Abstract
The blood-testis barrier (BTB) is formed by tight junctions between Sertoli cells. Results of previous studies suggested that the barrier is deficient in ets variant 5 (ETV5) gene-deleted mice; therefore, microarray data were examined for changes in tight junction-associated genes. The tight junctional protein claudin 5 (CLDN5) was decreased in testes of 8-day-old Etv5−/− pups. The study reported herein examined the expression of CLDN5 in wild-type (WT) and Etv5−/− mice and evaluated its contribution to BTB function. CLDN5 protein expression was evaluated in 8-day-old WT and Etv5−/− and adult WT, Etv5−/−, and W/Wv testes by immunohistochemistry and in 8-day-old WT Sertoli cell-enriched and germ cell-enriched fractions by immunocytochemistry. Cldn5 mRNA expression was evaluated in 0- to 20-day-old and adult WT mice and in 8-day-old and adult Etv5−/− mice via quantitative PCR. Tracer studies were performed in adult WT, Etv5−/−, and W/Wv mice. The results indicate the following: 1) CLDN5 was expressed in Sertoli cells, spermatogonia, and preleptotene spermatocytes. 2) Seminiferous epithelial CLDN5 expression depended upon both the presence of germ cells and ETV5. 3) CLDN5 expression in testicular vascular endothelium and rete testis epithelium was ETV5 independent. 4) Cldn5 mRNA expression increased in the testes of juvenile mice at the time of BTB formation. 5) Testes of Etv5−/− and W/Wv mice, which are both deficient in seminiferous epithelial CLDN5 expression, had biotin tracer leakage from the interstitial space into the seminiferous tubule lumen. In conclusion, CLDN5 is expressed in the seminiferous epithelium, appears to be regulated by multiple influences, and contributes to BTB function.
Keywords: blood-testis barrier, ETS-related molecule (ERM), gametogenesis, Sertoli cells, Sertoli cell tight junction, spermatogenesis, testis, tracer study
Testicular claudin 5 expression is present in Sertoli cells, spermatogonia, preleptotene spermatocytes, and vascular endothelium; the seminiferous epithelial expression is transcription factor ets-variant 5-dependent and contributes to the integrity of the blood-testis barrier.
INTRODUCTION
The blood-testis barrier (BTB) is formed near the basement membrane of seminiferous tubules by adjacent Sertoli cells and separates the epithelium into basal and adluminal compartments [1]. This separation contributes to the establishment of specific microenvironments for spermatogonia and early spermatocytes beneath the BTB and for primary spermatocytes and haploid spermatids in the adluminal area [1, 2]. The BTB blocks the movement of intercellular molecules into the adluminal compartment, thus protecting spermatocytes and spermatids from potentially harmful chemicals [1]. In concert with other immunoprotective mechanisms, the BTB maintains testicular immune privilege to prevent immunological attack upon the “non-self” spermatids [3]. Genetic deletion of the tight junctional proteins occludin [4] and claudin 11 [5] results in a Sertoli cell-only phenotype, a finding that emphasizes the importance of tight junctions in BTB function and the requirement of this barrier for male fertility.
The transcription factor ets variant 5 (ETV5) is also essential for male fertility. ETV5 is a member of the PEA3 subfamily of ets transcription factors [6], which have roles in hematopoesis, angiogenesis, neuronal growth, cell cycle regulation, and metastatic ability of tumor cells [6]. In the testis, ETV5 is responsible for spermatogonial stem cell self-renewal and maintenance of the spermatogonial stem cell niche [7, 8]. Spermatogonial stem cells in Etv5−/− mice fail to self-renew [9] and undergo a sequential loss of germ cells starting with the spermatogonia and progressing through the spermatocytes and spermatids [7]. These mice undergo just the first wave of spermatogenesis and have a Sertoli cell-only phenotype as adults [7].
The BTB may be disrupted in Etv5−/− mice. When these mice received transplanted wild-type (WT) spermatogonial stem cells, there was an increased inflammatory response [10]. The increase in seminiferous tubular hydrostatic pressure that occurs during the germ cell infusion can be traumatic [11] and may be sufficient to induce inflammation in mice with a deficient BTB. Therefore, we examined microarray data from whole testes of 8-day-old WT and Etv5−/− mice for alterations in mRNA expression of proteins associated with tight junctions. There was no alteration in expression of the transmembrane proteins occludin, junctional adhesion molecules, or coxsackievirus and adenovirus receptor [12]. However, the expression of claudin 5 (CLDN5), another transmembrane tight junctional protein, was decreased 1.5-fold in Etv5−/− mice [12].
The claudin family of tight junction proteins has more than 20 members, with a structure consisting of four transmembrane domains, two extracellular domains, and both the C-terminus and N-terminus located cytoplasmically [13]. The claudin extracellular domains on adjacent cells can have homomeric (same claudin member) or heterotypic (different claudin member) interactions with each other to regulate permeability between cells [13]. CLDN5 is expressed in many vascular endothelial tissues throughout the body [14] and is important in regulating blood-brain barrier permeability [15].
In testes, other studies [14, 16] have reported CLDN5 to be present only in vascular endothelium. However, there were no changes observed in the vasculature of Etv5−/− mice; rather, the Sertoli cells and spermatogonial stem cell niche were disrupted. Therefore, the present study reexamined testes of WT and Etv5−/− mice for CLDN5 expression. The data presented demonstrate that CLDN5 is present in the seminiferous epithelium and that loss of ETV5 disrupts CLDN5 expression and normal function of the BTB.
MATERIALS AND METHODS
Mice
Etv5−/− mice on a 129Sv/Ev background were developed, bred, and genotyped as previously described [7, 9]. W/Wv mice were obtained from The Jackson Laboratory (100410l; www.jax.org). C57/B6 dams with pups were ordered from Charles River (www.criver.com) for StaPut (www.tecniglas.com) isolation of Sertoli cells and germ cells.
