Abstract
Inhibition of vascular endothelial growth factor A (VEGFA) signal transduction arrests vascular and follicle development. Because antiangiogenic VEGFA isoforms are proposed to block proangiogenic VEGFA isoforms from binding to their receptors, we hypothesized that proangiogenic isoforms promote and antiangiogenic isoforms inhibit these processes. The antiangiogenic isoforms Vegfa_165b and Vegfa_189b were amplified and sequenced from rat ovaries. The Vegfa_165b sequence was 90% homologous to human VEGFA_165B. Quantitative RT-PCR determined that Vegfa_165b mRNA was more abundant around Embryonic Day 18, but Vegfa_189b lacked a distinct pattern of abundance. Antiangiogenic VEGFA isoforms were localized to pregranulosa and granulosa cells of all follicle stages and to theca cells of advanced-stage follicles. To determine the effects of VEGFA isoforms in developing ovaries, Postnatal Day 3/4 rat ovaries were cultured with VEGFA_164 or an antibody to antiangiogenic isoforms (anti-VEGFAxxxB). Treatment with 50 ng/ml of VEGFA_164 resulted in a 93% increase in vascular density (P < 0.01), and treated ovaries were composed of fewer primordial follicles (stage 0) and more developing follicles (stages 1–4) than controls (P < 0.04). Ovaries treated with 5 ng/ml of VEGFAxxxB antibody had a 93% increase in vascular density (P < 0.02), with fewer primordial and early primary follicles (stage 1) and more primary, transitional, and secondary follicles (stages 2, 3, and 4, respectively) compared with controls (P < 0.005). We conclude that neutralization of antiangiogenic VEGFA isoforms may be a more effective mechanism of enhancing vascular and follicular development in perinatal rat ovaries than treatment with the proangiogenic isoform VEGFA_164.
Keywords: follicle, ovary, perinatal rat, vascular development, VEGF
Proangiogenic and antiangiogenic VEGFA isoforms are involved in both vascular development and follicle activation in the perinatal rat ovary.
INTRODUCTION
During late embryonic development, oocytes are organized in large clusters called oocyte cysts. After birth, oocyte cysts begin to develop into individual follicles known as primordial follicles. A primordial follicle contains an oocyte surrounded by a single layer of squamous pregranulosa cells and is considered the first stage of development in follicle progression [1–4]. By Postnatal Day 3/4 (P3/4) in the rat, 90% of the ovary consists of primordial follicles [2]. Only a small portion of primordial follicles will develop into a preovulatory follicle, while the majority of follicles will undergo atresia [5, 6]. Although much has been elucidated about factors involved in folliculogenesis, there is still a great deal to be determined. Vascular endothelial growth factor A (VEGFA) has been shown to be upregulated during the primordial to primary follicle transition [7]. VEGFA is a potent mitogen that is highly involved in angiogenesis, the formation and differentiation of blood vessels. It has also been shown that follicular development is accelerated in cycling female rats after administration of VEGFA and that injection of a VEGFA antibody into the ovary decreases follicular angiogenesis and completely inhibits ovulation [8].
The Vegfa gene is composed of eight exons and produces different mRNA splice variants. These splice variants are translated into VEGFA protein isoforms with different numbers of amino acids. The predominant isoforms expressed in most tissues throughout the body are VEGFA_188, VEGFA_164, and VEGFA_120 [9], and VEGFA_164 is the predominant isoform involved in the recruitment of endothelial cells and the formation of major blood vessels [10, 11]. The human VEGFA protein contains an additional amino acid residue on each isoform compared with the rodent; thus, the human VEGFA_165 isoform is homologous to the rodent VEGFA_164 isoform.
Until recently, all VEGFA isoforms were thought to be proangiogenic; however, antiangiogenic splice variants to VEGFA have been identified [12]. VEGFA_165B, the newly identified human antiangiogenic isoform, is formed by differential splicing from the end of exon 7 into what was thought to be the 3′ untranslated region of the gene. This region has been identified as exon 8b. Isoforms that contain exon 8b instead of exon 8a are antiangiogenic isoforms. It has been proposed that there is a proximal splicing site that allows for production of proangiogenic isoforms and a distal splicing site that results in antiangiogenic isoforms [13]. In addition to the VEGFA_165B isoform, it has been proposed that every proangiogenic isoform has a corresponding antiangiogenic isoform in which exon 8a has been substituted by exon 8b [14].
Previous studies [14] have shown that VEGFA_165B can bind kinase insert domain protein receptor (KDR, also known as VEGFR2 and FLK1) with the same affinity as VEGFA_165; however, it is incapable of activating or stimulating downstream signaling pathways. Furthermore, antiangiogenic VEGFA isoforms are downregulated in renal and prostate tumors, potentially allowing for enhanced tumor metastasis [14]. A possible mechanism for the antiangiogenic effects of the antiangiogenic isoforms is blocking the proangiogenic isoforms from binding to their receptors, FMS-like tyrosine kinase 1 (FLT1, also known as VEGFR1) and KDR.
Previous experiments in our laboratory demonstrated a novel role for VEGFA in the recruitment of primordial follicles into the growing follicle pool, as well as a potential survival factor for primary and later-stage follicles through vascular-dependent and vascular-independent mechanisms [15]. Our objective for the present study was to further investigate the role of VEGFA on vascular development and follicle progression in the perinatal rat ovary. We hypothesized that treatment with a recombinant VEGFA_164 or neutralization of antiangiogenic VEGFA isoforms via treatment with a VEGFAxxxB antibody would promote vascular and early follicular development.
MATERIALS AND METHODS
Animals
Embryonic, postnatal, and adult ovaries were obtained from our Sprague-Dawley rat colony at the University of Nebraska-Lincoln Department of Animal Science, with founders purchased from Charles River (Wilmington, MA). Ovaries were dissected from Embryonic Day 13 (E13) to P10 rats to evaluate ovaries across the following important developmental stages: the formation of oocyte cysts, the formation of primordial follicles, and the initiation of follicular activation and development. Embryonic age was calculated from days after coitus. Postnatal age was determined using day of birth as P0. All animal procedures were approved by the University of Nebraska Animal Care and Use Committee.
