Abstract
Our previous studies have shown that in long-term two-bottle preference tests, mice from the C57BL/6ByJ (B6) inbred strain drink more monosodium glutamate (MSG) and inosine monophosphate (IMP) than mice from the 129P3/J (129) inbred strain. The goal of this study was to examine whether this variation in consumption could be attributed to strain differences in perception of the taste quality of MSG and IMP. We developed a conditioned taste aversion (CTA) in B6 and 129 mice to 100 mM MSG or 10 mM IMP and used a brief-access taste assay to examine CTA generalization. B6 and 129 mice did not differ in the generalization patterns following CTA to MSG: mice from both strains generalized CTA from MSG to NaCl. In contrast, strain differences in the generalization patterns were evident following the CTA to IMP: while mice from both strains generalized CTA from IMP to MSG, 129 mice tended to have stronger CTA generalization to saccharin and D-tryptophan, both of which are perceived as sweet by humans. These data suggest that the strain differences in MSG consumption are not due to variation in perception of the taste quality of MSG. Instead, the differential intake of IMP likely reflects strain differences in the way the taste quality of IMP is perceived. Our data suggest that mice perceive MSG and IMP as complex taste stimuli: some taste components are shared between these two substances, but their relative intensity seems to be different for MSG and IMP. The amiloride-sensitive salt taste component is more prevalent in MSG than in IMP taste, and in B6 compared with 129 mice.
Keywords: Taste, Umami, Conditioned Taste Aversion, MSG, IMP, Amiloride, Genetics, Inbred Strains
1. Introduction
It was believed for many years that there are four basic taste qualities, namely sweet, bitter, salty and sour. However, a century ago Ikeda [1] reported that monosodium glutamate (MSG) has a unique taste which he believed was the fifth basic taste. He named this taste quality umami. Five years later, Kodama [2] discovered another substance that had an umami quality to it, inosine mono phosphoric acid (IMP). Since these discoveries, many amino acids and nucleotides have been found to have umami taste characteristics [3, 4]. Recently, the metabotropic glutamate receptors mGluR4 and mGluR1 [5-7] and the T1R1 + T1R3 heterodimer receptor [8, 9] have been proposed to function as umami taste receptors.
Although MSG is the prototypical umami taste stimulus, its taste is complex to humans and other animals. Studies with rodents suggest that in addition to a unique taste [10], MSG may also evoke NaCl-like [11, 12] and sucrose-like [13-16] taste sensations. It has been suggested that in humans, IMP itself is tasteless, even though aquatic IMP solutions appear to have a taste. This apparent contradiction has been explained by a hypothesis that IMP enhances the umami taste of the subthreshold concentrations of glutamate contained in saliva and thus can evoke a taste sensation [17, 18]. However in mice, oral application of water solutions of IMP activates afferent gustatory nerves presumably in the absence of salivary glutamate [19, 20], which suggests that IMP evokes its own taste in mice.
In long-term two-bottle preference tests, mice from the C57BL/6ByJ (B6) strain drink more MSG and IMP than do mice from the 129P3/J (129) strain [21]. The goal of the work reported here was to investigate whether this variation in consumption could be attributed to strain differences in perception of the taste quality of MSG and IMP. We focused on the analysis of taste quality perception based on the following reasoning. Overall taste perception has several distinct components, including hedonics (affective value, or “pleasantness”) and taste quality (i.e. sweet, sour, salty, bitter, umami and perhaps others), which are often considered as orthogonal stimulus dimensions. However, the hedonic and qualitative components of overall perception are not completely independent because some taste qualities are generally associated with positive hedonic value (e.g., sweet and umami), while other taste qualities are generally associated with negative hedonic value (e.g., bitter). MSG and IMP have been shown to have a complex taste consisting of taste components of different qualities [10-16]. Consequently, the hedonic value of MSG and IMP depends in part on the combined hedonic values of their qualitatively distinct taste components. Based on this reasoning, differences in how inbred mice perceive qualities of MSG and IMP may alter their perceived hedonic value and influence their consumption in preference tests. Our assumption was that if the strains differ in their CTA generalization patterns, this would provide evidence that they perceive MSG and IMP differently, and that this difference in taste quality perception could underlie differences in intake. If, on the other hand, generalization patterns are similar, then the variation in intake must arise from other factors affecting hedonic responses, for example strain differences in the post-ingestive consequences of consuming these substances or in associative learning that integrates taste and post-ingestive effects.
To achieve this goal, we conditioned B6 and 129 mice to avoid 100 mM MSG or 10 mM IMP, and then examined suppression of their licking responses in brief-access tests to taste stimuli representing different taste qualities. We also examined the salty (NaCl-like) component of MSG and IMP taste using mixtures of the taste stimuli with amiloride. Amiloride is an epithelial sodium channel (ENaC) blocker that inhibits taste responses to NaCl in several animal species [22-25]. We compared licking responses of mice, conditioned to avoid MSG or IMP, to NaCl, MSG and IMP alone and these same substances that were mixed with 30 μM amiloride.
2. Materials and methods
2.1. Subjects
Subjects were adult male mice from the C57BL/6ByJ (B6) and 129P3/J (129) strains. At the beginning of the experiments, the mice were on average 4.3 months old (range 1.9 - 6.8 months; 20 - 30 g body weight). There were no differences in ages of B6 and 129 mice (4.2 ± 1.7 and 4.4 ± 1.8 months, respectively; p = 0.75, t-test). Animals were divided into 8 groups (6-10 mice per group; see details in Tables 1 and 2), which included two conditioned (MSG-LiCl and IMP-LiCl) and two control (MSG-NaCl and IMP-NaCl) groups for each of the two strains. The animals were housed in individual cages in a temperature controlled room at 23°C on a 12 h light: 12 h dark cycle (7:00 a.m. on, 7:00 p.m. off). They had free access to pelleted Teklad Rodent Diet 8604 (Harlan, Madison, WI), but were given restricted access to water as discussed below.
Table 1.
