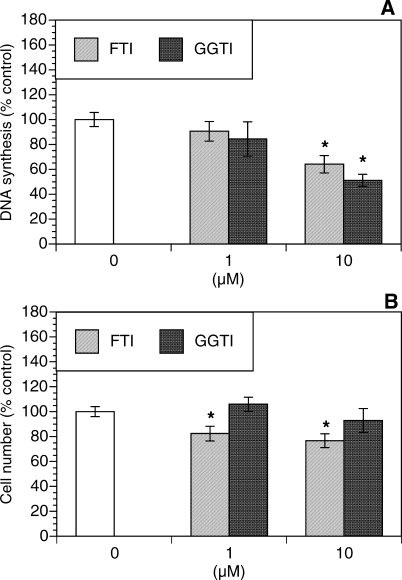
To further test the role of isoprenylation in the modulation of theca-interstitial growth, the effects of specific inhibitors of farnesylation and geranylgeranylation were evaluated. As shown in Figure 3, FTI (a selective inhibitor of farnesyl transferase) induced a significant decrease in DNA synthesis by 36% (P < 0.01) and reduced the number of viable cells by 23% (P < 0.05) below control values. Similarly, GGTI (a selective inhibitor of geranylgeranyl transferase) decreased DNA synthesis by up to 49% (P < 0.001) but had no statistically significant effect on the number of viable cells.
FIG. 3.
Effects of an inhibitor of farnesylation (FTI [1–10 μM]) and an inhibitor of geranylgeranylation (GGTI [1–10 μM]) on proliferation (DNA synthesis [A]) and the cell number (MTS assay [B]). The cells were cultured for 48 h in chemically defined media. Each bar represents the mean ± SEM (N = 8). *Denotes means significantly different from control (P < 0.05).
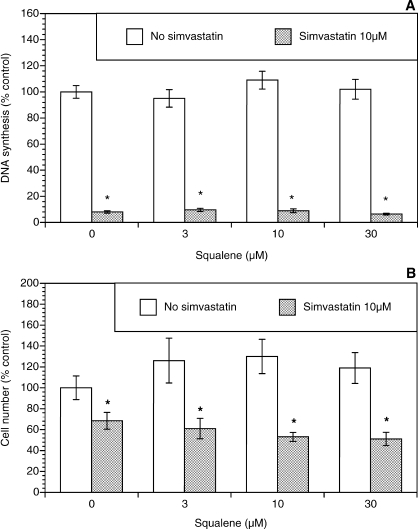
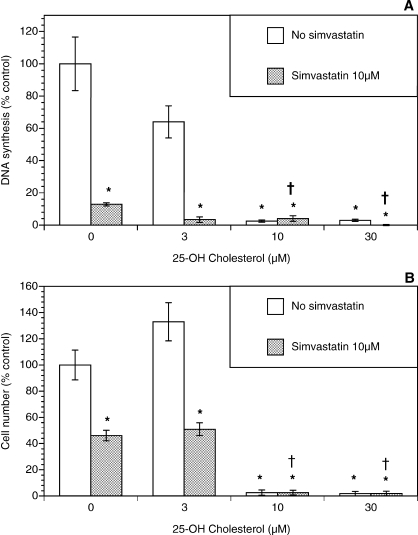
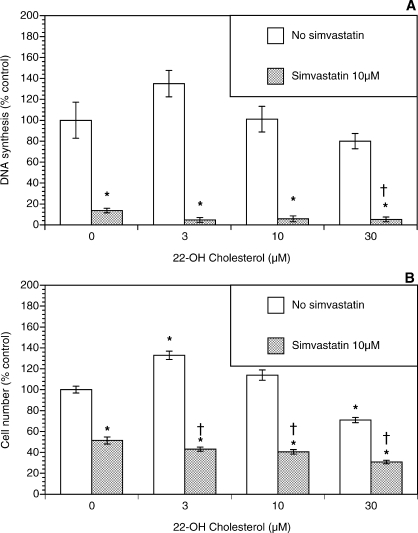
Because simvastatin-induced inhibition of growth of theca-interstitial cells may also be due to depletion of squalene and/or cholesterol, additional experiments were carried out evaluating the role of these compounds. As shown in Figure 4, squalene had no significant effect on DNA synthesis or the cell count in the absence or presence of simvastatin. To study the effects of cholesterol, two membrane-permeable analogs of cholesterol were used, namely, 25-hydroxycholesterol and 22-hydroxycholesterol [23, 24]. As shown in Figure 5, in the absence of simvastatin, 25-hydroxycholesterol alone induced profound inhibition of DNA synthesis and the viable cell number at doses of 10–30 μM. In the presence of simvastatin, the addition of 25-hydroxycholesterol also resulted in further suppression of cell growth above and beyond the effects of simvastatin. A similar but less potent pattern was observed in experiments using 22-hydroxycholesterol (Fig. 6).