Vegfa Antiangiogenic Isoforms RT-PCR and Quantitative RT-PCR
Total RNA from ovaries at different ages was isolated and converted to cDNA for subsequent RT-PCR according to previously reported methods [15]. The forward primer utilized for Vegfa antiangiogenic isoform conventional RT-PCR was previously used for Vegfa proangiogenic isoform RT-PCR in our laboratory [15]. The reverse primer (Table 1) was designed using the PrimerQuest primer design program (Integrated DNA Technologies, Coralville, IA). These primers were used with an annealing temperature of 54.5°C for 35 cycles to generate products of 220 base pair (bp) for Vegfa_165b and 292 bp for Vegfa_189b. Gapdh is a constitutively expressed gene and was used as a control for RNA isolation and amplification [16], and previously reported protocols [15] were utilized to produce a 460-bp product. All PCR products were subcloned and confirmed using restriction digest analysis. The PCR products were subcloned into pCRII (Invitrogen, San Diego, CA) using the TOPO TA Cloning kit (Invitrogen) and were sequenced with primers for the T7 promoter region (data not shown). The RT-PCR was conducted on three to five different samples for each time point. Quantitative RT-PCR (QRT-PCR) primers were designed using Primer Express 1.5 (software that accompanied the 7700 Prism sequence detector; Applied Biosystems, Foster City, CA) for rat Vegfa_165b and Vegfa_189b (Table 1). Fluorescent probe (Table 1) was obtained from Integrated DNA Technologies. Procedures and analyses for the antiangiogenic Vegfa QRT-PCR were performed as previously described [15]. At least three different pools of tissue from each age group were utilized to obtain these data.
TABLE 1.
Conventional and quantitative RT-PCR primers and probe sequences.
Embedding, Histology, and Immunohistochemistry
Ovaries were histologically prepared, and immunohistochemistry was performed according to standard protocols in our laboratory [15]. The primary antibody was a mouse polyclonal IgG1 raised against a peptide corresponding to the 9-amino acid C-terminus of human VEGFA_165B (Abcam, Cambridge, MA). This antibody has been characterized previously to bind VEGF_189B, VEGF_183B, VEGF_165B, VEGF_145B, and VEGF_121B [14, 17, 18]. Therefore, this antibody is collectively known as VEGFxxxB but is sold commercially (R&D Systems, Minneapolis, MN; and Abcam) as a VEGF_165B antibody. In other experiments in our laboratory, this VEGFAxxxB antibody has been shown to bind rat VEGFA_165B, VEGFA_189B, and VEGFA_121B via Western blot analysis in gonadal tissue (Baltes-Breitwisch et al, unpublished results). As a negative control, serial sections were processed without primary antibody. The biotinylated goat anti-mouse secondary antibodies were diluted 1:300. The secondary antibody was detected with aminoethyl carbazole chromagen substrate solution (ZYMED Laboratories, San Francisco, CA). Immunohistochemistry was performed on at least three different sections of tissue from each age group.
Organ Cultures
Whole ovaries from P3/4 rats were cultured for 14 days in accord with previously reported methods [15]. One ovary from each animal was designated as a control, while its pair was treated with 50 ng/ml of recombinant VEGFA_164 (R&D Systems), 5 ng/ml of VEGFAxxxB antibody, or 50 ng/ml of VEGFAxxxB antibody (the same antibody utilized for immunohistochemistry; Abcam). Doses of VEGFA_164 and VEGFAxxxB antibody (both diluted in PBS with 0.1% bovine serum albumin [BSA]) were added directly to the culture medium of the treated wells at the start of culture, and treatment was repeated daily. Similar doses of PBS with 0.1% BSA were added to the paired control wells for the VEGFA_164 cultures, and nonspecific IgG in PBS with 0.1% BSA was added to the paired control wells for the VEGFAxxxB antibody cultures. We cultured P3/4 ovaries because ovaries from rats of this age consist predominantly of primordial follicles [2] and because similar ovarian organ culture procedures have demonstrated that primordial follicles can spontaneously initiate development to early primary follicles [5].
Imaging and Area Analysis of Organ Cultures
After culture treatment, ovaries were imaged to obtain individual ovary areas as previously described [15]. The mean area of each control ovary was set to 100%, and the mean area of each treated ovary was calculated as a percentage of its paired control. In total, 12 (VEGFA_164 and 5 ng/ml of VEGFAxxxB antibody) and 15 (50 ng/ml of VEGFAxxxB antibody) ovary pairs were imaged for area measurements.
Whole-Mount Immunohistochemistry of Organ Cultures and Vascular Density Quantification
After imaging, ovaries were fixed in Bouins solution and paraffin embedded for histology (described herein) or fixed in 4% paraformaldehyde for whole-mount immunohistochemistry. Whole-mount immunohistochemistry for platelet endothelial cell adhesion molecule (PECAM1) utilized a mouse monoclonal IgG1 primary antibody raised against rat platelet endothelial cell adhesion molecule (BD Pharmingen, San Jose, CA) and followed the same procedures used for our previously reported V1 ovary culture experiments [15]. Confocal microscopy was performed, and red channel images were used to analyze the vascular density of control and treated organ culture ovaries as previously described for our V1 ovary culture experiments [15]. The mean vascular density of each control ovary was set to 100%, and the mean vascular density of each treated ovary was calculated as a percentage of its paired control. In total, 23 (VEGFA_164), 11 (5 ng/ml of VEGFAxxxB antibody), and 15 (50 ng/ml of VEGFAxxxB antibody) ovary pairs were analyzed for vascular density.