Mice were housed at 25°C with a 12L:12D photoperiod and were given water and standard rodent diet ad libitum. All experiments involving animals were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana-Champaign and were conducted in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
StaPut Isolation of Sertoli Cells and Germ Cells
Methods were adapted from those by Dym et al. [17]. Three groups of 8-day-old mice (24–30 mice/group) were euthanized by CO2 inhalation, and the testes were removed and decapsulated into ice-cold Dulbecco modified Eagle medium (DMEM)/F12 media. The testes were washed three times in 30–50 ml of media before a two-step enzymatic digestion process. For the first digest, testes were incubated in 15 ml of DMEM/F12 media containing collagenase IV (1 mg/ml) and DNase (80 μg/ml) with incubation in a 34°C water bath and shaking at 100 rpm for 20 min. The resulting seminiferous tubules suspension was washed with fresh media to eliminate Leydig cells and other interstitial cells. A second digest was performed to dissociate the seminiferous tubules and recover Sertoli cells and germ cells. The tubules were placed in 15 ml of DMEM/F12 media containing collagenase IV (1 mg/ml), hyaluronidase (1.5 mg/ml), trypsin (1 mg/ml), and DNase (80 μg/ml) and then incubated in a 34°C water bath with shaking at 100 rpm for 15 min. After the second digest, the cells were rinsed and resuspended in 2 ml of trypsin (1 mg/ml) and then pipetted for 2 min. A drop of the suspension was evaluated for clumps, with an additional 1 ml of trypsin and 1 min of pipetting performed if needed until complete dissociation. After washing, the cells were prepared for gravity sedimentation using the StaPut method in a 2%–4% bovine serum albumin (BSA) gradient [17] and were collected with a fraction collector (2110; Bio-Rad Laboratories, www.biorad.com).
Sertoli cell fractions were centrifuged and then rinsed twice in media, and the cells were suspended in PBS to make slide smears. Germ cell fractions were centrifuged, rinsed twice in media, and then resuspended in DMEM/F12 containing 10% NU serum, minimal media (www.bdbiosciences.ca). Residual Sertoli cells were removed by differential plating [18]. The cell suspension was transferred to cell culture plates that had been pretreated with 10% fetal calf serum and was incubated at 33°C with 5% CO2 for 3 h. The supernatant (germ cells) was collected, the cells were rinsed twice, and the cells were suspended in PBS to make slide smears. After being air dried, the cells were fixed with cold 4% paraformaldehyde in PBS for 5 min, rinsed in PBS and then 70% ethanol, allowed to air dry, and stored at 4°C until used.
Immunohistochemistry
Testes of adult (63- to 105-day-old) WT and Etv5−/− mice (n = 3 each) were fixed by perfusion [19] with cold neutral buffered 10% formalin (NBF). Testes of juvenile (8-day-old) WT and Etv5−/− mice (n = 2 each) were immersion fixed in cold NBF. Testes from both adult and juvenile mice were kept in NBF for 24 h before routine paraffin embedding and sectioning (5 μm). Archived blocks of W/Wv mouse testes were sectioned and processed in a similar manner [20].
All slides underwent antigen retrieval in 0.01 M citrate buffer (pH, 6.0) during microwaving for 10 min. Slides for light microscopy underwent a previously described protocol [19] for detection of immunostain via biotin signal amplification (PK-6100; Vector Laboratories, www.vectorlabs.com) and 3,3′-diaminobenzidine (DAB) substrate color development. The slides were incubated overnight at 4°C with the primary rabbit anti-CLDN5 polyclonal antibody (34–1600, 1:100; Invitrogen, www.invitrogen.com) and for 1 h at room temperature with the secondary goat anti-rabbit biotinylated polyclonal antibody (E0432, 1:100 with 5% normal goat serum; DAKO, www.dakousa.com). The tissues were counterstained with hematoxylin.
Slides for immunofluorescence were blocked with 10% normal goat serum after the antigen retrieval step. The slides were incubated with the anti-CLDN5 antibody (1:100) overnight at 4°C and were then treated with a secondary goat anti-rabbit Alexa Fluor 488-linked polyclonal antibody (A11034, 1:100 with 5% normal goat serum; Invitrogen). Slides were mounted with an aqueous mounting medium containing 4′,6′-diamidino-2-phenylindole (DAPI) (H-1200; Vector Laboratories).
Immunocytochemistry
Slides were quenched in 0.6% H2O2 in methanol, rinsed in PBS and then 0.1% Tween-20 in Tris-buffered saline (TBST), and then blocked with 10% normal goat serum. CLDN5 antibody (1:50) was applied, and the slides were incubated at 4°C overnight. Then, biotinylated goat anti-rabbit antibody (1:400 with 5% normal goat serum) was applied, followed by biotin signal amplification and color development in DAB solution for 10 min. After rinsing in tap water, the cells were counterstained with hematoxylin.
To confirm nuclear morphology and type of germ cells, DDX4 (VASA) immunocytochemistry (ICC) was performed using a primary rabbit polyclonal antibody at 1:800 (ab13840; Abcam, www.abcam.com) and a secondary goat anti-rabbit biotinylated antibody at 1:400. Sertoli cells were identified by ICC for Wilms tumor 1 transcription factor (WT1) using a primary rabbit polyclonal antibody at 1:400 (sc-192; Santa Cruz Biotechnology, Inc., www.scbt.com) and a secondary goat anti-rabbit biotinylated antibody at 1:400. The ICC procedure for these stains was the same as for the CLDN5 ICC.
CLDN5 antibody specificity was evaluated by preincubating the antibody at 8–10 M excess with the immunogenic peptide (kindly provided by Invitrogen) overnight at 4°C.
Western Blot
Adult mice were euthanized by CO2 inhalation, and the testes were collected and frozen in liquid nitrogen. The testes were homogenized, and protein extracts were collected. Sample aliquots of 10 μl (40 μg) were prepared and frozen at −70°C. Samples and the protein standard (LC5602; Invitrogen) were loaded onto a 15% Tris-HCl gel (161-1157; Bio-Rad Laboratories). After electrophoresis, the proteins were transferred to a polyvinylidene fluoride membrane (162-0177; Bio-Rad Laboratories).
After hydrating in methanol and then deionized H2O, the membrane was blocked with 10% skim milk in Tris-buffered saline (TBS), incubated with CLDN5 antibody (1:250 in 1% skim milk in TBS), rinsed three times in TBST, incubated with goat anti-rabbit horseradish peroxidase-linked polyclonal antibody (1:103 with 1% skim milk, P 0448; DAKO), and then rinsed three times in TBST with a final TBS rinse. All previous blocks, incubations, and rinses were performed on a rocking platform. The membrane was incubated 5 min with the signal substrate (SK-6604; Vector Laboratories), rinsed in 0.1 M Tris (pH, 9.5), wrapped in plastic wrap, and exposed to x-ray film (F-5513; Sigma-Aldrich, www.sigmaaldrich.com) for 1 min.
