Abstract
Important questions remain about the origin of the excitation that drives locomotion in vertebrates and the roles played by reticulospinal neurons. In young Xenopus tadpoles, paired whole-cell recordings reveal reticulospinal neurons that directly excite swimming circuit neurons in the brainstem and spinal cord. They form part of a column of neurons (dINs) with ipsilateral descending projections which fire reliably and rhythmically in time with swimming. We ask if, at this early stage of development, these reticulospinal neurons are themselves the primary source of rhythmic drive to spinal cord neurons on each cycle of swimming. Loose-patch recordings in the hindbrain and spinal cord from neurons active during fictive swimming distinguished dINs from other neurons by spike shape. These recordings showed that reticulospinal dINs in the caudal hindbrain (rhombomeres 7–8) fire significantly earlier on each swimming cycle than other, ipsilateral, swimming circuit neurons. Whole-cell recordings showed that fast EPSCs typically precede, and probably drive, spikes in most swimming circuit neurons. However, the earliest-firing reticulospinal dINs spike too soon to be driven by underlying fast EPSCs. We propose that rebound following reciprocal inhibition can contribute to early reticulospinal dIN firing during swimming and show rebound firing in dINs following evoked, reciprocal inhibitory PSPs. Our results define reticulospinal neurons that are the source of the primary, descending, rhythmic excitation that drives spinal cord neurons to fire during swimming. These neurons are an integral part of the rhythm generating circuitry. We discuss the origin of these reticulospinal neurons as specialised members of a longitudinally distributed population of excitatory interneurons extending from the brainstem into the spinal cord.
Introduction
It is widely accepted that excitatory reticulospinal neurons provide the major descending command that drives spinal cord locomotor systems in mammals and other vertebrates (Orlovsky et al. 1999; Grillner et al. 2008). Where locomotion is controlled from higher brain centres, areas like the basal ganglia and the midbrain locomotor region (MLR) route their input to the spinal cord through reticulospinal neurons (Grillner et al. 1997; Jordan et al. 2008; Goulding, 2009). In addition, work in the lamprey suggests that reticulospinal neurons are themselves able to directly transform sensory inputs into descending commands to drive ‘escape’ locomotion (Viana Di Prisco et al. 1997, 2000; Dubuc et al. 2008). Ascending signals from the spinal cord act on reticulospinal neurons either via spinobulbar neurons (Einum & Buchanan, 2004, 2005) or indirectly via the cerebellum (Arshavsky et al. 1986). These ascending signals lead to rhythmic reticulospinal firing (Drew et al. 1986; Kasicki & Grillner, 1986; Kasicki et al. 1989; Deliagina et al. 2000; Zelenin, 2005) which may help to synchronise activity between brainstem and spinal cord. In the lamprey, new evidence suggests that ascending excitatory input is actually needed to help sustain firing in reticulospinal neurons, and thereby provide command signals to drive extended locomotion over a longer time scale (Antri et al. 2009). However, even in this relatively simple vertebrate, there are >2000 reticulospinal neurons forming heterogeneous populations that are responsible for descending control of steering and posture as well as driving locomotion. (Buchanan & Cohen, 1982; McClellan & Grillner, 1984; Wannier et al. 1998; Zelenin et al. 2001; Deliagina et al. 2002; Zelenin, 2005). As a result of the complexity of adult vertebrates, important questions about the role of excitatory reticulospinal neurons during normal, ongoing locomotion remain only partially answered. To what extent does reticulospinal activity require excitatory input from other brain areas like the MLR or from the spinal cord? Does this requirement depend on the context of locomotion and how it is activated? Is their main role to provide descending tonic excitation (in the manner of Brown (1911), and often mimicked by NMDA applications in experiments (Cohen & Wallen, 1980; Barry & O’Donovan, 1987; Kudo & Yamada, 1987; Hernandez et al. 1991; Wheatley & Stein, 1992; Douglas et al. 1993; McDearmid & Drapeau, 2006; Gabriel et al. 2008)), or is it necessary that they provide some phasic excitation? Are reticulospinal neurons a separate source of descending excitation, or are they themselves components of the rhythm generating mechanism for locomotion? To address these questions, the fundamental requirement is a clear understanding of the origin and nature of the descending excitation to spinal neurons during locomotor activity.
By using a model vertebrate that is both simple and developmentally early, the young Xenopus tadpole at 2 days post-fertilisation, we can try to establish the origin of the excitation which drives spinal locomotor circuits before additional control systems have developed. In the young tadpole, sustained swimming can be initiated and maintained following a brief stimulus to the skin (Clarke et al. 1984) rather than artificially induced by sustained electrical stimulation or bath application of excitants like NMDA. A key feature is that this sustained swimming is not prevented by removal of the forebrain, midbrain and rostral hindbrain (Roberts & Alford 1986; Li et al. 2006), indicating that excitation from ‘higher’ brain centres is not required. The proposal to be tested here is that characterised reticulospinal neurons in the caudal hindbrain fire first at the start of each locomotor cycle, before other ipsilateral neurons, and that they are the source of the primary, descending, rhythmic excitation that drives spinal cord neurons to fire during swimming.