Follicle Staging and Quantification of Organ Cultures
Organ culture ovaries not used for whole-mount immunohistochemistry were histologically prepared and stained with hematoxylin-eosin as previously described [15]. With bright-field microscopy, follicles from each ovary were classified as stages 0–4. A stage 0 primordial follicle consists of an oocyte surrounded by a single layer of squamous pregranulosa cells. A stage 1 early primary follicle consists of an oocyte surrounded by a single layer of cells composed of a combination of pregranulosa and granulosa cells. A stage 2 primary follicle consists of an oocyte surrounded by a single layer of cuboidal granulosa cells. A stage 3 transitional follicle is developing a second layer of granulosa cells, while a stage 4 follicle has theca cells beginning to organize around the granulosa cell layers [5]. Follicles were staged and counted based upon previously reported methods [15]. Specifically for these experiments, three ovaries were imaged at 200× magnification, and the three middle histology sections were evaluated for each ovary. The mean number of follicles per area in each stage of development was statistically analyzed between control and treated ovaries. Additionally, the mean number of follicles from each treated ovary was expressed as a percentage of its paired control, and the percentages from all ovary pairs were analyzed. The mean number of each follicle stage was also expressed as a percentage of the total number of follicles from each area. These percentages were statistically analyzed between control and treated ovaries. Three ovary pairs were utilized for follicle quantification.
Statistical Analysis
All data were analyzed by one-way ANOVA using JMP software (SAS Institute, Cary, NC). Student t-test was used to compare the mean normalized QRT-PCR values between different developmental ages. Student t-test and Dunnett test were used to compare ovarian area, vascular density, and follicle counts between control and treated organs. Differences in data were considered statistically significant at P < 0.05 unless otherwise stated.
RESULTS
Vegfa Antiangiogenic Isoforms mRNA Expression During Ovarian Development
Through conventional RT-PCR and subcloning, our laboratory confirmed the presence of mRNA from the following two Vegfa antiangiogenic isoforms: Vegfa_165b (GenBank accession number EU040284.1 [Supplemental Fig. S1A available at www.biolreprod.org]) and Vegfa_189b (GenBank accession number EU040285.1 [Supplemental Fig. S1B]). A partial 3′ untranslated region was determined from the Rattus norvegicus vascular endothelial growth factor gene 3′ untranslated region (GenBank accession number U22372.1.) in developing and adult rat ovaries. We utilized these mRNA sequences to predict the protein sequence for rat VEGFA_165B (Supplemental Fig. S2A) and VEGFA_189B (Supplemental Fig. S2B). Notably, these protein sequences are 1 amino acid longer than rat VEGFA_164 (GenBank accession number AAI68708.1 [Supplemental Fig. S2A]) and VEGFA_188 (GenBank accession number AAF19211.1 [Supplemental Fig. S2B]). The rat Vegfa_165b mRNA sequence is 90% homologous to human VEGFA_165B (GenBank accession number AF430806.1 [Supplemental Fig. S3A]), and the predicted rat VEGFA_165B protein sequence is 88% homologous to human VEGFA_165B (GenBank accession number AAL27435.1 [Supplemental Fig. S3B]).
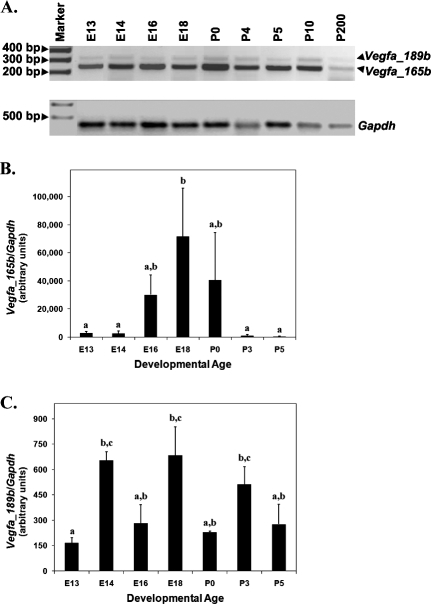
Conventional RT-PCR was used to evaluate Vegfa antiangiogenic isoform mRNA expression in developing and adult rat ovaries. Eight developmental time points (E13, E14, E16, E18, P0, P4, P5, and P10) and one adult time point (P200) were evaluated. Messenger RNA from both Vegfa_165b and Vegfa_189 was detected at all time points evaluated (Fig. 1A).
FIG. 1.
A) Conventional RT-PCR for Vegfa antiangiogenic isoforms from E13 to P10 developing ovaries and P200 adult ovaries. Gapdh served as a control for RNA isolation and amplification. Negative control samples (without template) did not produce a band (data not shown). B and C) Quantitative RT-PCR to detect Vegfa_165b and Vegfa_189b from E13 to P5 of ovarian development. Gapdh was used as an endogenous control to account for differences in starting material. These data are the result of at least three different pools of tissue from each age group. The mean ± SEM normalized values are presented, and different letters represent a statistically significant difference in means (P < 0.05).
Quantitative RT-PCR demonstrated differential abundance of Vegfa_165b and Vegfa_189b during prenatal and perinatal development of the ovary (Fig. 1, B and C). Specifically, Vegfa_165b levels increased from E13/14 to E18, but levels decreased again by P3/5 (Fig. 1B) (P < 0.05). Although a distinct Vegfa_189b mRNA pattern was not present, increased levels were detected at E14, E18, and P3 compared with E13 (Fig. 1C) (P < 0.05).
Localization of VEGFA Antiangiogenic Isoforms Within Developing Ovaries
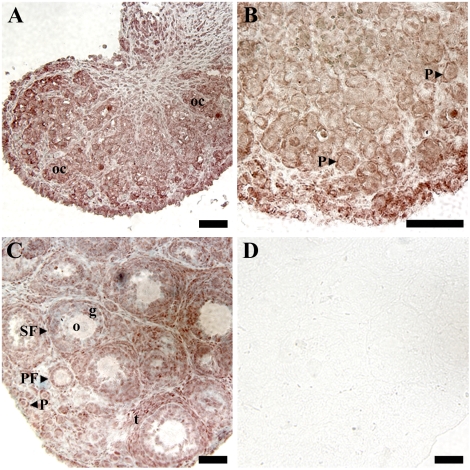
After confirmation of Vegfa_165b and Vegfa_189b mRNA expression during early ovarian development, immunohistochemistry was utilized to localize expression to specific cell types. Immunohistochemistry was performed on P0, P3, and P10 rat ovaries (Fig. 2). Staining for antiangiogenic isoforms was localized to pregranulosa and granulosa cells of follicles from primordial through antral stages and to theca cells of preantral and antral follicles (Fig. 2, A–C). Positive staining was also localized to oocyte cysts and to oocytes within primordial follicles (Fig. 2, A–C).
