Abstract
CYR61 is a secreted, cysteine-rich, heparin-binding protein encoded by a growth factor-inducible immediate–early gene. Acting as an extracellular, matrix-associated signaling molecule, CYR61 promotes the adhesion of endothelial cells through interaction with the integrin αVβ3 and augments growth factor-induced DNA synthesis in the same cell type. In this study, we show that purified CYR61 stimulates directed migration of human microvascular endothelial cells in culture through an αVβ3-dependent pathway and induces neovascularization in rat corneas. Both the chemotactic and angiogenic activities of CYR61 can be blocked by specific anti-CYR61 antibodies. Whereas most human tumor-derived cell lines tested express CYR61, the gastric adenocarcinoma cell line RF-1 does not. Expression of the CYR61 cDNA under the regulation of a constitutive promoter in RF-1 cells significantly enhances the tumorigenicity of these cells as measured by growth in immunodeficient mice, resulting in tumors that are larger and more vascularized than those produced by control RF-1 cells. Taken together, these results identify CYR61 as an angiogenic inducer that can promote tumor growth and vascularization; the results also suggest potential roles for CYR61 in physiologic and pathologic neovascularization.
Polypeptide growth factors regulate a diverse array of cellular processes, including cell migration, proliferation, and differentiation. In the organism, integration of growth factor signaling and environmental cues from the extracellular matrix specifies tissue and cellular identities during growth and development (1, 2). The genomic responses to growth factor stimulation include the rapid activation of a set of immediate–early genes, which encode proteins thought to mediate the biological effects of the growth factors (3, 4). One such immediate–early gene identified in fibroblasts is CYR61 (5), which encodes a secreted, 40-kDa, cysteine-rich and heparin-binding protein that associates with the extracellular matrix and cell surface (6). In recent studies, both recombinant human and mouse forms of CYR61 were purified and found to promote cell adhesion and augment growth factor-induced DNA synthesis in fibroblasts and endothelial cells and to stimulate cell migration in fibroblasts (7–9). Furthermore, CYR61 mediates the adhesion of human umbilical vein endothelial cells through interaction with the integrin αVβ3 (10), an adhesion receptor known to be involved in signaling that regulates a myriad of cellular processes, many of which also are regulated by signaling through growth factor receptors (11).
Angiogenesis, the formation of new blood vessels from existing vessels, is essential for a variety of normal biological events, including embryonic and placental development, wound healing, ovulation, and lactation (12). In addition, abnormal angiogenesis is an important factor in diseases such as diabetic retinopathy, arthritis, psoriasis, atherosclerosis, and cancer (12, 13). In particular, growth of solid tumors beyond a few millimeters in diameter is absolutely dependent on vascularization, which supplies nutrients to tumor cells and also may provide a conduit for the circulation of metastatic cells (14, 15).
We hypothesized that CYR61 may play a role in angiogenesis for several reasons: (i) CYR61 mediates endothelial cell adhesion and augments growth factor-induced DNA synthesis in the same cell type (7); (ii) CYR61 is expressed at sites of neovascularization, including the developing blood vessels of the mouse embryo (16), the trophectodermal and the placental vasculature (8, 16, 17), hypertrophic cartilage (17), and granulation tissues, during wound healing (17); and (iii) CYR61 is a ligand of the integrin αVβ3 (10), which is known to be involved in angiogenesis (18, 19). In this report, we demonstrate that purified CYR61 protein stimulates directed migration of human microvascular endothelial cells in culture through an αVβ3-dependent pathway and induces neovascularization in rat corneas. Furthermore, expression of CYR61 in tumor cells promotes tumor growth and vascularization. These results identify CYR61 as an angiogenic factor and suggest potential roles for CYR61 in both physiologic and pathologic angiogenesis.
MATERIALS AND METHODS
Cell Culture, Plasmids, and Transfection.
Primary human dermal microvascular endothelial cells (HMVECs) isolated from a single neonatal donor were obtained from a commercial source (Clonetics, San Diego) and were grown in endothelial cell basal medium (EBM, Clonetics). All other human cell lines were obtained from and grown according to the specifications of the American Type Culture Collection; these cell lines include RF-1 (CRL-1864) and RF-48 (CRL-1863) gastric adenocarcinoma, RT-4 (HTB-2) bladder papilloma, HT-29 (HTB-38) colon adenocarcinoma, SK-MEL-2 (HTB-68) melanoma, TE-671 (CRL-8805) medulloblastoma, MCF-7 (HTB-22) mammary adenocarcinoma, and HT-1080 (CCL-121) fibrosarcoma cells. The expression vector pL61SN (a generous gift of T. P. O’Brien, The Jackson Laboratory) was constructed by placing the full length mouse cyr61 cDNA (5) under the control of the retroviral long terminal repeat in the vector pLXSN (20). Transfection of RF-1 cells was carried out by electroporation as described (21), and transfectants were selected for neomycin resistance in the presence of 350 μg/ml G418.