Normalized licking rates (%) in 129 and B6 mice conditioned to avoid 100 mM MSG (means ± SE)
| Solution | 129 strain | B6 strain | ||
|---|---|---|---|---|
| control (NaCl-treated, n = 7) | conditioned (LiCl-treated, n = 9-10)a | control (NaCl-treated, n = 6) | conditioned (LiCl-treated, n = 8) | |
| TS without amiloride | ||||
| 300 mM sucrose | 99 ± 2 | 98 ± 1 | 97 ± 2 | 90 ± 5 |
| 0.3 mM quinine | 64 ± 12 | 26 ±7 | 52 ± 16 | 27 ± 8 |
| 0.03 mM quinine | 70 ± 11 | 54 ± 9 | 83 ± 10 | 64 ± 12 |
| 0.01 mM quinine | 85 ± 10 | 89 ± 8 | 95 ± 2 | 79 ± 8 |
| 0.003 mM quinine | 99 ± 1 | 96 ± 1 | 95 ± 2 | 86 ± 7 |
| 0.1 mM HCl | 99 ± 4 | 79 ± 6 | 93 ± 3 | 68 ± 10 |
| 0.1 mM Citric acid | 101 ± 1 | 96 ± 3 | 96 ± 4 | 80 ± 6 |
| 100 mM NaCl# | 98 ± 2 | 15 ± 6 | 93 ± 4 | 43 ± 12 |
| 1000 mM MSG# | 95 ± 2 | 10 ± 3 | 90 ± 4 | 15 ± 4 |
| 300 mM MSG# | 93 ± 3 | 17 ± 5 | 92 ± 4 | 23 ± 6 |
| 100 mM MSG# | 99 ± 1 | 9 ± 2 | 98 ± 1 | 17 ± 5 |
| 1 mM MSG | 99 ± 1 | 95 ± 2 | 99 ± 2 | 99 ± 1 |
| 10 mM IMP | 97 ± 1 | 47 ± 14 | 99 ± 1 | 50 ± 11 |
| 0.1 mM IMP | 100 ± 0.5 | 99 ± 1 | 97 ± 1 | 98 ± 3 |
| 100 mM D-phenylalanine | 95 ± 5 | 77 ± 7 | 97 ± 1 | 89 ± 4 |
| 30 mM D-tryptophan | 95 ± 3 | 87 ± 8 | 96 ± 2 | 86 ± 5 |
| 100 mM Glycine | 99 ± 1 | 94 ± 4 | 98 ± 2 | 95 ± 6 |
| 100 mM L-alanine | 99 ± 1 | 81 ± 7 | 98 ± 2 | 81 ± 6 |
| 100 mM L-glutamic acid | 25 ± 5 | 21 ± 6 | 35 ± 13 | 16 ± 5 |
| 10 mM L- glutamic acid | 66 ± 10 | 56 ± 10 | 79 ± 12 | 60 ± 7 |
| 1 mM L- glutamic acid | 93 ± 5 | 91 ± 2 | 98 ± 2 | 86 ± 4 |
| 100 mM L-valine | 99 ± 1 | 85 ± 6 | 97 ± 1 | 86 ± 7 |
| 100 mM L-leucine | 98 ± 1 | 90 ± 8 | 98 ± 2 | 82 ± 5 |
| 100 mM L-arginine | 99 ± 1 | 82 ± 10 | 96 ± 2 | 52 ± 10 |
| 20 mM saccharin | 92 ± 5 | 58 ± 12 | 98 ± 2 | 72 ± 9 |
| TS with amiloride | ||||
| 0.03 mM amiloride | 99 ± 1 | 99 ± 1 | 98 ± 2 | 92 ± 2 |
| 100 mM NaCl | 99 ± 2 | 73 ± 10* | 98 ± 1 | 88 ± 5* |
| 1000 mM MSG | 86 ± 7 | 26 ± 6 | 90 ± 3 | 64 ± 6* |
| 300 mM MSG | 100 ± 1 | 34 ± 13 | 100 ± 1 | 82 ± 5* |
| 100 mM MSG | 100 ± 0.5 | 44 ± 12* | 97 ± 1 | 91 ± 4* |
| 1 mM MSG | 100 ± 1 | 96 ± 3 | 101 ± 1 | 97 ± 1 |
| 10 mM IMP | 99 ± 2 | 59 ± 11 | 97 ± 1 | 83 ± 6* |
| 0.1 mM IMP | 101 ± 1 | 98 ± 2 | 99 ± 1 | 94 ± 3 |
The order (top to bottom) of taste stimuli (TS) is the same as the order of their presentation.
n = 10 for TS without amiloride, and n = 9 for TS with amiloride.
TS without amiloride: Significant difference between conditioned and control groups pooled between the two strains; p<0.05; Tukey post-hoc tests for a two-way (TS × treatment) interaction. The post-hoc tests were based on results of three-way ANOVA of normalized licking rates to TS without amiloride: effects of strain [B6 vs. 129; F(1,27) = 0.002, p = 1.0]; treatment [LiCl vs. NaCl; F(1,27) = 83.2, p < 0.001]; TS [F(24,648) = 43.2, p < 0.001]; strain × treatment [F(1,27) = 0.014, p = 0.9]; strain × TS [F(24,648) = 1.2, p = 0.2]; treatment × TS [F(24,648) = 18.0, p < 0.001]; strain × treatment × TS [F(24,648) = 1.0, p = 0.5].
TS with amiloride: Significant difference between licking rates for TS with and without amiloride; p<0.05; Tukey post-hoc tests for a three-way (strain × amiloride × TS) interaction. The post-hoc tests were based on results of three-way ANOVA of normalized licking rates to TS (NaCl, MSG and IMP) without and with amiloride in conditioned groups of mice: effects of strain [B6 vs. 129; F(1,15) = 11.8, p = 0.004]; amiloride [with or without; F(1,15) = 99.5, p < 0.001]; TS [F(6,90) = 55.3, p < 0.001]; strain × amiloride [F(1,15) = 8.6, p = 0.01]; strain × TS [F(6,90) = 2.4, p = 0.03]; amiloride × TS [F(6,90) = 16.5, p < 0.001]; strain × amiloride × TS [F(6,90) = 4.2, p = 0.0009].
Table 2.
