Abstract
Recent phylogenic studies indicate that DNA recombination could have occurred in ancient papillomaviruses types. However, no experimental data is available to demonstrate this event because of the lack of human papillomavirus infection models. We have used the cottontail rabbit papillomavirus (CRPV)/rabbit model to study pathogenesis and immunogenicity of different mutant genomes in vivo. Although the domestic rabbit is not a natural host for CRPV infection, it is possible to initiate infection with naked CRPV DNA cloned into a plasmid and monitor papilloma outgrowth on these animals. Taking advantage of a large panel of mutants based on a CRPV strain (Hershey CRPV), we tested the hypothesis that two non-viable mutant genomes could induce papillomas by either recombination or complementation. We found that co-infection with a dysfunctional mutant with an E2 transactivation domain mutation and another mutant with an E7 ATG knock out generated papillomas in rabbits. DNA extracted from these papillomas contained genotypes from both parental genomes. Three additional pairs of dysfunctional mutants also showed similar results. Individual wild type genes were also shown to rescue the function of corresponding dysfunctional mutants. Therefore, we suggest that complementation occurred between these two non-viable mutant PV genomes in vivo.
Keywords: CRPV, dysfunctional genomes, in vivo, DNA recombination, DNA complementation, rabbits, DNA infection
1. Introduction
The cottontail rabbit papillomavirus (CRPV)/ rabbit model has been used extensively in pathogenesis and vaccine development studies (Nicholls & Stanley, 2000;Christensen, 2005). This model has also played an important role in the development of virus-like particle (VLP) vaccines for clinical application (Breitburd, Kirnbauer et al., 1995;Christensen, Reed et al., 1996;Lowy & Schiller, 2006). One unique characteristic of this model system is that the infection can be initiated with CRPV DNA (Kreider, Cladel et al., 1995;Brandsma & Xiao, 1993). This feature has allowed us and others to generate large numbers of mutants for pathogenesis investigations (Nasseri, Meyers et al., 1989;Wu, Xiao et al., 1994;Salmon, Nonnenmacher et al., 2000;Jeckel, Huber et al., 2002;Hu, Cladel et al., 2002). Our previous studies have demonstrated a high capacity of the CRPV genome for modification without loss of viability (Hu, Cladel et al., 2007). However, some mutant genomes were defective and incapable of tumor formation in rabbits. These mutants were identified as non-viable or dysfunctional genomes.
A novel virus that was recently detected in papillomas and carcinomas of the endangered western barred bandicoot exhibits genomic features of both papillomaviridae and polyomaviridae. This observation suggests a recombination event might have occurred between these two ancient viruses (Woolford, Rector et al., 2007). Other phylogenetic studies have suggested that recombination may have occurred in variants from the same papillomavirus type. (Pushko, Sasagawa et al., 1994;Varsani, van der et al., 2006;Narechania, Chen et al., 2005;Angulo & Carvajal-Rodriguez, 2007;Bravo & Alonso, 2004). To demonstrate recombination of human papillomaviruses in vivo is challenging because these viruses are generally considered to be highly specific for their hosts although bovine papillomaviruses are able to cause nonproductive infections in horses and other only distantly related mammals(Bloch, Breen et al., 1994;Koller & Olson, 1972). To date, the lack of immunocompetent in vivo laboratory animal models has hindered the direct studies of HPV infection. Our dysfunctional mutants generated from the Hershey progressive strain of CRPV (H. CRPV, also identified as wild type CRPV) are all members of the same papillomavirus type. We postulated that one dysfunctional mutant CRPV genome (with a mutation in one gene) could rescue the function of another dysfunctional CRPV genome (with a mutation in another gene) by co-infection in vivo. The readout would be papilloma formation in rabbits. To avoid the argument that infections might have arisen from wild type CRPV contamination, we used mutants that contained a functional point mutation in the E6 gene in addition to defective mutations in either the E2 or E7 genes. These E6 point mutant genomes have been shown to be functional in previous studies (Hu, Cladel, et al., 2002). Among the pairs of dysfunctional genomes tested in New Zealand White (NZW) rabbits, four pairs were able to induce papillomas. DNA sequencing demonstrated that the genotype of the viral DNA extracted from these papillomas showed both parental genotypes. Therefore, we propose that in vivo complementation events have occurred between these two non-viable papillomavirus DNAs.
2. Materials and methods
2.1 Constructs
Hershey progressive strain CRPV (H.CRPV) cloned into PUC19 at SalI was identified as wild type CRPV and used as the backbone for all mutants (Hu, Cladel, et al., 2002). For convenience of cloning and handling, each selected individual viral gene was cloned into PUC19 for subsequent modification. Point mutations and deletions were introduced into each gene by using a QuickChange™ site-directed mutagenesis kit (Stratagene, La Jolla, CA.). Other insertions and replacements were made with routine restriction digest and ligation technology as described previously (Hu, Cladel,et al., 2007). All the mutations were confirmed by DNA sequence analysis at the Core Facility of Pennsylvania State University Hershey Medical Center. To avoid possible contamination by the wild type DNA, the mutants used for the complementation/recombination studies all contained a point mutation in E6 as an extra biomarker to distinguish them from the wild type E6. H.CRPVE6, E7 and E2 were also individually cloned into the expression vector PCR3 containing a CMV promoter (Invitrogen) for complementation studies. A tandem repeat construct that contained a dysfunctional mutant genome in sense orientation within a BglII fragment of CRPV DNA that contained the functional site was also generated (Figure 1). The constructs were purified by ultra-centrifugation on cesium chloride gradients and adjusted to 200μg/ml in 1× TE buffer (Hu, Cladel, et al., 2002;Hu, Cladel, et al., 2007) prior to challenge on animals.
