Abstract
While the human hand has an extraordinary capacity to manipulate objects, movement of its digits is usually not completely independent. These limits have been documented for extrinsic flexor muscles, although hand skills also require selectivity for extension movements. Hence, we measured the degree of independent control of the major extrinsic extensor (extensor digitorum, ED). Subjects grasped a cylinder, with the thumb perpendicular to the fingers. Load cells were connected to the proximal phalanges of the fingers and the thumb's distal phalanx. Intramuscular recordings using needle electrodes were made from the individual digital compartments of ED. Subjects were instructed to extend each digit isometrically in a voluntary ramp contraction to 50% maximal force. In total, the behaviour of 283 single motor units was analysed. More than half of the units associated with one ‘test’ finger were recruited inadvertently when another digit contracted to 50% maximum, with most units being recruited by extension of the adjacent digits. Usually, test motor units were recruited at higher forces by extension of fingers further from the test finger. Unexpectedly, extension of the thumb recruited many motor units acting on the little finger. Across tasks, at recruitment of the test motor units, the force produced by the test finger often differed between the voluntary and inadvertent contractions. Overall, the independent control of the output of ED is limited; this may reflect ‘spill-over’ of motor commands to other digital extensor compartments. This level of control of the extrinsic extensor muscles is more independent than the control of the deep extrinsic flexor muscle but less independent than that of the superficial extrinsic flexor muscle.
Introduction
Compared to non-human primates, humans have a superior ability to flex digits of the hand independently (Wood-Jones, 1949; Napier, 1980). This facilitates grasping, object manipulation, and finger-specific tasks such as typing (e.g. Schieber, 1995; Dennerlein et al. 1998; Slobounov et al. 2002; Schieber & Santello, 2004). The capacity to extend the digits is crucial too. This allows fingers to be removed from an object so that they can be accurately repositioned with the sensitive finger pad engaged in tactile exploration for functional tasks requiring participation of one or more fingers.
Despite the advantage over non-human primates, humans have a limited ability to flex the digits independently due to anatomical links between the multi-tendon extrinsic muscles and neural constraints linking output from motoneurone pools innervating digital compartments of the extrinsic muscles (e.g. Wood-Jones, 1949; Napier, 1980; Kilbreath & Gandevia, 1994; Zatsiorsky et al. 1998, 2000; Keen & Fuglevand, 2003; Lang & Schieber, 2004). Two approaches have exposed these constraints for the long extrinsic flexor muscles.
First, maximal flexion of one finger is accompanied by unintentional force production of other fingers. This has been termed ‘enslavement’ (e.g. Zatsiorsky et al. 1998, 2000). Enslavement has also been shown during submaximal contractions lasting 5 s (Reilly & Hammond, 2000; Slobounov et al. 2002) and during ballistic submaximal contractions (e.g. Reilly & Hammond, 2004). Enslavement is not restricted to the fingers; it has also been shown in the thumb (Olafsdottir et al. 2005). Most of these enslavement studies focused on flexion, even though unintentional force production in extension has been noted (Reilly & Hammond, 2000). However, in a recent study we showed that enslavement forces produced during digit extension were relatively higher than those produced during digit flexion (Van Duinen et al. 2009).
Second, for the long flexors, single motor units in digital compartments are recruited during flexion of other digits. For most fingers, not even half of the ‘test’ motor units of flexor digitorum superficialis (FDS) acting on one finger were recruited when another finger flexes voluntarily at the proximal interphalangeal joint (Butler et al. 2005). The closer the commanded finger to the test finger, the greater the number of motor units recruited (and at lower forces; Butler et al. 2005). The same phenomenon, but at much lower forces, occurs for motor units of flexor digitorum profundus (FDP) during flexion of the distal joint of the fingers. Commonly, it is impossible to flex one distal joint to 5% maximal force without recruitment of units acting on adjacent digits including the thumb (Kilbreath & Gandevia, 1994). This effect also extends to FPL. This link has been shown with measures of short-term synchronization (Hockensmith et al. 2005), although the mechanical linkage between FPL and the index portion of FDP is small with hand muscles at their common lengths (Yu et al. 2007). The lower recruitment thresholds in FDP could be the result of high common input to the different compartments of the FDP, as there is stronger synchronization between muscle compartments in FDP compared to FDS (Winges & Santello, 2004; McIsaac & Fuglevand, 2007).
Although crucial for manual dexterity, little is known about the selectivity of motor unit recruitment for voluntary extension of the individual digits. For voluntary extension of one digital compartment, there is a systematic relationship between the sizes of motor units, their recruitment, and their firing behaviour (Monster & Chan, 1977). Furthermore, Keen & Fuglevand (2004b) have shown that intraneural microstimulation can selectively activate different compartments of extensor digitorum (ED), but during voluntary extension the individual control is less selective. Leijnse & colleagues (2008b) also showed electromyographic activity (EMG) in neighbouring digital compartments of ED during finger tapping, using surface electrodes. However, they suggested this activity in neighbouring compartments resulted from ‘crosstalk’ between recordings instead of ‘spill-over’ of neural drive, as proposed for the finger flexors (Kilbreath & Gandevia, 1994).