Normalized licking rates (%) in 129 and B6 mice conditioned to avoid 10 mM IMP (means ± SE)
| Solution | 129 strain | B6 strain | ||
|---|---|---|---|---|
| control (NaCl-treated, n = 7) | conditioned (LiCl-treated, n = 7) | control (NaCl-treated, n = 6) | conditioned (LiCl-treated, n = 8) | |
| TS without amiloride | ||||
| 300 mM sucrose | 97 ± 1 | 43 ± 12 | 98 ± 1 | 70 ±12 |
| 0.3 mM quinine | 67 ± 13 | 15 ± 4 | 48 ± 13 | 23 ± 6 |
| 0.03 mM quinine | 75 ± 15 | 40 ± 4 | 94 ± 4 | 56 ± 11 |
| 0.01 mM quinine | 93 ± 11 | 57 ± 12 | 99 ± 2 | 77 ± 8 |
| 0.003 mM quinine | 98 ± 2 | 81 ± 10 | 94 ± 5 | 95 ± 3 |
| 0.1 mM HCl | 89 ± 11 | 68 ± 10 | 94 ± 3 | 84 ± 6 |
| 0.1 mM Citric acid | 98 ± 2 | 85 ± 14 | 98 ± 1 | 81 ± 8 |
| 100 mM NaCl | 97 ± 1 | 26 ± 7 | 98 ± 1 | 64 ± 11 |
| 1000 mM MSG# | 96 ± 2 | 10 ± 2 | 99 ± 2 | 41 ± 8 |
| 300 mM MSG# | 97 ± 1 | 30 ± 11 | 98 ± 1 | 43 ± 11 |
| 100 mM MSG# | 98 ± 1 | 48 ± 12 | 96 ± 1 | 31 ± 10 |
| 1 mM MSG | 101 ± 1 | 91 ± 7 | 98 ± 1 | 93 ± 3 |
| 10 mM IMP# | 99 ± 1 | 10 ± 3 | 100 ± 0.4 | 16 ± 4 |
| 0.1 mM IMP | 98 ±1 | 95 ± 3 | 98 ± 1 | 88 ± 4 |
| 100 mM D-phenylalanine | 93 ± 3 | 55 ± 17 | 99 ± 1 | 81 ± 6 |
| 30 mM D-tryptophan | 97 ± 2 | 25 ± 7 | 96 ± 2 | 69 ± 10 |
| 100 mM Glycine | 100 ± 1 | 35 ± 11 | 101 ± 2 | 75 ± 10 |
| 100 mM L-alanine | 96 ± 2 | 92 ± 5 | 99 ± 3 | 80 ± 9 |
| 100 mM L-glutamic acid | 39 ± 16 | 12 ± 3 | 54 ± 15 | 16 ± 4 |
| 10 mM L- glutamic acid | 80 ± 9 | 43 ± 14 | 80 ± 10 | 44 ± 8 |
| 1 mM L- glutamic acid | 95 ± 2 | 86 ± 5 | 99 ± 1 | 83 ± 5 |
| 100 mM L-valine | 101 ± 1 | 60 ± 14 | 100 ± 2 | 85 ± 9 |
| 100 mM L-leucine | 99 ± 2 | 68 ± 11 | 98 ± 1 | 80 ± 8 |
| 100 mM L-arginine | 98 ± 1 | 45 ± 14 | 96 ± 1 | 68 ± 9 |
| 20 mM saccharin# | 97 ± 1 | 12 ± 3 | 98 ± 1 | 64 ± 11 |
| TS with amiloride | ||||
| 0.03 mM amiloride | 102 ± 1 | 101 ± 1 | 100 ± 1 | 98 ± 2 |
| 100 mM NaCl | 100 ± 1 | 73 ± 14* | 99 ± 2 | 90 ± 3* |
| 1000 mM MSG | 90 ± 6 | 30 ± 7* | 97 ± 3 | 77 ± 6* |
| 300 mM MSG | 100 ± 0.4 | 59 ± 12* | 99 ± 1 | 89 ± 4* |
| 100 mM MSG | 101 ± 1 | 74 ± 11* | 99 ± 1 | 86 ± 5* |
| 1 mM MSG | 102 ± 1 | 98 ± 2 | 100 ± 1 | 97 ± 1 |
| 10 mM IMP | 100 ± 1 | 28 ± 6 | 98 ± 1 | 40 ± 10 |
| 0.1 mM IMP | 100 ± 1 | 98 ± 2 | 101 ± 1 | 96 ± 2 |
The order (top to bottom) of TS is the same as the order of their presentation.
TS without amiloride: Significant difference between conditioned and control groups pooled between the two strains; p<0.05; Tukey post-hoc tests for a two-way (TS × treatment) interaction. The post-hoc tests were based on results of three-way ANOVA of normalized licking rates to TS without amiloride: effects of strain [F(1,24) = 11.1, p = 0.003]; treatment [F(1,24) = 213.4, p < 0.001]; TS [F(24,576) = 19.5, p < 0.001]; strain × treatment [F(1,24) = 7.8, p = 0.01]; strain × TS [F(24,576) = 1.6, p = 0.03]; treatment × TS [F(24,576) = 8.0, p < 0.001]; strain × treatment × TS [F(24,576) = 1.5, p = 0.06].
TS with amiloride: Significant difference between licking rates for TS with and without amiloride pooled between the two strains; p<0.05; Tukey post-hoc tests for a two-way (amiloride × TS) interaction. The post-hoc tests were based on results of three-way ANOVA of normalized licking rates to TS (NaCl, MSG and IMP) without and with amiloride in conditioned groups of mice: effects of strain [F(1,13) = 22.8, p < 0.001]; amiloride [F(1,13) = 51.2, p < 0.001]; TS [F(6,78) = 56.2, p < 0.001]; strain × amiloride [F(1,13) = 1.0, p = 0.3]; strain × TS [F(6,78) = 5.7, p < 0.001]; amiloride × TS [F(6,78) = 4.1, p = 0.001]; strain × amiloride × TS [F(6,78) = 1.1, p = 0.4].
2.2. Taste stimuli
Mice were presented with deionized water or solutions of taste stimuli in deionized water. The conditioned stimuli (CS) were 100 mM MSG or 10 mM IMP (disodium salt). These particular solution concentrations were chosen because in our previous studies they evoked strong behavioral [21] and neural [20] responses in B6 and 129 mice, suggesting that these solutions are salient stimuli for conditioning. The test stimuli (TS) were MSG (0.1-1000 mM), IMP (0.01-10 mM), sucrose (Suc: 300 mM), quinine hydrochloride (Qui: 0.003-0.3 mM), HCl (0.1 mM), citric acid (Citric: 0.1 mM), NaCl (100 mM), D-phenylalanine (D-Phe: 100 mM), D-tryptophan (D-Trp: 30mM), glycine (Gly: 100 mM), L-alanine (L-Ala: 100 mM), L-glutamic acid hydrochloride (L-Glu: 1, 10 and 100 mM), L-valine (L-Val: 100 mM), L-leucine (L-Leu: 100 mM), L-arginine (L-Arg: 100 mM), saccharin (sodium salt; Sac: 20 mM), amiloride (30 μM) and mixtures of 30 μM amiloride with MSG (1-1000 mM), IMP (0.1 and 10 mM) or NaCl (100 mM). The 30 μM amiloride concentration was chosen because it was frequently used in previous studies and was shown to suppress NaCl responses in taste bud cells [26] and the chorda tympani nerve [27, 28], and disrupt discrimination between NaCl and KCl in behavioral tests [28, 29]. All chemicals were purchased from the Sigma-Aldrich Co. (St. Louis, MO).
2.3. Apparatus
The number of licks was recorded using the Davis MS-160 gustometer (DiLog Instruments, Tallahassee, FL) or the Licking counter (Neuro Science Idea, Osaka, Japan). The procedures of counting licks using these devices were similar to those described by Glendinning et al. [30] and Murata et al. [31], respectively. Both lickometers are designed to record lick numbers from a single tube. The lick detection principle of the Davis MS-160 gustometer is that a high frequency electric signal is applied to the sipper tube, and a phase sensitive demodulator detects the animal’s capacitance when it contacts the sipper tube (no direct current is passed through the tongue). The Licking counter detects licks by intercepting a laser beam during contact of the mouse tongue with fluid. Taste solution presentation is automatic in the Davis gustometer and manual in the Licking counter. For both instruments, timing of solution presentation and inter-trial intervals were similar, and the numbers of licks were counted during the 10-sec period starting from the animal’s first lick after test tube presentation. In each group, 2-3 animals were tested in the Davis Rig and the rest were tested in the Licking counter. We tested each mouse in only one type of lickometer.