Figure 1.
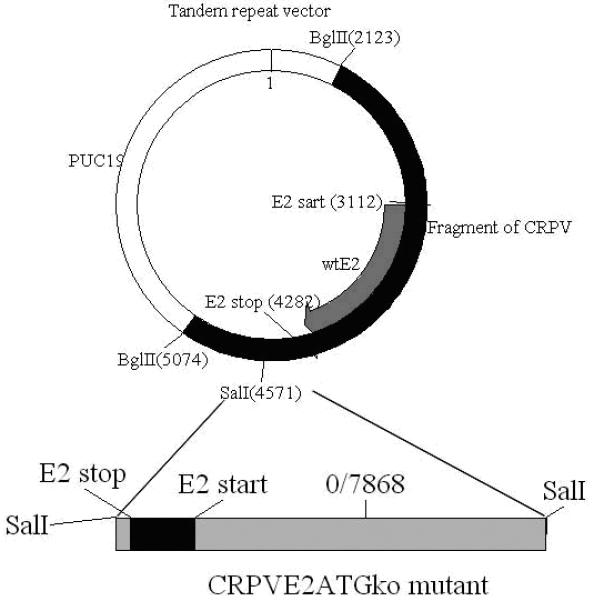
The BglII fragment (2123-5074bp) from the wild type CRPV genome cloned into PUC19 was used as the template for insertion of a whole CRPVE2ATGko mutant genome. A wild type CRPV E2 (3112-4282bp) gene was located inside this fragment. The CRPV E2ATGko dysfunctional genome was inserted in sense orientation at enzyme site SalI (4571bp) included inside this BglII fragment. The final construct contains one wild type CRPVE2 and one CRPVE2ATGko in sense orientation.
2.2 DNA challenge and monitoring of tumors
New Zealand White (NZW) rabbits were maintained in the animal facility of the Pennsylvania State University College of Medicine. The studies were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University. For application of viral DNA and viruses, rabbits were sedated using ketamine / xylazine anesthesia (Cladel, Hu et al., 2008). Back skin of the animals was scarified with a scalpel blade and superficially scratched. Three days later, again under sedation, each site was then challenged with 10 μg Hershey progressive CRPV DNA (wtCRPV) or a combination of two dysfunctional mutants (5 μg /5 μg ) in 50μl of 1×TE buffer. The infected sites were then scratched 20 times with a 21G needle to introduce small abrasions into the scab (Cladel, Hu, et al., 2008). Monitoring of papilloma outgrowth began three weeks after the infection and continued weekly until week 12. Papillomas were harvested for DNA isolation and histological examination.
2.3 DNA sequence confirmation from papillomas
Total genomic DNA was isolated from 20mg of each papilloma biopsy using the DNeasy Tissue Kit (QIAGEN) according to the manufacture's protocol. DNA extracts were then used as templates to amplify specific regions and also the region from the E6 to the E2 gene. The primers for the E6 and E7 region were: upstream primer 5′ GAC-AAC-TGC-CTG-CCG-CGG-TCG-CTA-GAG-AAG and down stream primer 5′ CCG-GAT-CCC-AGT-CAT-CGA-TAG-GGT-CTG-TAC and the primer pairs to amplify the E2 region were: upstream primer 5′ GGT-GAC-GAT-GGA-GGC-TCT-CA and down stream primer 5′ GTT-TTC-GTT-GCT-TTG-CCG. The primers for E6-E2 large fragment (about 4kb) were: upstream primer 5′ GAC-AAC-TGC-CTG-CCG-CGG-TCG-CTA-GAG-AAG and down stream primer 5′ GCGGAATTCCTAAAGCCCATAAAAATTCCC 3′. The E6-E2 long fragment was also cloned into PUC19. Clones from each mixture were sequenced to analyze for recombination. The PCR products for E6, E7 and E2 regions were purified with PCR purification kit (QIAGEN) and also sent for sequencing at the Core Facility of Pennsylvania State University Hershey Medical Center. The sequences were analyzed using the DNAMAN software (Lynnon BioSoft) and representative sequence regions were cropped with Chromas 2 (www.technelysium.com.au).
2.4 Histology
Papillomas induced by wild type CRPV DNA and mutant mixtures were harvested and fixed in 10% formalin. The tissue was processed as reported previously (Hu, Cladel et al., 2006).