Hence, we used single motor unit recording to measure any spill-over of neural drive to neighbouring compartments of ED during voluntary extension of the fingers and thumb, and assessed the recruitment thresholds of motor units in each digital compartment of ED. Finally, we examined whether the force produced by the test finger at the time of motor unit recruitment was the same during voluntary and inadvertent contractions. Such forces have not been reported for contractions of FDS and FDP. We found that ED motor units were recruited at force levels produced by the neighbouring digits that fell in between those of FDP and FDS. The force produced by the test finger at the time of recruitment of the test motor units differed between voluntary and unintentional contractions. Furthermore, there was an unexpected link between extension of the thumb and recruitment of motor units acting on the little finger compartment.
Methods
Participants
Ten subjects (6 males, 4 females; age: 24–54 years) participated in one or more experimental sessions, after giving informed written consent. The studies were conducted in accordance with the Declaration of Helsinki, with the procedures approved by the local Human Research Ethics Committee at the University of New South Wales.
Experimental design
Force measurements
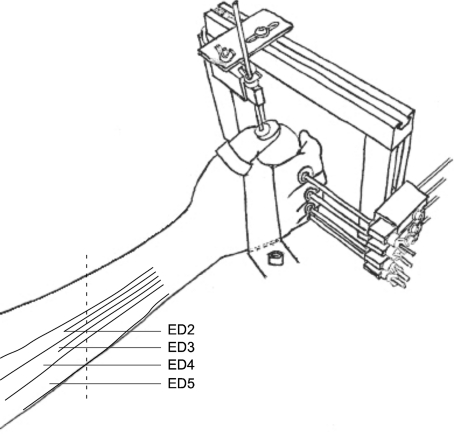
The task involved voluntary isometric extension of the digit(s). The subject sat comfortably in a chair with the right forearm resting on a table. The wrist was at ∼30 deg extension and the forearm at ∼45 deg to the coronal plane. The right hand was positioned in mid-pronation to hold a cylinder (65 mm diameter and 100 mm height) that was fixed to a table (Fig. 1). The dorsal surface of the proximal phalanx of each finger was placed against a load cell mounted horizontally on a vertical post. The dorsal surface of the distal phalanx of the thumb was aligned horizontally against a load cell which measured vertical force. With this arrangement, the thumb force was perpendicular to the forces exerted by the fingers. The positions of the load cells were adjusted for each subject.
Figure 1. Experimental set-up.
A concentric needle electrode was inserted into extensor digitorum (ED) ∼1 cm proximal to the middle of the forearm (vertical dotted line) and slightly to the ulnar side of the middle of the forearm for index finger recordings. To record activity from units of the other digital compartments of ED the needle was re-inserted at more proximal and ulnar sites.
The subjects performed three brief maximal voluntary contractions (MVCs) of each digit in extension. During the MVCs, the subjects were instructed to focus on extension of the contracting digit, without paying attention to the other digits. They received visual feedback of the extension force produced by the test digit. To minimize fatigue, there was at least 1 min between two contractions of the same digit. For each digit, we determined the highest force in the attempted MVCs of the individual digits. This highest MVC was used for further analysis and to express all other forces as a percentage of the maximal voluntary contraction force (% MVC) and to calculate the submaximal force level for the ramp contractions (see ‘Experimental protocol’). The average MVC values (±s.d.) were 19.7 ± 4.2, 30.3 ± 8.4, 23.9 ± 7.6, 17.4 ± 8.1, and 19.1 ± 7.7 N for the thumb, index, middle, ring and little fingers, respectively.
EMG measurements
The major extrinsic muscle which extends the fingers is extensor digitorum (ED), which has multiple compartments and separate tendons to each finger. Additional extrinsic extensor muscles are extensor indicis (EI) and extensor digiti minimi (EDM), which extend the index and little fingers, respectively. Intrinsic hand muscles (interossei and lumbricals) extend interphalangeal but not metacarpophalangeal joints (Chao et al. 1989; Brand & Hollister, 1999; Zilber & Oberlin, 2004; Leijnse et al. 2008a,b;). A concentric needle electrode (0.46 mm diameter, Medelec, CareFusion, Warwick, UK, no. S53155) was inserted into the ED ‘compartment’ of the test finger from the dorsal surface about 1–2 cm proximal to the mid-point of the forearm (Fig. 1); we will refer to these compartments for the index, middle, ring and little fingers as ED2, ED3, ED4 and ED5, respectively. The little finger compartments of the ED and the EDM are often closely connected and difficult to separate from each other. In the present study, the ED5 is likely to refer to the combination of the EDM and the ED5.
After insertion of the needle, subjects were instructed to extend each of the digits separately with a weak but steady force to verify the electrode position in the compartment of interest. This position was confirmed by the presence of reproducible electromyographic activity (EMG) involving one or more low-threshold motor units recruited by extension of the MCP joint of the ‘test’ finger. Auditory feedback of the EMG was provided along with visual feedback of single motor unit activity on a digital oscilloscope. With the aid of this feedback, subjects were asked to slowly increase the extension force of the ‘test’ digit to recruit a motor unit. After selection of this test motor unit, we started the experimental protocol.
Experimental protocol
The protocol consisted of isometric extension of the digits, following a ramp using visual feedback (Fig. 2). Subjects were instructed to focus on the digit they had to contract and to follow the ramp as closely as possible, but to stop when the selected motor unit became active, when there were too many motor units such that the selected unit could not be clearly detected, or when the site of needle insertion became painful. The protocol started with one ramp for the test digit up to maximal 10% MVC in 5 s, followed by three ramps of another digit up to ∼50% MVC in 5 s. This ramp rate for contractions of the non-test digits was the same as in the previous study of flexor digitorum superficialis (FDS; Butler et al. 2005). To ensure that the position of the electrode and the activity in the test unit remained stable, a 10% MVC ramp of the test finger was repeated after each set of ramp extensions. The protocol was terminated after all digits had performed the sets of ramp contractions, or when the motor unit of interest was lost. Typically, more units than just the test motor unit were recorded during the protocol. If possible, these units were also analysed (see below).