Quantitative PCR
Testes from 0-, 4-, 8-, 12-, 16-, and 20-day-old and adult (86- to 150-day-old) WT mice and from 8-day-old and adult (86- to 139-day-old) Etv5−/− mice were collected, decapsulated, placed in a stabilizing solution (AM7021; Applied Biosystems Ambion, www.ambion.com), and frozen at −20°C. Total RNA was extracted with a commercial kit (74104; Qiagen, www.qiagen.com). RNA was quantified by a spectrophotometer (NanoDrop ND-1000; Thermo Scientific, www.nanodrop.com), and purity was assessed by the ratio of absorbance at 260 and 280 nm. All samples had ratios from 1.9 to 2.2. First-strand cDNA was synthesized from 1 μg of total RNA using an RT (56575; Invitrogen) and random primers (48190–011; Invitrogen). Real-time quantitative PCR was performed on a sequence detection system (ABI Prism 7000; Applied Biosystems, www.appliedbiosystems.com) using the following three gene expression assays (Applied Biosystems): Mm00727012_s1 (Cldn5), Mm00802636_m1 (Gata6), and Hs99999901_s1 (Rn18s [18s rRNA]). Expression of Cldn5 and Gata6 mRNA in each sample was standardized to 18s rRNA expression using the ΔΔCT method [21].
Cldn5 expression was determined relative to Gata6 mRNA, a marker for Sertoli cells that has been used previously to adjust for the expression of other testicular claudins [22]. This provides an adjustment for changes in RNA due to different numbers of germ cells with age and differences seen in spermatogenic versus aspermatogenic testes.
Developmental expression of Cldn5 among WT mice was analyzed by ANOVA. Cldn5 expression between Etv5−/− and WT mice was analyzed by Student t-test. Significance was set at P < 0.05.
Biotin Tracer
The BTB was assessed in WT (68- to 105-day-old), Etv5−/− (68- to 94-day-old), and W/Wv (72-day-old) (n = 3 each) using a method adopted from that by Meng et al. [23]. Mice were anesthetized i.p. with ketamine (65 mg/kg) and xylazine (6.5 mg/kg). The testes were sequentially exteriorized and injected with 10–20 μl of 7.5 mg/ml of EZ-Link Sulfo-NHS-LC-Biotin (molecular weight, 557 Da) (21335; Thermo Scientific, www.piercenet.com) in freshly diluted PBS containing 1 mM CaCl2 using a 30-gauge needle into the interstitial space. Mice were kept anesthetized for 30 min and then given an overdose of pentobarbital and perfused with cold NBF, and the testes were removed. The testes were left immersed in cold NBF for 24 h and then processed for paraffin embedding. The slides were deparaffinized, rehydrated, rinsed in PBS, and incubated with Alexa Fluor 568-linked streptavidin (S-11226, 1:100; Invitrogen) at room temperature for 1 h. After rinsing in PBS, the slides were mounted with medium containing DAPI.
RESULTS
CLDN5 Protein Expression in Mouse Testis
Adult WT mice.
To evaluate the role of ETV5 as an upstream mediator of CLDN5 expression, it was necessary to confirm the expression of CLDN5 in WT mice. Immunohistochemistry revealed the presence of CLDN5 in the seminiferous epithelium of WT mice (Fig. 1). It was primarily expressed during stage VIII and surrounded the preleptotene and leptotene spermatocytes. Protein staining was greatly reduced in other stages of spermatogenesis but when present was associated with spermatogonia. Additionally, there was positive immunostaining in the vasculature and rete testes epithelia. Vasculature staining was expected, as CLDN5 is primarily identified for its contribution to tight junction permeability in the vascular endothelium [16]. Thus, vasculature staining served as a positive control, indicating that the CLDN5 antibody was recognizing the target peptide sequence.
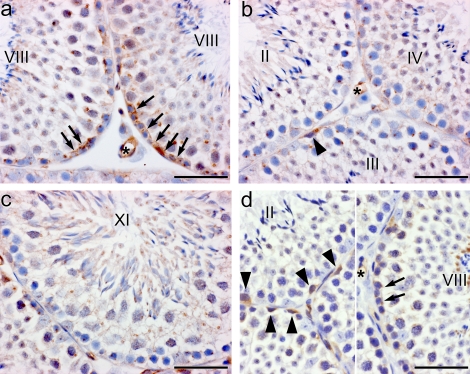
FIG. 1.
a) CLDN5 expression in adult WT mouse testes. CLDN5 expression in the seminiferous epithelium was highest at the level of the BTB during stage VIII (arrows). b, c, and d) Its expression was decreased in other stages but was observed in the cytoplasm of spermatogonia (arrowheads). a, b, and d) CLDN5 was also highly expressed in vascular endothelium. d) Lower antibody concentrations resulted in less background staining, but immunoreaction remained in the vasculature (*), the BTB area of stage VIII (arrows), and in germ cells of other stages (arrowheads). DAB immunostaining with hematoxylin counterstain. Bar = 50 μm.
Adult WT, Etv5−/−, and W/Wv CLDN5 expression.
Once CLDN5 was determined to be expressed in WT seminiferous epithelia, its expression was then evaluated in adult Etv5−/− mice. W/Wv mouse testes that were recipients for WT spermatogonial stem cells were used as controls for potential germ cell effects, as a mixture of aspermic and spermatogenic tubules was present in these samples. CLDN5 expression was absent from the aspermatogenic tubules of Etv5−/− and W/Wv mice (Fig. 2). However, CLDN5 was present at the BTB of W/Wv mice in which spermatogenesis had been established. Thus, germ cells are necessary for CLDN5 protein expression. CLDN5 was also present in the vascular endothelium (Fig. 2d) and rete testes epithelium (Fig. 2e) of all mouse genotypes evaluated.
FIG. 2.