2.5 Statistics
Papilloma size was determined by calculating the cubic root of the product of length × width × height of individual papillomas in millimeters to obtain a geometric mean diameter (GMD). Data were represented as the means (± SEMs) of the GMDs for each test group. Statistical significance was determined by unpaired t-test comparison (P<0.05 was considered significant).
3. Results
3.1 Dysfunctional mutant genome mixtures induced persistent papillomas in rabbits
Among the dysfunctional mutant genomes we tested, four combinations were able to induce papillomas in rabbits (Table 1). One combination [PJF192 (E6/G907A and E2/CTC del, a deletion in the E2 transactivation domain) plus PJF185 (E7/ATGko)] has been tested on 16 animals in total and 65% of the challenge sites grew papillomas. Some of the papillomas induced by these mixed mutants were comparable in size to papillomas grown from wild type CRPV DNA infection. However, the mean papilloma size induced by the mixture was significantly smaller when compared to that induced by the wild type CRPV DNA (Figure 2, P<0.05, unpaired student t test) at all the time points monitored. Similar results were found in the other combination infections (Figure 2, P<0.05, unpaired student t test). Most papillomas were persistent but some papillomas did regress on some animals (Table 2). The regression sites usually had small papillomas (GMD<3mm). We also noticed 2 tiny papillomas (GMD=1mm) that grew on two different animals that were infected with another mutant mixture (an E6 early stop mutant together with the E7ATGko mutant) but these sites quickly regressed.
Table 1.
Dysfunctional mutant CRPV genomes
| Constructs ID | Mutation sites causing Dysfunctional |
Additional mutation sites |
|---|---|---|
| PJF192 | E2/ 518-520bpdel | E6/G907A |
| PJF170 | E2/ 518-520bpdel | E6/G974A |
| PJF75 | E6/ 456bpstop | |
| PJF185 | E7ATGko |
Figure 2.
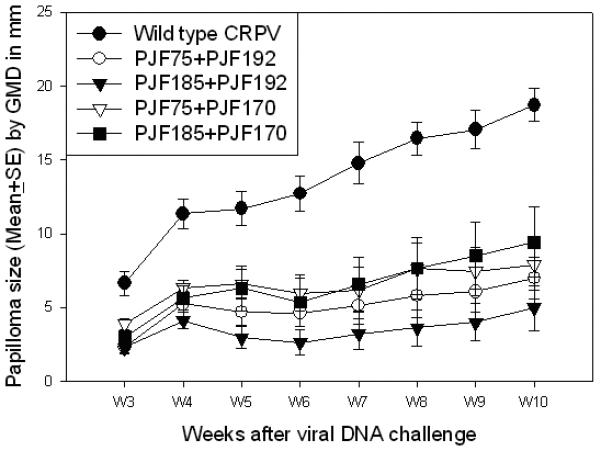
Papilloma outgrowth by four pairs of two dysfunctional CRPV mutant genomes. Significantly smaller papillomas were found at sites challenged with four pairs of genome mixtures: 1) CRPV with an early stop codon in E6 (PJF75, 5μg) together with CRPV with 3bp deletion in E2 transactivation domain plus G907A in E6 (PJF192, 5μg) ; 2) PJF75 (5μg) with 3bp deletion in E2 transactivation domain plus G974A (PJF170, 5μg); 3) CRPV with E7ATGko (PJF185, 5μg) with PJF170 (5μg) ; 4) PJF185 (5μg) with PJF192 (5μg) when compared to those challenged with wild type CRPV DNA (P<0.05, unpaired student T test). No significant difference in papilloma size was found between these four pairs of mixtures (P>0.05, unpaired student T test).
Table 2.