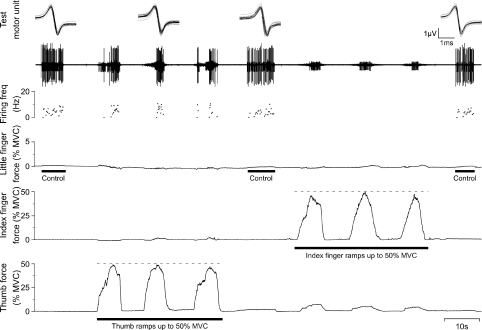
Figure 2. Example of extension ramp contractions by thumb and index finger while recording from ED5.
From top to bottom: superimposed action potentials from the single motor unit of interest (test motor unit); the intramuscular EMG recording (iEMG); the instantaneous firing frequency of the test unit (firing freq.); little finger force; index finger force; and thumb force. All forces are shown as a percentage of maximal voluntary contraction force (% MVC). In this example, a weak control extension force produced by the little finger was followed by three ramps of increasing force produced by extension of the thumb up to 50% MVC, a control little-finger contraction, and three ramps performed by the index finger. Finally, there is another control extension contraction of the little finger. The test unit was recruited as expected by weak extension contractions of the little finger, but it was also recruited during extension contractions of the thumb.
To test the next compartment, the concentric needle electrode was reinserted in a new position. The order of test compartments was random, and so was the order of isometric contractions of the non-test digits.
Data acquisition and analysis
All data were recorded and analysed offline using Spike2 (CED 1401, Cambridge Electronic Design, Cambridge, UK). The force signals from the five load cells were each sampled at 1 kHz. The EMG signal was band-pass filtered (16–3000 Hz), amplified (3000–30 000 times), and sampled at 6 kHz.
Single motor units were identified using template matching combined with manual sorting (see Butler et al. 2005). Then, we determined the force levels at which each single motor unit was recruited, both during the ramp contraction of the test finger and during the ramp contractions of the other digits. The mean values from these ramp contractions were used in the analyses. Not all test units were recruited during the ramp contractions; to be able to use all test units in the statistical analyses an arbitrary recruitment value had to be assigned to these non-recruited units. In our previous study on FDS recruitment thresholds, we used 60% MVC, which was the peak contraction force of the ramp (50% MVC) plus 10% MVC to allow for the fact that the unit was not yet recruited (Butler et al. 2005). However in the present experiment, not all ramps were continued to 50% MVC (see above), and thus using 60% MVC would lead to artificially high forces at the time of recruitment. Therefore, we decided to use the peak ramp force of the test finger plus 10% MVC.
SPSS (v. 15.0, Chicago, IL, USA) was used for the statistical analyses (unless otherwise specified). We performed a one-way analysis of variance (ANOVA) to test whether the recruitment thresholds were the same for the four test fingers. As the variances were not homogeneous, we used Dunnett's T3 post hoc analysis to determine which thresholds differed significantly. A chi-square (χ2) test was used to test whether the percentages of recruited motor units of a specific finger differed depending on the distance between this finger and the commanded digit (SigmaStat, v. 2.03). Furthermore, we tested whether the force levels of the digits were different at the time of recruitment of the test motor units, using a repeated-measures ANOVA for the motor units acting on index, middle, ring and little fingers separately. In this test, we included the estimated values of the motor units that were not recruited, so that all units were included in the analyses. We repeated this test without data from the test finger in the analysis, so that the low recruitment thresholds of the test finger did not exaggerate the statistical results. Post hoc analyses were performed to assess which digits differed significantly from the other digits (Bonferroni corrected). We also tested whether there were differences in the force produced by the test finger at the time of recruitment of the test motor units during the extension contractions of each digit (repeated measures ANOVA; within-subjects factor: digit).
Results
In total, the activity of 283 low-threshold ‘test’ motor units was recorded from the digital components of the extensor digitorum (ED). Activity of additional motor units in the test finger compartment was recorded during voluntary contractions of ‘non-test’ digits, but this was not studied further. Of the test motor units, 66 acted on the index finger, 80 on the middle finger, 65 on the ring finger, and 72 on the little finger. The mean voluntary contraction forces (± SD) produced by the test fingers at recruitment of the units were 2.91 ± 2.61, 2.49 ± 2.17, 1.80 ± 1.90 and 0.77 ± 0.85% MVC for index, middle, ring and little fingers, respectively. These recruitment forces differed significantly from each other (one-way ANOVA: F3,279= 15.708; P < 0.001). Post hoc analyses showed that the mean recruitment force of ED5 motor units was significantly lower than recruitment forces for motor units of other digits (P < 0.001), and that the mean recruitment force of ED2 units was significantly higher than the recruitment force of ED4 units (P < 0.05).