Expression of CYR61.
Expression of CYR61 in various cell lines was determined by reverse transcriptase–PCR by using total RNA isolated as described (22). cDNA synthesis was carried out by using the SuperScript preamplification system (GIBCO/BRL) followed by PCR using Taq polymerase to yield a 731-bp CYR61-specific fragment using the primers 5′-GGCTGCGGCTGCTGTAAGGTC-3′ and 5′-GTTCTGGGGATTTCTTGGTCT-3′. Expression of glyceraldehyde-3-phosphate dehydrogenase was detected simultaneously by the addition of specific primers as an internal control. Immunoprecipitations were carried out as described (23).
Proteins and Antibodies.
Recombinant human basic fibroblast growth factor (bFGF) was obtained from GIBCO/BRL; vascular endothelial growth factor (VEGF) was obtained from R & D Systems. Recombinant human CYR61 protein was purified from serum-free conditioned media of Sf-9 insect cells programmed for synthesis of the CYR61 protein via a baculovirus expression vector (7, 9). The purified CYR61 protein was nearly homogeneous, as judged by SDS/PAGE. Affinity-purified anti-CYR61 antibodies (7) were prepared from polyclonal rabbit antisera raised against a TrpE–CYR61 fusion protein (5, 6). The antibodies recognize both the human and mouse forms of CYR61, which share a 91% amino acid sequence identity but are highly specific for CYR61 and do not cross react with the structurally related family member Fisp12, which is purified in a similar manner (8). mAb against the integrin αVβ3 (LM609) was obtained from Chemicon.
Chemotaxis of Microvascular Endothelial Cells.
The effect of CYR61 and bFGF on cell migration was assessed in 48-well modified Boyden chambers (Neuroprobe, Cabin John, MD) as described (24). HMVECs were serum-starved in EBM for 24 hr, resuspended (7 × 105 cells/ml) in EBM plus 0.1% BSA, and plated on the lower surface of a gelatinized polycarbonated membrane of 5-μm pore size (Costar). Cells were allowed 2 hr to attach; the chambers were inverted thereafter, and test substances were added to the upper chamber. The membrane was removed after 4 hr of incubation and was stained with Diff-Quik (Baxter Diagnostics, McGraw Park, IL). Cells that migrated through the membrane to the upper chamber were counted under the microscope. Each experimental sample was tested in quadruplicate. To determine basal migration, plating medium (EBM plus 0.1% BSA) plus the corresponding amount of the CYR61 buffer (50 mM Mes, pH 6.0/2 mM EDTA/0.5 mM phenylmethylsulfonyl fluoride/0.6 M NaCl) was used as a test substance. Where indicated, bFGF was added at 10 ng/ml in the same medium containing CYR61 buffer. Toxicity of various test substances was evaluated by treating cells with each test substance in parallel in 24-well plates and measuring trypan blue exclusion at the end of the experiment; in no cases was toxicity observed (data not shown).
Rat Corneal Pocket Angiogenesis Assay.
Neovascularization in vivo was examined by implanting samples into the rat cornea (24). Male Sprague–Dawley rats were anesthetized by i.v. injection of sodium pentobarbital, and ≈5-μl Hydron pellets (Interferon Sciences, New Brunswick, NJ) containing test samples were implanted into pockets made in normal avascular corneal stroma 1–1.5 mm from the corneal limbus. Animals were perfused with colloidal carbon after 7 days, and corneal responses were scored as positive if vessel ingrowth was sustained and vigorous and if most vessels reached the pellet.
Tumor Growth in Nude Mice.
Stably transfected pools of human gastric adenocarcinoma RF-1 cells (5 × 106 cells/site) were injected s.c. into severe combined immunodeficient/beige mice (Harlan–Sprague–Dawley). Each animal was injected at two sites in the flanks, and tumors were harvested 32 days thereafter. Samples were individually weighed after removal and before fixation, and results were expressed as mean ± SEM. Statistical analysis was performed by using the Student’s t test and by ANOVA followed by the Tukey–Kramer multiple comparison test.
Immunoblotting and Immunohistochemistry.
For immunoblotting, RF-1 cells transfected with pL61SN or pLXSN were lysed in RIPA buffer [150 mM NaCl/1.0% Nonidet P-40/0.5% deoxycholate/0.1% SDS/50 mM Tris, pH 8.0/10 μl/ml Protease inhibitor mixture (Sigma)]. Total protein (100 μg) from each lysate was resolved by SDS/PAGE, was electroblotted (25), was probed with anti-CYR61 antibodies and peroxidase-labeled goat anti-rabbit IgG (Kirkegaard & Perry Laboratories), and was detected via enhanced chemiluminescence reagent (Amersham). For immunohistochemistry, frozen sections of tumors were incubated for 16 hr at 4°C with biotinylated rat anti-mouse CD31 (MEC 13.3; PharMingen) or affinity-purified anti-CYR61 antibody or with the same antibody preincubated with purified CYR61 protein for 1 h. Slides were washed in PBS and were incubated for 1 h with biotinylated rabbit anti-rat IgG (Zymed Laboratories) or goat anti-rabbit IgG (Vector Laboratories), then were developed with a Renaissance indirect tyramide signal amplification (TSA) kit (NEN) and were counterstained with hematoxylin before mounting. Vascular density was determined by two independent observers counting vessels in the nine most highly vascularized fields (×500) per tumor sample (excluding necrotic areas). Results were expressed as mean ± SEM; statistical analysis was performed as above.