FIG. 2.
Immunohistochemistry for VEGFA antiangiogenic (xxxB) isoforms in P0 (A), P3 (B), and P10 (C and D) ovaries. A and C were lightly counterstained with hematoxylin. D) Postnatal Day 10 ovarian sections with no primary antibody served as negative controls. These data are the result of at least three different ovaries from each developmental time point. Bar = 50 μm. oc, oocyte cyst; P, primordial follicle; PF, primary follicle; SF, secondary follicle; o, oocyte; g, granulosa cells; t, theca cells.
Effects of Recombinant VEGFA_164 on Rat Ovarian Organ Cultures
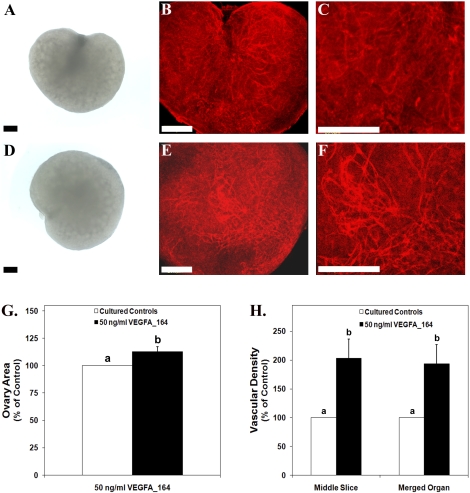
Ovarian organ cultures were utilized to determine the effects of treatment with recombinant VEGFA_164 on vascular development and follicle progression in the perinatal rat ovary. Ovaries treated with 50 ng/ml of VEGFA_164 (Fig. 3D) had a 13% increase in ovarian area compared with their paired controls (Fig. 3, A and G) (P < 0.006). The depth of treated ovaries was increased by 14% compared with controls (P < 0.008) (data not shown). Whole-mount immunohistochemistry with PECAM1 staining was conducted to determine the effects of VEGFA_164 treatment on vasculature. PECAM1 staining localizes endothelial cells [19] and thus identifies vasculature within the ovaries. Cultured ovaries treated with VEGFA_164 (Fig. 3, E and F) had a 93% increase in overall vascular density and a 103% increase in vascular density within the middle ovarian slice compared with their paired controls (Fig. 3, B, C, and H) (P < 0.01).
FIG. 3.
Postnatal Day 3/4 ovarian organ cultures treated with vehicle control (A–C) or 50 ng/ml of recombinant VEGFA_164 (D–F). A and D) Bright-field images. B, C, E, and F) Confocal images of whole-mount immunohistochemistry staining for PECAM1 (red indicates endothelial cell marker) to localize vasculature. G) Effect of VEGFA_164 treatment on ovarian area expressed as a percentage of control organs. H) Effect of VEGFA_164 on vascular density (middle organ slice and total merged organ) expressed as a percentage of control organs. Bar = 150 μm. Data are the result of 12 (G) and 23 (H) ovary pairs. G and H) The mean ± SEM areas are presented, and different letters represent a statistically significant difference between treated and control groups (P < 0.01).
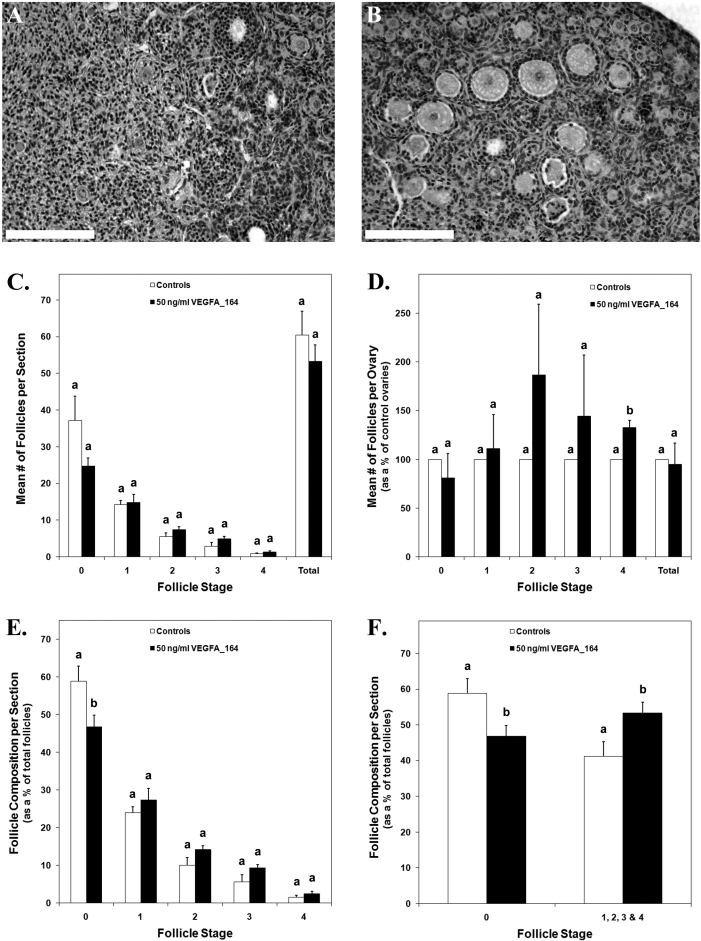
The number of follicles in cultured control ovaries (Fig. 4A) was compared with that in recombinant VEGFA_164-treated ovaries (Fig. 4B) to determine differences due to treatment. The mean number of follicles from each stage of development was not different between treated and control ovaries (Fig. 4C). However, evaluation of the mean follicle counts from treated ovaries as a percentage of control ovaries revealed a 33% increase in secondary follicles in VEGFA_164-treated ovaries (Fig. 4D) (P < 0.02). Furthermore, treated ovaries consisted of 47% primordial follicles and 53% developing follicles (stages 1–4), while control ovaries had 59% primordial follicles and 41% developing follicles (Fig. 4, E and F) (P < 0.04).
FIG. 4.
