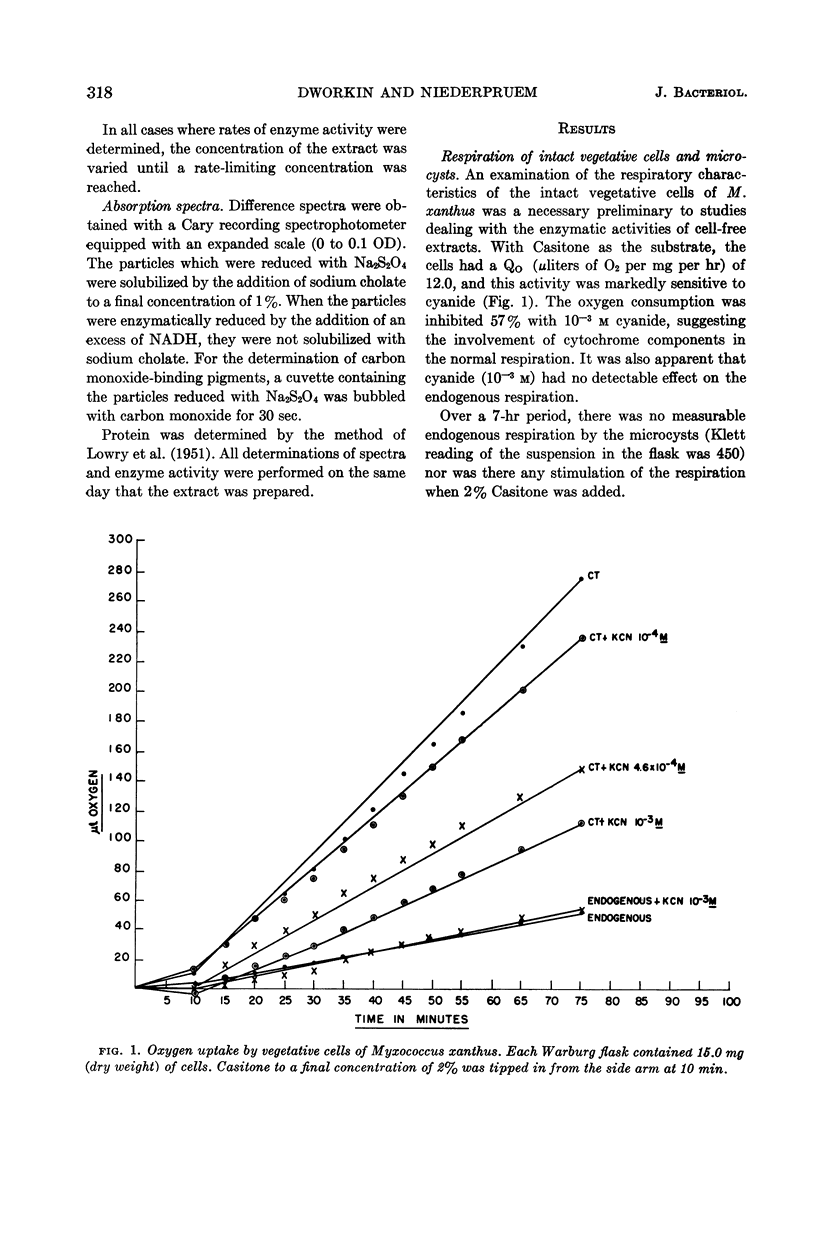
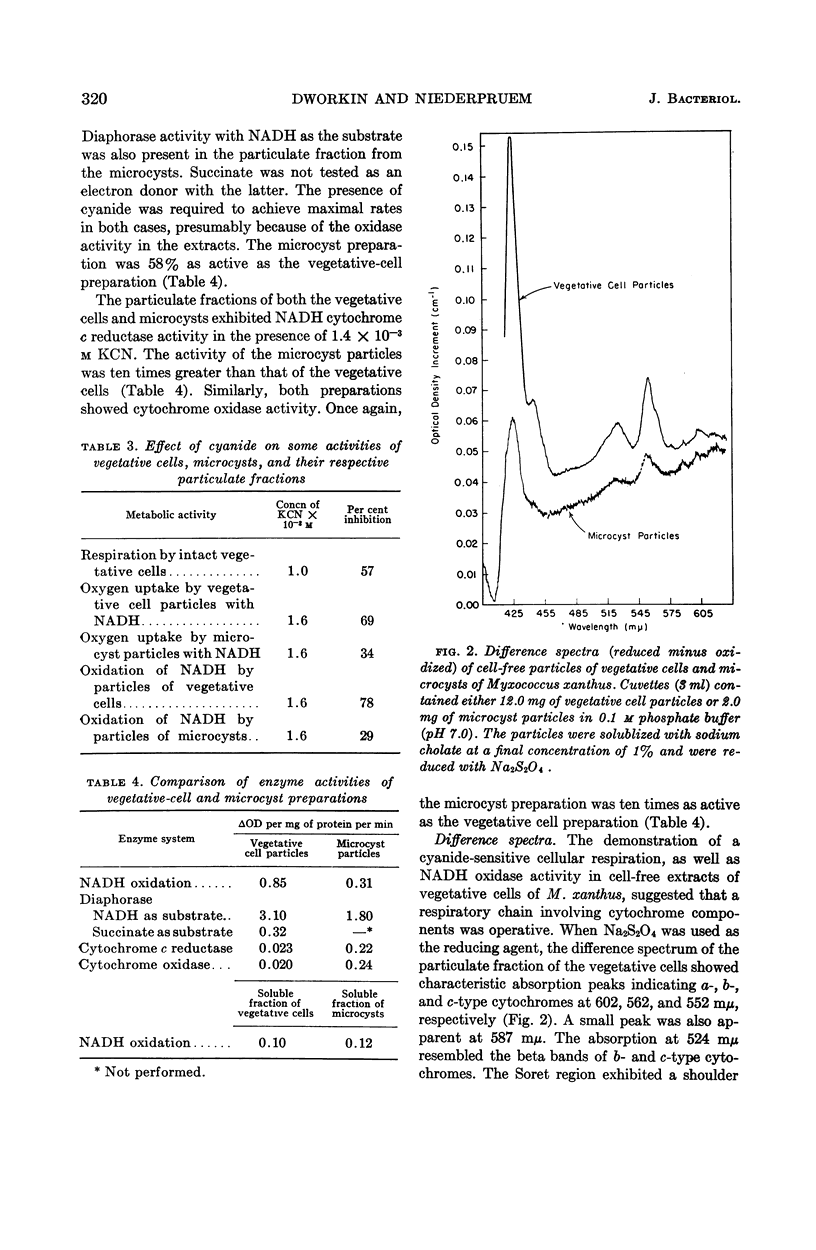
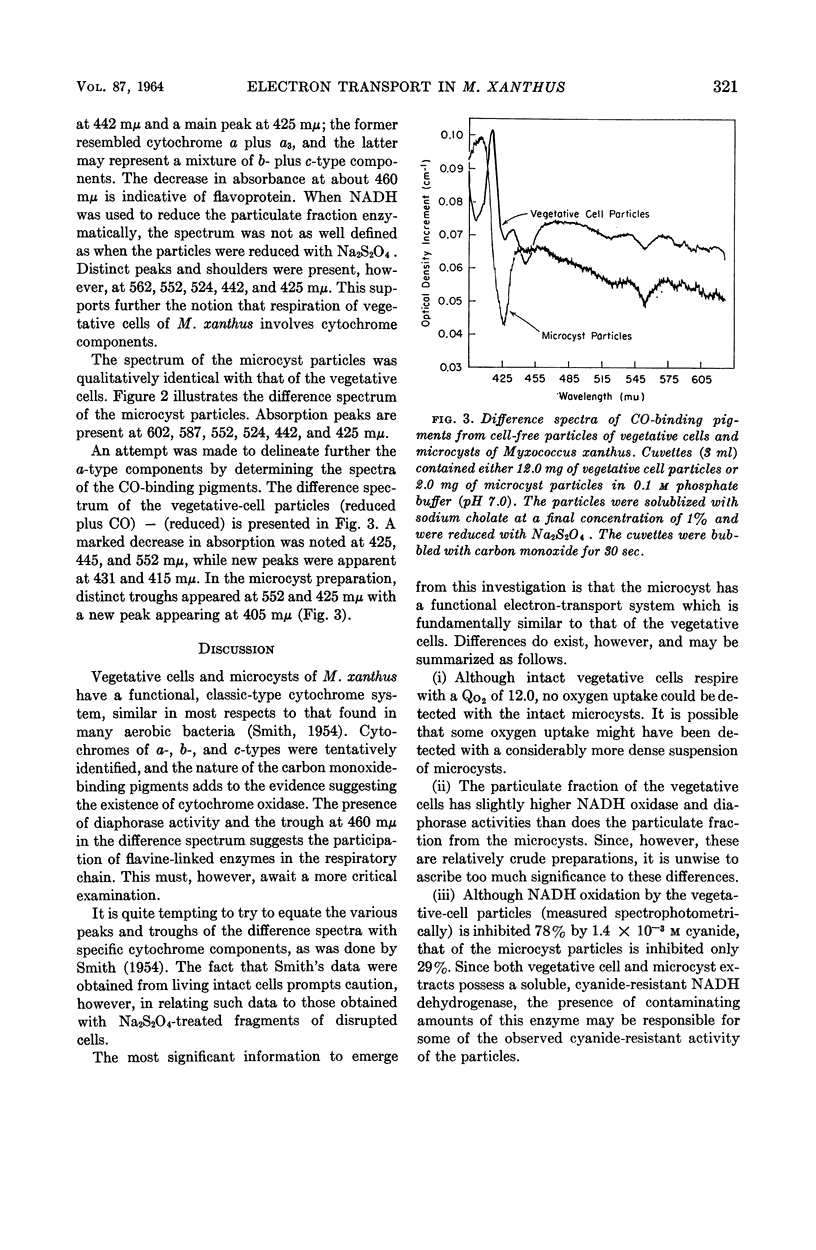
Abstract
Dworkin, Martin (University of Minnesota, Minneapolis), and Donald J. Niederpruem. Electron transport system in vegetative cells and microcysts of Myxococcus xanthus. J. Bacteriol. 87:316–322. 1964.—Respiration by intact cells of the fruiting myxobacterium Myxococcus xanthus is cyanide-sensitive and can be demonstrated in the vegetative cells but not in the microcysts. Cell-free particles from both vegetative cells and microcysts have cyanide-sensitive reduced nicotinamide adenine dinucleotide (NADH) oxidase, diaphorase, NADH cytochrome c reductase, and cytochrome oxidase activities. While the vegetative cell specific activities