RESULTS
CYR61 Stimulates Directed Migration of HMVEC.
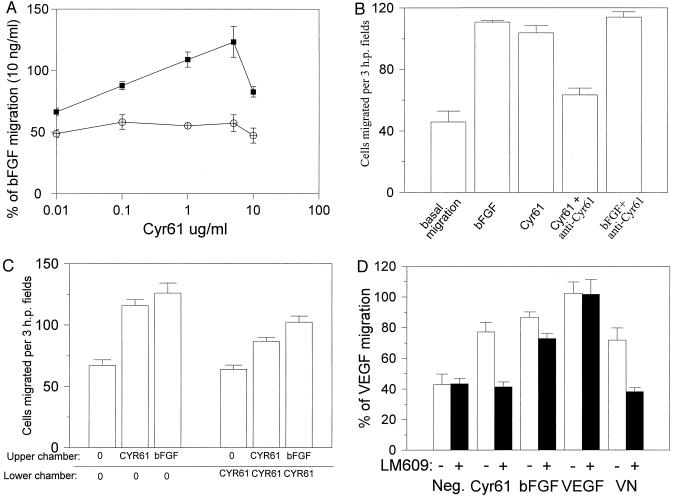
To assess the potential angiogenic activity of CYR61, we first evaluated its ability to induce microvascular endothelial cell migration. As shown in Fig. 1A, CYR61-stimulated HMVEC migration was dose-dependent and detectable when CYR61 was present at 10 ng/ml, reaching maximal activity at 5 μg/ml. A higher concentration of CYR61 was less effective in inducing HMVEC migration, resulting in a bell-shaped dose response curve observed for many chemotactic factors. Anti-CYR61 antibodies inhibited stimulation of cell migration by CYR61 but not by bFGF, confirming that this activity can be attributed to the CYR61 protein (Fig. 1B).
Figure 1.
Chemotactic activity of CYR61. The migration of HMVECs was measured in a modified Boyden chamber assay. The cells placed in the lower chamber that migrated into the upper chamber were counted in three high power fields for each condition after a 4-hr incubation at 37°C. CYR61 was used at 1 μg/ml (except for dose response in A), vitronectin was used at 5 μg/ml, bFGF was used at 10 ng/ml, and VEGF was used at 1 ng/ml as chemoattractants. (A) CYR61-stimulated cell migration is dose-dependent. Cells that migrated into the upper well, where the indicated amount of purified CYR61 (▪) or the equivalent amount of CYR61 storage buffer (○) was placed, were counted. In parallel, HMVEC migration was measured by using bFGF as a chemoattractant, placed in the upper well with various amounts of CYR61 storage buffer corresponding to the amounts used in CYR61-stimulated migration experiment. Cells migrated are expressed as a percentage of bFGF-induced migration ± SD (B) Specific inhibition of CYR61-stimulated cell migration by anti-Cyr61 antibodies (25 μg/ml). HMVEC migration was monitored as described above by using either CYR61 or bFGF as a chemoattractant. Where indicated, these proteins were preincubated with anti-Cyr61 antibodies before use. (C) Directed migration of HMVEC tested in a checkerboard-type analysis. CYR61 or bFGF was added to the upper chamber, the lower chamber, neither chamber, or both chambers as indicated. In B and C, results are shown as the number of cells that migrated per three high power fields ± SD (D) Specific inhibition of CYR61-stimulated migration by an antibody against integrin αVβ3 but not αVβ5. HMVEC migration was monitored by using CYR61, vitronectin, bFGF, or VEGF as chemoattractants. Where indicated, cells were preincubated with 50 μg/ml LM609 for 1 hr before addition to the lower chamber. Results are expressed as percentage of cells migrated to VEGF. Neg., background migration in the absence of chemoattractant.
To determine whether CYR61 stimulated chemotaxis (directed cell migration) or chemokinesis (random cell movement), we performed a checkerboard analysis. In the modified Boyden chamber, CYR61 was placed in the upper chamber (no cells), in the lower chamber (with cells), in neither chamber, or in both chambers (Fig. 1C). HMVEC migration into the upper chamber was not enhanced when CYR61 was incubated with cells in the lower chamber, indicating that CYR61 does not cause a chemokinetic response. CYR61 stimulated cell migration most effectively when placed in the upper chamber only, a fact that is consistent with CYR61 exerting a directed chemotactic activity. Cell migration was reduced by the presence of CYR61 in both the upper and lower chambers, indicating that HMVECs were sensitive to a concentration gradient of CYR61 in the two chambers. Moreover, CYR61 in the lower chamber reduced cell migration toward bFGF, a known chemotactic agent. Together, these results show that CYR61 stimulates directed chemotaxis of HMVEC.