Papilloma outgrowth by two dysfunctional CRPV mutant genomes
| Constructs | Number of Rabbits |
Challenge sites/ rabbit |
Appearance rate |
Papilloma regressed |
|---|---|---|---|---|
| WtCRPV (10μg or 5μg) | 16 | 2 | 32/32 | 0 |
| PJF185(E7ATGko,10μg) | 6 | 2 | 0/12 | NA |
| PJF75(E6/456bpstop,10μg) | 3 | 2 | 0/6 | NA |
| PJF170(E2/518-520bp del+E6/G974A,10μg) |
4 | 2 | 0/8 | NA |
| PJF192(E2/518-520bp del+E6/G907A 10μg) |
8 | 2 | 0/16 | NA |
| PJF185+PJF170 (5μg+5μg) |
4 | 2 | 8/8 | 2 |
| PJF185+PJF192 (5μg+5μg) |
16 | 2 | 21/32 | 3 |
| PJF75+PJF192 (5μg+5μg) |
8 | 2 | 16/16 | 3 |
| PJF75+PJF170 (5μg+5μg) |
8 | 2 | 14/16 | 4 |
3.2 Papillomas induced by two mutant mixtures contained sequences from both parent DNA
To determine the mechanism for papilloma growth following infection with two dysfunctional mutant genomes, viral DNA from regions that discriminated between both mutants were checked for possible recombination. We amplified E6, E7 and a portion of the E2 gene that retained the mutation and deletion regions in order to perform sequencing. Chromatic features of two mixed populations of DNA sequences identical to the parent mutants are shown in supplementary Figure A-C. For example, DNA amplified from papillomas initiated with the two dysfunctional mutant genomes PJF185 (CRPVE7ATGko) and PJF192(CRPVE2CTCdel) showed a mixed population of wtE7, E7ATGko, wt E6, E6/G907A, wt E2 and E2CTCdel that represented the sequences from both parental genomes
To further determine if a recombinant genotype was present in the papillomas, a DNA fragment containing E6 through E2 was amplified from DNA extracted from the papillomas induced by the mixture of PJF185 plus PJF192 and was cloned into PUC19 vector. E6, E7 and E2 regions from these clones were sequenced using corresponding primers (Figure 3A-C). In addition to the clones that maintained parental genotypes, 3 out of 21 sequenced clones also contained a new genotype in which E6 and E7 was from PJF192 but E2 was from PJF185 (summarized in Table 3). To investigate whether this new genotype was an artifact generated from PCR amplification, eqimolar and nonequimolar amounts of PJF192 and PJF185 plasmid were used as templates for PCR amplification. The corresponding E6 through E2 fragment was subsequently cloned and sequenced. Interestingly, in contrast to what we have found from the papilloma DNA extract, the majority of the clones from these PCR products showed a genotype identical to that of PJF185 (15/20). In addition, a new genotype that contained E7ATGko and E2/518-520bpdel was identified. Therefore we concluded that the new genotype detected from the papilloma DNA extract might be a PCR amplification artifact (data not shown).
Figure 3.
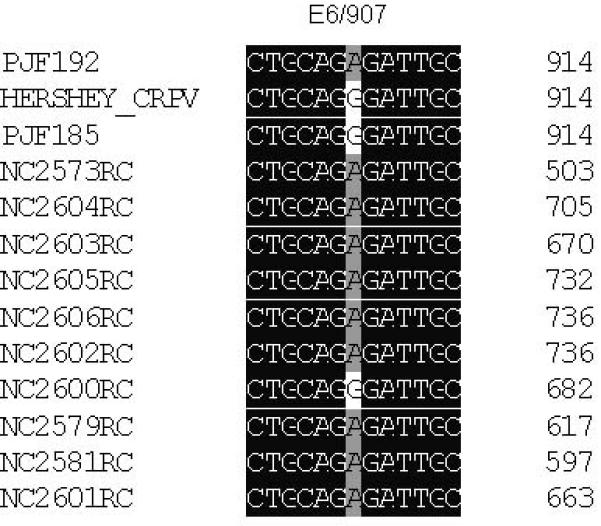
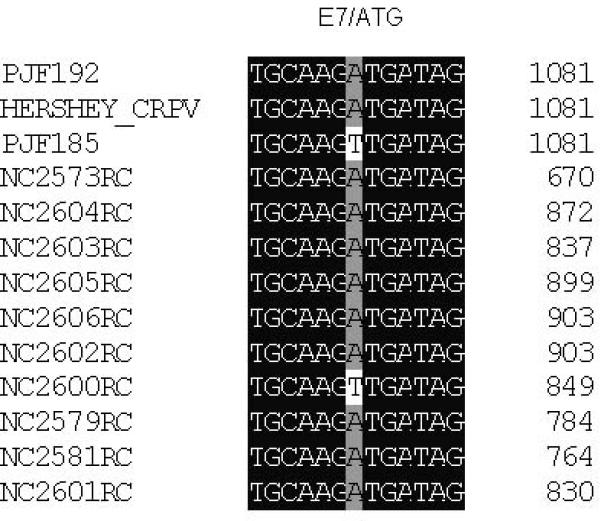
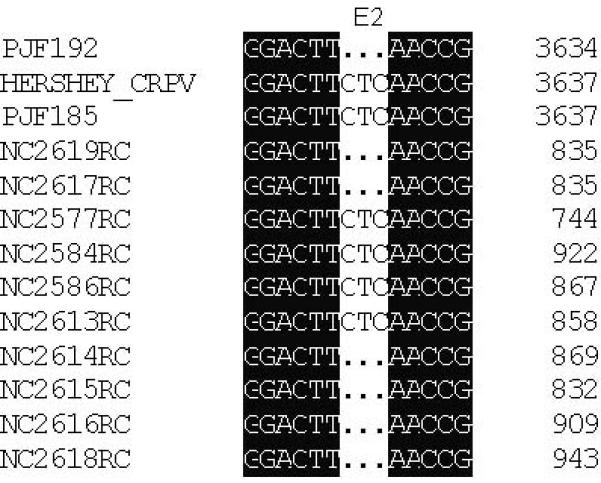
DNA alignment of clones amplified by PCR from papilloma DNA extracted from papillomas derived from mixtures of PJF192 and PJF185. The majority of clones showed an E6/G907A (A), a wild type E7 (B) and also E2/518-520bp del (C) that were identical to the sequence found in PJF192. Three clones (NC2573, NC2579, NC2581) out of the 31 clones which showed an identical mutation in E6 to that of PJF192 also had a wild type E2 (NC2577, NC2584, NC2586) to that of PJF185. The clone (NC2600) that has an E7ATGko mutation and a wild type E6 gene also has a wild type E2 (NC2613) that was identical to the sequence of PJF185.