Motor unit behaviour – force thresholds and recruitment
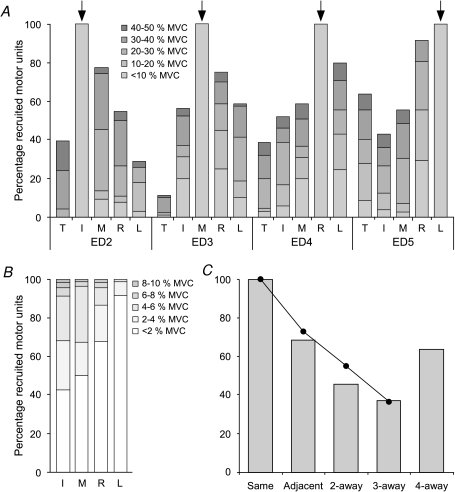
During recordings from the ED compartment of a test finger, subjects performed ramp contractions with the extensors of the other digits. Figure 3A shows the percentage of motor units acting on index, middle, ring and little that were recruited during contractions of the digits. In general, extension of the other fingers up to 50% MVC recruited more than half of the test motor units in the other fingers. However, voluntary isometric extensions of the thumb up to 50% MVC only recruited 40% or less of the test motor units, except for the little finger units. More than 60% of these ED5 units were recruited by voluntary extension of the thumb. More motor units were recruited in ramp contractions of adjacent digits than more remote digits (Fig. 3C; χ2= 282.39, df = 4 including data for the thumb, grey columns; or χ2= 230.85, df = 3 excluding data for the thumb, black circles and continuous line; both P < 0.001). As expected, all motor units acting on the test finger were recruited at force levels below 10% MVC in the contractions produced by the test finger (Fig. 3A and B).
Figure 3. Percentages of motor units recruited by the different tasks.
A, the percentages of motor units recruited during extension of thumb, index, middle, ring and little fingers (T, I, M, R, L) were determined for the motor units of each ED compartment (ED2, 3, 4, 5). The degree of shading of the grey bars indicates the force of the active digit at the time of recruitment (in blocks of 10% MVC; with darker shading signifying a higher recruitment threshold). When the instructed finger is the test finger (arrows), all motor units were recruited below 10% MVC, and further detail is shown in panel B. B, the columns of the test fingers in A are divided in lighter shades of grey to distinguish the recruitment thresholds of the test motor units in blocks of 2% MVC. C, the columns show the effect of ‘distance’ on the percentage of recruited motor units, including the data from extension of the thumb. The filled circles, connected by the line, show the same data without the data from thumb extension. Adjacent digits recruited more motor units than digits further away. However, for digits ‘4-away’ (thumb and little finger) there was major recruitment of little finger units by thumb extension.
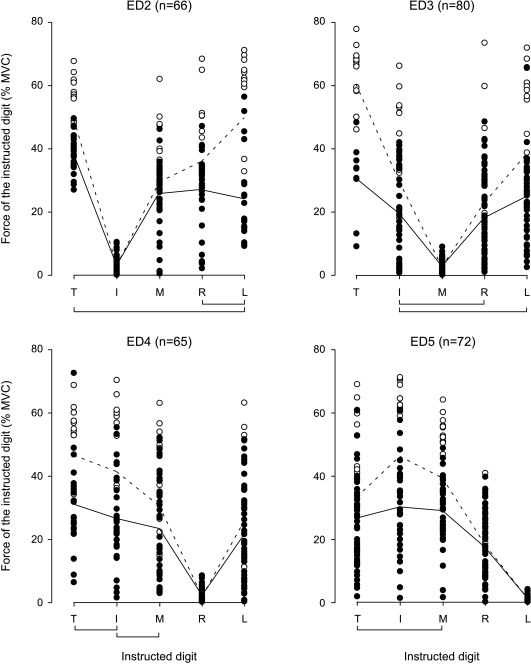
The force produced by the active digit at the time of recruitment of the motor unit acting on the test finger (recruitment threshold) differed between the digits. Figure 4 shows the recruitment thresholds (filled circles) for each motor unit and for all digits. Threshold forces are plotted for ED2, ED3, ED4 and ED5 units separately. Test motor units that were not recruited by a ramp contraction of a particular digit are plotted at their estimated value (open circles; see Methods). The continuous lines in Fig. 4 depict the mean recruitment thresholds for units that were recruited, and the dotted lines depict the mean recruitment thresholds for all test motor units. More than 90% of the individual motor units showed the same ‘V-shaped’ pattern of recruitment forces as depicted for the mean responses in Fig. 4. The recruitment thresholds differed significantly between the digits for all test fingers (index: F4,260= 123.73; P < 0.001; middle: F4,312= 106.81; P < 0.001; ring: F4,256= 85.10; P < 0.001; little: F4,284= 131.49; P < 0.001). As expected, the test motor units were recruited at the lowest force by the test finger (post hoc analyses: P < 0.001 for all test digits). However, even without data from the test finger in the analysis, the recruitment thresholds differed significantly between the digits. In general, recruitment thresholds were lower for neighbouring fingers than for fingers further away, and they were lower for the fingers than for the thumb. The exception to this general pattern was that low voluntary extension forces at the thumb were associated with recruitment of test motor units in ED5.
Figure 4. Force at the time of motor unit recruitment for each digital compartment of ED.
The four panels show all test motor units at their motor unit recruitment force produced by the other digits for a particular compartment of ED. The filled circles represent the actual recruitment thresholds of the motor units that were recruited. The open circles represent the estimated recruitment thresholds of the motor units that were not recruited during the extension ramp contractions of the instructed digits. These values were estimated as the average peak ramp force of that digit plus 10% MVC (see Methods). The continuous lines join the mean recruitment threshold of the motor units that were recruited by the test digits. The dashed lines represent the estimated mean recruitment thresholds of all motor units, including the non-recruited units. The lines below the plots link the digits of which the average force at time of recruitment of all test motor units (including the estimated values of the non-recruited motor units) was not different (P > 0.05).