CLDN5 expression in testes from adult (a–e) WT, W/Wv, and Etv5−/− mice and from 8-day-old (f and g) WT and Etv5−/− mice. a) In WT testes, CLDN5 was expressed in the seminiferous epithelial in the BTB region (arrows). The enlargement shows intense staining of preleptotene spermatocytes (arrow), the BTB area (arrowhead), and an endothelium (*). b) Seminiferous tubule from W/Wv spermatogonial stem cell transplant recipient testes shows CLDN 5 staining of the BTB area (arrows), although with less intensity than in WT. c) Aspermatogenic tubules of Etv5−/− testes do not express CLDN5. The enlargement shows staining of the vasculature (*), but the seminiferous epithelium is negative (arrow). d) Seminiferous tubules of the W/Wv mouse testis also show no CLDN5 staining, but the vascular endothelium (*) is strongly positive. e) Rete testes (RT) epithelium is also strongly positive in the W/Wv mouse testis. In a transitional area (Tr) between seminiferous tubule and rete testis, CLDN5 expression is starting to appear between Sertoli cells (arrowheads). Vascular endothelium and rete testis epithelium were positive for CLDN5 in all genotypes examined. f) Wild-type 8-day-old testis showing intense CLDN5 staining surrounding all germ cells (arrows) in all seminiferous tubules. g) Etv5−/− 8-day-old testes show intense staining of the interstitial space but greatly reduced or absent CLDN5 staining surrounding the germ cells (*). Bar = 50 μm.
Eight-day-old WT and Etv5−/− CLDN5 expression.
To evaluate a potential role of ETV5 in controlling CLDN5 expression, 8-day-old mice were examined because germ cells are present in Etv5−/− pups at this age and because previous microarray data [12] had detected a difference in testicular Cldn5 expression between WT and Etv5−/− 8-day-old pups. In 8-day-old WT mice, CLDN5 was present throughout the seminiferous epithelium surrounding germ cells both at the basement membrane and toward the lumen (Fig. 2f). In 8-day-old Etv5−/− mice, CLDN5 expression was absent (Fig. 2g) or greatly reduced in the seminiferous tubules, thus indicating that ETV5 is necessary for optimal CLDN5 expression in the seminiferous epithelium.
Isolated Sertoli cell and germ cell expression of CLDN5.
In adult WT mice, cytoplasm of spermatogonia and preleptotene spermatocytes stained positive for CLDN5 (Fig. 1b). These results were at first presumed to be due to technical difficulties with immunohistochemistry. Various techniques using lower antibody concentrations were evaluated; however, germ cells still appeared to express CLDN5. To differentiate between potential CLDN5 protein diffusion during fixation and actual expression of CLDN5 in germ cells, isolated Sertoli cell and germ cell populations from 8-day-old WT mice were evaluated.
CLDN5 was present in germ cells and Sertoli cells isolated from 8-day-old WT mice (Fig. 3). Staining was variable, with some cells more positive than others within each isolated population. When the antibody was incubated with excess CLDN5 peptide, the cells displayed no immunostain (Fig. 3, d–f). Cells were identified by size, via nuclear characteristics, and with specific staining for germ cells (VASA) and for Sertoli cells (WT1).
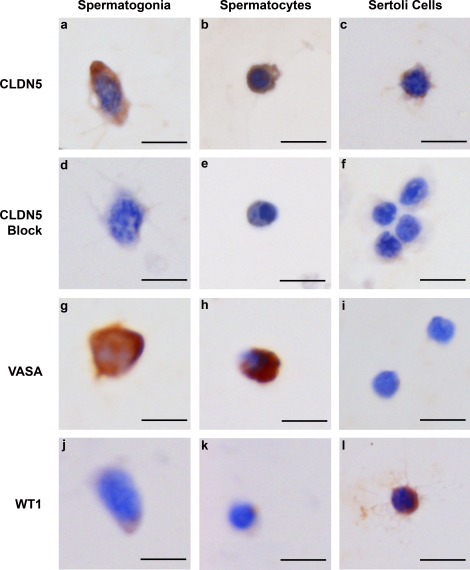
FIG. 3.
CLDN5 expression in germ cells and Sertoli cells isolated from 8-day-old WT mice. CLDN 5 is expressed in spermatogonia (a), preleptotene spermatocytes (b), and Sertoli cells (c). d–f) When the CLDN5 antibody was preincubated with excess immunogenic peptide, all cell types showed no immunostaining. Germ cells were identified by the marker VASA (g and h), which was not present in Sertoli cells (i). Sertoli cells were identified by the marker WT1 (l), which was not present in germ cells (j and k). Bar = 10 μm.
CLDN5 Western blot.
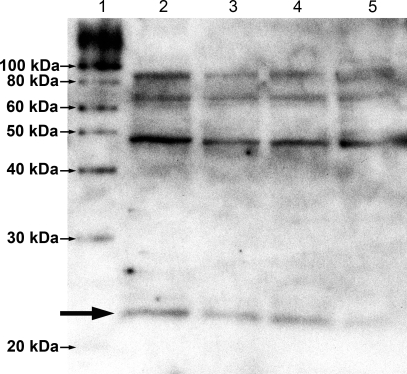
As an additional control for the CLDN5 antibody specificity, Western blot analysis was performed on whole testes extracts. A 23-kDa band was recognized in each extract (Fig. 4). Additional bands were present at about 46, 69, and 92 kDa, which may represent claudin multimers [24, 25].
FIG. 4.
Western blot analysis for CLDN5 from four adult testes extracts (lanes 2–5), with approximately 40 μg of total protein per lane. A 23-kDa band is seen in all testes samples (large arrow). The additional bands at 46, 69, and 92 kDa may represent claudin multimers [24, 25].
Developmental and Adult mRNA Expression
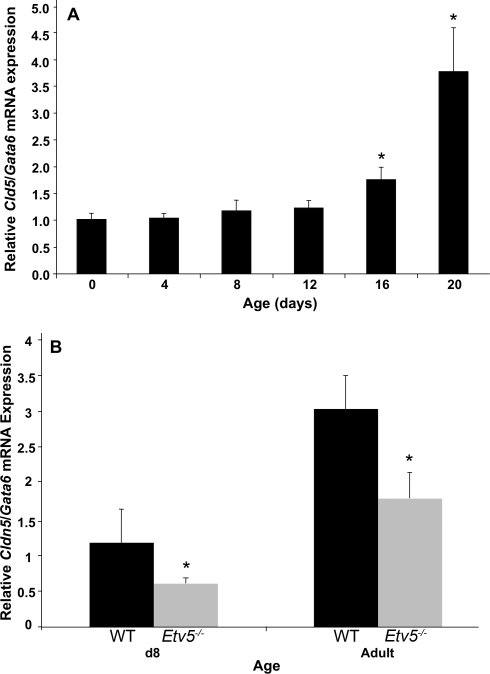
The developmental expression of Cldn5 was evaluated by quantitative PCR. Cldn5 mRNA expression remained constant in WT whole testis from birth through Postnatal Day 12. Starting on Postnatal Day 16 and continuing to Postnatal Day 20, this expression increased, becoming 1.75-fold (P = 0.014) and 3.75-fold (P = 0.038), respectively, over Postnatal Day 0 levels (Fig. 5).