Table 3.
Clones from papillomas induced by two dysfunctional CRPV mutant genomes
| Constructs | PJF185 | PJF192 | New genotype |
|---|---|---|---|
| PJF185+PJF192 | wtE6, E7ATGko and wtE2 (3 /31) |
E6/907A, wtE7 and E2/518-520bp del (25/31) |
E6/907A ,wtE7 and wtE2 (3 /31) |
3.3 Single gene products restored the function of the dysfunctional mutants
Because a majority of the clones from mixed dysfunctional mutant genomes maintained the parental DNA sequences, this indicated that in addition to the possibility of a recombination event, complementation may have contributed to the restored growth of the dysfunctional mutant CRPV genomes. To test this hypothesis, we infected rabbits with constructs that expressed the corresponding wild type gene product together with the dysfunctional CRPV genome mutant. Very tiny papillomas grew on three mixtures [CRPVE2ATGko plus PCR3E2 (2 papillomas/10 challenge sites), CRPVE6 early stop mutant plus PCR3E6 (2 papillomas/10 challenge sites) and CRPVE2CTCdel plus PCR3E2 (3 papillomas/10 challenge sites)] and half of these regressed rapidly. Although very few sites grew papillomas (when compared to those sites infected with wild type CRPV DNA), the presence of these papillomas further confirmed a role for complementation.
3.4 CRPVE2ATGko function was rescued when inserted into a plasmid containing wild type CRPV E2
Our previous studies have shown that a genome fragment cloned together with a full length CRPV genome in sense orientation increased the growth rate of papillomas when compared to single copy CRPV genome (unpublished observations). To investigate whether this strategy would rescue the function of a dysfunctional mutant genome, we cloned a CRPVE2ATGko mutant genome in sense orientation within a fragment of CRPV (Bgl II fragment from 2123bp to 5074bp) that contains a wild type E2 ORF (Figure 1). Papillomas grew at all sites that were challenged with this tandem repeat construct, but the size was significantly smaller when compared with those sites induced by wild type CRPV DNA (Figure 4, P<0.05, unpaired student t test). This study further confirmed that complementation could rescue a dysfunctional mutant genome.
Figure 4.
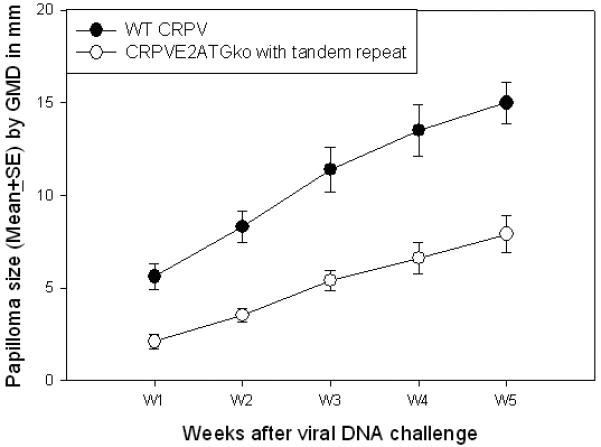
Papilloma outgrowth in rabbits challenged with wild type CRPV DNA and an E2ATGko mutant genome inserted as a tandem repeat plasmid that additionally contains a wild type E2 gene in sense orientation (see Materials and Methods and Figure 1). Significantly smaller tumors were found at sites challenged with the mutant when compared with those induced by wild type CRPV (P<0.05, unpaired student T test).
3.5 Histological analysis
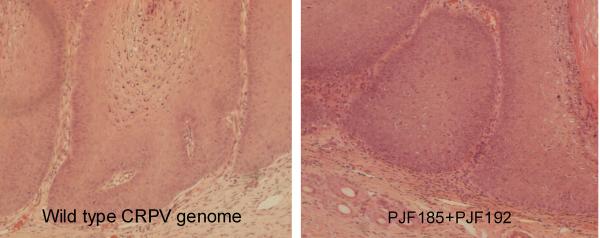
Papillomas induced by infectious wild type CRPV DNA and mixed mutant genomes were harvested at the termination of the study. The tissues were fixed with 10% formalin and processed with hematoxylin and eosin stain (H&E stain). Very similar histopathology was found for the papillomas induced by wild type DNA and two dysfunctional mutant mixtures (Figure 5).
Figure 5.
Hematoxylin and eosin (H&E) stain of papillomas induced by wild type CRPV DNA and a two dysfunctional mutant genome mixture. Very similar histological features were found for these two papilloma samples.