In Fig. 4, data from the motor units were plotted from a ‘unit perspective’, meaning that we followed a test unit during ramp contractions of the other digits. However, we re-plotted the data of the recruited motor units from a ‘digit perspective’ in Fig. 5. It shows the behaviour of motor units recorded from the four finger components of ED when recruited during the ramp contractions of each digit separately. This reveals what happens to the motor units of all fingers, during voluntary extension of one digit. Again this shows that more than half of the units were recruited during ramp contractions performed by other fingers, that voluntary extension of a finger recruited more motor units acting on adjacent fingers than units acting at fingers further away, and that the recruitment thresholds for the units acting on adjacent fingers were lower.
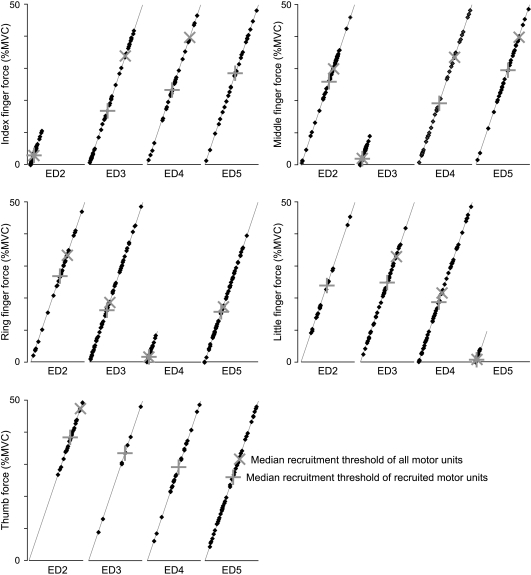
Figure 5. Force at the time of motor unit recruitment plotted along a virtual ramp of voluntary force for each digit.
For each digit, the thresholds are plotted for the motor units that were recruited in the test fingers (ED2, 3, 4, 5) along a virtual ramp contraction up to 50% MVC (or 10% MVC when the instructed finger was the test finger). This illustrates how motor units acting on the four fingers are recruited during a ramp contraction of each of the digits. In these ramps the median recruitment thresholds are plotted to show at which force level half of the recruited motor units are recruited (‘+’), and when half of all motor units are recruited (‘×’). If less than half of the units are recruited, the × is missing. When the active finger is the test finger, the + and × are the same and are superimposed.
Force produced by the test finger at the time of motor unit recruitment
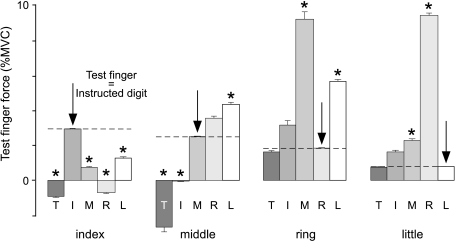
When the same motor units are recruited during a voluntary contraction and during an unintentional contraction of a test finger, one might expect that the test finger produces a similar amount of force at the time of recruitment. However, this did not usually occur, as in Fig. 6, which shows the mean force produced by the test finger at the time of motor unit recruitment. For example, ED2 motor units were usually recruited at ∼2.9% MVC in a voluntary extension of the index finger, but in voluntary extension of the other digits, the same ED2 units were recruited at lower index finger forces (P < 0.001). ED3 units were recruited during thumb and index finger extension at a lower force level than in voluntary middle finger extension (P < 0.01), but at a higher level in little finger extension (P < 0.001) and a similar level during ring finger extension. During ramp extensions of the thumb, subjects tended to flex both index and middle fingers; this resulted in a release from the transducers eliciting a negative force. ED4 units were recruited at a lower force in voluntary extension of the ring finger than in extension of the middle and little fingers (P < 0.005). ED5 units were recruited at a lower force in voluntary extension of the little finger force than in extension of the middle and ring fingers (P < 0.01). Overall, force produced by the test finger at the time of recruitment of the test motor unit differed between voluntary and inadvertent contractions.
Figure 6. Force produced by the test finger at time of motor unit recruitment.
The mean (unintentional) force (±s.e.m.) of the test fingers (index, middle, ring and little fingers) is plotted at the time of recruitment during ramp extension contractions of the digits (T, I, M, R and L). The arrows indicate the condition in which the test finger is the instructed digit. Here, the force is the mean voluntary motor unit recruitment threshold and this force is also indicated by the horizontal dashed lines. Commonly there was a difference between the voluntary motor unit recruitment threshold and the unintentional force at the time of recruitment. When this difference is significant, it is indicated by an asterisk (P < 0.01).
Discussion
The present study investigated motor unit recruitment in the different compartments of extensor digitorum (ED). There were several novel findings. First, voluntarily extension of one finger (up to 50% of maximal force) recruited single motor units that act on the extending finger and also many of the motor units that act on other fingers. Second, more motor units acting on the adjacent fingers were recruited than units acting on more distant fingers, and these units acting on adjacent fingers were recruited at lower forces. This pattern of recruitment of ED units was commonly intermediate between those previously published for the two multi-tendoned flexor muscles (FDP and FDS; see below). Third, an unexpected finding was that, unlike for the flexors, contraction of the thumb extensor recruited more than half of the motor units acting on the little finger, but less than half of those acting on index and ring fingers, and only ∼10% of units acting on the middle finger. Finally, the force at which a motor unit was recruited during a voluntary contraction of one digital compartment of ED commonly differed from the force at the time of recruitment during an inadvertent contraction.