for NADH oxidase and diaphorase are slightly higher than those for the microcysts, the microcysts have ten times the cytochrome c reductase and cytochrome oxidase activities of the vegetative cells. Furthermore, the respiration of the microcyst particles is considerably less cyanide-sensitive than is that of the vegetative-cell particles. Difference spectra of the cell-free particles of vegetative cells and microcysts are qualitatively identical, showing the presence of b- and c-type cytochrome and flavoprotein. The a-type pigments are clearly present in the extracts of the vegetative cells and are suggested by the spectrum of the microcyst particles. The cytochrome oxidase activity of both extracts is consistent with the presence of a-type pigments in both. The spectra of the carbon monoxide-binding pigments were determined and, by this parameter, qualitative differences appear between the vegetative cells and the microcysts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DWORKIN M. NUTRITIONAL REGU.ATION OF MORPHOGENESIS IN MYXOCOCCUS XANTHUS. J Bacteriol. 1963 Jul;86:67–72. doi: 10.1128/jb.86.1.67-72.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SMITH L. Bacterial cytochromes; difference spectra. Arch Biochem Biophys. 1954 Jun;50(2):299–314. doi: 10.1016/0003-9861(54)90045-4. [DOI] [PubMed] [Google Scholar]
- VOELZ H., DWORKIN M. Fine structure of Myxococcus xanthus during morphogenesis. J Bacteriol. 1962 Nov;84:943–952. doi: 10.1128/jb.84.5.943-952.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


