We used a transgenic mouse model of breast cancer to investigate contrast enhancement of ductal carcinoma in situ (DCIS) on clinical dynamic contrast material–enhanced (DCE) MR images of the breast, and we have shown via two independent routes—DCE MR imaging and x-ray fluorescence microscopy—that after injection of gadodiamide, there is gadolinium uptake inside ducts distended with murine DCIS.
Abstract
Purpose:
To combine dynamic contrast material–enhanced (DCE) magnetic resonance (MR) imaging with x-ray fluorescence microscopy (XFM) of mammary gland tissue samples from mice to identify the spatial distribution of gadolinium after intravenous injection.
Materials and Methods:
C3(1) Sv-40 large T antigen transgenic mice (n = 23) were studied with institutional animal care and use committee approval. Twelve mice underwent DCE MR imaging after injection of gadodiamide, and gadolinium concentration–time curves were fit to a two-compartment pharmacokinetic model with the following parameters: transfer constant (Ktrans) and volume of extravascular extracellular space per unit volume of tissue (ve). Eleven mice received gadodiamide before XFM. These mice were sacrificed 2 minutes after injection, and frozen slices containing ducts distended with murine ductal carcinoma in situ (DCIS) were prepared for XFM. One mouse received saline and served as the control animal. Elemental gadolinium concentrations were measured in and around the ducts with DCIS. Hematoxylin-eosin–stained slices of mammary tissues were obtained after DCE MR imaging and XFM.
Results:
Ducts containing DCIS were unambiguously identified on MR images. DCE MR imaging revealed gadolinium uptake along the length of ducts with DCIS, with an average Ktrans of 0.21 min−1 ± 0.14 (standard deviation) and an average ve of 0.40 ± 0.16. XFM revealed gadolinium uptake inside ducts with DCIS, with an average concentration of 0.475 mmol/L ± 0.380; the corresponding value for DCE MR imaging was 0.30 mmol/L ± 0.13.
Conclusion:
These results provide insight into the physiologic basis of contrast enhancement of DCIS lesions on DCE MR images: Gadolinium penetrates and collects inside neoplastic ducts.
© RSNA, 2009
Introduction
The sensitivity and specificity of dynamic contrast material–enhanced (DCE) magnetic resonance (MR) imaging in the early detection of invasive cancers have been shown to be equal or superior to those of x-ray mammography (1). However, this has not been consistently demonstrated for ductal carcinoma in situ (DCIS), which is a nonobligate precursor to invasive breast cancer, in which cancer cells are still confined by the basement membrane of mammary ducts. Because DCIS is the earliest stage of breast cancer and has the best prognosis, it is likely that further improvements in the detection of breast cancers at a preinvasive stage may improve patient outcomes. Some reports have shown decreased diagnostic accuracy of DCE MR imaging for DCIS (2,3), while others have shown comparable or even higher performance compared with that of x-ray mammography (4,5). The sensitivity of DCE MR imaging for detection of DCIS may be compromised if the lesion does not exhibit sufficient gadolinium uptake or if it is obscured by strongly enhancing parenchyma (6,7). Even when DCIS is detected with DCE MR imaging, it can still be misidentified because of tumor morphology (the characteristic non-masslike enhancement of DCIS could be mistaken for enhancing normal parenchyma) and tumor kinetics (DCIS often exhibits plateau or persistent kinetic curve types, which are not typical of malignant lesions) (8). Thus, there is a need to improve the diagnostic accuracy of DCE MR imaging in the detection of DCIS (9).
A better understanding of the mechanism of contrast enhancement of DCIS may be helpful for improving quantitative analysis of DCE MR imaging data. Invasive tumors demonstrate uptake of gadolinium chelates used as MR contrast agents because of their dense and leaky neovasculature. Contrast enhancement kinetics are often modeled as contrast material exchange between two physiologic compartments: the vascular and extracellular extravascular space within the tumor (10). However, DCIS lesions are not always associated with dense vasculature, especially at their early stages; thus, the mechanism of gadolinium distribution is not well understood. In particular, it is unknown whether gadolinium penetrates the basement membrane to enter mammary ducts distended with DCIS.
Unfortunately, it is difficult to perform direct and detailed measurement of gadolinium concentrations in women with DCIS lesions, as it is challenging to isolate signal from individual ducts that are known to contain DCIS. Thus, the purpose of our study was to combine DCE MR imaging with x-ray fluorescence microscopy (XFM) of mammary gland tissue samples from mice to identify the spatial distribution of gadolinium after intravenous injection.