Because CYR61 is a ligand of the integrin αVβ3 (10), which is known to be involved in chemotaxis (11), it is of interest to determine whether CYR61 stimulates chemotaxis through this integrin receptor. As shown in Fig. 1D, CYR61-stimulated chemotaxis was inhibited completely when HMVECs were preincubated with LM609, a well characterized anti-αVβ3 mAb (26). By contrast, preincubation of HMVECs with LM609 had no effect on either basal migration or VEGF-stimulated cell migration. This finding is consistent with the observation that VEGF-induced angiogenesis is independent of αVβ3 (19). LM609 inhibited bFGF-stimulated migration by ≈30%; this result might be explained by the recent finding that bFGF itself can bind to the integrin αVβ3 in addition to its specific high affinity receptors (27), and thus bFGF interaction with αVβ3 might contribute partially to the chemotactic activity of bFGF. As expected, LM609 inhibited vitronectin-stimulated cell migration completely because αVβ3 is a known receptor for vitronectin. Taken together, these results show that CYR61, a ligand of αVβ3, induces vascular endothelial cell chemotaxis through an αVβ3-dependent pathway.
CYR61 Induces Neovascularization in Rat Cornea.
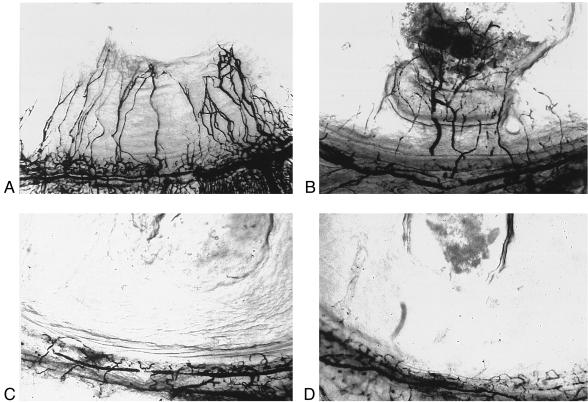
The ability of CYR61 to induce directed migration of microvascular endothelial cells supported the notion that it also may induce neovascularization in vivo. To assess this possibility, we carried out a rat corneal pocket angiogenesis assay (Fig. 2 and Table 1). Hydron pellets containing test substances were implanted into rat corneas. Purified CYR61 protein was able to induce neovascularization, as was bFGF, whereas the vehicle control did not elicit any response. Furthermore, preincubation of CYR61 with anti-CYR61 antibodies before adsorption to Hydron pellets abolished neovascularization entirely. These results show that CYR61 can induce neovascularization in vivo.
Figure 2.
CYR61 stimulates neovascularization in rat cornea. Hydron pellets containing test (or control) samples were formulated and implanted into corneas of rats as described in Materials and Methods (also see Table 1). New blood vessels that formed were visualized by perfusion with colloidal carbon 7 days after implantation. Hydron pellets contained (A) CYR61; (B) bFGF; (C) CYR61 storage buffer; or (D) CYR61 protein preincubated with anti-Cyr61 antibodies.
Table 1.
The effect of CYR61 protein on corneal neovascularization
| Test substance | Vascularized (+) and unvascularized (−) corneas
|
|
|---|---|---|
| + | − | |
| CYR61 | 6 | 1 |
| bFGF | 4 | 0 |
| CYR61 buffer | 0 | 6 |
| CYR61 + anti-CYR61 antibody | 0 | 4 |
Hydron pellets containing CYR61 storage buffer, CYR61 (50 ng), bFGF (0.5 ng), or CYR61 with anti-CYR61 antibodies (1 μg) were implanted into rat corneas. Corneal vascularization was scored after 7 days (see Fig. 2).
Expression of CYR61 Promotes Tumor Growth and Vascularization.
We investigated the ability of CYR61 to promote tumor vascularization and tumor growth. We first screened a panel of human tumor cell lines for expression of CYR61. Most of the cell lines tested expressed CYR61 as judged by reverse transcription–PCR analysis (including SK-MEL-2, MCF-7, RT-4, TE-671, and HT-29 cells) or by immunoprecipitation (HT-1080 cells) (data not shown), suggesting a correlation between CYR61 expression and tumorigenesis. We found two cell lines that did not express CYR61: RF-1, a gastric adenocarcinoma cell line isolated from a primary tumor, and RF-48, the metastatic variant of RF-1 (28) (data not shown). These cell lines supported the development of only small tumors in immunodeficient mice (see below) whereas some of the other cells lines tested that express CYR61 are strongly tumorigenic (29, 30). These findings prompted us to investigate whether the expression of CYR61, by virtue of its angiogenic activity, might convert a cell line of low tumorigenicity to one of high tumorigenicity.