4. Discussion
Recombination in ancestors of the alpha papillomaviruses and between ancestors of papillomaviruses and polyomaviruses has been reported in several recent studies (Chan, Bernard et al., 1992;Schlecht, Burk et al., 2005;Angulo & Carvajal-Rodriguez, 2007) }. In vitro co-infection studies using primary keratinocytes have suggested evidence of co-existence and replication of two HPV DNAs in the same cell (McLaughlin-Drubin & Meyers, 2004). However, no in vivo experimental data has been reported to support evidence for recombination. One barrier for this is that no immunocompetent animal model can be used to directly study human papillomavirus infection (Fausch, Da Silva et al., 2003). Our previous study showed that co-infection with two HPV types (HPV40 and HPV11) resulted in regional separation or exclusion in human xenografts grown in athymic mice (Christensen, Koltun et al., 1997). However, whether mutants from the same HPV types can complement and /or recombine in vivo has not been studied. Taking advantage of dysfunctional mutant genomes generated from Hershey CRPV, we demonstrated for the first time that complementation between two CRPV genomes can occur in vivo.
The CRPV/rabbit model is an excellent model to investigate viral pathogenesis because this model mimics high-risk HPV induced malignancy in humans (Breitburd, Salmon et al., 1997;Campo, 2002;Brandsma, 2005). We have found that CRPV DNA has a high capacity to be modified without loss of function in vivo (Hu, Cladel et al., et al., 2007). Some of our mutants, however, did fail to induce papillomas on animals. These mutants had deletions or mutations in different early genes that are essential for papilloma outgrowth (Wu, Xiao, & Brandsma, 1994;Harry & Wettstein, 1996). To test whether one dysfunctional mutant DNA can rescue the function of a second dysfunctional genome, we mixed two mutants and tested for papilloma growth which is an excellent readout on rabbits. Most mixtures we checked retained their dysfunctional status. These results suggest that either complementation or recombination events do not occur frequently in vivo. However, four pairs of mutant mixtures were able to induce papillomas in animals at a growth rate that was significantly slower than papilloma growth from wild type DNA. One of the dysfunctional constructs had mutations in either the E6 or E7 gene while the other had mutations in the E2 gene. Several lines of evidence support the complementation of these two dysfunctional mutants in vivo. First, two parental DNA sequences coexisted in the papillomas suggesting that the two parent genomes were replicated; second, individual intact genes could rescue the function of the mutant; third, the successful application of tandem repeat strategy suggested that complementation from the wild type gene product rescued the dysfunctional mutant. Taken together, these data indicate that DNA complementation contributed to the rescue of one dysfunctional mutant genome by the other dysfunctional mutant genome.
It was interesting to observe an imbalance of replication of the two dysfunctional mutant genomes in their induced papillomas. In the mixture of PJF192 plus PJF185 induced papillomas, the PJF192 genome had a frequency of 80% while the PJF185 genome was found in only 10% of the clones. This result was not found when equimolar or non-equimolar amounts of PJF185 and PJF192 plasmid DNA were used as templates for PCR amplification. A replication advantage of one HPV type over the other type was also found in previous in vitro studies (McLaughlin-Drubin & Meyers, 2004). The reason that one mutant would have an advantage over the other in replication in situ is unclear and requires further investigation.
We observed that it took longer for most sites challenged with the mixed dysfunctional mutants to produce papillomas compared to those produced from wild type CRPV DNA. In addition, a slower growth rate of papillomas was observed. Because complementation requires both mutant genomes to be present in the same cell or that the gene products from both mutants be present in the same cell, the slow growth and longer delay by these mixtures might result from inefficient co-delivery. The observation that the tandem repeat genome, in which both wild type gene and dysfunctional genome were delivered into the same cell, increased the frequency of tumor appearance supports this hypothesis. Papilloma outgrowth also depends on the availability of the wild type gene product produced by the second dysfunctional mutant which might not be as vigorous as production from the wild type genome. Our previous studies also demonstrated that the genetic background of the host could play an important role in the outcome of tumor growth (Hu, Cladel et al., 2002;Hu, Peng et al., 2005). In these studies, we used outbred NZW rabbits which have a highly diverse genetic background. Although we achieved very consistent results with wild type CRPV DNA infection from experiment to experiment, the results with the mixed infections of dysfunctional CRPV genomes were more variable. Nevertheless, some papillomas from infection with dysfunctional DNA mixtures grew at a comparable rate and size to those from wild type CRPV infections and lesions remained persistent.
In summary, we report that two dysfunctional CRPV mutant genomes showed complementation in vivo to produce papillomas on animals. These results represent the first study to demonstrate papillomavirus complementation in vivo.
Supplementary Material
DNA sequence profile for DNA amplified from the papillomas induced by mixed mutant CRPV genomes. Two populations of DNA identical to the parent DNA were found in the papillomas. A) Two different DNA sequences in E6 representing two parental DNA sequences [E6/G974A, E6/G907A, E6/465bp stop (CCG/TAG) or wild type]; B) Two different DNA sequences in E7 representing two parental DNA sequences were found [E7ATG ko (TTG) and wild type (ATG)]; C) Two different DNA sequences in E2 representing two parental DNA sequences (E2 CTC deletion causing a shift in the sequence and wild type).