Materials and Methods
Animals
The C3(1) SV-40 large T antigen mouse model was used in this study. Female mice develop spontaneous orthotopic mammary cancer that resembles human ductal carcinoma, including progression through atypical ductal hyperplasia (at approximately 8 weeks of age), DCIS (at approximately 12 weeks of age), and invasive ductal carcinoma (at approximately 16 weeks of age) (11,12). A total of 23 SV-40 large T antigen mice were selected for this study: Twelve mice between 12 and 16 weeks of age were selected for in vivo MR imaging, and 11 mice between 12 and 14 weeks of age were selected for XFM. All procedures were performed in accordance with the approval of the University of Chicago institutional animal care and use committee.
MR Experiments
Imaging was performed with a 4.7-T magnet (Bruker Biospin, Billerica, Mass) equipped with a self-shielded gradient set that delivered a maximum gradient strength of 0.002 T/cm. A homebuilt eight-leg low-pass half-open birdcage coil (3.0-cm length, 3.0-cm width, 2.0-cm height) (13) was used for in vivo imaging. Multisection axial gradient-echo images (repetition time msec/echo time msec, 675/7; field of view, 3.0 × 3.0 cm; two signals acquired; section thickness, 0.5 mm; number of sections acquired, 42; in-plane spatial resolution, 117 μm; flip angle, 30°) with fat suppression were acquired to localize lesions (14). Subsequently, three sections that contained lesions of interest were selected for DCE MR imaging (30/3.5; section thickness, 1.0 mm; in-plane spatial resolution, 256 μm; flip angle, 20°). Because DCE MR images were obtained in only three sections through the mammary gland, we were not able to evaluate contrast kinetics in every detected lesion. Baseline images (n = 5) were acquired before contrast material injection, and 128 images were acquired after contrast material injection at a temporal resolution of 3.84 seconds; therefore, gadolinium uptake and washout were followed for a total of 8.2 minutes.
Animals were anesthetized prior to imaging, and anesthesia was maintained during imaging with 1.5% isoflorane. Each animal's temperature, heart rate, and respiration rate were monitored, and the respiration rate was used to obtain gated images. A 24-gauge angiocatheter was implanted into the tail vein to inject 0.2 mL of 0.0184 mol/L gadodiamide (Ominscan; Nycomed Amersham, Princeton NJ), resulting in an injected dose of between 0.15 and 0.16 mmol per kilogram of body weight.
Histologic Evaluation
Hematoxylin-eosin–stained slices of all imaged mammary glands were obtained (5-μm-thick hematoxylin-eosin–stained slices were obtained every 50 μm) and evaluated by a breast and mouse mammary gland pathologist (T.K.) with more than 20 years of experience. Intramammary lymph nodes, invasive tumors, and ducts distended with DCIS that had a diameter larger than 300 μm were identified and used as the reference standard. MR images were correlated with hematoxylin-eosin–stained slices by using an agar grid, as detailed in a prior report (14), to enable us to identify DCIS, invasive tumors, lymph nodes, and areas of normal mammary gland on the images.
DCE MR Imaging Analysis
All data analysis was performed with software written in Interactive Data Language (Research Systems, Boulder, Colo). Signal intensities were converted to gadodiamide concentration (which is equivalent to gadolinium concentration) by using muscle as a reference tissue. A graduate student (S.A.J., 3 years of experience analyzing MR images of early murine mammary cancer) generated gadolinium concentration–time curves for the following regions of interest (ROIs) after extensive training by an experienced pathologist (T.K.): DCIS (n = 9), invasive tumors (n = 3), lymph nodes, (n = 11), muscle (n = 12), and normal mammary gland (n = 11). Overall, the average ROI size was 0.32 mm3 ± 0.33 (standard deviation) (range, 0.03–1.33 mm3) (Table 1). The mean gadolinium concentration 2 minutes after injection was calculated.
Table 1.
Ktrans and ve in Various ROIs

Note.—Unless otherwise indicated, data are averages ± standard deviations. Data in parentheses are ranges.
A simple two-compartment model (TCM) of blood plasma versus extravascular extracellular space was used to describe the distribution of gadodiamide after bolus injection. Gadolinium concentration–time curves were excluded from TCM analysis if they did not exhibit sufficient gadolinium uptake or if they were too noisy because of motion artifacts. Three of the 46 gadolinium concentration–time curves (all three were from normal mammary gland ROIs) were excluded from TCM fitting because they did not exhibit sufficient gadolinium uptake. An additional five curves (one from a normal mammary gland ROI, one from a lymph node ROI, and three from DCIS ROIs) were excluded because of motion artifacts. The TCM is used to predict the change in the gadolinium concentration–time curve as follows: dC(t)/dt = Ktrans · [Cp(t) − C(t)/ve], where C(t) is the tissue gadolinium concentration, dc(t)/dt is the rate of change of tissue gadolinium concentration as a function of time, Ktrans is the volume transfer constant between blood plasma and extravascular extracellular space, ve is the volume of extravascular extracellular space per unit volume of tissue, and Cp(t) is the gadolinium concentration in blood plasma (calculated by using a reference tissue approach) (15). To determine how well the TCM fit the ROI concentration curves, the goodness of fit parameter R2 was used. For those curves fit by the TCM, Ktrans and ve were compared. Further details on this model and its analysis can be found in Appendix E1 (online).