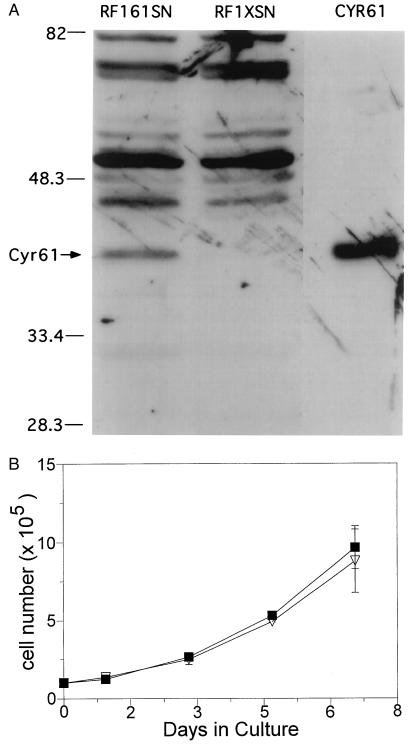
To address the above question, we first established RF-1 cells that express CYR61. RF-1 cells were transfected stably with either an expression vector for CYR61 (pL61SN) or the empty vector (pLXSN) as control. As expected, CYR61 protein was detected only in pL61SN-transfected cells (RF1–61SN) but not in pLXSN-transfected cells (RF1-XSN)(Fig. 3A). Expression of CYR61 in RF-1 cells did not change the growth rate of these cells in culture (Fig. 3B).
Figure 3.
Expression of CYR61 and effect in cell growth. (A) Immunoblot analysis of lysates from RF-1 cells transfected with a cyr61-expression vector (RF1–61SN) or with the empty vector (RF1-XSN). Lysates were electrophoresed on a 10% SDS/PAGE gel followed by immunoblotting with anti-CYR61 antibodies. The lane marked “CYR61” displays a sample of purified CYR61 protein. Molecular weights of marker proteins are indicated on the left in kDa. (B) The growth of RF1–61SN (▪) and RF1-XSN (▿) was monitored in culture. Cells were seeded at 1 × 105 cells per well in 24-well plates, and the medium was changed every 3 days. Cell numbers were counted in triplicates on indicated days.
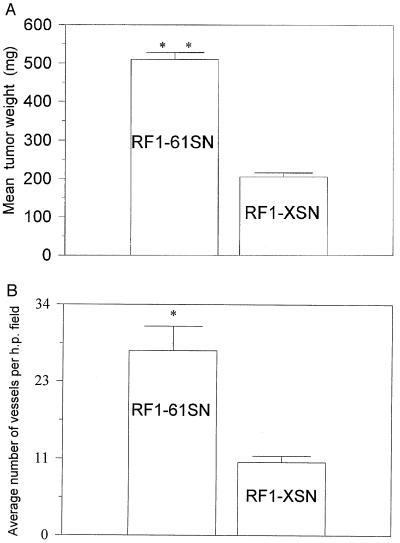
Pilot experiments indicated that RF-1 cells formed tumors inconsistently when injected into nude mice whereas the severe combined immunodeficiency/beige mice were more permissive hosts in which small tumors formed consistently (data not shown). Thus, stably transfected pools of RF1–61SN and RF1-XSN cells were injected s.c. into severe combined immunodeficiency/beige mice, generating tumors at the sites of injection. Tumors were harvested 32 days after injection and were analyzed (Fig. 4). Tumors derived from RF1–61SN cells grew significantly larger (0.50 ± 0.02 g; n = 10, P < 0.001) than control tumors derived from RF1-XSN cells (0.20 ± 0.01 g; n = 10). A duplicate set of experiments was performed in which independently transfected pools of RF-1 cells were injected into immunodeficient mice, and similar results were obtained. Immunohistological analysis of tumors derived from RF1–61SN cells confirmed that they expressed CYR61 protein in vivo whereas tumors from RF1-XSN cells did not (Fig. 5 A and B). Immunostaining revealed clusters of cells throughout the RF1–61SN-derived tumors that stained intensely for CYR61. The immunostaining was abolished when the anti-Cyr61 antibodies were preincubated with purified CYR61 protein. Cells in the tumor that did not stain for CYR61 may represent stromal cells or RF1–61SN cells that express low levels of CYR61 because the injected cells were composed of a pool of stable transfectants.
Figure 4.
Effect of cyr61 expression on tumor size and vessel density. Tumors grown 32 days after s.c. injection of RF-1 cells, which were transfected either with a cyr61-expressing vector (RF1–61SN) or with the empty vector (RF1-XSN), into severe combined immunodeficiency/beige mice were harvested and examined. (A) Tumor weights and (B) number of blood vessels per high power field were determined as described in Materials and Methods. Histograms show the mean values, and error bars show the SEM. Statistical analysis was performed by using the Student’s t test, as well as ANOVA and the Tukey–Kramer multiple comparison test. ∗, P < 0.05; ∗∗, P < 0.001.