Acknowledgements
We thank Martin Pickel for excellent help with the animals. This work was supported by the National Cancer Institute grant RO1 CA47622 from the National Institutes of Health and the Jake Gittlen Memorial Golf Tournament.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angulo M, Carvajal-Rodriguez A. Evidence of recombination within human alpha-papillomavirus. Virol.J. 2007;4:33. doi: 10.1186/1743-422X-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch N, Breen M, Spradbrow PB. Genomic sequences of bovine papillomaviruses in formalin- fixed sarcoids from Australian horses revealed by polymerase chain reaction. Vet.Microbiol. 1994;41:163–172. doi: 10.1016/0378-1135(94)90145-7. [DOI] [PubMed] [Google Scholar]
- Brandsma JL. The cottontail rabbit papillomavirus model of high-risk HPV-induced disease. Methods Mol.Med. 2005;119:217–235. doi: 10.1385/1-59259-982-6:217. [DOI] [PubMed] [Google Scholar]
- Brandsma JL, Xiao W. Infectious virus replication in papillomas induced by molecularly cloned cottontail rabbit papillomavirus DNA. Journal of Virology. 1993;67:567–571. doi: 10.1128/jvi.67.1.567-571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo IG, Alonso A. Mucosal human papillomaviruses encode four different E5 proteins whose chemistry and phylogeny correlate with malignant or benign growth. Journal of Virology. 2004;78:13613–13626. doi: 10.1128/JVI.78.24.13613-13626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. Journal of Virology. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitburd F, Salmon J, Orth G. The rabbit viral skin papillomas and carcinomas: a model for the immunogenetics of HPV-associated carcinogenesis. Clin.Dermatol. 1997;15:237–247. doi: 10.1016/s0738-081x(97)00009-6. [DOI] [PubMed] [Google Scholar]
- Campo MS. Animal models of papillomavirus pathogenesis. Virus Research. 2002;89:249–261. doi: 10.1016/s0168-1702(02)00193-4. [DOI] [PubMed] [Google Scholar]
- Chan S-Y, Bernard H-U, Ong C-K, Chan S-P, Hofmann B, Delius H. Phylogenetic analysis of 48 papillomavirus types and 28 subtypes and variants: A showcase for the molecular evolution of DNA viruses. Journal of Virology. 1992;66:5714–5725. doi: 10.1128/jvi.66.10.5714-5725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ND. Cottontail rabbit papillomavirus (CRPV) model system to test antiviral and immunotherapeutic strategies. Antivir.Chem.Chemother. 2005;16:355–362. doi: 10.1177/095632020501600602. [DOI] [PubMed] [Google Scholar]
- Christensen ND, Koltun WA, Cladel NM, Budgeon LR, Reed CA, Kreider JW, Welsh PA, Patrick SD, Yang H. Coinfection of human foreskin fragments with multiple human papillomavirus types (HPV-11, -40, -LVX82/MM7) produces regionally separate HPV infections within the same athymic mouse xenograft. Journal of Virology. 1997;71:7337–7344. doi: 10.1128/jvi.71.10.7337-7344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ND, Reed CA, Cladel NM, Han R, Kreider JW. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. Journal of Virology. 1996;70:960–965. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cladel NM, Hu J, Balogh K, Mejia A, Christensen ND. Wounding prior to challenge substantially improves infectivity of cottontail rabbit papillomavirus and allows for standardization of infection. J.Virol.Methods. 2008;148:34–39. doi: 10.1016/j.jviromet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausch SC, Da Silva DM, Eiben GL, Le Poole IC, Kast WM. HPV protein/peptide vaccines: from animal models to clinical trials. Front Biosci. 2003;8:s81–s91. doi: 10.2741/1009. [DOI] [PubMed] [Google Scholar]
- Harry JB, Wettstein FO. Transforming properties of the cottontail rabbit papillomavirus oncoproteins LE6 and SE6 and of the E8 protein. Journal of Virology. 1996;70:3355–3362. doi: 10.1128/jvi.70.6.3355-3362.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Cladel NM, Balogh K, Budgeon L, Christensen ND. Impact of genetic changes to the CRPV genome and their application to the study of pathogenesis in vivo. Virology. 2007;358:384–390. doi: 10.1016/j.virol.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Cladel NM, Budgeon LR, Reed CA, Pickel MD, Christensen ND. Protective cell-mediated immunity by DNA vaccination against Papillomavirus L1 capsid protein in the Cottontail Rabbit Papillomavirus model. Viral Immunology. 2006;19:492–507. doi: 10.1089/vim.2006.19.492. [DOI] [PubMed] [Google Scholar]
- Hu J, Cladel NM, Pickel MD, Christensen ND. Amino Acid residues in the carboxy-terminal region of cottontail rabbit papillomavirus e6 influence spontaneous regression of cutaneous papillomas. Journal of Virology. 2002;76:11801–11808. doi: 10.1128/JVI.76.23.11801-11808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Peng X, Cladel NM, Pickel MD, Christensen ND. Large cutaneous rabbit papillomas that persist during cyclosporin A treatment can regress spontaneously after cessation of immunosuppression. Journal of General Virology. 2005;86:55–63. doi: 10.1099/vir.0.80448-0. [DOI] [PubMed] [Google Scholar]