XFM Analysis
To depict (with micron resolution) the spatial distribution of gadolinium in mouse mammary glands with DCIS, 10 mice were injected with 0.2 mL of 0.0184 mol/L gadodiamide and sacrificed 2 minutes after injection. Inguinal mammary glands were excised immediately, embedded in Tissue-Tek optimal cutting temperature compound (Miles, Elkhart, Ind), and frozen in liquid nitrogen. In addition, one mouse was injected with 0.2 mL of saline to serve as a control. After cryoslicing, frozen 7-μm-thick slices of portions of mammary glands with DCIS, lymph nodes, or tumors were (a) mounted on silicon nitride windows (area, 3.0 × 3.0 mm; thickness, 200 nm) (Silson, Blisworth, England), air dried, and imaged or (b) imaged as frozen-hydrated samples with a cryostage. Adjacent hematoxylin-eosin–stained slices were acquired to aid in lesion identification. Specimens were imaged with the scanning x-ray microprobe at beam line 2-ID-E at the Advanced Photon Source (Argonne National Laboratory, Argonne, Ill). Specimens were raster scanned with 10-keV incident x-rays in steps of 3.0–5.0 μm, and fluorescence spectra were collected for 1- to 2-second dwell times.
Elemental Concentrations from XFM
Image processing and elemental concentration analysis was performed with MAPS software (Stefan Vogt, Argonne, Ill) (16). The fluorescence spectra were converted from counts to a two-dimensional concentration in micrograms per square centimeter by fitting against the spectra derived from thin-film standards NBS-1832 and NBS-1833 from the National Bureau of Standards (Gaithersburg, Md). Two-dimensional concentrations were converted to three-dimensional concentrations (measured in millimoles per liter) by using the thickness of each section (7 μm) and the molecular weight of gadolinium (157.3 g/mol). Elemental concentration maps for phosphorus, iron, and gadolinium were derived according to their Kα- or L-characteristic x-ray fluorescence. Phosphorus concentration maps are often used to locate cell nuclei and were used along with adjacent hematoxylin-eosin–stained slices to determine cellularity and locate mammary ducts, lymph nodes, and tumors. Iron concentration maps can serve as potential indicators of the location of blood cells, vessels, or both. We used the phosphorous concentration maps to draw ROIs in which the concentration of gadolinium was quantified in (a) ducts with DCIS, (b) lymph nodes, and (c) invasive tumors. The average gadolinium concentration in ducts with DCIS in the control mouse was used to determine the measurement error and was subtracted from the measured gadolinium concentration in mice that received gadodiamide.
Statistical Analysis
A paired t test was used to compare the values of Ktrans and ve in ducts with DCIS with those in other ROIs in the same animal.
Results
DCE MR Imaging of Murine DCIS
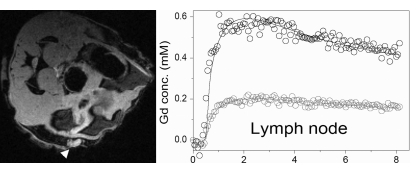
DCIS lesions and early invasive tumors appeared clearly on unenhanced gradient-echo MR images against a darker background of mammary glandular tissue and fat (Fig 1). On the basis of histologic analysis, 11 lymph nodes, one large (5-mm-diameter) tumor, eight small (0.5–3-mm-diameter) tumors, and 15 ducts distended with DCIS that were larger than 300 μm in diameter were detected on unenhanced gradient-echo MR images.
Figure 1a:
Axial high-spatial-resolution gradient-echo fat-suppressed MR sections (2.0 × 2.4-cm field of view) obtained through the mammary glands of three mice, with corresponding gadolinium concentration–time curves and TCM fits displayed in black show (a) a normal mammary gland (darker area outlined in white), (b) an intramammary lymph node (arrowhead), (c) DCIS (arrows), and (d) a tumor (arrowhead). The lymph node, DCIS, and tumor appear clearly against the darker background of the normal mammary gland. In each image, the gray curve represents the gadolinium concentration–time curve in an ROI drawn on the back muscle for the sake of comparison. All plots are scaled from −0.1 to 0.6 mmol/L.
Figure 1b:
Axial high-spatial-resolution gradient-echo fat-suppressed MR sections (2.0 × 2.4-cm field of view) obtained through the mammary glands of three mice, with corresponding gadolinium concentration–time curves and TCM fits displayed in black show (a) a normal mammary gland (darker area outlined in white), (b) an intramammary lymph node (arrowhead), (c) DCIS (arrows), and (d) a tumor (arrowhead). The lymph node, DCIS, and tumor appear clearly against the darker background of the normal mammary gland. In each image, the gray curve represents the gadolinium concentration–time curve in an ROI drawn on the back muscle for the sake of comparison. All plots are scaled from −0.1 to 0.6 mmol/L.