FIG. 5.
Cldn5 mRNA expression (n = 3–4 mice/group). A) Cldn5 expression remained constant from birth through Postnatal Day 12 and then increased at Postnatal Days 16 and 20 (*P < 0.05 compared with Postnatal Day 0). B) Cldn5 expression was decreased in 8-day-old (d8) and adult Etv5−/− mice (*P < 0.05 compared with WT mice at the same age). To adjust for a germ cell dilution effect that occurs with testicular development, Cldn5 expression was determined relative to Gata6, a gene expressed in Sertoli cells but not in germ cells [22].
In Etv5−/− whole testis, Cldn5 expression on Postnatal Day 8 was reduced to 50% of WT (t = 0.021). In the adult Etv5−/−, Cldn5 expression was reduced to 59% of WT whole testis (t = 0.012). In all samples, Cldn5 mRNA was determined relative to the Gata6 signal, which provided an adjustment for changes in germ cell numbers with age and differences seen in spermatogenic versus aspermatogenic tubules.
Biotin Tracer in WT, Etv5−/−, and W/Wv Mice
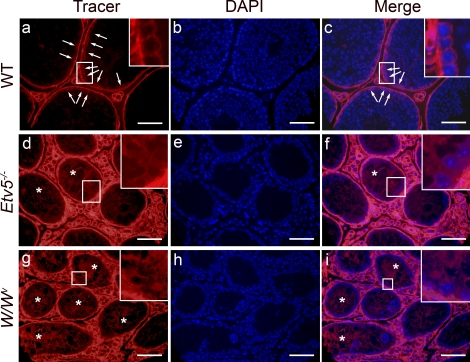
The integrity of the BTB was determined in adult WT, Etv5−/−, and W/Wv mice using a biotin tracer. In WT mice testes, the tracer was restricted to testicular interstitium and the basal compartment of the seminiferous tubules, stopping at the site of the BTB, with no tracer observed in the tubular lumen (Fig. 6). However, in both Etv5−/− and W/Wv mice, tracer was present along the Sertoli cell plasma membranes from the basement membrane to the lumen. In all genotypes, some tubules had more tracer than other seminiferous tubules, most likely due to a difference in distance from the injection sites.
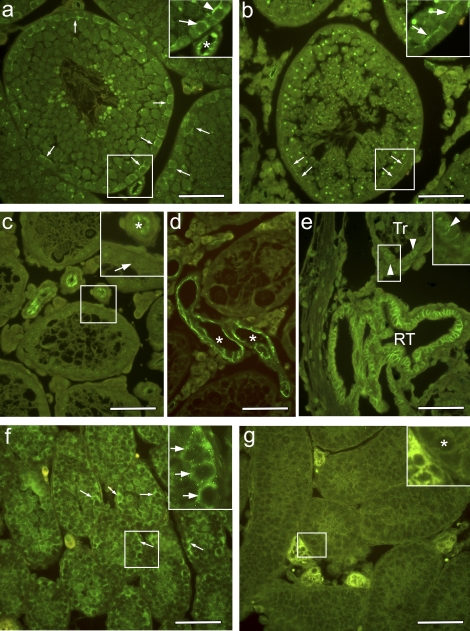
FIG. 6.
Biotin tracer localization in adult WT, Etv5−/−, and W/Wv mice. A biotin tracer was injected into testicular interstitial space of adult mice and was allowed to penetrate the tissue for 30 min. b, e, and h) Cell nuclei are visualized with DAPI stain, and merged images of tracer and DAPI show the penetration of tracer into the seminiferous epithelium and lumen. Enlargements are shown of areas outlined by white boxes. a–c) In WT mice, the tracer stopped at the level of the BTB (arrows). Tracer is seen surrounding only cells lining the basement membrane. d–f) In Etv5−/− mice, tracer is observed surrounding all cells and along strands of Sertoli cell membranes (*) and reaching the tubule lumen. g–i) In W/Wv mice, biotin tracer surrounds all cell types and extends into the lumen along Sertoli cell membranes (*). Bar = 50 μm.
DISCUSSION
To our knowledge, this is the first report of CLDN5 expression in murine seminiferous epithelium. The study revealed the following multifaceted aspects of testicular CLDN5 expression: 1) CLDN5 was expressed in Sertoli cells, spermatogonia, and preleptotene spermatocytes. 2) Seminiferous epithelial CLDN5 expression depended upon both the presence of germ cells and ETV5. 3) CLDN5 expression in testicular vascular endothelium and rete testis epithelium was ETV5 independent. 4) Cldn5 mRNA expression increased in the testes of juvenile mice at the time of BTB formation. 5) Testes of Etv5−/− and W/Wv mice, which were deficient in seminiferous epithelial CLDN5 expression, had biotin tracer leakage from the interstitial space into the seminiferous tubule lumen.
The developmental expression of Cldn5 remained low in whole testis from birth through Postnatal Day 12 in WT mice, but expression by Postnatal Days 16–20 had increased more than 3-fold and remained elevated throughout adulthood. This pattern of expression coincides with development of tight junctions between adjacent Sertoli cells, as the BTB forms between Postnatal Days 16 and 19 in rodents [26], and numerous other tight junctional proteins increase during this time [23, 27, 28].
The expression of CLDN5 in germ cells was unexpected. Claudins are best known as tight junctional proteins that mediate paracellular molecular transport and barrier tightness [13]. Current models of claudin-mediated passage are dependent upon claudin-×-claudin extracellular domain interactions [29]. These claudin-×-claudin extracellular interactions bring the opposing cell membranes into close apposition and obliterate the intermembrane space, thus forming the tight junction [29].
However, germ cells lack tight junctions [1, 30]. Cardiac myocytes are the only other cell type without tight junctions that have been identified as expressing CLDN5 [31]. Thus, the presence of a tight junctional protein in germ cells raises two important questions for future studies: 1) Why are tight junctions not observed in the germ cell ultrastructure [30]? 2) What function would CLDN5 have in germ cells?