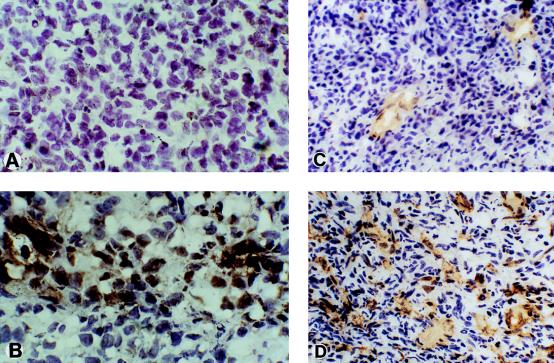
Figure 5.
Immunohistochemical analysis. Detection of CYR61 in tumors derived from (A) RF1-XSN or (B) RF1–61SN was carried out by immunostaining with anti-Cyr61 antibodies. Immunostaining for CYR61 in an RF1–61SN-derived tumor was abolished when the anti-CYR61 antibodies were preincubated with purified CYR61 protein (not shown). To visualize blood vessels, (C) RF1-XSN and (D) RF1–61SN tumor sections were stained with anti-CD31 antibodies.
To address whether the increased size of CYR61-expressing tumors might be related to the angiogenic activity of CYR61, we stained sections of tumors with antibodies for the vascular endothelial cell marker CD31. As shown in Fig. 5 C and D, CYR61-expressing tumors showed a much higher vascular density than did the control-derived tumors, as indicated by vessels comprised of CD31-staining endothelial cells. Direct counting of blood vessels in tissue sections from randomly chosen tumors demonstrated a significantly higher vascular density in CYR61-expressing tumors (27.2 ± 3.6, P < 0.05) than in control tumors (10.8 ± 0.9) (Fig. 4B). Taken together, these results show that expression of CYR61 promotes tumor growth and vascularization.
DISCUSSION
CYR61, initially identified as a growth factor-inducible immediate–early gene, encodes an extracellular matrix-associated signaling molecule whose expression is both tissue-specific and temporally regulated during development (5, 6, 16, 31). A member of a larger gene family, CYR61 has relatives from Drosophila to man (32–35). Recent purification and characterization of the CYR61 protein have shown that it is a regulator of remarkable versatility: CYR61 promotes cell adhesion, migration, proliferation, and chondrogenic differentiation (7–10, 31). In this report, we show that CYR61 is an angiogenic inducer that is capable of promoting tumor growth and vascularization in an experimental tumor model.
We concluded that CYR61 is an angiogenic factor based on the observation that purified CYR61 protein stimulates chemotaxis of microvascular endothelial cells in culture (Fig. 1) and induces neovascularization in vivo (Fig. 2). Both of these activities were blocked by specific anti-CYR61 antibodies, indicating that they can be ascribed to the CYR61 protein. Angiogenesis requires the coordinated execution of a series of cellular processes, initiating with the degradation of the basement membrane and extracellular matrix surrounding the parent vessel; endothelial cells then migrate from the parent vessel and proliferate along a gradient of a chemoattractant toward an angiogenic signal, resulting in the formation of nascent capillary sprouts (36–40). In culture, CYR61 promotes cell adhesion, enhances growth factor-induced DNA synthesis (7), and stimulates directed migration of endothelial cells (Fig. 1). Thus, the action of CYR61 is capable of inducing principal aspects of the angiogenic process in culture.
Although the mechanism by which CYR61 induces angiogenesis is currently unknown, two lines of evidence implicate the involvement of the integrin αVβ3, which has been shown to be required for neovascularization (18). First, CYR61 is a ligand of this integrin, which mediates endothelial cell adhesion to CYR61 (10). Second, CYR61 induces chemotaxis of HMVECs through an αVβ3-dependent pathway (Fig. 1D). It is thus an intriguing possibility that CYR61 might induce angiogenesis through interaction with integrin αVβ3.
The ability of CYR61 to promote tumor growth suggests that it may play a role in pathologic angiogenesis. Whereas most human tumor cell lines tested express CYR61, the RF-1 gastric adenocarcinoma cells do not. Expression of the CYR61 cDNA in RF-1 cells under the control of a constitutive promoter enhances tumorigenicity of these cells in immunodeficient mice, resulting in tumors that grow significantly larger in size and are more vascularized than the non-CYR61-expressing control (Figs. 4 and 5). Because expression of CYR61 in RF-1 cells did not confer a growth advantage on these cells in culture (Fig. 3B), it is unlikely that CYR61 expression enhances tumor growth through cell cycle shortening. Because CYR61 is capable of inducing angiogenesis in vivo (Fig. 2) and because the CYR61-expressing tumors are more vascularized, the most direct interpretation of these results is that CYR61 promotes tumor growth through induction of neovascularization. However, we cannot rule out the possibility that CYR61 may possess activities other than angiogenesis that also contribute to the enhancement of tumor growth.