ED recruitment thresholds
ED2 motor units had higher recruitment thresholds than ED4 and ED5 units. This may result from simultaneous activation of the extensor indicis (EI) during extension of the index finger. Subjects preferentially contract EI for extension of the index finger alone but also contract ED2 for the extension of the index finger in combination with other fingers (Fuglevand, personal communication). This differential activation will contribute to the discrepancy in ED2 recruitment thresholds during voluntary and unintentional force production (see below).
While it is possible to contract single intrinsic hand muscles selectively (e.g. Gandevia & Rothwell, 1987; Gandevia et al. 1990) and to extend fingers selectively at low force levels using intraneural microstimulation, voluntary control of finger extension is less selective (Keen & Fuglevand, 2004b). We now show that it is difficult to extend one digit up to 50% MVC without recruiting motor units that extend the other digits. The closer these other digits are to the extending digit, the more units are recruited and the lower force of the extending digit at the time of recruitment of those units. This pattern of recruitment fits with studies on recruitment thresholds of long flexor muscles, short-term synchronization between units of extensors and flexors, and the spread of force in flexion and extension (Kilbreath & Gandevia, 1994; Zatsiorsky et al. 2000; Slobounov et al. 2002; Keen & Fuglevand, 2003, 2004a,b; Butler et al. 2005; Van Duinen et al. 2009). The message from these studies is that there is more common input (or more spill-over of neural drive) to more adjacent fingers than to fingers that are further away.
One exception to this pattern was that voluntary extension of the thumb recruited more ED5 motor units than other motor units, even though the thumb and little finger are maximally separated. This coupling between thumb and little finger was also shown in a vertical thumb tapping task, even though the authors suggested that this resulted from crosstalk between the surface EMG electrodes of the extensor pollicis longus and the little finger extensors which are anatomically close together (Leijnse et al. 2008b). Another interaction between the thumb and little finger has been observed in monkeys during flexion and extension of the digits (Schieber, 1995). One reason for the recruitment of ED5 units during thumb extension in the present study may be the secondary requirement to stabilize the wrist.
Comparison with FDS and FDP
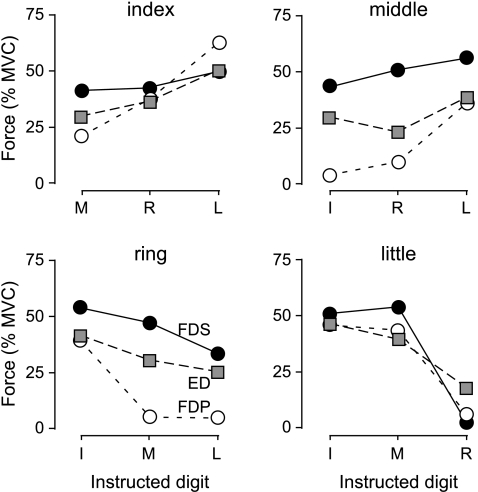
The ED recruitment thresholds in the present study fell between the thresholds recorded in FDP and FDS (Fig. 7; Kilbreath & Gandevia, 1994; Butler et al. 2005). When the recruitment threshold of the two long flexors differed significantly (see fig. 6 of Butler et al. 2005), the motor unit recruitment thresholds of ED were higher than those of FDP and lower than those of FDS. This suggests that during voluntary contractions up to 50% of maximal force, extension of the fingers is more independent than flexion of the fingertips (FDP), but less independent than flexion at the proximal interphalangeal joint (FDS).
Figure 7. Comparison of motor unit recruitment thresholds of the long multi-tendoned flexors and extensor of the human hand.
The mean motor unit recruitment thresholds during voluntary contractions of non-test fingers are plotted for all index, middle, ring and little finger units of the flexor digitorum superficialis (FDS; filled circles), flexor digitorum profundus (FDP; open circles) and extensor digitorum (ED; grey squares). Data for FDS and FDP are adapted from Butler et al. 2005.
As noted in the Introduction, the higher recruitment thresholds of motor units in neighbouring compartments of FDS compared to FDP would fit with the lower short-term synchronization of FDS compared to FDP (Kilbreath & Gandevia, 1994; Winges & Santello, 2004; Butler et al. 2005; McIsaac & Fuglevand, 2007; see also Reilly et al. 2004). However, the level of short-term synchronization of motor units of ED did not fall in between that for FDS and FDP (Keen & Fuglevand, 2004a). While short-term synchronization and motor unit recruitment studies reveal the common drive to two compartments, this might differ with attempts to extend two digits at very low force levels (which is required during studies which assess short-term synchronization) compared with attempts to extend only one digit (as in the present study).
Test finger force at time of motor unit recruitment
Force produced by the test finger at the time of motor unit recruitment differed between voluntary contractions and unintentional force production (Fig. 6). The force produced by the index finger at the time of motor unit recruitment was lower during voluntary extension of the other digits than during voluntary extension of the index finger itself. The most likely explanation for this lower force at the time of inadvertent recruitment is the fact that there are two muscles for extension of the index finger: the index finger compartment of the extensor digitorum (ED2) and the extensor indicis (EI). The use of ED2, EI, or both varies between and within people (Leijnse et al. 2008b); EI is contracted more during extension of the index finger alone and ED2 is used more in combination with extension of other fingers (Fuglevand, personal communication). Co-contraction of EI during intentional extension of the index finger might result in relatively high index finger forces at the time of ED2 motor unit recruitment. Contraction of ED during voluntary extension of other fingers is more likely to result in spread of neural drive to ED2, showing subsequently a lower force produced by the index finger at recruitment of the same ED2 test motor unit.