2.4. Procedures
Training and testing procedures were identical for mice tested in both devices. All fluids were always presented in a single tube in a test cage (with the exception of water presented in the home cage on recovery days after conditioning). On the first and second days of training, each water-deprived animal was placed in a test cage and given free access to water presented in a single drinking tube for 30 min without interruptions. From the third to the fifth day, each animal was trained to drink water on an interval schedule consisting of 10-sec periods of presentation of water alternating with 20 sec intertrial intervals. Mice were given 1.0 - 1.5 ml of water in a drinking tube, and the 10-s presentations continued until all water was consumed. On the sixth day, the trained mice were placed in the test cage and presented with a CS for 30 min without interruptions (during this period, all mice drank at least 0.5 ml of CS). Immediately after that, mice were given an intraperitoneal (i.p.) injection of 230 mg/kg 240 mM LiCl (conditioned group) or 230 mg/kg 240 mM NaCl (control group). The seventh day was a recovery period, during which the mice remained in their home cages and were given water to drink for 20-30 min.
Starting from the eighth day, test sessions began. During the test sessions, each fluid was presented in a single bottle for 10 sec alternated with 20 sec intertrial intervals. Presentations of taste solutions (CS and TSs) were alternated with presentations of water. For conditioned mice, each test day started with two or three presentations of the CS. This was done to examine retention of the CTA. If the animal avoided the CS (i.e., licking rate of the CS did not exceed 20% of the licking rate of water presented between the CS presentations), then the TSs were presented. If a mouse did not avoid the CS (i.e., licking rate of the CS exceeded 20% of the licking rate of water), no other TSs were presented, and the mouse was injected with 230 mg/kg of LiCl i.p. (i.e., the CS-unconditioned stimulus pairing was repeated). After a recovery day, this mouse was again tested with the CS and if it displayed CS avoidance, TSs were presented. The CS-unconditioned stimulus pairings needed to be repeated for 12 out of 16 B6 mice (75%) and for 5 out of 17 129 mice (29%; p = 0.01, t-test). There were no differences in frequency of additional conditioning between mice conditioned with MSG (11 out of 18; 61%) and IMP (6 out of 15; 40%, p = 0.24). Groups conditioned only once or more than once did not differ in licking rates or licking suppression rates of the CS (effects of strain, additional conditioning and their interaction were not significant; F(1,29) < 3.9, p > 0.05; two-way ANOVA). In all cases, if a mouse has reached the conditioning criterion, it maintained aversion in the remaining test sessions (on subsequent test days). As a result of using our criterion for adequate conditioning, mean licking suppression rates for CS exceeded 80% and were often close to 90%. This is comparable with the CS suppression rates reported in other studies (e.g., [10, 32]).
The TSs were presented in the same fixed order in mice from all groups, with one exception: Testing of conditioned (but not control) mice started with series of concentrations of MSG (presented to mice conditioned to avoid MSG) or IMP (presented to mice conditioned to avoid IMP) (data not shown). Next, we presented the following 33 TSs to both MSG- and IMP-conditioned and control mice. We started with presenting five prototypical taste stimuli (sucrose, quinine, HCl, citric acid, NaCl), MSG, IMP, amino acids and saccharin; this was followed by amiloride alone, and its mixtures with NaCl, MSG and IMP (the order of presentation of these TSs was the same as the top to bottom order shown in Tables 1 and 2, and the left to right order shown on the X-axis in Figures 1 and 2). When more than one concentration of a tastant was tested, the solutions were presented in the descending order of concentration. Testing the whole set of TSs was completed in several test sessions (usually in 2-4 test sessions conducted daily). During each session, 13-25 stimuli were tested. The session was stopped after completion of 25 trials or sooner if licking rate for water fell below 50% of water licking rate at the beginning of the session. When testing of the whole battery of TSs was not completed during a session, it continued the next day. Each TS was tested in each mouse only once.
Fig. 1.
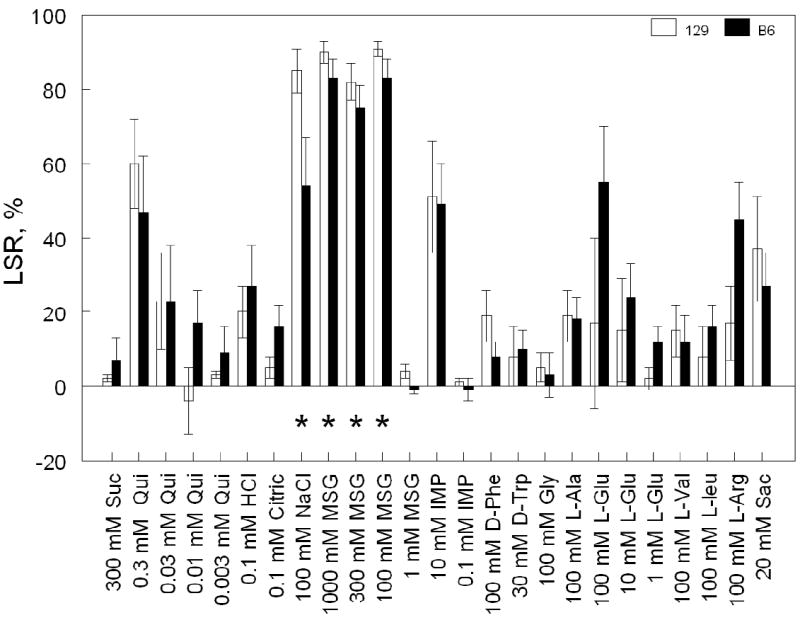
Licking suppression ratios (LSR; means ± SE) of 129 and B6 mice conditioned to avoid 100 mM MSG. The LSR “100%” indicates complete aversion and “0%” indicates licking rate similar to that in the control group. The order of TS (left to right) is the same as the order of their presentation. Suc, sucrose; Qui, quinine hydrochloride; Citric, citric acid; D-Phe, D-phenylalanine; D-Trp, D-tryptophan; Gly, glycine; L-Ala, L-alanine; L-Glu, L-glutamic acid; L-Val, L-valine; L-Leu, L-leucine; L-Arg, L-arginine; Sac, saccharin. Results of two-way ANOVA: effects of strain [F(1,16) = 0.14, p = 0.7]; TS [F(24,384) = 23.6, p < 0.001]; strain × TS [F(24,384) = 1.5, p = 0.06]. *Significant licking suppression indicated by significantly lower normalized licking rates of conditioned mice relative to control mice (p < 0.05; Tukey post-hoc tests of licking rates pooled between the two strains; see Table 1).
Fig. 2.