The association between CLDN5 expression and formation of tight junction strands is variable. Endogenous CLDN5 was associated with lung endothelial tight junction strands in mice in vivo [14] and in primary cultures of human dermal endothelial cells [32]. However, endogenous CLDN5 in cultured human umbilical vein endothelial cells was unable to form tight junction strands or any other identifiable membrane ultrastructure [33]. Mouse CLDN5 transfection into mouse l-fibroblasts [14] or human embryonal kidney (HEK 292) neuronal cells [34] formed well-developed networks of discontinuous strands. Human CLDN5 transfection into mouse NIH/3T3 fibroblast cells formed strands of particles and arrays that are similar in appearance to gap junction ultrastructure [25]. Thus, variability of CLDN5 tight junction morphology among these models makes it difficult to predict which, if any, of the germ cell membrane ultrastructures are associated with CLDN5.
CLDN5 and other claudins have been detected at non-tight junctional locations in other epithelial cells along the basolateral membrane [35–37]. Their function at these locations is unknown. One hypothesis suggests that claudins may aid in cellular adhesion in these locations [35]. Indeed, fibroblasts transfected with claudin 1, claudin 2, or claudin 3 reaggregate after dissociation, a functional measure of cellular adhesiveness [38]. However, claudin-claudin adhesive strength is weaker than that resulting from adhesive junctional transmembrane molecules such as cadherins [38, 39]. Assuming poor adhesive qualities are a claudin-wide trait, it is doubtful that germ cell CLDN5 would significantly contribute to germ cell adhesion to Sertoli cells, especially as spermatogonia [40] and preleptotene spermatocytes [41] contain multiple adhesion molecules.
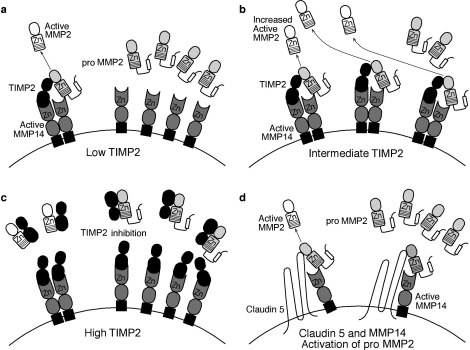
A potential function for germ cell CLDN5 may be mediated via its ability to assist membrane-type (MT) matrix metalloproteinases (MMPs) in activating soluble-type MMP2 [42]. Current models of MMP2 activation describe an intricate balance between the expression levels of pro-MMP2, MMP14 (MT1-MMP), and the soluble tissue inhibitor of MMPs (TIMP2) and the subsequent activation of pro-MMP2 (Fig. 7) [43, 44]. In these models, TIMP2 is a necessary component to activate pro-MMP2; TIMP2 acts as a bridge between pro-MMP2 and MMP14, thus allowing an adjacent unoccupied MMP14 to activate pro-MMP2 to MMP2. CLDN5 and MMP14 can function together independent of TIMP2 to activate MMP2 [42]. It was proposed that the CLDN5 extracellular loops act as the bridge and connect pro-MMP2 to the active site of MMP14. This model for CLDN5 function in spermatogonia and preleptotene spermatocytes is dependent upon the basal compartment expression of MMP2 and MMP14. While Mmp2 and Mmp14 mRNA are strongly expressed in mouse testis [45], the cellular distribution of the enzymes is not known.
FIG. 7.
Proposed model of metalloproteinase activation as a role for CLDN5 in germ cells. The activation of pro-MMP2 under conditions of low (a), optimal (b), and high (c) levels of soluble TIMP2 are illustrated. a) At low levels, there are not enough TIMP2 bridges formed, resulting in low activation of pro-MMP2. b) At intermediate levels, TIMP2 acts as a bridge between pro-MMP2 and MMP14, thus allowing MMP14 to activate pro-MMP2 to MMP2. c) At high TIMP2 levels, all of the active sites on MMP14 are occupied, thus inhibiting the activation of pro-MMP2 to MMP2. d) Data by Miyamori et al. [42] support the hypothesis of claudins and MMP14 activation of pro-MMP2 independent of TIMP2. Claudins are proposed to act as the bridge that links pro-MMP2 to MMP14 to allow activation. Both of claudin's extracellular loops, particularly the first, appear to be involved in this process. Such a mechanism could account for the delicate balance between the germ cells' regulation of their migration through and the Sertoli cells' maintenance of the tight junctional complexes. Adapted with permission from Macmillan Publishers Ltd. [43], with information by Miyamori et al. [42]. Zn, Zinc (2+) ion.
The aforementioned model describes a role for germ cell CLDN5 at the cell membrane; however, CLDN5 was also expressed diffusely throughout the cytoplasm and concentrated in perinuclear spots in spermatogonia and spermatocytes. It is unknown what organelle or other ultrastructure feature is represented by the perinuclear spots. Whether these cytoplasmic forms of CLDN5 have a functional role, represent storage depots of CLDN5, or indicate areas of CLDN5 degradation and recycling remain to be investigated.
While a germ cell role for CLDN5 is unknown, Sertoli cell CLDN5 appears to modulate BTB integrity. Both Etv5−/− and W/Wv mice lacked seminiferous epithelial CLDN5 and were vulnerable to tracer penetration across the BTB. The hypothesis that CLDN5 has a role in BTB formation is also supported by its immunohistochemical expression over the luminal side of preleptotene spermatocytes in germ cells transplanted into W/Wv testes. Preleptotene spermatocytes are associated with stage VIII seminiferous tubules and the BTB [46] and with other BTB proteins [23, 27, 28]. Cldn5 mRNA expression also increased between Postnatal Days 16 and 20, a period when the BTB is formed [26], representing a pattern that is observed with other BTB proteins [23, 27, 28]. It should be noted that the Gata6 adjustment normalized expression of Cldn5 mRNA at the level of the seminiferous tubule and not at the level of the Sertoli cell. Therefore, increases in Cldn5 expression in the germ cell compartment may also have contributed to the increased Cldn5 expression between Postnatal Days 16 and 20.
CLDN5 protein expression was dramatically increased in stage VIII of the seminiferous cycle, the stage during which preleptotene spermatocytes start to migrate across the BTB [47]. CLDN3 expression also peaks in stage VIII [23], thus permitting possible CLDN3 and CLDN5 trans-interactions between adjacent Sertoli cells. CLDN3 and CLDN5 trans-interactions can dramatically change tight junction permeability [25]. The coexpression of CLDN3 and CLDN5 at the BTB during spermatocyte migration strongly suggests a potential role for CLDN3-×-CLDN5 interactions in maintaining BTB integrity during this time of dynamic junctional restructuring. It is noteworthy that mice lacking Sertoli cell CLDN3 protein also have BTB leakiness [23].