Another explanation for the lower force during the voluntary extension of the other digits is co-activation of antagonist muscles, producing a lower net extension force. This co-activation of the flexor muscles could also contribute to the low middle finger force during extension of thumb and index finger up to 50% MVC, by counterbalancing the recruitment of ED3 units. During maximal voluntary extensions of the thumb or index finger, some subjects show indeed a net flexor torque by the middle finger (Van Duinen et al. 2009).
At the time of motor unit recruitment, the forces produced by the middle, ring and little fingers were generally higher during inadvertent contractions of these three fingers than during voluntary extension of these fingers. While we cannot exclude mechanical linkages between the fingers in contributing to the ‘inadvertent’ forces or extension of the wrist, other units were often already recruited in the test ED compartment before recruitment of ‘test’ units. This suggests that the motor unit recruitment order in the test finger compartment was not unique, but depended on the task (extending the test finger or another digit). Motor unit recruitment order is considered to proceed according to the size principle (e.g. Henneman, 1957; Henneman & Mendell, 1981) and is considered to be stable. There are few major exceptions in recruitment order, depending on task or fatigue (e.g. Desmedt & Godaux, 1981; Thomas et al. 1987; Enoka et al. 1989; Nardone et al. 1989; Howell et al. 1995; Butler et al. 1999). It may be that the central nervous system can achieve the same outcome with the fingers by recruitment of different sets of motor units in the various subvolumes of ED or as well as by the use of different muscles. The possibility that the order is altered in inadvertent contractions deserves further study.
This study shows that voluntary extension of one finger usually recruits more than half of the motor units that are acting on the other fingers. Hence, the individual control of the digits is limited in the extension as well as in the flexion direction. Furthermore, the closer the other fingers are to the extending finger the less independently they act. Adjacent digits recruit more motor units and at lower force levels. Interestingly, voluntary extension of the thumb recruited more motor units acting on the little finger than on other fingers. The data show that a spill-over of neural drive from one digit to the others is a major limit to individuation of digit extension.
Acknowledgments
This work was supported by the National Health and Medical Research Council. We are grateful to Anna Hudson and Chris McNeil for comments on the manuscript.
Glossary
Abbreviations
- ED
extensor digitorum
- EDM
extensor digiti minimi
- ED2
index finger compartment of ED
- ED3
middle finger compartment of ED
- ED4
ring finger compartment of ED
- ED5
little finger compartment of ED
- FDP
flexor digitorum profundus
- FDS
flexor digitorum superficialis
- FPL
flexor pollicis longus
- I
index finger
- iEMG
intramuscular EMG
- L
little finger
- M
middle finger
- MCP
metacarpophalangeal
- MVC
maximal voluntary contraction
- R
ring finger
- T
thumb
Author contributions
All authors were involved in the conception and conduct of the studies. HvD and SG performed the intramuscular recordings.
References
- Brand P, Hollister A. Clinical Mechanisms of the Hand. St Louis, MO: Mosby; 1999. [Google Scholar]
- Butler JE, McKenzie DK, Gandevia SC. Discharge properties and recruitment of human diaphragmatic motor units during voluntary inspiratory tasks. J Physiol. 1999;518:907–920. doi: 10.1111/j.1469-7793.1999.0907p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TJ, Kilbreath SL, Gorman RB, Gandevia SC. Selective recruitment of single motor units in human flexor digitorum superficialis muscle during flexion of individual fingers. J Physiol. 2005;567:301–309. doi: 10.1113/jphysiol.2005.089201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao EYS, An K-N, Cooney WP, III, Linschied RL. Biomechanics of the Hand: A Basic Research Study. Singapore: World Scientific; 1989. [Google Scholar]
- Dennerlein JT, Mote CD, Jr, Rempel DM. Control strategies for finger movement during touch-typing. The role of the extrinsic muscles during a keystroke. Exp Brain Res. 1998;121:1–6. doi: 10.1007/s002210050430. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Spinal motoneuron recruitment in man: rank deordering with direction but not with speed of voluntary movement. Science. 1981;2141:933–936. doi: 10.1126/science.7302570. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Robinson GA, Kossev AR. Task and fatigue effects on low-threshold motor units in human hand muscle. J Neurophysiol. 1989;62:1344–1359. doi: 10.1152/jn.1989.62.6.1344. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Macefield G, Burke D, McKenzie DK. Voluntary activation of human motor axons in the absence of muscle afferent feedback. The control of the deafferented hand. Brain. 1990;113:1563–1581. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC. Knowledge of motor commands and the recruitment of human motoneurons. Brain. 1987;110:1117–1130. doi: 10.1093/brain/110.5.1117. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of the motoneuron pool and its inputs. In: Brooks VB, editor. Handbook of Physiology, section 1, The Nervous System, vol. II, Motor Control. Bethesda, MD: American Physiological, Society; 1981. pp. 423–507. [Google Scholar]