Postnatal Day 3/4 cultured control ovaries (A) or ovaries treated with 50 ng/ml of recombinant VEGFA_164 (B) stained with hematoxylin-eosin. C) The mean number of follicles per section from control and VEGFA_164 ovary pairs. D) The mean number of follicles for VEGFA_164-treated ovaries expressed as a percentage of their paired controls. E and F) The mean number of follicles per section for control and treated ovaries expressed as a percentage of the total number of follicles per section. Bar = 150 μm. C–F) Data are the result of three ovary pairs and three histological sections evaluated at 200× magnification per ovary. The mean ± SEM numbers of follicles are presented, and different letters within each follicle stage represent a statistically significant difference (P < 0.04).
Effects of Antiangiogenic VEGFAxxxB Antibody on Rat Ovarian Organ Cultures
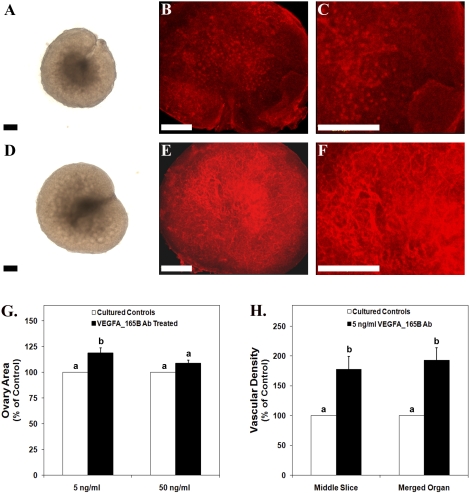
Ovarian organ cultures were utilized to determine the effects of antiangiogenic VEGFA isoform blockage on vascular development and follicle progression in the perinatal rat ovary. Ovaries treated with 5 ng/ml of VEGFAxxxB antibody (Fig. 5D) had a 19% increase in ovarian area compared with their paired controls (Fig. 5, A and G) (P < 0.005), while ovaries treated with 50 ng/ml of VEGFAxxxB antibody had a 9% increase in ovarian area compared with controls (Fig. 5G) (P < 0.004). The depth of treated ovaries was increased by 26% and 13% for 5 ng/ml and 50 ng/ml of VEGFAxxxB antibody treatment, respectively, compared with controls (P < 0.004) (data not shown).
FIG. 5.
Postnatal Day 3/4 ovarian organ cultures treated with vehicle control (A–C) or 5 ng/ml of VEGFAxxxB antibody (D–F). A and D) Bright-field images. B, C, E, and F) Confocal images of whole-mount immunohistochemistry staining for PECAM1 (red indicates endothelial cell marker) to localize vasculature. G) Effect of VEGFAxxxB antibody treatment on ovarian area expressed as a percentage of control organs. H) Effect of 5 ng/ml of VEGFAxxxB antibody on vascular density (middle organ slice and total merged organ) expressed as a percentage of control organs. Bar = 150 μm. Data are the result of 12 (G [5 ng/ml]), 15 (G [50 ng/ml]), and 11 (H) ovary pairs. G and H) The mean ± SEM areas are presented, and different letters represent a statistically significant difference between treated and control groups (P < 0.02). Ab, antibody.
Ovaries treated with 5 ng/ml of VEGFAxxxB antibody (Fig. 5, E and F) had a 93% increase in overall vascular density and a 77% increase in vascular density within the middle ovarian slice compared with their paired controls (Fig. 5, B, C, and H) (P < 0.02). The vascular density of ovaries treated with 50 ng/ml of VEGFAxxxB was not statistically different from controls (data not shown).
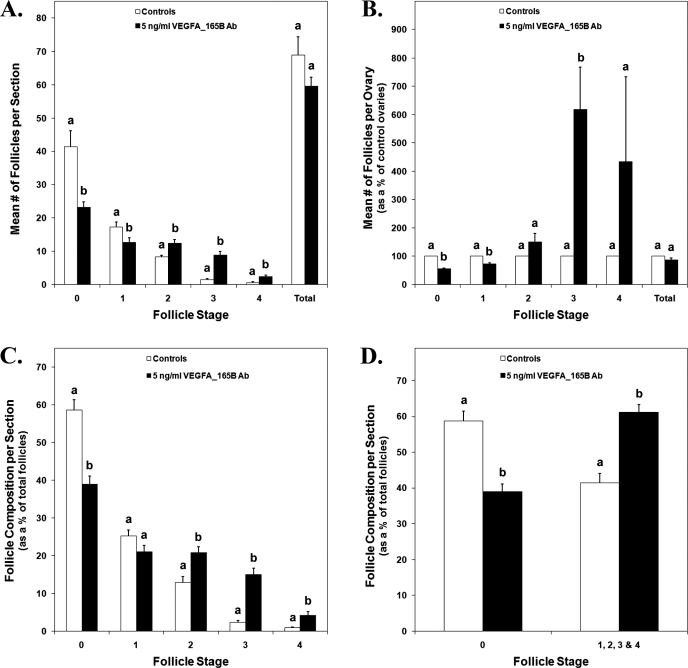
Ovaries treated with 5 ng/ml of VEGFAxxxB antibody had 44% less primordial (stage 0) follicles and 27% less early primary (stage 1) follicles per section than control ovaries (Fig. 6A) (P < 0.05). Treatment with 5 ng/ml of VEGFAxxxB antibody also resulted in 50%, 493%, and 378% more primary (stage 2), transitional (stage 3), and secondary (stage 4) follicles per section, respectively (Fig. 6A) (P < 0.006). Evaluation of the mean follicle counts from treated ovaries as a percentage of control ovaries revealed 44% and 27% reductions in primordial and early primary follicles, respectively, and a 518% increase in transitional follicles in VEGFAxxxB antibody-treated ovaries (Fig. 6B) (P < 0.03). In addition, treated ovaries consisted of 39% primordial follicles and 61% developing follicles (stages 1–4), while control ovaries had 59% primordial follicles and 41% developing follicles (Fig. 6D) (P < 0.0001). Specifically, ovaries treated with 5 ng/ml of VEGFAxxxB antibody consisted of 20% less primordial follicles, 8% more primary follicles, 13% more transitional follicles, and 3% more secondary follicles (Fig. 6C) (P < 0.02).