FIG. 4.
Effect of squalene (3–30 μM) on proliferation of ovarian theca-interstitial cells in the absence and presence of simvastatin (10 μM). Cells were cultured as described for Figure 1. A) Effects on DNA synthesis. B) Effects on the number of viable cells. Each bar represents the mean ± SEM (N = 8). *Denotes means significantly different from control in the absence of squalene (P < 0.05).
FIG. 5.
Effect of 25-hydroxycholesterol (22-OH cholesterol) (3–30 μM) on proliferation of ovarian theca-interstitial cells in the absence and presence of simvastatin (10 μM). The cells were cultured as described for Figure 1. A) Effects on DNA synthesis. B) Effects on the number of viable cells. Each bar represents the mean ± SEM (N = 8). *Denotes means significantly different from control in the absence of 25-hydroxycholesterol (P < 0.05). †Denotes means significantly different from simvastatin alone (P < 0.05 [applies only to comparison among cultures containing simvastatin]).
FIG. 6.
Effect of 22-hydroxycholesterol (22-OH cholesterol) (3–30 μM) on proliferation of ovarian theca-interstitial cells in the absence and presence of simvastatin (10 μM). The cells were cultured as described for Figure 1. A) Effects on DNA synthesis. B) Effects on the number of viable cells. Each bar represents the mean ± SEM (N = 8). *Denotes means significantly different from control in the absence of 22-hydroxycholesterol (P < 0.05). †Denotes means significantly different from simvastatin alone (P < 0.05 [applies only to comparison among cultures containing simvastatin]).
DISCUSSION
In the present study, we have shown (1) that ovarian theca-interstitial cell growth is profoundly inhibited by simvastatin, (2) that this suppressive effect of simvastatin is partly reversed by the precursors of isoprenylation but not by squalene or cholesterol, and (3) that cell growth is also inhibited by inhibitors of isoprenylation.
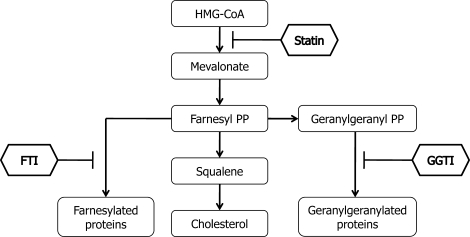
Conceptually, the inhibitory effects of statins on cell growth may be mediated by various pathways and mechanisms related to inhibition of HMG-CoA reductase and hence by several biologically active products of the mevalonate pathway (Fig. 7). Because growth of tissues requires availability of cholesterol, statin-induced reduction of cholesterol levels may be a potentially important explanation for reduced DNA synthesis and the number of viable cells. However, in this study, squalene (a precursor of cholesterol) and two cell membrane- and mitochondrion-permeable analogs of cholesterol did not reverse the inhibitory effects of simvastatin, indicating that a limited supply of cholesterol is not a likely factor in the observed effects of simvastatin. Furthermore, both 25-hydroxycholesterol and 22-hydroxycholesterol reduced theca-interstitial cell growth in the absence and presence of simvastatin.
FIG. 7.
Outline of the mevalonate pathway. Conversion of acetoacetyl-coenzyme A to HMG-CoA to mevalonic acid is regulated by HMG-CoA reductase, the rate-limiting step of cholesterol synthesis. Statins competitively inhibit HMG-CoA reductase. Products of mevalonic acid include FPP (farnesyl PP) and GGPP (geranylgeranyl PP). Farnesyl pyrophosphate is required for farnesylation; this step is inhibited by FTI. Geranylgeranyl pyrophosphate is required for geranylgeranylation; this step is inhibited by GGTI.