Figure 1c:
Axial high-spatial-resolution gradient-echo fat-suppressed MR sections (2.0 × 2.4-cm field of view) obtained through the mammary glands of three mice, with corresponding gadolinium concentration–time curves and TCM fits displayed in black show (a) a normal mammary gland (darker area outlined in white), (b) an intramammary lymph node (arrowhead), (c) DCIS (arrows), and (d) a tumor (arrowhead). The lymph node, DCIS, and tumor appear clearly against the darker background of the normal mammary gland. In each image, the gray curve represents the gadolinium concentration–time curve in an ROI drawn on the back muscle for the sake of comparison. All plots are scaled from −0.1 to 0.6 mmol/L.
Figure 1d:
Axial high-spatial-resolution gradient-echo fat-suppressed MR sections (2.0 × 2.4-cm field of view) obtained through the mammary glands of three mice, with corresponding gadolinium concentration–time curves and TCM fits displayed in black show (a) a normal mammary gland (darker area outlined in white), (b) an intramammary lymph node (arrowhead), (c) DCIS (arrows), and (d) a tumor (arrowhead). The lymph node, DCIS, and tumor appear clearly against the darker background of the normal mammary gland. In each image, the gray curve represents the gadolinium concentration–time curve in an ROI drawn on the back muscle for the sake of comparison. All plots are scaled from −0.1 to 0.6 mmol/L.
The average gadolinium concentration measured with DCE MR imaging in DCIS lesions 2 minutes after injection was 0.30 mmol/L ± 0.13 (Table 1, Fig 2). The average gadolinium concentration in the normal mammary gland 2 minutes after injection was 0.09 mmol/L ± 0.11 (range, 0.01–0.32 mmol/L). DCIS lesions fit with the TCM exhibited a wide range of kinetic curve shapes (Fig E1 [online]) and corresponding Ktrans and ve values (Table 1). Gadolinium concentration–time curves obtained in the normal mammary gland also exhibited considerable variability (Fig E1 [online]). The goodness of fit of the TCM was lowest for normal mammary gland ROIs.
Figure 2a:

Precontrast and 2-minute postcontrast subtraction DCE MR images show an early invasive tumor, lymph node, and DCIS.
Figure 2b:

Precontrast and 2-minute postcontrast subtraction DCE MR images show an early invasive tumor, lymph node, and DCIS.
Figure 2c:

Precontrast and 2-minute postcontrast subtraction DCE MR images show an early invasive tumor, lymph node, and DCIS.
Figure 2d:

Precontrast and 2-minute postcontrast subtraction DCE MR images show an early invasive tumor, lymph node, and DCIS.
Figure 2e:

Precontrast and 2-minute postcontrast subtraction DCE MR images show an early invasive tumor, lymph node, and DCIS.
Figure 2f:

Precontrast and 2-minute postcontrast subtraction DCE MR images show an early invasive tumor, lymph node, and DCIS.
There was considerable overlap in the Ktrans and ve values in DCIS lesions, invasive tumors, and lymph nodes (Table 1). There was a trend for DCIS lesions to have a lower Ktrans when compared with the Ktrans of lymph nodes and tumors; however, only the difference between Ktrans of DCIS lesions and that of lymph nodes was significant (P < .02). TCM parameters for back muscle clustered near a Ktrans of 0.11 min−1 and a ve of 0.20, while normal mammary gland areas had considerably lower Ktrans and ve values than did DCIS lesions, lymph nodes, or tumors (Table 1). The ve values were high in DCIS lesions and tumors.
XFM of Murine DCIS
XFM maps of DCIS (n = 26), lymph nodes (n = 2), and a tumor (n = 1) were obtained with micron resolution. In mice that received gadodiamide, the contrast agent was detected in regions outside the neoplastic ducts; these regions generally comprise fat, stroma, normal ducts, or blood vessels. XFM also revealed gadolinium uptake in tumors and lymph nodes and inside ducts distended with DCIS. The average concentration of gadolinium inside ducts at XFM was 0.475 mmol/L ± 0.380 (Table 2) after correcting for the background concentration of gadolinium inside ducts with DCIS. Gadolinium uptake within ducts was demonstrated both for larger ducts distended to a few hundred microns (Fig 3), as well as for smaller ducts with DCIS approximately 100 μm in size. On the other hand, iron did not have a strong presence in the mammary ducts; this suggests that no red blood cells accumulate inside the ducts and that gadolinium diffuses from blood vessels into ducts with DCIS. Lymph nodes and a tumor also exhibited concentrations of gadolinium comparable with those in DCIS lesions.