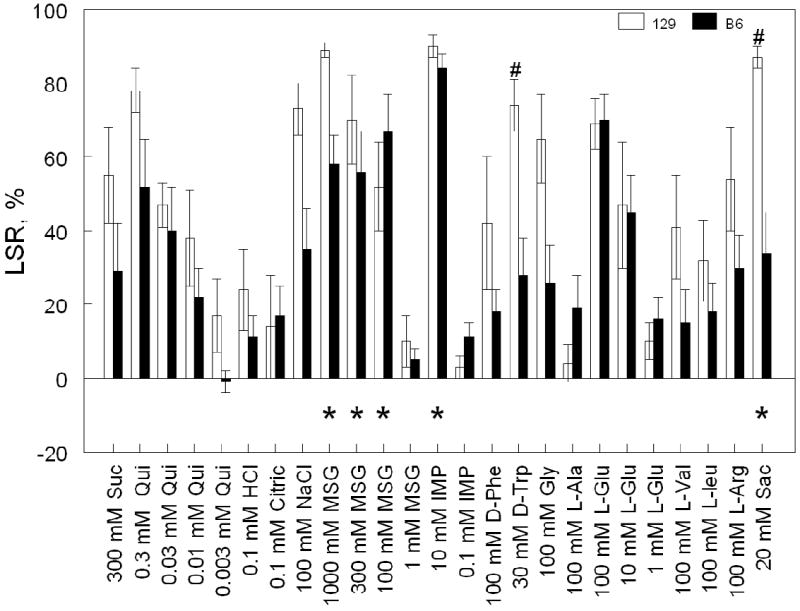
Licking suppression ratios (LSR; means ± SE) of 129 and B6 mice conditioned to avoid 10 mM IMP. #Significant differences between B6 and 129 mice; p < 0.05; LSD post-hoc tests (more stringent Tukey post-hoc tests did not detect significant differences between 129 and B6 mice). The post-hoc tests were based on results of two-way ANOVA: effects of strain [F(1,13) = 14.1, p = 0.002]; TS [F(24,312) = 12.6, p < 0.001]; strain × TS [F(24,312) = 2.0, p = 0.006]. *Significant licking suppression indicated by significantly lower normalized licking rates of conditioned mice relative to control mice (p < 0.05; Tukey post-hoc tests of licking rates pooled between the two strains; see Table 2). Other explanations are the same as in Fig. 1.
2.5. Data analysis
The raw licks were counted by the lickometer during the 10-s period starting from the first lick after test tube presentation. To avoid any systematic differences between apparatus and between animals, unrelated to MSG or IMP tastes, we calculated normalized licking rates of individual mice for each stimulus according to the following formula: Normalized licking rate (%) = (number of licks of the CS or TS /10 sec)/(mean number of licks of water presented in the same session before and after presenting the corresponding CS or TS /10 sec) × 100. To assess the strength of the CTA, we calculated licking suppression ratios (LSR) of individual mice for each stimulus according to the following formula (see [33]): LSR (%) = [1- (normalized licking rate to a TS of an individual from the conditioned group)/(mean licking rate to the same TS in the control group)] × 100. The LSR “100%” indicates complete aversion and “0%” indicates licking rate similar to that in the control group.
Data were analyzed using two- or three-way ANOVA. When there was a significant effect of a factor, Tukey HSD or LSD post-hoc tests were carried out to evaluate differences between individual means. For TSs without amiloride, normalized licking rates were analyzed using three-way ANOVA (with TS as a within-subject factor, and strain and treatment as between-subject factors). The significance of licking suppression was assessed by comparing licking rates in control and experimental groups using post-hoc tests. LSR were analyzed using two-way ANOVA (with TS as a within-subject factor, and strain as a between-subject factor). To analyze effects of amiloride on CTA, we examined normalized licking rates and LSR of conditioned mice using three-way ANOVA (with TS and addition of amiloride as within-subject factors, and strain as a between-subject factor). The effect of amiloride was assessed by comparing licking rates for TSs mixed with and without amiloride using post-hoc tests in conditioned mice.
3. Results
3.1. Generalization of MSG aversions
Licking rates of mice conditioned to avoid 100 mM MSG and control mice are shown in Table 1. An absence of significant strain × treatment × TS interaction or any other effects involving strain (three-way ANOVA) indicates that B6 and 129 mice had similar CTA generalization patterns. Significant treatment × TS interaction (p < 0.001) indicates that CTA to 100 mM MSG generalized to some but not all TSs tested. Conditioned mice significantly suppressed licking rates for MSG (100, 300 and 1000 mM) and NaCl (100 mM), but not for any of the other TSs tested. Consistent with these results, LSR did not differ between the two strains (Fig. 1; non-significant effects of strain and strain × TS interaction).
3.2. Generalization of IMP aversions
Licking rates of mice conditioned to avoid 10 mM IMP and control mice are shown in Table 2. Strain × treatment × TS interaction did not reach the level of statistical significance (p = 0.06, three-way ANOVA), but treatment × TS interaction was significant (p < 0.001) indicating that CTA to 10 mM IMP generalized to some but not all TSs tested. Conditioned mice significantly suppressed licking rates for IMP (10 mM), MSG (100, 300 and 1000 mM) and saccharin (20 mM), but not for any of the other TSs tested. Although the effect of the three-way interaction (strain × treatment × TS) on licking rates only approached but did not reach the threshold of statistical significance, several other results suggest that there were strain differences in the CTA generalization patterns. First, several strain-related effects significantly affected licking rates (strain; strain × treatment; strain × TS; p ≤ 0.03; see Table 2 caption). Second, LSR were significantly affected by strain × TS interaction (p = 0.006; Fig. 2).
3.3. Effects of amiloride on CTA generalization patterns
The experiments described above have shown that the licking rates to NaCl were significantly suppressed after CTA with 100 mM MSG. This suggests that MSG has salty (NaCl-like) taste to mice. The NaCl-like taste could also have been evoked by IMP because we used a disodium salt of IMP in our experiments. The goal of this experiment was to examine the relative contribution of the amiloride-blockable NaCl taste component to qualitative taste perception of MSG and IMP.
We measured licking responses of control and conditioned 129 and B6 mice to NaCl, MSG and IMP mixed with amiloride and compared them with licking responses to the same TSs without amiloride. There was little variation in licking responses to TSs mixed with amiloride in control mice: their normalized licking rates were similar to licking rates of water and ranged from 86 to 102% (Tables 1 and 2). Therefore, our analyses focused on comparisons of licking responses in conditioned groups.
In mice conditioned to avoid 100 mM MSG, normalized licking rates (Table 1) and LSR (Fig. 3) were significantly affected by amiloride (with or without) × strain × TS interaction, which indicates that there were strain differences in effects of amiloride on responses to some but not all TSs. In both strains, suppression of licking to 100 mM NaCl and 100 mM MSG was significantly attenuated after addition of amiloride. However, suppression of licking to 300 and 1000 mM MSG, and to 10 mM IMP was significantly attenuated after addition of amiloride only in B6 mice.
Fig. 3.