The finding that CYR61 is an angiogenic inducer provides insights into its biological functions in growth and development. Because the expression of CYR61 is induced in granulation tissues during wound healing (17), CYR61 may promote neovascularization during wound repair. During placental development, CYR61 is expressed highly in trophoblastic giant cells and trophoblasts of the ectoplacental cone (8, 16, 17). These trophoblasts produce a rich complement of angiogenic factors, including bFGF, VEGF (41), the urokinase-type plasminogen activator (42), and proliferin (43). These invasive trophoblasts help mediate nutrient exchange with maternal blood, and CYR61 expression in these cells may help to promote uterine vessel growth toward the embryo. During embryonic development, CYR61 is localized in hypertrophic cartilage (17), where vessel invasion produces conduits for osteoblasts to settle, leading to endochondral ossification (44). The presence of CYR61 may help to induce angiogenesis as a prelude to replacement of cartilage by bone. Additionally, CYR61 expression during embryogenesis in vessels such as the dorsal aorta, the bronchial arch, and the umbilical artery (16) suggests that CYR61 might also play a role in vasculogenesis.
The expression of CYR61 in most tumor cell lines examined and the ability of CYR61 to promote tumor growth and vascularization suggest that CYR61 expression may serve as a tumor progression marker. As the vasculature is normally quiescent, a tumor must acquire the ability to induce neovascularization during its development to grow beyond a certain size. This angiogenic switch involves the up-regulation of angiogenic inducers and/or down-regulation of angiogenic inhibitors (15, 40). Some oncogenes also act to induce the production of angiogenic factors (15). For example, v-src is known to induce the production of prostaglandin (45) and to up-regulate plasminogen activator (46), both of which are angiogenic inducers. In this regard, v-src has been shown to activate transcriptionally cef10, the chicken homolog of CYR61, in chicken embryo fibroblasts (47). Thus, activation of CYR61 expression may constitute part of the angiogenic switch. Consistent with a possible role of CYR61 in tumor progression is the observation that the expression of integrin αVβ3, a receptor for CYR61, is correlated with tumor vascularization and progression (48, 49). Antagonists of integrin αVβ3 significantly decrease tumor vascularization and tumor size (50). Although the potential role of CYR61 in metastasis has not been examined, it is clear that adhesion molecules, including integrins, play important roles in this process (51). The possibilities that Cyr61 may serve as a tumor progression and/or metastatic marker and as a possible target for anti-tumor therapy clearly merit further investigation.
Acknowledgments
We are greatly indebted to Drs. Noël Bouck and Olga V. Volpert of Northwestern University Medical School for teaching us the in vitro and in vivo angiogenesis assays and for many helpful suggestions. We also thank Anupama Koures and Eric Schmidt for technical assistance, Dr. Jeffrey A. Greenspan for assistance, and Drs. Cho Yeung and Stephen Lam and members of the laboratory for helpful discussions. This work was supported by grants from the National Institutes of Health (CA46565), and the Munin Corporation.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: HMVEC, human dermal microvascular endothelial cells; bFGF, basic fibroblast growth factor; VEGF, vascular endothelial growth factor.
References
- 1.Gumbiner B M. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 2.Sastry S K, Horwitz A F. Dev Biol. 1996;180:455–467. doi: 10.1006/dbio.1996.0319. [DOI] [PubMed] [Google Scholar]
- 3.Lau L F, Nathans D. In: Genes Induced by Serum Growth Factors. Cohen P, Foulkes J G, editors. Amsterdam: Elsevier; 1991. 257–293. [Google Scholar]
- 4.Herschman H R. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien T P, Yang G P, Sanders L, Lau L F. Mol Cell Biol. 1990;10:3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang G P, Lau L F. Cell Growth Differ. 1991;2:351–357. [PubMed] [Google Scholar]
- 7.Kireeva M L, Mo F-E, Yang G P, Lau L F. Mol Cell Biol. 1996;16:1326–1334. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kireeva M L, Latinkic B V, Kolesnikova T V, Chen C-C, Yang G P, Abler A S, Lau L F. Exp Cell Res. 1997;233:63–77. doi: 10.1006/excr.1997.3548. [DOI] [PubMed] [Google Scholar]
- 9.Kolesnikova T V, Lau L F. Oncogene. 1998;16:747–754. doi: 10.1038/sj.onc.1201572. [DOI] [PubMed] [Google Scholar]