Investigation is facilitated in the young Xenopus tadpole because we have a very detailed knowledge of the neuron types within the 0.1 mm diameter spinal cord, including those active and contributing to a robust swimming motor pattern, which we refer to here as swimming circuit neurons. At this early stage of development, detailed evidence indicates that there are only 10 types of spinal neuron of which only four are swimming circuit neurons. These include motoneurons and three types of premotor interneurons: reciprocal inhibitory commissural interneurons (cINs), recurrent inhibitory ascending interneurons (aINs), and the excitatory descending interneurons (dINs) (Roberts, 2000; Sautois et al. 2007). All of these form longitudinally distributed columns which extend from the spinal cord into the hindbrain with typically 5–15 of each type per 0.1 mm on each side (Dale et al. 1986; Roberts & Alford, 1986; Roberts et al. 1987; van Mier & ten Donkelaar, 1989; Roberts et al. 1999). The low numbers of these and other neurons in the hindbrain of this small, developing animal allow confidence in interpretation of experimental results that is not possible in larger, more mature nervous systems.
The excitatory reticulospinal neurons that we examine here are the rostral members of one of the four Xenopus tadpole swimming circuit neuron types: the dINs, which form a continuous ventrolateral column of neurons lying in the hindbrain and spinal cord. The basic anatomy, properties and connections of dINs have been described (Li et al. 2004, 2006). Their features are consistent across the whole dIN population and distinguish them from other swimming circuit neurons. The reticulospinal dINs we examine here (termed hdINs in Li et al. 2006) are the rostral members of the dIN column lying within the hindbrain.
The timing of neuron activity during swimming in the young Xenopus tadpole is rather strictly defined. Swimming circuit neurons on one side of the CNS, including dINs, typically fire a single spike per cycle approximately in-phase with ipsilateral ventral root discharge. This discharge also shows a reliable rostro-caudal progression (Kahn & Roberts, 1982; Tunstall & Roberts, 1991). As a result, we can compare in detail the timing of rhythmic firing and its underlying excitation in reticulospinal dINs and other swimming circuit neurons on the same side of the CNS during swimming. If the reticulospinal members of the dIN population are, as we propose, the primary excitatory drivers of swimming, then we predict firstly that they will be the earliest neurons on a particular side to fire on each swimming cycle, and secondly that their spikes will precede the fast rhythmic excitation which underlies rhythmic firing in other swimming neurons and which comes from the dINs. If their spikes are not being driven by yet earlier firing excitatory neurons, then we also predict that their firing must depend on other mechanisms such as post-inhibitory rebound during steady depolarisation. We have therefore compared the timing of spikes and underlying EPSCs in dINs and other swimming circuit neurons on one side of the tadpole hindbrain and spinal cord to establish where activity begins at the start of each cycle of swimming, using ventral root discharge as a reference. Because uncertainties remain about the processes involved in the initiation of swimming, we have looked specifically at activity once swimming has become established. Our results lead us to conclude that reticulospinal dINs in the caudal hindbrain are indeed the origin of the primary descending excitation that drives spinal cord neurons to fire during swimming, that they are integral members of the rhythm generating circuitry, and that their maintained activity may result from post inhibitory rebound following reciprocal inhibition.
Methods
Experiments were carried out on Xenopus laevis tadpoles at developmental stage 37/38 (Nieuwkoop & Faber, 1956). Procedures for obtaining the tadpoles comply with UK Home Office regulations and all experiments have been approved following local (Bristol) ethical committee review. Tadpoles were briefly anaesthetised with 0.1% MS-222 (3-aminobenzoic acid ester, Sigma, UK) prior to experiments so a cut could be made in the skin to allow access of 10 μm α-bungarotoxin (Sigma, Gillingham, UK) in saline. Anaesthetics were not used during subsequent experiments because at stage 37/38 these tadpoles are considered to be insentient.