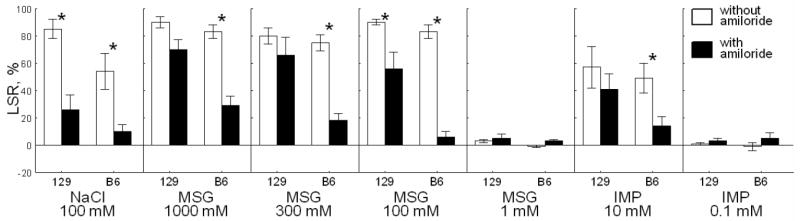
Effects of amiloride on licking suppression ratios (LSR; means ± SE) of 129 and B6 mice conditioned to avoid 100 mM MSG. *Significant difference between LSR for TS with and without amiloride; p<0.05; Tukey post-hoc tests for a three-way (strain × amiloride × TS) interaction. The post-hoc tests were based on results of three-way ANOVA: effects of strain [F(1,15) = 13.0 p = 0.003]; amiloride [F(1,15) = 96.2, p < 0.001]; TS [F(6,90) = 50.0, p < 0.001]; strain × amiloride [F(1,15) = 8.7, p = 0.01]; strain × TS [F(6,90) = 2.4, p = 0.04]; amiloride × TS [F(6,90) = 16.7, p < 0.001]; strain × amiloride × TS [F(6,90) = 4.4, p < 0.001].
In mice conditioned to avoid 10 mM IMP, normalized licking rates (Table 2) and LSR (Fig. 4) were not significantly affected by amiloride × strain × TS and amiloride × strain interaction, which indicates that there were no strain differences in effects of amiloride. A significant amiloride × TS interaction indicates that amiloride affected licking rates for some but not all TSs. Licking rates to NaCl (100 mM) and MSG (100, 300 and 1000 mM) were significantly increased after addition of amiloride.
Fig. 4.
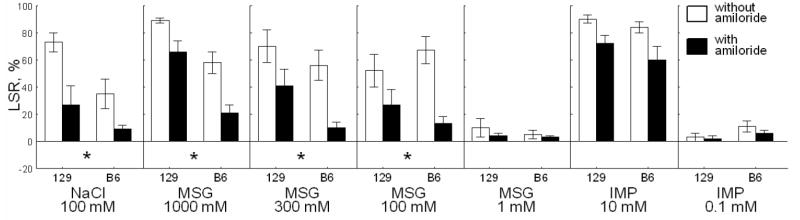
Effects of amiloride on licking suppression ratios (LSR; means ± SE) of 129 and B6 mice conditioned to avoid 10 mM IMP. *Significant difference between LSR for TS with and without amiloride pooled between the two strains; p<0.05; Tukey post-hoc tests for a two-way (amiloride × TS) interaction. The post-hoc tests were based on results of three-way ANOVA: effects of strain [F(1,13) = 23.8, p < 0.001]; amiloride [F(1,13) = 47.6, p < 0.001]; TS [F(6,78) = 53.3, p < 0.001]; strain × amiloride [F(1,13) = 0.9, p = 0.4]; strain × TS [F(6,78) = 5.3, p < 0.001]; amiloride × TS [F(6,78) = 4.4, p < 0.001]; strain × amiloride × TS [F(6,78) = 1.1, p = 0.4].
4. Discussion
Previous studies have shown that in the long-term two-bottle tests, B6 mice drink more MSG and IMP than do 129 mice [21]. To assess whether strain differences in consumption might be due to differential perception of the taste quality of MSG and IMP, we examined patterns of generalization of CTA to 100 mM MSG or 10 mM IMP in B6 and 129 mice.
4.1. Strain differences in taste perception of MSG
B6 and 129 mice that were conditioned to avoid MSG had similar patterns of CTA generalization. Mice from both strains generalized CTA from 100 mM MSG to other MSG solutions and NaCl. CTA generalization between MSG and NaCl was also shown in previous studies with mice [10], rats [11] and hamsters [12]. Qualitative taste similarity between MSG and NaCl was also shown in two other types of experiments. First, MSG and NaCl evoked similar patterns of responses in single fibers of the chorda tympani gustatory nerve in mice [19] and hamsters [12]. Second, MSG was shown to have a salty taste quality component in psychophysical experiments with humans [34, 35]. Similarity of MSG and NaCl tastes is probably attributed to presence of sodium in both compounds. In addition to the taste component attributed to sodium, MSG evokes other taste sensations, which must be attributed to glutamate. For example when rats were conditioned to avoid a mixture of MSG with amiloride, they generalized the CTA to sucrose [13-16]. Besides NaCl-like and sucrose-like taste components, MSG was also shown to have a unique taste to mice [10], which is probably equivalent to umami taste in humans.
In our experiments, addition of amiloride attenuated MSG and NaCl licking suppression of MSG-conditioned mice from both strains. This is consistent with the existence of an amiloride-blockable NaCl-like taste component of MSG in both B6 and 129 mice. However, effects of amiloride were stronger in B6 mice than in 129 mice. In MSG-conditioned B6 mice, addition of amiloride diminished suppression of licks for a wider range of MSG concentrations compared with 129 mice, and it also attenuated suppression of IMP licking rate. This is consistent with results of electrophysiological studies showing that amiloride-induced suppression of activity in the chorda tympani gustatory nerve in response to lingual application of NaCl was greater in B6 mice compared with 129 mice [27, 36, 37]. Overall, these data suggest that an amiloride-blockable NaCl-like taste is a prominent component of MSG taste, and that it is quantitatively more prevalent (more salient) in B6 than in 129 mice. Nevertheless, these quantitative strain differences in strength of the amiloride-sensitive salt taste component did not affect qualitative taste perception of MSG by B6 and 129 mice: mice from both strains generalized CTA from MSG to NaCl to the same degree.
We conclude that B6 and 129 mice have a similar perception of the taste quality of MSG, albeit with quantitative differences in strength of the amiloride-sensitive salt taste component. This is consistent with the similar responses to glutamate salts in gustatory nerves of these mice [20]. In addition, taste-naive B6 mice have similar or lower MSG licking rates in brief-access tests compared with 129 mice (Zolotarev and Bachmanov, unpublished data). Therefore, strain differences in MSG consumption in the long-term tests are unlikely to be explained by differences in qualitative or hedonic aspects of taste perception.
We propose that strain differences in MSG consumption in the long-term two-bottle tests are likely due to differences in postingestive effects of MSG. This conclusion is entirely consistent with two other lines of evidence: strain-specific effects of prior experience with MSG on its intake [21], and strain differences in the metabolism of orally administered MSG [38]. In the latter study [38], we found that after intragastric administration of MSG B6 mice preferentially metabolize it through gluconeogenesis, whereas the predominant process for 129 mice is thermogenesis. This suggests that a process related to gluconeogenesis of the ingested glutamate generates a rewarding stimulus, and that the glutamate-induced post-ingestive thermogenesis generates an aversive stimulus. These post-ingestive effects could be associated with MSG taste through learning and consequently influence MSG ingestion. We propose a hypothesis that these strain-specific rewarding or aversive postingestive effects of glutamate correspondingly stimulate (in B6 mice) or suppress (in 129 mice) MSG consumption in long-term tests. However, additional studies are needed to test this hypothesis.