- 10.Kireeva M L, Lam S C T, Lau L F. J Biol Chem. 1998;273:3090–3096. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- 11.Felding-Habermann B, Cheresh D A. Curr Opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J. In: Tumor Angiogenesis. Mendelsohn J, Howley P M, Israel M A, Liotta L A, editors. Philadelphia: Saunders; 1995. pp. 206–232. [Google Scholar]
- 15.Bouck N, Stellmach V, Hsu S C. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien T P, Lau L F. Cell Growth Differ. 1992;3:645–654. [PubMed] [Google Scholar]
- 17.Latinkic B V. Ph.D. thesis. Chicago: Univ. of Illinois; 1994. [Google Scholar]
- 18.Brooks P C, Clark R A F, Cheresh D A. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 19.Friedlander M, Brooks P C, Shaffer R W, Kincaid C M, Varner J A, Cheresh D A. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 20.Miller A D, Rosman G J. Biotechniques. 1989;7:980–982. [PMC free article] [PubMed] [Google Scholar]
- 21.Baum C, Forster P, Hegewisch-Becker S, Harbers K. Biotechniques. 1994;17:1058–1062. [PubMed] [Google Scholar]
- 22.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab Press; 1988. [Google Scholar]
- 24.Polverini P J, Bouck N P, Rastinjad F. Methods Enzymol. 1991;198:440–441. doi: 10.1016/0076-6879(91)98044-7. [DOI] [PubMed] [Google Scholar]
- 25.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- 26.Cheresh D A, Spiro R C. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- 27.Rusnati M, Tanghetti E, Dell’Era P, Gualandris A, Presta M. Mol Biol Cell. 1997;8:2449–2461. doi: 10.1091/mbc.8.12.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salesiotis A N, Wang C K, Wang C D, Burger A, Li H, Seth A. Cancer Lett. 1995;91:47–54. doi: 10.1016/0304-3835(95)03717-b. [DOI] [PubMed] [Google Scholar]
- 29.Marshall C J, Franks L M, Carbonell A W. J Clin Invest. 1977;58:1743–1747. doi: 10.1093/jnci/58.6.1743. [DOI] [PubMed] [Google Scholar]
- 30.Rasheed S, Nelson-Rees W A, Toth E M, Arnstein P, Garner M B. Cancer. 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 31.Wong M, Kireeva M L, Kolesnikova T V, Lau L F. Dev Biol. 1997;192:492–508. doi: 10.1006/dbio.1997.8766. [DOI] [PubMed] [Google Scholar]
- 32.Bork P. FEBS Lett. 1993;327:125–130. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- 33.Mason E D, Konard K D, Webb C D, Marsh J L. Genes Dev. 1994;8:1489–1501. doi: 10.1101/gad.8.13.1489. [DOI] [PubMed] [Google Scholar]
- 34.Francois V, Bier E. Cell. 1995;80:19–20. doi: 10.1016/0092-8674(95)90446-8. [DOI] [PubMed] [Google Scholar]
- 35.Ying Z, King M L. Gene. 1996;171:243–248. doi: 10.1016/0378-1119(95)00891-8. [DOI] [PubMed] [Google Scholar]
- 36.Risau W. Nature (London) 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 37.Bussolino F, Mantovni A, Persico G. Trends Biochem Sci. 1997;22:251–256. doi: 10.1016/s0968-0004(97)01074-8. [DOI] [PubMed] [Google Scholar]
- 38.Folkman J. Cell. 1997;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- 39.Varner J A. In: The Role of Vascular Cell Integrins αvβ3 and αvβ5 in angiogenesis. Goldberg I D, Rosen E M, editors. Basel: Birkhauser; 1997. pp. 361–390. [Google Scholar]
- 40.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 41.Jackson M R, Carney E W, Lye S J, Ritchie J W K. Placenta. 1994;15:341–353. doi: 10.1016/0143-4004(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 42.Sappino A P, Huarte J, Belin D, Vassalli D. J Cell Biol. 1989;109:2471–2479. doi: 10.1083/jcb.109.5.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S J, Talamantes F, Wilder E, Linzer D I, Nathans D. Endocrinology. 1988;122:1761–1768. doi: 10.1210/endo-122-5-1761. [DOI] [PubMed] [Google Scholar]
- 44.Alini M, Marriott A, Chen T, Abe S, Poole A R. Dev Biol. 1996;176:124–132. doi: 10.1006/dbio.1996.9989. [DOI] [PubMed] [Google Scholar]
- 45.Xie W, Chipman J G, Robertson D L, Erikson R L, Simmons D L. Proc Natl Acad Sci USA. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berkenpas M B, Quigley J P. Proc Natl Acad Sci USA. 1991;88:7768–7772. doi: 10.1073/pnas.88.17.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simmons D L, Levy D B, Yannoni Y, Erikson R L. Proc Natl Acad Sci USA. 1989;86:1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albelda S M, Mette S A, Elder D A, Stewart R, Damjanovich L, Herlyn M, Buck C A. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 49.Feldding-Habermann B, Mueller B M, Romerdahl C A, Cheresh D A. J Clin Invest. 1992;89:2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks P C, Montgomery A M P, Rosenfeld M, Reisfel R, Hu T, Klier G, Cheresh D. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 51.Nip J, Brodt P. Cancer Metastasis Rev. 1995;14:241–252. doi: 10.1007/BF00690295. [DOI] [PubMed] [Google Scholar]