- Hockensmith GB, Lowell SY, Fuglevand AJ. Common input across motor nuclei mediating precision grip in humans. J Neurosci. 2005;25:4560–4564. doi: 10.1523/JNEUROSCI.0046-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JN, Fuglevand AJ, Walsh ML, Bigland-Ritchie B. Motor unit activity during isometric and concentric-eccentric contractions of the human first dorsal interosseus muscle. J Neurophysiol. 1995;74:901–904. doi: 10.1152/jn.1995.74.2.901. [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Role of intertendinous connections in distribution of force in the human extensor digitorum muscle. Muscle Nerve. 2003;28:614–622. doi: 10.1002/mus.10481. [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol. 2004a;91:57–62. doi: 10.1152/jn.00650.2003. [DOI] [PubMed] [Google Scholar]
- Keen DA, Fuglevand AJ. Distribution of motor unit force in human extensor digitorum assessed by spike-triggered averaging and intraneural microstimulation. J Neurophysiol. 2004b;91:2515–2523. doi: 10.1152/jn.01178.2003. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J Physiol. 1994;479:487–497. doi: 10.1113/jphysiol.1994.sp020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Human finger independence: limitations due to passive mechanical coupling versus active neuromuscular control. J Neurophysiol. 2004;92:2802–2810. doi: 10.1152/jn.00480.2004. [DOI] [PubMed] [Google Scholar]
- Leijnse JNAL, Carter S, Gupta A, McCabe S. Anatomic basis for individuated surface EMG and homogeneous electrostimulation with neuroprostheses of the extensor digitorum communis. J Neurophysiol. 2008a;100:64–75. doi: 10.1152/jn.00706.2007. [DOI] [PubMed] [Google Scholar]
- Leijnse JNAL, Campbell-Kyureghyan NH, Spektor D, Quesada PM. Assessment of individual finger muscle activity in the extensor digitorum communis by surface EMG. J Neurophysiol. 2008b;100:3225–3235. doi: 10.1152/jn.90570.2008. [DOI] [PubMed] [Google Scholar]
- McIsaac TL, Fuglevand AJ. Motor-unit synchrony within and across compartments of the human flexor digitorum superficialis. J Neurophysiol. 2007;97:550–556. doi: 10.1152/jn.01071.2006. [DOI] [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol. 1977;40:1432–1443. doi: 10.1152/jn.1977.40.6.1432. [DOI] [PubMed] [Google Scholar]
- Nardone A, Romano C, Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol. 1989;409:451–471. doi: 10.1113/jphysiol.1989.sp017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier JR. Hands. New York: Pantheon Books; 1980. [Google Scholar]
- Olafsdottir H, Zatsiorsky VM, Latash ML. Is the thumb a fifth finger? A study of digit interaction during force production tasks. Exp Brain Res. 2005;160:203–213. doi: 10.1007/s00221-004-2004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly KT, Hammond GR. Independence of force production by digits of the human hand. Neurosci Lett. 2000;290:53–56. doi: 10.1016/s0304-3940(00)01328-8. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Hammond GR. Human handedness: Is there a difference in the independence of the digits on the preferred and non-preferred hands? Exp Brain Res. 2004;156:255–262. doi: 10.1007/s00221-003-1783-z. [DOI] [PubMed] [Google Scholar]
- Reilly KT, Nordstrom MA, Schieber MH. Short-term synchronization between motor units in different functional subdivisions of the human flexor digitorum profundus muscle. J Neurophysiol. 2004;92:734–742. doi: 10.1152/jn.00027.2004. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Individuated movements of rhesus monkeys: means of quantifying the independence of digits. J Neurophysiol. 1995;65:1381–1391. doi: 10.1152/jn.1991.65.6.1381. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Santello M. Hand function: peripheral and central constraints on performance. J Appl Physiol. 2004;96:2293–2300. doi: 10.1152/japplphysiol.01063.2003. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Johnston J, Chiang H, Ray W. The role of sub-maximal force production in the enslaving phenomenon. Brain Res. 2002;954:212–219. doi: 10.1016/s0006-8993(02)03288-2. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Ross BH, Calancie B. Human motor-unit recruitment during isometric contractions and repeated dynamic movements. J Neurophysiol. 1987;57:311–324. doi: 10.1152/jn.1987.57.1.311. [DOI] [PubMed] [Google Scholar]
- Van Duinen H, Yu WS, Gandevia SC. Society for the Neural Control of Movement; Individual control of digits in flexion and extension. Annual meeting poster no. H50. [Google Scholar]
- Winges SA, Santello M. Common input to motor units of digit flexors during multi-digit grasping. J Neurophysiol. 2004;92:3210–3220. doi: 10.1152/jn.00516.2004. [DOI] [PubMed] [Google Scholar]
- Wood-Jones F. The Principles of Anatomy as Seen in the Hand. London: Balliere, Tindall and Cox; 1949. [Google Scholar]
- Yu WS, Kilbreath SL, Fitzpatrick RC, Gandevia SC. Thumb and finger forces produced by motor units in the long flexor of the human thumb. J Physiol. 2007;583:1145–1154. doi: 10.1113/jphysiol.2007.135640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatsiorsky VM, Li ZM, Latash ML. Coordinated force production in multifinger tasks: finger interaction and neural network modelling. Biol Cybern. 1998;79:139–150. doi: 10.1007/s004220050466. [DOI] [PubMed] [Google Scholar]
- Zatsiorsky VM, Li ZM, Latash ML. Enslaving effects in multi-finger force production. Exp Brain Res. 2000;131:187–195. doi: 10.1007/s002219900261. [DOI] [PubMed] [Google Scholar]
- Zilber S, Oberlin C. Anatomical variations of the extensor tendons to the fingers over the dorsum of the hand: a study of 50 hands and a review of the literature. Plast Reconstr Surg. 2004;113:214–221. doi: 10.1097/01.PRS.0000091163.86851.9C. [DOI] [PubMed] [Google Scholar]