4.2. Strain differences in taste perception of IMP
It has been suggested that in humans, IMP itself is tasteless, but it enhances the umami taste of the subthreshold concentrations of glutamate contained in saliva and thus can evoke a taste sensation [17, 18]. However, mouse gustatory nerves respond to lingual application of water solutions of IMP [19, 20], which suggests that IMP has a taste to mice. Consistent with this, in our current study we successfully conditioned mice to avoid IMP. We also found that patterns of CTA generalization were not identical in MSG- and IMP-conditioned mice, and therefore IMP taste cannot be explained only by enhancing taste of glutamate.
CTA to IMP generalized to MSG and saccharin solutions. There were some differences between B6 and 129 mice in CTA generalization patterns: compared with IMP-conditioned B6 mice, 129 mice had stronger licking suppression in response to saccharin and D-tryptophan. Amiloride had similar effects on licking rates in IMP-conditioned 129 and B6 mice: in mice from both strains, adding amiloride attenuated licking suppression for NaCl and MSG, but not for IMP.
These results suggest that 129 and B6 mice differ in their perception of IMP taste. While mice from both strains generalized CTA from IMP to MSG, 129 mice tended to have stronger CTA generalization to saccharin and D-tryptophan, both of which are perceived as sweet by humans. This suggests that in addition to MSG-like taste, 129 mice may also perceive IMP as having a sweet taste component. Several studies have shown perceptual [13-16] and neurophysiological [39] similarities between tastes of MSG and sucrose in rats. Our data suggest that a commonality of perception of umami and sweet taste stimuli may also exist in mice, but it is strain specific.
The difference between B6 and 129 mice in perception of IMP taste corresponds to differences between these strains in the chorda tympani nerve responses to IMP [20]. A larger neural response to IMP in 129 mice [20] may reflect a broader range of taste qualities evoked by IMP in these mice. The differential perception of IMP taste between 129 and B6 strains may contribute to the strain differences in IMP preference. However, this does not completely rule out a contribution of post-ingestive effects or their interactions with taste to the strain differences in IMP preference. Thus, further studies are needed to clarify the contributions of taste and post-ingestive effects to strain differences in IMP consumption.
4.3. Comparison of taste perception of MSG and IMP
Our finding that CTA generalized from IMP to MSG suggests that these two compounds evoke a shared taste quality. This is consistent with results of another CTA generalization study in mice [10]. However, there are also some perceptual differences between MSG and IMP. First, CTA of MSG did not significantly generalize to IMP. Second, CTA of IMP but not MSG generalized to saccharin. Third, addition of amiloride attenuated MSG licking suppression in MSG-conditioned mice, but it did not significantly affect IMP licking suppression in IMP-conditioned mice. This suggests that amiloride-blockable taste component is more prominent in MSG taste than in IMP taste (this could reflect either the five times greater Na+ concentration in 100 mM MSG relative to 10 mM IMP, or a different relative strength of salt and non-salt components of MSG and IMP tastes). The differences between tastes of MSG and IMP could be due not only to differences in cation (Na+) concentrations, but also to differential activation of umami taste receptors. There is evidence that multiple receptor mechanisms underlie umami taste (e.g., [5-7, 40-45]) and that glutamate and purine 5’-nucleotides may activate different receptors [20, 46].
4.4. Concluding remarks
In summary, our data suggest that mice perceive MSG and IMP as complex taste stimuli. Some taste components are shared between these two compounds, but their relative intensity is probably different for MSG and IMP. We found differences between 129 and B6 mice in generalization patterns of CTA to IMP, which suggests that the strain differences in IMP intake may depend on differences in the way the taste quality of IMP is perceived. However, there were no strain differences in generalization patterns of CTA to MSG. This suggests that the strain differences in MSG consumption cannot be attributed to variation in perception of the taste quality of MSG and must therefore arise from other factors, such as variation in the post-ingestive consequences of consuming MSG or differences in associative learning that integrates MSG taste and post-ingestive effects.
Acknowledgments
This study was supported by Fisheries Research Agency in Japan, Research Overseas Program (YM), NIH grant DC00882 (AB and GKB) and the Ajinomoto Amino Acid Research Program (AB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ikeda K. On a new seasoning. J Tokyo Chem Soc. 1909;30:820–836. [Google Scholar]
- 2.Kodama S. On the isolation of inosinic acid. J Tokyo Chem Soc. 1913;34:751–753. [Google Scholar]
- 3.Kuninaka A. Studies on taste of ribonucleic acid derivatives. J Agric Chem Soc Jpn. 1960;34:489–492. [Google Scholar]
- 4.Yamaguchi S. The synergistic taste effect of monosodium glutamate and disodium 5′ inosinate. J Food Sci. 1967;32:473–478. [Google Scholar]
- 5.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci. 2000;3:113–9. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- 6.San Gabriel A, Uneyama H, Yoshie S, Torii K. Cloning and characterization of a novel mGluR1 variant from vallate papillae that functions as a receptor for L-glutamate stimuli. Chem Senses. 2005;30(Suppl 1):i25–i26. doi: 10.1093/chemse/bjh095. [DOI] [PubMed] [Google Scholar]
- 7.Toyono T, Seta Y, Kataoka S, Kawano S, Shigemoto R, Toyoshima K. Expression of metabotropic glutamate receptor group I in rat gustatory papillae. Cell Tissue Res. 2003;313:29–35. doi: 10.1007/s00441-003-0740-2. [DOI] [PubMed] [Google Scholar]
- 8.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–6. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ninomiya Y, Funakoshi M. Behavioural discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol. 1989;92:365–370. doi: 10.1016/0300-9629(89)90577-x. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. III. Neural and behavioral measures compared. J Neurophysiol. 1985;53:1370–86. doi: 10.1152/jn.1985.53.6.1370. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste effects of ‘umami’ substances in hamsters as studied by electrophysiological and conditioned taste aversion techniques. Brain Res. 1988;451:147–162. doi: 10.1016/0006-8993(88)90759-7. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Matsuo R, Fujimoto Y, Fukanaga I, Miyasaka A, Imoto T. Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol Behav. 1991;49:919–925. doi: 10.1016/0031-9384(91)90204-2. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper S. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci. 1996;16:3817–3826. doi: 10.1523/JNEUROSCI.16-12-03817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stapleton JR, Roper SD, Delay ER. The taste of monosodium glutamate (MSG), L-aspartic acid, and N-methyl-D-aspartate (NMDA) in rats: are NMDA receptors involved in MSG taste? Chem Senses. 1999;24:449–57. doi: 10.1093/chemse/24.4.449. [DOI] [PubMed] [Google Scholar]