This study revealed two aspects of CLDN5 regulation. The first aspect of CLDN5 regulation is that germ cells are needed for seminiferous epithelial CLDN5 expression, as seminiferous tubular expression of CLDN5 was absent in both aspermic Etv5−/− and W/Wv mice testes. However, in W/Wv tubules showing limited spermatogenesis, CLDN5 was expressed at the BTB, which demonstrates not only that germ cells are necessary for Sertoli cell CLDN5 expression but also that lack of CLDN5 at tight junctions between Sertoli cells may mediate W/Wv BTB leakiness. The barrier effectiveness is not simply due to the physical presence of Sertoli cell-germ cell contacts, as tracers surrounded the germ cells up to the level of the barrier when introduced either through the vasculature or through the tubule lumen [48]. Rather, germ cells signal the Sertoli cells, with resultant tight junction modulation between Sertoli cells, ectoplasmic specialization, or both [1].
The second aspect of CLDN5 regulation is its ETV5 dependence for optimal expression in seminiferous epithelium but its ETV5 independence in vascular endothelium. CLDN5 expression was absent from Etv5−/− seminiferous tubules in 8-day-old pups even in the presence of germ cells, yet there was intense staining in the vasculature. In contrast, 8-day-old WT tubules showed intense staining for CLDN5 surrounding all the germ cells. The decreased Cldn5 mRNA expression in 8-day-old and adult Etv5−/− mice supports the conclusion that ETV5 is required for CLDN5 expression in the seminiferous epithelium.
To our knowledge, this is the first study to detect CLDN5 in seminiferous tubules. A major difference between this study and prior studies [14, 16] was the fixation method. Prior studies used cryopreservation, followed by alcohol and acetone fixation. In this study, testes were perfusion fixed with cold NBF and embedded in paraffin. It is well known that tissue fixation is a major determinant for how well a particular protein retains its antigen expression in processed tissues [49]. There also appears to be a cell type-×-fixation method interaction for the detection of CLDN5, as CLDN5 was expressed in vascular endothelium with both fixation methods but was only observed in Sertoli cells with NBF fixation.
In addition to fixation, the antibody used in immunohistochemistry can affect results [49]. Although the antibodies in this and the other studies [14, 16] were from different sources, they were all rabbit polyclonal antibodies from rabbits immunized with peptides from the C-terminal end of CLDN5. Therefore, similar spectra of specificity among these antibodies may be expected. Regarding the antibody used in this study, localization of the immunostain in the region of the BTB and recognition of a 23-kDa band on Western blot imply that it recognizes a claudin protein. Decreased Cldn5 mRNA expression in Etv5−/− mice and expression of immunosignal in vascular endothelium are consistent with CLDN5 specificity of the antibody used.
In conclusion, this study revealed new and unique factors of murine testicular CLDN5 expression. CLDN5 was found in seminiferous epithelial cells, including spermatogonia and preleptotene spermatocytes, germ cells that do not form tight junctions, as well as in Sertoli cells. Although a function for its presence in germ cells is not known, CLDN5 contributes to BTB function in Sertoli cells. Seminiferous epithelial expression was dependent upon the presence of both germ cells and ETV5. However, vascular endothelial CLDN5 expression was ETV5 independent.
Acknowledgments
This article represents a portion of a thesis submitted by Dr. Morrow to the University of Illinois at Urbana-Champaign Graduate College as partial fulfillment of the requirements for a PhD.
Footnotes
Supported by the Billie A. Field Endowment, University of Illinois (P.S.C.), CICCR, a program of CONRAD, Eastern Virginia Medical School (R.A.H.), NIEHS training grant T32 ES07326 (C.M.K.M.), and the NIH Contraception and Infertility Loan Repayment Program (C.M.K.M.). The work at the University of Illinois at Urbana-Champaign was conducted in a facility constructed with support from Research Facilities Improvement Program grant number C06 RR16515 from the NIH National Center for Research Resources.
REFERENCES
- Mruk DD, Cheng CY.Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25: 747–806.. [DOI] [PubMed] [Google Scholar]
- Onoda M, Suarez-Quian CA, Djakiew D, Dym M.Characterization of Sertoli cells cultured in the bicameral chamber system: relationship between formation of permeability barriers and polarized secretion of transferrin. Biol Reprod 1990; 43: 672–683.. [DOI] [PubMed] [Google Scholar]
- Fijak M, Meinhardt A.The testis in immune privilege. Immunol Rev 2006; 213: 66–81.. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S.Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 2000; 11: 4131–4142.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA.CNS myelin and Sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 1999; 99: 649–659.. [DOI] [PubMed] [Google Scholar]
- de Launoit Y, Baert JL, Chotteau-Lelievre A, Monte D, Coutte L, Mauen S, Firlej V, Degerny C, Verreman K.The Ets transcription factors of the PEA3 group: transcriptional regulators in metastasis. Biochim Biophys Acta 2006; 1766: 79–87.. [DOI] [PubMed] [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, Cheng AM, Hassell JA, et al. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature 2005; 436: 1030–1034.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Brinster RL.Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem 2007; 282: 25842–25851.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesser HN, Simon L, Hofmann MC, Murphy KM, Murphy T, Hess RA, Cooke PS.Effects of ETV5 (ets variant gene 5) on testis and body growth, time course of spermatogonial stem cell loss, and fertility in mice. Biol Reprod 2008; 78: 483–489.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C, Hostetler C, Griswold M, Hofmann MC, Murphy K, Cooke PS, Hess RA.ETV5 is required for continuous spermatogenesis in adult mice and may mediate blood-testes barrier function and testicular immune privilege. Ann N Y Acad Sci 2007; 1120: 144–151.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreira GG, Ogawa T, Avarbock MR, Franca LR, Brinster RL, Russell LD.Development of germ cell transplants in mice. Biol Reprod 1998; 59: 1360–1370.. [DOI] [PubMed] [Google Scholar]
- Tyagi G. Mechanism of spermatogonial stem cell regulation by ETV5. Urbana:: University of Illinois at Urbana-Champaign;; 2009. Dissertation. [Google Scholar]