Table 2.
Gadolinium Concentration in Various ROIs

Note.—NA = not applicable.
* Data are averages ± standard deviations.
Figure 3a:

(a) Hematoxylin-eosin–stained tissue sample and (b–d) XFM images of ducts with DCIS show concentration maps for phosphorus (b), iron (c) and gadolinium (d). On the rainbow scale, blue indicates a lower concentration; green, a higher concentration; and red, the highest concentration. The images are scaled as follows: for phosphorus, 0–15 μg/cm2; for iron, 0–0.7 μg/cm2; and for gadolinium, 0–0.1 μg/cm2 (which is equivalent to 0.91 mmol/L per pixel). Gadolinium uptake can be seen within the duct. The scale in b also applies to a.
Figure 3b:

(a) Hematoxylin-eosin–stained tissue sample and (b–d) XFM images of ducts with DCIS show concentration maps for phosphorus (b), iron (c) and gadolinium (d). On the rainbow scale, blue indicates a lower concentration; green, a higher concentration; and red, the highest concentration. The images are scaled as follows: for phosphorus, 0–15 μg/cm2; for iron, 0–0.7 μg/cm2; and for gadolinium, 0–0.1 μg/cm2 (which is equivalent to 0.91 mmol/L per pixel). Gadolinium uptake can be seen within the duct. The scale in b also applies to a.
Figure 3c:

(a) Hematoxylin-eosin–stained tissue sample and (b–d) XFM images of ducts with DCIS show concentration maps for phosphorus (b), iron (c) and gadolinium (d). On the rainbow scale, blue indicates a lower concentration; green, a higher concentration; and red, the highest concentration. The images are scaled as follows: for phosphorus, 0–15 μg/cm2; for iron, 0–0.7 μg/cm2; and for gadolinium, 0–0.1 μg/cm2 (which is equivalent to 0.91 mmol/L per pixel). Gadolinium uptake can be seen within the duct. The scale in b also applies to a.
Figure 3d:

(a) Hematoxylin-eosin–stained tissue sample and (b–d) XFM images of ducts with DCIS show concentration maps for phosphorus (b), iron (c) and gadolinium (d). On the rainbow scale, blue indicates a lower concentration; green, a higher concentration; and red, the highest concentration. The images are scaled as follows: for phosphorus, 0–15 μg/cm2; for iron, 0–0.7 μg/cm2; and for gadolinium, 0–0.1 μg/cm2 (which is equivalent to 0.91 mmol/L per pixel). Gadolinium uptake can be seen within the duct. The scale in b also applies to a.
There was a heterogeneous distribution of gadolinium throughout the ducts (Fig E2 [online]). Gadolinium concentration was highest around the cancer cells near the interface of the duct and stroma, it decreased around the cells further into the duct, and it collected once again in the lumen.
Discussion
We used a transgenic mouse model of breast cancer to investigate contrast enhancement of DCIS on clinical DCE MR images of the breast, and we have shown via two independent routes—DCE MR imaging and XFM—that after injection of gadodiamide, there is gadolinium uptake inside ducts distended with murine DCIS. XFM and DCE MR imaging revealed the average concentration of gadolinium inside mammary ducts 2 minutes after injection was 0.475 mmol/L and 0.30 mmol/L, respectively. DCE MR imaging data showed cancer-containing ducts were fairly effectively isolated from surrounding tissue; however, there may have been some partial volume effects due to section thickness. However, XFM revealed gadolinium penetrates inside ducts with DCIS. To our knowledge, this is a new insight into the physiologic basis for contrast enhancement of these lesions.
Several groups have investigated the distribution of gadolinium in tissue or cells, often by using x-ray fluorescence spectroscopy. De Stasio et al found gadolinium uptake in glioblastoma cells after exposure to gadopentetate dimeglumine both in vitro and in vivo for the purpose of neutron capture therapy (17,18). Gadolinium uptake is of particular interest given the concern that uptake of dissociated gadolinium by the kidney can result in nephrogenic systemic fibrosis (19). In our study, it was not clear whether the gadolinium detected with XFM in tissue remained a part of the chelate or had dissociated from the chelate. Future work with x-ray absorption spectroscopy will be performed to investigate the chelation status of the gadolinium detected inside the ducts. In addition, XFM of individual cancer cells should be performed to determine whether gadolinium penetrated cells, as has been done elsewhere in the context of following targeted nanoparticles (20,21).