- 16.Heyer BR, Taylor-Burds CC, Tran LH, Delay ER. Monosodium glutamate and sweet taste: generalization of conditioned taste aversion between glutamate and sweet stimuli in rats. Chem Senses. 2003;28:631–41. doi: 10.1093/chemse/bjg056. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi S. Basic properties of umami and effects on humans. Physiol Behav. 1991;49:833–841. doi: 10.1016/0031-9384(91)90192-q. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi S. Basic properties of umami and its effects on food flavor. Food Review International. 1998;14:139–176. [Google Scholar]
- 19.Ninomiya Y, Funakoshi M. Peripheral neural basis for behavioural discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol. 1989;92:371–376. doi: 10.1016/0300-9629(89)90578-1. [DOI] [PubMed] [Google Scholar]
- 20.Inoue M, Beauchamp GK, Bachmanov AA. Gustatory neural responses to umami taste stimuli in C57BL/6ByJ and 129P3/J mice. Chem Senses. 2004;29:789–95. doi: 10.1093/chemse/bjh083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachmanov AA, Tordoff MG, Beauchamp GK. Intake of umami-tasting solutions by mice: a genetic analysis. J Nutr. 2000;130:935S–41S. doi: 10.1093/jn/130.4.935S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 23.Brand J, Teeter J, Silver W. Inhibition by amiloride of chorda tympani responses evoked by monovalent salts. Brain Res. 1985;334:207–214. doi: 10.1016/0006-8993(85)90212-4. [DOI] [PubMed] [Google Scholar]
- 24.Avenet P, Lindemann B. Amiloride-blockable sodium currents in isolated taste receptor cells. The Journal of membrane biology. 1988;105:245–55. doi: 10.1007/BF01871001. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya Y. Reinnervation of cross-regenerated gustatory nerve fibers into amiloride-sensitive and amiloride-insensitive taste receptor cells. Proc Natl Acad Sci USA. 1998;95:5347–50. doi: 10.1073/pnas.95.9.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida R, Horio N, Murata Y, Yasumatsu K, Shigemura N, Ninomiya Y. NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience. 2009;159:795–803. doi: 10.1016/j.neuroscience.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 27.Ohkuri T, Yasumatsu K, Shigemura N, Yoshida R, Ninomiya Y. Amiloride inhibition on NaCl responses of the chorda tympani nerve in two 129 substrains of mice, 129P3/J and 129X1/SvJ. Chem Senses. 2006;31:565–72. doi: 10.1093/chemse/bjj061. [DOI] [PubMed] [Google Scholar]
- 28.Yasumatsu K, Katsukawa H, Sasamoto K, Ninomiya Y. Recovery of amiloride-sensitive neural coding during regeneration of the gustatory nerve: behavioral-neural correlation of salt taste discrimination. J Neurosci. 2003;23:4362–8. doi: 10.1523/JNEUROSCI.23-10-04362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eylam S, Spector AC. Taste discrimination between NaCl and KCl is disrupted by amiloride in inbred mice with amiloride-insensitive chorda tympani nerves. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1361–8. doi: 10.1152/ajpregu.00796.2004. [DOI] [PubMed] [Google Scholar]
- 30.Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 2002;27:461–74. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- 31.Murata Y, Nakashima K, Yamada A, Shigemura N, Sasamoto K, Ninomiya Y. Gurmarin suppression of licking responses to sweetener-quinine mixtures in C57BL mice. Chem Senses. 2003;28:237–43. doi: 10.1093/chemse/28.3.237. [DOI] [PubMed] [Google Scholar]
- 32.Ninomiya Y, Kajiura H, Ishibashi T, Imai Y. Different responsiveness of the chorda tympani and glossopharyngeal nerves to L-lysine in mice. Chem Senses. 1994;19:617–26. doi: 10.1093/chemse/19.6.617. [DOI] [PubMed] [Google Scholar]
- 33.Manita S, Bachmanov AA, Li X, Beauchamp GK, Inoue M. Is glycine “sweet” to mice? Mouse strain differences in perception of glycine taste. Chem Senses. 2006;31:785–93. doi: 10.1093/chemse/bjl020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida M, Saito S. Multidimensional scaling of the taste of amino acids. Jpn Psychol Res. 1969;11:149–166. [Google Scholar]
- 35.Bartoshuk LM, Cain WS, Cleveland CT, Grossman LS, Marks LE, Stevens JC, Stolwijk JA. Saltiness of monosodium glutamate and sodium intake. J Am Med Assoc. 1974;230:670. doi: 10.1001/jama.1974.03240050018008. [DOI] [PubMed] [Google Scholar]
- 36.Ninomiya Y, Fukami Y, Yamazaki K, Beauchamp GK. Amiloride inhibition of chorda tympani responses to NaCl and its temperature dependency in mice. Brain Res. 1996;708:153–8. doi: 10.1016/0006-8993(95)01218-4. [DOI] [PubMed] [Google Scholar]
- 37.Gannon K, Contreras RJ. Sodium intake linked to amiloride-sensitive gustatory transduction in C57BL/6J and 129/J mice. Physiol Behav. 1993;57:231–239. doi: 10.1016/0031-9384(94)00279-e. [DOI] [PubMed] [Google Scholar]
- 38.Ji H, Bachmanov AA. Differences in postingestive metabolism of glutamate and glycine between C57BL/6ByJ and 129P3/J mice. Physiol Genomics. 2007;31:475–82. doi: 10.1152/physiolgenomics.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Formaker BK, Stapleton JR, Roper SD, Frank ME. Responses of the rat chorda tympani nerve to glutamate-sucrose mixtures. Chem Senses. 2004;29:473–482. doi: 10.1093/chemse/bjh049. [DOI] [PubMed] [Google Scholar]
- 40.Brand JG. Receptor and transduction processes for umami taste. J Nutr. 2000;130:942S–5S. doi: 10.1093/jn/130.4.942S. [DOI] [PubMed] [Google Scholar]
- 41.Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses. 2006 doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhari N, Maruyama Y, Roper S, Trubey K. Multiple pathways for signaling glutamate taste in rodents. Chem Senses. 2005;30(Suppl 1):i29–30. doi: 10.1093/chemse/bjh097. [DOI] [PubMed] [Google Scholar]
- 43.Maruyama Y, Pereira E, Margolskee RF, Chaudhari N, Roper SD. Umami responses in mouse taste cells indicate more than one receptor. J Neurosci. 2006;26:2227–34. doi: 10.1523/JNEUROSCI.4329-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasuo T, Kusuhara Y, Yasumatsu K, Ninomiya Y. Multiple receptor systems for glutamate detection in the taste organ. Biological & pharmaceutical bulletin. 2008;31:1833–7. doi: 10.1248/bpb.31.1833. [DOI] [PubMed] [Google Scholar]
- 45.Rong M, He W, Yasumatsu K, Kokrashvili Z, Perez CA, Mosinger B, Ninomiya Y, Margolskee RF, Damak S. Signal transduction of umami taste: insights from knockout mice. Chem Senses. 2005;30(Suppl 1):i33–4. doi: 10.1093/chemse/bjh099. [DOI] [PubMed] [Google Scholar]
- 46.Lin W, Ogura T, Kinnamon SC. Responses to di-sodium guanosine 5’-monophosphate and monosodium L-glutamate in taste receptor cells of rat fungiform papillae. J Neurophysiol. 2003;89:1434–9. doi: 10.1152/jn.00994.2002. [DOI] [PubMed] [Google Scholar]