FIG. 6.
A) The mean number of follicles per section from control and 5 ng/ml of VEGFAxxxB antibody-treated P3/4 cultured ovary pairs. B) The mean number of follicles for VEGFAxxxB antibody-treated ovaries expressed as a percentage of their paired controls. C and D) The mean number of follicles per section for control and treated ovaries expressed as a percentage of the total number of follicles per section. A–D) Data are the result of three ovary pairs and three histological sections evaluated at 200× magnification per ovary. The mean ± SEM numbers of follicles are presented, and different letters within each follicle stage represent a statistically significant difference (P < 0.05). Ab, antibody.
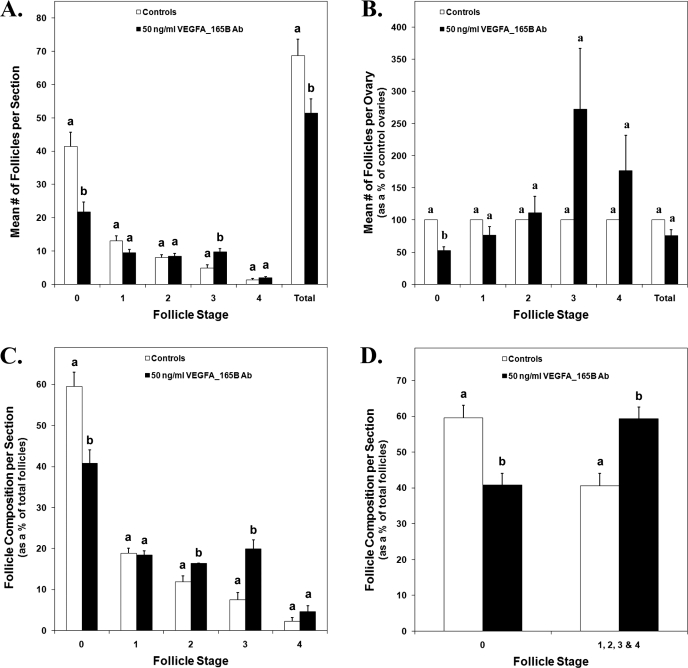
Organ culture ovaries treated with 50 ng/ml of VEGFAxxxB antibody resulted in 48% less primordial follicles, 25% less total follicles, and 102% more transitional follicles per section than controls (Fig. 7A) (P < 0.02). Evaluation of the mean follicle counts from treated ovaries as a percentage of control ovaries revealed a 47% reduction in primordial follicles in VEGFAxxxB antibody-treated ovaries (Fig. 7B) (P < 0.002). Treated ovaries consisted of 41% primordial follicles and 59% developing follicles (stages 1–4), while control ovaries had 60% primordial follicles and 40% developing follicles (Fig. 7D) (P < 0.002). Specifically, ovaries treated with 50 ng/ml of VEGFAxxxB antibody consisted of 4% and 12% more primary and transitional follicles, respectively, and 19% less primordial follicles (Fig. 7C) (P < 0.03).
FIG. 7.
A) The mean number of follicles per section from control and 50 ng/ml VEGFAxxxB antibody-treated P3/4 cultured ovary pairs. B) The mean number of follicles for VEGFAxxxB antibody-treated ovaries expressed as a percentage of their paired controls. C and D) The mean number of follicles per section for control and treated ovaries expressed as a percentage of the total number of follicles per section. A–D) Data are the result of three ovary pairs and three histological sections evaluated at 200× magnification per ovary. The mean ± SEM numbers of follicles are presented, and different letters within each follicle stage represent a statistically significant difference (P < 0.03). Ab, antibody.
DISCUSSION
The present study is the first (to our knowledge) to identify mRNA expression of antiangiogenic Vegfa isoforms in rat ovarian tissue. By utilizing the mRNA sequences to predict the antiangiogenic VEGFA protein sequences, we also determined that the rat antiangiogenic isoforms VEGFA_165B and VEGFA_189B are 1 amino acid longer than their comparable proangiogenic isoforms VEGFA_164 and VEGFA_188. Furthermore, neutralizing antiangiogenic VEGFA isoforms with a VEGFAxxxB antibody revealed a role for the antiangiogenic isoforms in the regulation of vascular and follicular development in perinatal rat ovaries. Notably, the neutralization of the antiangiogenic isoforms had a more dramatic effect on follicle development than treatment with a proangiogenic isoform.
In previous experiments in our laboratory, we detected mRNA expression for three proangiogenic Vegfa isoforms (Vegfa_120, Vegfa_164, and Vegfa_188) and the Vegfa receptors Kdr and Flt1 in developing rat ovaries [15]. In the present study, mRNA expression was also detected for Vegfa_165b and Vegfa_189b in developing and adult rat ovaries. Analysis using QRT-PCR has demonstrated that Vegfa_188 mRNA is most abundant at E16 and that there are elevated amounts of Vegfa_164 and Vegfa_120 after E18 and P0, respectively [15]. For the antiangiogenic isoforms, QRT-PCR revealed that Vegfa_165b mRNA is most abundant around E18, but there is not a distinct pattern of abundance for Vegfa_189b.
Around E18 in the rat, primordial germ cells have formed germline cysts that are ceasing to proliferate and are undergoing meiosis. Germline cysts are composed of clusters of oogonia connected by intracellular bridges formed through incomplete cytokinesis. These cell clusters synchronously proliferate, and waves of oogonia enter meiosis in a nonsynchronous manner until they are arrested around birth [20, 21]. During embryonic development, the actions of VEGFA_188 and the antiangiogenic actions of VEGFA_165B may be important in regulating VEGFA-mediated vascular development and reorganization or other nonvascular functions of VEGFA that may affect oocyte maintenance or meiosis.
Between E18 and P3/4, the developing rat ovary transitions from being composed predominantly of oocyte cysts to being composed predominantly of primordial follicles [2, 22]. Oocyte cysts undergo a programmed breakdown in which pregranulosa cells invade, divide cytoplasm, and surround oocytes to form primordial follicles [3, 20]. During late embryonic and early postnatal development, VEGFA_120 and VEGFA_164 may act via vascular or nonvascular mechanisms to regulate the oocyte cyst to primordial follicle transition.