A possible explanation for the observed suppression of cell growth by cholesterol is the well-described inhibitory effect of cholesterol and its analogs on HMG-CoA reductase [25–28]. These actions of cholesterol are related to suppression of the cleavage of sterol regulatory element-binding proteins, which in turn leads to reduced gene transcription and ultimately to decreased levels of enzymes controlling cholesterol synthesis, including HMG-CoA reductase and HMG-CoA synthase. Ultimately, the mevalonate pathway is suppressed, including decreased levels of the precursors of isoprenylation (FPP and GGPP).
This explanation fits well with the present observations that FPP and GGPP significantly ameliorated statin-induced suppression of cell proliferation in a concentration-dependent fashion. In addition, the relevance of isoprenylation pathways is supported by the observation that selective inhibitors of farnesyl transferase and geranylgeranyl transferase also reduced theca-interstitial proliferation.
Notably, the addition of FPP alone or GGPP alone had no consistent significant effect on proliferation except for a modest stimulatory effect of GGPP at 30 μM (Fig. 2B). We speculate that, under basal conditions in the absence of inhibition by statin, cells may have a sufficient supply of substrates for isoprenylation; hence, a further increase in the supply of FPP and GGPP may have no significant effect.
Farnesylation and geranylgeranylation are likely relevant because of their role in posttranslational lipid modifications of several proteins that are important to regulation of tissue growth [18, 19, 29]. Specifically, interruption of farnesylation leads to reduction of membrane-bound RAS and hence diminished activity of mitogen-activated protein kinase 1 (MAPK1). In our previous studies [9, 11, 30], we demonstrated that insulin and moderate oxidative stress induce theca-interstitial proliferation and that these effects are mediated, at least in part, by MAPK1. More recently, we showed that another statin (mevastatin) inhibits growth of theca-interstitial cells and reduces activity of MAPK1 [13].
Another relevant protein that undergoes farnesylation is RHEB, a small guanosine triphosphatase (GTPase) that positively regulates the mammalian target of rapamycin (FRAP1, also known as mTOR) upstream of RPS6KB1 [31]. Our previous findings have shown that RPS6KB1 activity is important in induction of proliferation of theca-interstitial cells by insulin and moderate oxidative stress [30]. Recently, one of the statins (atorvastatin) was shown to inhibit growth of tuberous sclerosis complex 2-null cells by mechanisms involving impaired function of RHO and RHEB [32].
In the present study, GGPP appeared to be at least as potent as FPP in reversing simvastatin-induced inhibition; hence, it is likely that geranylgeranylation may also be of importance in the modulation of theca-interstitial growth. Indeed, geranylgeranylation regulates function of several small GTPases, including RHO, RAC, and CDC42, which may have a significant role in regulation of theca-interstitial growth. RHO, RAX, and CDC42 are involved in regulation of organization of the actin cytoskeleton; furthermore, microinjection of these proteins into fibroblasts induces cell cycle progression via pathways independent of MAPK1 [33]. One of the statins (cervistatin) was shown to reduce proliferation and invasion of a breast cancer cell line via mechanisms involving inhibition of the RHO pathway [34].
Another potentially important pathway that may explain the role of geranylgeranylation in regulating theca-interstitial growth involves the modulation of NADPH oxidase (a multicomponent enzyme), which may consist of several subunits, including RAC1, CYBA (also known as p22phox), and NOX1 [35, 36]. The modulation of subunit expression is important for the activity of NADPH oxidase, a major intracellular source of reactive oxygen species (ROS). Because induction of oxidative stress by ROS may promote proliferation of theca-interstitial cells, it may be tempting to speculate that the actions of statins may involve reduction of oxidative stress by decreasing NADPH oxidase activity by mechanisms that possibly involve reduction of geranylgeranylation of RAC1 and reduction of availability of other subunits. Indeed, consistent with this concept, findings in vascular smooth muscle have demonstrated that one of the statins (atorvastatin) reduces mRNA expression of several subunits of NADPH oxidase, as well as reduced RAC1 membrane translocation and activity [37].
Most important, in this study, the evaluation of growth of theca-interstitial cells was carried out by two distinctly different approaches, namely, assessment of DNA synthesis and indirect counting of viable cells by MTS assay. Close inspection of our findings (e.g., Figs. 1 and 2) indicates that the inhibitory action of simvastatin appeared greater in assays evaluating DNA synthesis than in assays evaluating the cell number. Furthermore, the effectiveness of FPP and GGPP in the restoration of the cell number was greater than that in the restoration of DNA synthesis. The differences in the observed effects for the two methods may be related to at least two factors. First, the relationship of the cell count and DNA synthesis depends on the proportion of cells undergoing division; thus, for example, when a small proportion of cells undergoes division, the effects on DNA synthesis are greater than the effects on the total cell number. Second, the number of viable cells depends not only on the rate of proliferation and DNA synthesis but also on the rate of cell death (e.g., apoptosis).