To our knowledge, our study represents the first functional characterization of murine DCIS with DCE MR imaging, which has an advantage over characterization of DCIS in humans: Ducts with DCIS can be clearly depicted without contrast material injection; therefore, an ROI can be placed directly on the neoplastic duct to enable one to more accurately measure its kinetic parameters, thereby minimizing the partial voluming that complicates human kinetic data. We found the ve of DCIS was relatively high compared with that in other reports of rodent tumors, which are usually large and at an advanced stage (22,23). The XFM observation of gadolinium penetration and accumulation in the lumen of neoplastic mammary ducts is consistent with a higher ve. However, it also implies that the two-compartment model, which is widely used to model contrast kinetics in patients with cancer (24), may not be valid for use in patients with DCIS since contrast material exchange into mammary ducts represents a third compartment. Thus, for accurate physiologic modeling, the path that gadolinium takes from blood vessels to mammary ducts needs to be elucidated. A likely explanation of the mechanism underlying our findings is that gadolinium diffuses out of capillaries into the extraductal space, reaches leaky duct basement membranes, and collects and distributes in the largely unobstructed duct lumen. The variable kinetic curve shapes of DCIS found in this study can yield insights into the heterogeneous physiology of DCIS and surrounding tissue. The distance of blood vessels to the ducts, the permeability of basement membranes, and the volume of duct lumen available for gadolinium accumulation are physiologic factors that can directly affect the contrast enhancement kinetic curves of these lesions. Further investigation of the distribution of gadolinium in the extraductal space and blood vessels is needed.
We can translate the murine results to women if we assume certain physiologic similarities between mammary glands across species, in particular that the permeability of the mammary duct basement membranes to gadolinium is similar in both species. This permeability needs further exploration. It might be due to protease secretion by cancer cells, and it could prove to be a marker for DCIS lesions that are likely to become invasive, perhaps leading to improvements in the clinical management of DCIS. The average concentration of gadolinium in ducts measured in our study was high enough to be a substantial source of measured signal in clinical DCE MR imaging. This may partly explain the typical morphology of DCIS lesions on clinical DCE MR images: non-masslike ductal rather than periductal enhancement in a segmental and/or linear distribution (4,8,25). In addition, the higher ve of DCIS and the likely longer timeframe of gadolinium exchange into and out of ducts may explain the persistent and plateau curve types often found for DCIS (25–29). Enhancement of the normal mammary glandular tissue was also observed in some cases, several with a “persistent” curve shape, which is consistent with parenchymal enhancement observed in women (6). More detailed study of gadolinium accumulation within normal ducts with XFM is an important area for future research.
There were several limitations to this study. First, the small number of lesions limited the relevance of our findings, particularly for DCE MR imaging analysis. Second, because of the small lesion size, the gadolinium concentration–time curves were subject to noise and motion artifacts, which potentially compromised the TCM fits. Third, our application of the TCM involved several approximations and should be refined in future studies (30). Fourth, a concern may arise that the gadolinium detected with XFM had diffused from its original location at the time of tissue harvesting. XFM is a technique with minimal artifacts, provided there is good sample preparation (31,32), which we aimed to achieve in this study. Thus, we do not anticipate that our results suffered from extensive artifacts, particularly in light of certain internal controls—for example, that air-dried and frozen-hydrated tissue samples provided similar gadolinium distribution patterns. Thus, while some gadolinium “motion” in samples could be possible due to sample preparation, it is likely not visible at the resolutions used in these studies (3–5-μm step scans). Fifth, the seemingly high concentration of background gadolinium (0.011 mmol/L ± 0.004) is likely due to considerable overlap of the gadolinium L-shell fluorescence peak with the iron K-shell fluorescence peak, and improved accuracy of the gadolinium concentration is desirable. Sixth, because of the spontaneous nature of in situ cancer development in this mouse model, the slightly different age ranges used in XFM and DCE MR imaging studies may compromise comparisons made between the two. Seventh, we did not perform histologic evaluation of the entire specimen to determine whether the ducts imaged were pure DCIS or whether microinvasion that could be the entry point of the gadolinium was present in other slices. However, several smaller ducts with DCIS in which microinvasion was not likely also exhibited gadolinium uptake. With these limitations noted, our preliminary study requires further validation and improvements. For example, future XFM and DCE MR imaging correlative studies should be performed in this same type of animal. In addition, the distribution of a contrast agent with a greater molecular weight that is less likely to penetrate the duct basement membrane should be evaluated.
We have presented a general approach for the use of mouse models of breast cancer to better understand DCIS in women. Prior work has shown that MR imaging enables excellent morphologic evaluation of early murine mammary cancers (14). We have used DCE MR imaging to perform functional characterization of the contrast kinetics of murine DCIS, and—along with use of XFM—have demonstrated gadolinium uptake inside and along neoplastic mammary ducts. In future work, we plan to use serial MR imaging to characterize both the morphologic and functional changes that occur during the development and progression of DCIS into invasive carcinoma.