Previous immunohistochemical findings in developing rat ovaries have localized VEGFA to oocyte cysts, pregranulosa cells, granulosa cells, theca cells, and the cytoplasm of oocytes of preantral and antral follicles [15]. FLT1 and KDR have also been localized to oocyte cysts, pregranulosa cells, granulosa cells, theca cells, and oocytes of all follicle stages [15]. In the present study, antiangiogenic VEGFA isoforms were localized to oocyte cysts, pregranulosa cells, granulosa cells, theca cells, and oocytes within primordial follicles. Antiangiogenic VEGFA isoforms were localized to similar nonvascular (granulosa and oocyte) and vascular (theca) follicular cells as proangiogenic VEGFA isoforms and receptors. However, proangiogenic isoforms were localized to oocytes of advanced follicle stages (preantral and antral follicles), while antiangiogenic isoforms were localized to oocytes of nondeveloping follicles (primordial follicles). This suggests that the antiangiogenic isoforms may be important in dampening the proangiogenic and nonangiogenic functions of VEGFA signaling in the regulation of follicle development.
VEGFA_164 is the most potent VEGFA isoform that is capable of recruiting endothelial cells for the formation of large blood vessels within the body [10, 23]. Previous experiments demonstrated that VEGFA_164 was the only VEGF isoform capable of fully restoring tumorigenic capacity to VEGFA-deficient transformed cells [10]. In another study [24], VEGFA gene fragments were injected into the ovaries of miniature gilts, resulting in increased Vegfa_120 and Vegfa_164 mRNA in granulosa cells and VEGFA protein content in follicular fluid. Furthermore, there was increased capillary density within the theca interna and increased number of preovulatory follicles [24]. In our ovarian organ cultures, administration of recombinant VEGFA_164 stimulated an increase in vascular development and an increase in the percentage of developing follicles (stages 1–4) in treated ovaries. The increased amount of vasculature that resulted from VEGFA_164 treatment may be responsible for promoting follicle development in the perinatal rat ovary, or the VEGFA_164-mediated follicle progression may be the result of vascular-independent mechanisms. In mice lacking PTEN (a suppressor of PIK3) within their oocytes, all primordial follicles are activated during early adulthood, which results in premature ovarian failure [25]. Because VEGFA has been shown to activate the PIK3 signaling pathway [26–28] and because VEGFA and its receptors have been localized to primordial follicles [15], VEGFA signaling through the PIK3 pathway may be involved in promoting primordial follicle activation.
The human antiangiogenic VEGFA_165B isoform has been shown to inhibit tumor growth by blocking VEGFA-induced angiogenesis [12]. Because VEGFA_165B can bind KDR but does not activate downstream signaling [14], a possible mechanism for this reduced angiogenesis is through the blocking of proangiogenic isoforms from binding to their receptors. It has also been shown that treatment with human VEGFA_165B can significantly reduce preretinal neovascularization in mice with oxygen-induced retinopathy, while still allowing normal physiologic retinal angiogenesis [29]. These studies demonstrated that administration of an antiangiogenic VEGFA isoform can regulate vascular development.
We hypothesized that a reduction in active VEGFA antiangiogenic isoforms would allow for greater action of the proangiogenic isoforms, thus increasing vascular density and stimulating ovarian follicle development. Treatment of organ culture ovaries with VEGFAxxxB antibody supported our hypothesis. The binding of antiangiogenic VEGFA isoforms to antibody blocks their binding to either VEGFA receptor. The vascular density of ovaries treated with 50 ng/ml of VEGFAxxxB antibody was not different from control ovaries; however, treatment with 5 ng/ml of VEGFAxxxB antibody had similar effects on vascular density as treatment with the proangiogenic isoform VEGFA_164. This suggests that blocking antiangiogenic isoforms from binding to VEGFA receptors allows for increased VEGFA-induced angiogenesis throughout the whole organ.
Both doses of VEGFAxxxB antibody resulted in a reduction in primordial (stage 0) follicle numbers and in an increase in transitional (stage 3) follicle numbers, but the lower (5 ng/ml) dose also produced a reduction in early primary (stage 1) follicles and an increase in primary (stage 2) and secondary (stage 4) follicles. We conclude that the antiangiogenic VEGFA isoforms maintain primordial follicles in an arrested state and that removal of this inhibition allows for increased progression of primordial follicles to the developing follicle pool.
In our experiments, treatment with 5 ng/ml of VEGFAxxxB antibody was more effective at promoting vascular development and follicle progression than 50 ng/ml. Compared with their paired controls, organs treated with 50 ng/ml of antibody also had a smaller increase in ovarian area and depth than organs treated with 5 ng/ml. These differences suggest that 50 ng/ml of VEGFAxxxB antibody may hinder ovarian development and may be too high for use in ovarian organ cultures.
Ovaries treated with both VEGFA_164 and the VEGFAxxxB antibody had an overall increase in ovarian area. We speculate that this change in area is the result of a greater composition of developing follicles, which are larger than primordial follicles, in treated ovaries compared with controls. Although treatment with recombinant VEGFA_164 and 5 ng/ml of VEGFAxxxB antibody produced similar effects on vascular density in cultured perinatal rat ovaries, VEGFAxxxB antibody treatment had a more profound effect on follicle development. It has been proposed that there is an antiangiogenic VEGFA isoform that corresponds to each proangiogenic VEGFA isoform [12]. Because the VEGFAxxxB antibody we utilized in our experiments was raised against a peptide corresponding to the 9-amino acid C-terminus of human VEGFA_165B, the antibody is likely blocking all VEGFA antiangiogenic isoforms from binding to their receptors, not just VEGFA_165B. Therefore, the more pronounced follicle progression seen with VEGFAxxxB antibody treatment compared with VEGFA_164 treatment may be the result of antibody binding to multiple antiangiogenic VEGFA isoforms. Another possible explanation for this difference is that the antiangiogenic isoforms may regulate follicle progression to a larger extent then the proangiogenic isoforms.