- Koval M.Claudins: key pieces in the tight junction puzzle. Cell Commun Adhes 2006; 13: 127–138.. [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furpuse M, Tsukita S.Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 1999; 147: 185–194.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S.Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161: 653–660.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura Y, Chiba H, Utsumi H, Gotoh T, Tobioka H, Sawada N.Barrier function of microvessels and roles of glial cell line-derived neurotrophic factor in the rat testis. Med Electron Microsc 2002; 35: 139–145.. [DOI] [PubMed] [Google Scholar]
- Dym M, Jia MC, Dirami G, Price JM, Rabin SJ, Mocchetti I, Ravindranath N.Expression of c-kit receptor and its autophosphorylation in immature rat type A spermatogonia. Biol Reprod 1995; 52: 8–19.. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC.Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev Biol 2007; 304: 34–45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie R, Zhou Q, Jassim E, Saunders PT, Hess RA.Differential expression of estrogen receptors alpha and beta in the reproductive tracts of adult male dogs and cats. Biol Reprod 2002; 66: 1161–1168.. [DOI] [PubMed] [Google Scholar]
- Tyagi G, Carnes K, Morrow C, Kostereva N, Ekman GC, Meling DD, Hostetler C, Griswold M, Murphy KM, Hess RA, Hofmann MC, Cooke PS.Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis. Biol Reprod 2009; 81: 258–266.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD.Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–408.. [DOI] [PubMed] [Google Scholar]
- Florin A, Maire M, Bozec A, Hellani A, Chater S, Bars R, Chuzel F, Benahmed M.Androgens and postmeiotic germ cells regulate claudin-11 expression in rat Sertoli cells. Endocrinology 2005; 146: 1532–1540.. [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE.Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A 2005; 102: 16696–16700.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasig IE, Winkler L, Lassowski B, Mueller SL, Zuleger N, Krause E, Krause G, Gast K, Kolbe M, Piontek J.On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci 2006; 63: 505–514.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG.Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol 2003; 285: L1166–L1178.. [DOI] [PubMed] [Google Scholar]
- Vitale R, Fawcett DW, Dym M.The normal development of the blood-testis barrier and the effects of clomiphene and estrogen treatment. Anat Rec 1973; 176: 331–344.. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Hermo L, Egenberger N, Mertineit C, Trasler JM, Laird DW.Cellular immunolocalization of occludin during embryonic and postnatal development of the mouse testis and epididymis. Endocrinology 1999; 140: 3815–3825.. [DOI] [PubMed] [Google Scholar]
- Hellani A, Ji J, Mauduit C, Deschildre C, Tabone E, Benahmed M.Developmental and hormonal regulation of the expression of oligodendrocyte-specific protein/claudin 11 in mouse testis. Endocrinology 2000; 141: 3012–3019.. [DOI] [PubMed] [Google Scholar]
- Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE.Structure and function of claudins. Biochim Biophys Acta 2008; 1778: 631–645.. [DOI] [PubMed] [Google Scholar]
- Russell LD, Peterson RN.Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 1985; 94: 177–211.. [DOI] [PubMed] [Google Scholar]
- Sanford JL, Edwards JD, Mays TA, Gong B, Merriam AP, Rafael-Fortney JA.Claudin-5 localizes to the lateral membranes of cardiomyocytes and is altered in utrophin/dystrophin-deficient cardiomyopathic mice. J Mol Cell Cardiol 2005; 38: 323–332.. [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse K, Furuse M, Tsukita S, Miyachi Y.Expression of claudin-5 in dermal vascular endothelia. Exp Dermatol 2003; 12: 289–295.. [DOI] [PubMed] [Google Scholar]
- Fontijn RD, Rohlena J, van Marle J, Pannekoek H, Horrevoets AJ.Limited contribution of claudin-5-dependent tight junction strands to endothelial barrier function. Eur J Cell Biol 2006; 85: 1131–1144.. [DOI] [PubMed] [Google Scholar]
- Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE.Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 2008; 22: 146–158.. [DOI] [PubMed] [Google Scholar]
- Gregory M, Dufresne J, Hermo L, Cyr D.Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology 2001; 142: 854–863.. [DOI] [PubMed] [Google Scholar]
- Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S.Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 2002; 13: 875–886.. [DOI] [PubMed] [Google Scholar]
- Rahner C, Mitic LL, Anderson JM.Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 2001; 120: 411–422.. [DOI] [PubMed] [Google Scholar]
- Kubota K, Furuse M, Sasaki H, Sonoda N, Fujita K, Nagafuchi A, Tsukita S.Ca(2+)-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Curr Biol 1999; 9: 1035–1038.. [DOI] [PubMed] [Google Scholar]
- Lim TS, Vedula SR, Kausalya PJ, Hunziker W, Lim CT.Single-molecular-level study of claudin-1-mediated adhesion. Langmuir 2008; 24: 490–495.. [DOI] [PubMed] [Google Scholar]
- Tokuda M, Kadokawa Y, Kurahashi H, Marunouchi T.CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod 2007; 76: 130–141.. [DOI] [PubMed] [Google Scholar]
- Wang CQ, Cheng CY.A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J Cell Biol 2007; 178: 549–556.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamori H, Takino T, Kobayashi Y, Tokai H, Itoh Y, Seiki M, Sato H.Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J Biol Chem 2001; 276: 28204–28211.. [DOI] [PubMed] [Google Scholar]
- Lu KV, Jong KA, Rajasekaran AK, Cloughesy TF, Mischel PS.Upregulation of tissue inhibitor of metalloproteinases (TIMP)-2 promotes matrix metalloproteinase (MMP)-2 activation and cell invasion in a human glioblastoma cell line. Lab Invest 2004; 84: 8–20.. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z.How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001; 17: 463–516.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR.Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett 2004; 563: 129–134.. [DOI] [PubMed] [Google Scholar]
- Dym M, Fawcett DW.The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 1970; 3: 308–326.. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Hikim APS, Clegg ED.Mammalian spermatogenesis. Russell LD.Histological and Histopathological Evaluation of the Testis Clearwater, FL:Cache River Press;1990: 1–40.. [Google Scholar]
- Cavicchia JC, Sacerdote FL.Topography of the rat blood-testis barrier after intratubular administration of intercellular tracers. Tissue Cell 1988; 20: 577–586.. [DOI] [PubMed] [Google Scholar]
- Ramos-Vara JA.Technical aspects of immunohistochemistry. Vet Pathol 2005; 42: 405–426.. [DOI] [PubMed] [Google Scholar]