The present observations have potentially important clinical implications. Because PCOS is characterized by excessive growth of ovarian theca-interstitial cells and hyperandrogenism, statin-mediated reduction of growth of these tissues may be important in decreasing ovarian androgen production. Indeed, our previous studies [14, 15] have shown that simvastatin significantly reduces androgen levels. More recently, marked reduction of androgen levels was observed in patients with PCOS using atorvastatin [16]. However, it should be noted that in reproductively healthy women statins may have potentially adverse effects on reproductive function (e.g., by interfering with normal folliculogenesis).
In conclusion, the present findings suggest that simvastatin inhibits growth of ovarian theca-interstitial cells by mechanisms involving reduction of farnesylation and geranylgeranylation but not reduction of the availability of cholesterol. These actions may explain clinically observed improvement of ovarian hyperandrogenism in women with PCOS.
Footnotes
Supported by grant R01-HD050656 from the Eunice Shriver National Institute of Child Health and Human Development (to A.J.D.).
REFERENCES
- Hughesdon PE.Morphology and morphogenesis of the Stein-Leventhal ovary and of so called “hyperthecosis.” Obstet Gynecol Surv 1982; 37: 59–77.. [DOI] [PubMed] [Google Scholar]
- Burghen GA, Givens JR, Kitabchi AE.Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab 1980; 50: 113–116.. [DOI] [PubMed] [Google Scholar]
- Chang RJ, Nakamura RM, Judd HL, Kaplan SA.Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab 1983; 57: 356–359.. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A.Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab 1987; 65: 499–507.. [DOI] [PubMed] [Google Scholar]
- Iwashita M, Mimuro T, Watanabe M, Setoyama T, Matsuo A, Adachi T, Takeda Y, Sakamoto S.Plasma levels of insulin-like growth factor-I and its binding protein in polycystic ovary syndrome. Horm Res 1990; 33: 21–26.. [DOI] [PubMed] [Google Scholar]
- Thierry van Dessel HJ, Lee PD, Faessen G, Fauser BC, Giudice LC.Elevated serum levels of free insulin-like growth factor I in polycystic ovary syndrome. J Clin Endocrinol Metab 1999; 84: 3030–3035.. [DOI] [PubMed] [Google Scholar]
- Sabuncu T, Vural H, Harma M.Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem 2001; 34: 407–413.. [DOI] [PubMed] [Google Scholar]
- Fenkci V, Fenkci S, Yilmazer M, Serteser M.Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril 2003; 80: 123–127.. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR.Effects of insulin and insulin-like growth factors on proliferation of rat ovarian theca-interstitial cells. Biol Reprod 1997; 56: 891–897.. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Spaczynski RZ, Olive DL.Insulin and insulin-like growth factor I stimulate the proliferation of human ovarian theca-interstitial cells. Fertil Steril 1998; 69: 335–340.. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Foyouzi N, Karaca M, Pehlivan T, Kwintkiewicz J, Behrman HR.Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod 2004; 19: 1519–1524.. [DOI] [PubMed] [Google Scholar]
- Izquierdo D, Foyouzi N, Kwintkiewicz J, Duleba AJ.Mevastatin inhibits ovarian theca-interstitial cell proliferation and steroidogenesis. Fertil Steril 2004; 82(suppl 3):1193–1197.. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Foyouzi N, Piotrowski P, Rzepczynska I, Duleba AJ.Mevastatin inhibits proliferation of rat ovarian theca-interstitial cells by blocking the mitogen-activated protein kinase pathway. Fertil Steril 2006; 86(suppl 4):1053–1058.. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Banaszewska B, Spaczynski RZ, Pawelczyk L.Simvastatin improves biochemical parameters in women with polycystic ovary syndrome: results of a prospective, randomized trial. Fertil Steril 2006; 85: 996–1001.. [DOI] [PubMed] [Google Scholar]
- Banaszewska B, Pawelczyk L, Spaczynski RZ, Dziura J, Duleba AJ.Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome: prospective, randomized, crossover trial. J Clin Endocrinol Metab 2007; 92: 456–461.. [DOI] [PubMed] [Google Scholar]
- Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL.The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study. J Clin Endocrinol Metab 2009; 94: 103–108.. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS.Regulation of the mevalonate pathway. Nature 1990; 343: 425–430.. [DOI] [PubMed] [Google Scholar]
- Rombouts K, Kisanga E, Hellemans K, Wielant A, Schuppan D, Geerts A.Effect of HMG-CoA reductase inhibitors on proliferation and protein synthesis by rat hepatic stellate cells. J Hepatol 2003; 38: 564–572.. [DOI] [PubMed] [Google Scholar]
- Danesh FR, Sadeghi MM, Amro N, Philips C, Zeng L, Lin S, Sahai A, Kanwar YS.3-Hydroxy-3-methylglutaryl CoA reductase inhibitors prevent high glucose-induced proliferation of mesangial cells via modulation of Rho GTPase/ p21 signaling pathway: implications for diabetic nephropathy. Proc Natl Acad Sci U S A 2002; 99: 8301–8305.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoffin DA, Erickson GF.Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology 1988; 122: 2345–2347.. [DOI] [PubMed] [Google Scholar]
- Capasso JM, Cossio BR, Berl T, Rivard CJ, Jimenez C.A colorimetric assay for determination of cell viability in algal cultures. Biomol Eng 2003; 20: 133–138.. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG.The use of transformation when comparing two means. BMJ 1996; 312: e1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman HR, Aten RF.Evidence that hydrogen peroxide blocks hormone-sensitive cholesterol transport into mitochondria of rat luteal cells. Endocrinology 1991; 128: 2958–2966.. [DOI] [PubMed] [Google Scholar]
- Musicki B, Aten RF, Behrman HR.Inhibition of protein synthesis and hormone-sensitive steroidogenesis in response to hydrogen peroxide in rat luteal cells. Endocrinology 1994; 134: 588–595.. [DOI] [PubMed] [Google Scholar]
- Kandutsch AA, Chen HW.Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J Biol Chem 1974; 249: 6057–6061.. [PubMed] [Google Scholar]
- Brown MS, Goldstein JL.Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem 1974; 249: 7306–7314.. [PubMed] [Google Scholar]
- Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM.Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem 1997; 272: 3137–3140.. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL.The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997; 89: 331–340.. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ.Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 1996; 65: 241–269.. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Spaczynski RZ, Foyouzi N, Pehlivan T, Duleba AJ.Insulin and oxidative stress modulate proliferation of rat ovarian theca-interstitial cells through diverse signal transduction pathways. Biol Reprod 2006; 74: 1034–1040.. [DOI] [PubMed] [Google Scholar]
- Basso AD, Mirza A, Liu G, Long BJ, Bishop WR, Kirschmeier P.The farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits Rheb farnesylation and mTOR signaling: role in FTI enhancement of taxane and tamoxifen anti-tumor activity. J Biol Chem 2005; 280: 31101–31108.. [DOI] [PubMed] [Google Scholar]
- Finlay GA, Malhowski AJ, Liu Y, Fanburg BL, Kwiatkowski DJ, Toksoz D.Selective inhibition of growth of tuberous sclerosis complex 2 null cells by atorvastatin is associated with impaired Rheb and Rho GTPase function and reduced mTOR/S6 kinase activity. Cancer Res 2007; 67: 9878–9886.. [DOI] [PubMed] [Google Scholar]
- Olson MF, Ashworth A, Hall A.An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science 1995; 269: 1270–1272.. [DOI] [PubMed] [Google Scholar]
- Denoyelle C, Albanese P, Uzan G, Hong L, Vannier JP, Soria J, Soria C.Molecular mechanism of the anti-cancer activity of cerivastatin, an inhibitor of HMG-CoA reductase, on aggressive human breast cancer cells. Cell Signal 2003; 15: 327–338.. [DOI] [PubMed] [Google Scholar]
- Lassegue B, Clempus RE.Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 2003; 285: R277–R297.. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Laufs U, Muller K, Konkol C, Ahlbory K, Baumer AT, Linz W, Bohm M, Nickenig G.Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol 2002; 22: 300–305.. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Laufs U, Baumer AT, Muller K, Konkol C, Sauer H, Bohm M, Nickenig G.Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol Pharmacol 2001; 59: 646–654.. [DOI] [PubMed] [Google Scholar]