The results from the present study, along with findings from previous experiments in our laboratory [15], demonstrate a dramatic role for proangiogenic and antiangiogenic VEGFA isoforms in the formation and maintenance of vasculature, as well as the regulation of follicle progression, in perinatal rat ovaries. However, blockage of VEGFA antiangiogenic isoforms from binding to VEGFA receptors is more effective at promoting vascular and follicle development than treatment with the proangiogenic VEGFA_164 isoform. In conclusion, VEGFA isoforms are involved in vascular development and follicle activation and may be manipulated to regulate follicle development in the perinatal rat ovary.
Supplementary Material
Footnotes
Supported in part by NIH 5R01HD051979 and by Tobacco Funds for Biomedical Research from the state of Nebraska. A contribution of the University of Nebraska Agriculture Research Division, Lincoln, Nebraska.
REFERENCES
- Hirshfield AN.Heterogeneity of cell populations that contribute to the formation of primordial follicles in rats. Biol Reprod 1992; 47: 466–472.. [DOI] [PubMed] [Google Scholar]
- Rajah R, Glaser EM, Hirshfield AN.The changing architecture of the neonatal rat ovary during histogenesis. Dev Dyn 1992; 194: 177–192.. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC.Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 2001; 234: 339–351.. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Skinner MK.Cellular interactions that control primordial follicle development and folliculogenesis. J Soc Gynecol Investig 2001; 8: S17–S20.. [DOI] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK.Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology 1999; 140: 4262–4271.. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien M, Wigglesworth K.Mammalian oocyte growth and development in vitro. Mol Reprod Dev 1996; 44: 260–273.. [DOI] [PubMed] [Google Scholar]
- Kezele PR, Ague JM, Nilsson E, Skinner MK.Alterations in the ovarian transcriptome during primordial follicle assembly and development. Biol Reprod 2005; 72: 241–255.. [DOI] [PubMed] [Google Scholar]
- Iijima K JJ, Shimizu T, Sasada H, Sato E.Acceleration of follicular development by administration of vascular endothelial growth factor in cycling female rats. J Reprod Dev 2005; 51: 161–168.. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Collen D.Role of vascular endothelial growth factor and vascular endothelial growth factor receptors in vascular development. Curr Top Microbiol Immunol 1999; 237: 133–158.. [DOI] [PubMed] [Google Scholar]
- Grunstein J, Masbad JJ, Hickey R, Giordano F, Johnson RS.Isoforms of vascular endothelial growth factor act in a coordinate fashion to recruit and expand tumor vasculature. Mol Cell Biol 2000; 20: 7282–7291.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J.The biology of VEGF and its receptors. Nat Med 2003; 9: 669–676.. [DOI] [PubMed] [Google Scholar]
- Bates D, Cui TG, Doughty J, Winkler M, Sugiono M, Shields J, Peat D, Gillatt D, Harper S.VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 2002; 62: 4123–4131.. [PubMed] [Google Scholar]
- Harper S, Bates D.VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer 2008; 8: 880–887.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolard J, Wang WY, Bevan H, Qui Y, Morbidelli L, Pritchard-Jones R, Cui TG, Sugiono M, Waine E, Perrin R, Foster R, Digby-Bell J, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res 2004; 64: 7822–7835.. [DOI] [PubMed] [Google Scholar]
- McFee RM, Artac RA, McFee RM, Clopton DT, Longfellow Smith RA, Rozell TG, Cupp AS.Inhibition of vascular endothelial growth factor receptor signal transduction blocks follicle progression but does not necessarily disrupt vascular development in perinatal rat ovaries. Biol Reprod 2009; 81: 966–977.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski SR, Allan MF, Nielsen MK, Pomp D.Evaluation of hypothalamic gene expression in mice divergently selected for heat loss. Physiol Genomics 2003; 13: 129–137.. [DOI] [PubMed] [Google Scholar]
- Perrin R, Konopatskaya O, Qiu Y, Harper S, Bates D, Churchill A.Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia 2005; 48: 2422–2427.. [DOI] [PubMed] [Google Scholar]
- Bates D, MacMillan P, Manjaly J, Qiu Y, Hudson S, Bevan H, Hunter A, Soothill P, Read M, Donaldson L, Harper S.The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term. Clin Sci (Lond) 2006; 110: 575–585.. [DOI] [PubMed] [Google Scholar]
- Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A, Dejana E.Monoclonal antibodies specific for endothelial cells of mouse blood vessels: their application in the identification of adult and embryonic endothelium. Eur J Cell Biol 1994; 63: 247–254.. [PubMed] [Google Scholar]
- Pepling ME, Spradling AC.Female mouse germ cells form synchronously dividing cysts. Development 1998; 125: 3323–3328.. [DOI] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK.Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol 2006; 298: 132–148.. [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK.Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology 2003; 144: 3329–3337.. [DOI] [PubMed] [Google Scholar]
- Springer ML, Hortelano G, Bouley DM, Wong J, Kraft PE, Blau HM.Induction of angiogenesis by implantation of encapsulated primary myoblasts expressing vascular endothelial growth factor. J Gene Med 2000; 2: 279–288.. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Jiang JY, Iijima K, Miyabayashi K, Ogawa Y, Sasada H, Sato E.Induction of follicular development by direct single injection of vascular endothelial growth factor gene fragments into the ovary of miniature gilts. Biol Reprod 2003; 69: 1388–1393.. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng S, Lan Z, Cooney A, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008; 319: 611–613.. [DOI] [PubMed] [Google Scholar]
- Abid M, Guo S, Minami T, Spokes K, Ueki K, Skurk C, Walsh K, Aird W.Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler Thromb Vasc Biol 2004; 24: 294–300.. [DOI] [PubMed] [Google Scholar]
- Fujio Y, Walsh K.Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem 1999; 274: 16349–16354.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Jia Q, Song H, Warren R, Donner D.Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains: association with endothelial cell proliferation. J Biol Chem 1995; 270: 6729–6733.. [DOI] [PubMed] [Google Scholar]
- Konopatskaya O, Churchill A, Harper S, Bates D, Gardiner T.VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol Vis 2006; 12: 626–632.. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.