Understanding the uptake of gadolinium by mammary ducts may help to improve the sensitivity and specificity of DCE MR imaging by improving the interpretation and modeling of existing data and designing new acquisition techniques targeted for DCIS. For example, clinical DCE MR imaging data obtained in patients has relatively coarse spatial resolution, and individual voxels may contain DCIS and blood vessels. One could decompose gadolinium concentration–time curves for DCIS voxels into a fast component (representing gadolinium in blood vessels) and a slow component with a large ve (representing gadolinium in ducts).
Advances in Knowledge.
Despite its small size, murine ductal carcinoma in situ (DCIS) can be reliably depicted with MR imaging.
Elemental mapping of samples with x-ray fluorescence microscopy and in vivo dynamic contrast material–enhanced (DCE) MR imaging of murine DCIS independently shows that gadolinium is present in high concentrations inside and along mammary ducts.
There is a high extravascular extracellular space fraction in DCIS lesions.
Implications for Patient Care.
Understanding the uptake of gadolinium in mammary ducts may lead to improvements in imaging methods, mathematic modeling of kinetic data, and interpretation of DCE MR imaging data.
The two-compartment pharmacokinetic model of blood plasma versus extravascular extracellular space may not be valid for modeling the kinetics of DCIS, since contrast material exchange with mammary ducts represents a third compartment.
Leakiness of the duct basement membrane may prove to be a marker for DCIS lesions that are likely to become invasive, which could lead to improvements in the clinical management of DCIS.
Supplementary Material
Acknowledgments
We thank Erica J. Markiewicz, BS, University of Chicago, for her help in performing the mouse imaging experiments.
Received November 15, 2008; revision requested January 8, 2009; revision received April 17; accepted May 8; final version accepted June 1.
From the 2008 RSNA Annual Meeting.
Supported by the Segal Foundation, the Florsheim Foundation, the University of Chicago Cancer Center, and Department of Defense Award W81XWH-06-1-0329. Use of Advanced Photon Source was funded by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under contract no. DE-AC02-06CH11357. G. M. N. supported by Philips Medical USA.
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.2533082026/-/DC1
Funding: This research was supported by the National Institutes of Health (grants R21 CA104774-01A2 and 1 R01 EB003108-04).
Authors stated no financial relationship to disclose.
See also Science to Practice in this issue.
Abbreviations:
- DCE
- dynamic contrast material–enhanced
- DCIS
- ductal carcinoma in situ
- Ktrans
- transfer constant
- ROI
- region of interest
- TCM
- two-compartment model
- ve
- volume of extravascular extracellular space per unit volume of tissue
- XFM
- x-ray fluorescence microscopy
References
- 1.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57:75–89 [DOI] [PubMed] [Google Scholar]
- 2.Gilles R, Meunier M, Lucidarme O, et al. Clustered breast microcalcifications: evaluation by dynamic contrast-enhanced subtraction MRI. J Comput Assist Tomogr 1996;20:9–14 [DOI] [PubMed] [Google Scholar]
- 3.Westerhof JP, Fischer U, Moritz JD, Oestmann JW. MR imaging of mammographically detected clustered microcalcifications: is there any value? Radiology 1998;207:675–681 [DOI] [PubMed] [Google Scholar]
- 4.Esserman LJ, Kumar AS, Herrera AF, et al. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J Clin Oncol 2006;24:4603–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet 2007;370:485–492 [DOI] [PubMed] [Google Scholar]
- 6.Kuhl CK, Bieling HB, Gieseke J, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology 1997;203:137–144 [DOI] [PubMed] [Google Scholar]
- 7.Weinreb JC, Newstead G. MR imaging of the breast. Radiology 1995;196:593–610 [DOI] [PubMed] [Google Scholar]
- 8.Jansen SA, Newstead GM, Abe H, Shimauchi A, Schmidt RA, Karczmar GS. Pure ductal carcinoma in situ: kinetic and morphologic MR characteristics compared with mammographic appearance and nuclear grade. Radiology 2007;245:684–691 [DOI] [PubMed] [Google Scholar]
- 9.Harms SE. The use of breast magnetic resonance imaging in ductal carcinoma in situ. Breast J 2005;11:379–381 [DOI] [PubMed] [Google Scholar]
- 10.Armitage P, Behrenbruch C, Brady M, Moore N. Extracting and visualizing physiological parameters using dynamic contrast-enhanced magnetic resonance imaging of the breast. Med Image Anal 2005;9:315–329 [DOI] [PubMed] [Google Scholar]