- Jeckel S, Huber E, Stubenrauch F, Iftner T. A transactivator function of cottontail rabbit papillomavirus e2 is essential for tumor induction in rabbits. Journal of Virology. 2002;76:11209–11215. doi: 10.1128/JVI.76.22.11209-11215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller LD, Olson C. Attempted transmission of warts from man, cattle and horses and of deer fibroma to selected hosts. Journal of Investigative Dermatology. 1972;58:366–368. doi: 10.1111/1523-1747.ep12540579. [DOI] [PubMed] [Google Scholar]
- Kreider JW, Cladel NM, Patrick SD, Welsh PA, DiAngelo SL, Bower JM, Christensen ND. High efficiency induction of papillomas in vivo using recombinant cottontail rabbit papillomavirus DNA. J.Virol.Methods. 1995;55:233–244. doi: 10.1016/0166-0934(95)00062-y. [DOI] [PubMed] [Google Scholar]
- Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines 1. J.Clin.Invest. 2006;116:1167–1173. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Meyers C. Evidence for the coexistence of two genital HPV types within the same host cell in vitro. Virology. 2004;321:173–180. doi: 10.1016/j.virol.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Narechania A, Chen Z, DeSalle R, Burk RD. Phylogenetic incongruence among oncogenic genital alpha human papillomaviruses. Journal of Virology. 2005;79:15503–15510. doi: 10.1128/JVI.79.24.15503-15510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri M, Meyers C, Wettstein FO. Genetic analysis of CRPV pathogenesis: The L1 open reading frame is dispensable for cellular transformation but is required for papilloma formation. Virology. 1989;170:321–325. doi: 10.1016/0042-6822(89)90388-7. [DOI] [PubMed] [Google Scholar]
- Nicholls PK, Stanley MA. The immunology of animal papillomaviruses. Vet.Immunol.Immunopathol. 2000;73:101–127. doi: 10.1016/s0165-2427(99)00165-8. [DOI] [PubMed] [Google Scholar]
- Pushko P, Sasagawa T, Cuzick J, Crawford L. Sequence variation in the capsid protein genes of human papillomavirus type 16. Journal of General Virology. 1994;75(Pt 4):911–916. doi: 10.1099/0022-1317-75-4-911. [DOI] [PubMed] [Google Scholar]
- Salmon J, Nonnenmacher M, Caze S, Flamant P, Croissant O, Orth G, Breitburd F. Variation in the nucleotide sequence of cottontail rabbit papillomavirus a and b subtypes affects wart regression and malignant transformation and level of viral replication in domestic rabbits. Journal of Virology. 2000;74:10766–10777. doi: 10.1128/jvi.74.22.10766-10777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht NF, Burk RD, Palefsky JM, Minkoff H, Xue X, Massad LS, Bacon M, Levine AM, Anastos K, Gange SJ, Watts DH, Da Costa MM, Chen Z, Bang JY, Fazzari M, Hall C, Strickler HD. Variants of human papillomaviruses 16 and 18 and their natural history in human immunodeficiency virus-positive women. Journal of General Virology. 2005;86:2709–2720. doi: 10.1099/vir.0.81060-0. [DOI] [PubMed] [Google Scholar]
- Varsani A, van der WE, Heath L, Rybicki EP, Williamson AL, Martin DP. Evidence of ancient papillomavirus recombination. Journal of General Virology. 2006;87:2527–2531. doi: 10.1099/vir.0.81917-0. [DOI] [PubMed] [Google Scholar]
- Woolford L, Rector A, Van Ranst M, Ducki A, Bennett MD, Nicholls PK, Warren KS, Swan RA, Wilcox GE, O'Hara AJ. A novel virus detected in papillomas and carcinomas of the endangered western barred bandicoot (Perameles bougainville) exhibits genomic features of both the Papillomaviridae and Polyomaviridae. Journal of Virology. 2007;81:13280–13290. doi: 10.1128/JVI.01662-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Xiao W, Brandsma J. Papilloma formation by cottontail rabbit papillomavirus requires E1 and E2 regulatory genes in addition to E6 and E7 transforming genes. Journal of Virology. 1994;68:6097–6102. doi: 10.1128/jvi.68.9.6097-6102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DNA sequence profile for DNA amplified from the papillomas induced by mixed mutant CRPV genomes. Two populations of DNA identical to the parent DNA were found in the papillomas. A) Two different DNA sequences in E6 representing two parental DNA sequences [E6/G974A, E6/G907A, E6/465bp stop (CCG/TAG) or wild type]; B) Two different DNA sequences in E7 representing two parental DNA sequences were found [E7ATG ko (TTG) and wild type (ATG)]; C) Two different DNA sequences in E2 representing two parental DNA sequences (E2 CTC deletion causing a shift in the sequence and wild type).