- 11.Green JE, Shibata MA, Yoshidome K, et al. The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene 2000;19:1020–1027 [DOI] [PubMed] [Google Scholar]
- 12.Maroulakou IG, Anver M, Garrett L, Green JE. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci U S A 1994;91:11236–11240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X, Markiewicz EJ, Zamora M, Karczmar GS, Roman BB. Comparison and evaluation of mouse cardiac MRI acquired with open birdcage, single loop surface and volume birdcage coils. Phys Med Biol 2006;51:N451–N459 [DOI] [PubMed] [Google Scholar]
- 14.Jansen SA, Conzen SD, Fan X, et al. Detection of in situ mammary cancer in a transgenic mouse model: in vitro and in vivo MRI studies demonstrate histopathologic correlation. Phys Med Biol 2008;53:5481–5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovar DA, Lewis M, Karczmar GS. A new method for imaging perfusion and contrast extraction fraction: input functions derived from reference tissues. J Magn Reson Imaging 1998;8:1126–1134 [DOI] [PubMed] [Google Scholar]
- 16.Vogt S. MAPS: a set of software tools for analysis and visualization of 3D x-ray fluorescence data sets. J Phys IV 2003;104:635–638 [Google Scholar]
- 17.De Stasio G, Casalbore P, Pallini R, et al. Gadolinium in human glioblastoma cells for gadolinium neutron capture therapy. Cancer Res 2001;61:4272–4277 [PubMed] [Google Scholar]
- 18.De Stasio G, Rajesh D, Casalbore P, et al. Are gadolinium contrast agents suitable for gadolinium neutron capture therapy? Neurol Res 2005;27:387–398 [DOI] [PubMed] [Google Scholar]
- 19.Sieber MA, Lengsfeld P, Frenzel T, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol 2008;18:2164–2173 [DOI] [PubMed] [Google Scholar]
- 20.Paunesku T, Ke T, Dharmakumar R, et al. Gadolinium-conjugated TiO2-DNA oligonucleotide nanoconjugates show prolonged intracellular retention period and T1-weighted contrast enhancement in magnetic resonance images. Nanomedicine 2008;4:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endres PJ, Paunesku T, Vogt S, Meade TJ, Woloschak GE. DNA-TiO2 nanoconjugates labeled with magnetic resonance contrast agents. J Am Chem Soc 2007;129:15760–15761 [DOI] [PubMed] [Google Scholar]
- 22.Weidensteiner C, Rausch M, McSheehy PM, Allegrini PR. Quantitative dynamic contrast-enhanced MRI in tumor-bearing rats and mice with inversion recovery TrueFISP and two contrast agents at 4.7 T. J Magn Reson Imaging 2006;24:646–656 [DOI] [PubMed] [Google Scholar]
- 23.Yankeelov TE, DeBusk LM, Billheimer DD, et al. Repeatability of a reference region model for analysis of murine DCE-MRI data at 7T. J Magn Reson Imaging 2006;24:1140–1147 [DOI] [PubMed] [Google Scholar]
- 24.Furman-Haran E, Schechtman E, Kelcz F, Kirshenbaum K, Degani H. Magnetic resonance imaging reveals functional diversity of the vasculature in benign and malignant breast lesions. Cancer 2005;104:708–718 [DOI] [PubMed] [Google Scholar]
- 25.Neubauer H, Li M, Kuehne-Heid R, Schneider A, Kaiser WA. High grade and non-high grade ductal carcinoma in situ on dynamic MR mammography: characteristic findings for signal increase and morphological pattern of enhancement. Br J Radiol 2003;76:3–12 [DOI] [PubMed] [Google Scholar]
- 26.Menell JH, Morris EA, Dershaw DD, Abramson AF, Brogi E, Liberman L. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. Breast J 2005;11:382–390 [DOI] [PubMed] [Google Scholar]
- 27.Van Goethem M, Schelfout K, Kersschot E, et al. Comparison of MRI features of different grades of DCIS and invasive carcinoma of the breast. JBR-BTR 2005;88:225–232 [DOI] [PubMed] [Google Scholar]
- 28.Orel SG, Mendonca MH, Reynolds C, Schnall MD, Solin LJ, Sullivan DC. MR imaging of ductal carcinoma in situ. Radiology 1997;202:413–420 [DOI] [PubMed] [Google Scholar]
- 29.Groves AM, Warren RM, Godward S, Rajan PS. Characterization of pure high-grade DCIS on magnetic resonance imaging using the evolving breast MR lexicon terminology: can it be differentiated from pure invasive disease? Magn Reson Imaging 2005;23:733–738 [DOI] [PubMed] [Google Scholar]
- 30.Huang W, Li X, Morris EA, et al. The magnetic resonance shutter speed discriminates vascular properties of malignant and benign breast tumors in vivo. Proc Natl Acad Sci U S A 2008;105:17943–17948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zierold K. Comparison of cryopreparation techniques for electron probe microanalysis of cells as exemplified by human erythrocytes. Scanning Microsc 1992;6:1137–1143 [PubMed] [Google Scholar]
- 32.Ralle M, Lutsenko S. Quantitative imaging of metals in tissues. Biometals 2009;22:197–205 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.