Tadpoles immobilised with α-bungarotoxin were pinned to a rubber block in a bath of saline (concentrations in mm: NaCl 115, KCl 3, CaCl2 2, NaHCO3 2.4, MgCl2 1, Hepes 10, adjusted to pH 7.4 with NaOH). The methods for whole-cell patch recording, using electrodes containing 0.1% neurobiotin (Vector Laboratories, Burlingame, CA, USA) and Alexa Fluor-488 (Molecular Probes, Eugene, OR, USA), extracellular suction electrode recording from ventral roots, and processing for neuronal anatomy have been described recently (Li et al. 2009; based on Li et al. 2002). Patch pipettes contained (in mm): potassium gluconate 100, MgCl2 2, EGTA 10, Hepes 10, Na2ATP 3, NaGTP 0.5 adjusted to pH 7.3 with KOH. They had resistances of ∼10 MΩ. Fluorescence imaging was used after recording to check that neuron somata were intact after electrode withdrawal. For loose patch recording, pipettes were filled with the normal intracellular solution and gentle suction was applied once the tip touched the soma surface. The same pipette was often used for recording several somata (∼10 somata were recorded from each tadpole in this set of experiments). Somata were exposed randomly in dissection and only neurons which fired on the majority of swimming cycles were picked for analysis. Episodes of swimming were evoked by single electrical stimuli (∼1 ms) applied to the trunk skin by means of a suction electrode. Those neurons firing unreliably during swimming tended to fire later than the reliable firing ones (for example, some aINs: Li et al. 2002). They are excluded from this study. In experiments into the timing of firing, neuronal soma positions and the position of the ventral root suction electrode were measured in situ relative to the mid/hindbrain boundary, using a micrometer. One or two whole-cell recordings were made after the serial loose patch recordings. The locations of these neurobiotin labelled neurons were then used to confirm the in situ measurements. Shrinkage of tissue during neurobiotin processing was corrected by a factor of 1.28 (Roberts et al. 1999). The anatomical division between midbrain and hindbrain is clear. The division between hindbrain and spinal cord at the caudal end of the 8th rhombomere is less obvious, but is conventionally determined by the position of the obex. This is typically ∼0.85 mm from the midbrain–hindbrain boundary. We use this position to distinguish between hindbrain and spinal cord, and therefore between reticulospinal dINs and other dINs.
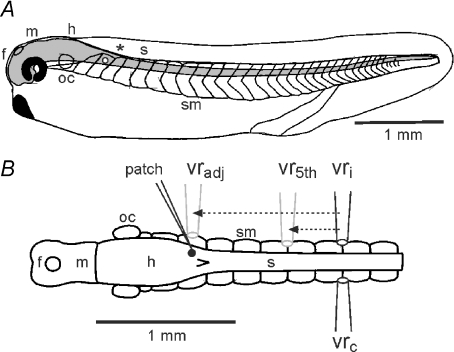
Timing of neuron activity during swimming (spikes and EPSCs) was measured relative to the start of ventral root bursts. Depending on the experiment, timing measurements were made with reference either to an adjacent ventral root or to a position equivalent to the 5th post-otic ventral root 1.36 mm caudal to the midbrain. In practice, it was generally not possible to make ventral root recordings at exactly the appropriate longitudinal level and on the same side of the animal as a particular recorded neuron. It was therefore necessary to make two kinds of corrections to the measured timing. Where the recordings were from opposite sides, timing was first corrected from the contralateral to an equivalent ipsilateral ventral root recording site (vrc to vri, Fig. 1). To do this, adjusted ventral root bursts were taken to start at the mid points of cycles measured on the opposite side (this assumes the typical strict alternation of activity between the two sides). Importantly, there is also a longitudinal, rostro-caudal delay in ventral root burst timing along the tadpole during swimming (Kahn & Roberts, 1982). This is typically constant across cycle periods and has a mean value of 3.5 ms mm−1 (∼0.6 ms per muscle segment; Tunstall & Roberts, 1991). To compensate for this delay, a second correction was made to adjust timing values according to the longitudinal distance separating the real ventral root recording site from the chosen reference (adjacent, vradj or 5th post-otic ventral root, vr5th, Fig. 1), so that timing was expressed as though measured relative to this reference. The longitudinal adjustment was: ta=tm− 3.5d, where ta is the adjusted and tm is the measured time in milliseconds, d is the separation distance in millimetres (either vradj– vri or vr5th– vri; Fig. 1), and where distances for relatively rostral neurons are negative and relatively caudal neurons are positive. Negative times indicate that activity (spike or EPSC) precedes the reference ventral root burst, positive values that activity follows the burst. This convention is used throughout this study.
Figure 1. The hatchling Xenopus tadpole and adjustments to timing measurements according to recording sites.
A, diagram of the tadpole in side view to show the CNS (grey) and its regions (f, m, h: fore-, mid- and hind-brain; s: spinal cord; oc: otic capsule;*: obex) with swimming muscles (sm) in chevron shaped segments. White dot indicates the neuron in B. B, diagram of the CNS and swimming muscles in dorsal view to show typical sites for electrodes to record from a single neuron (patch) and ventral roots on the ipsilateral (vri) or contralateral (vrc) side. Measured timings of neuron activity were adjusted as though relative to recordings from either an assumed, adjacent ventral root (vradj) or the 5th post-otic ventral root (vr5th) on the same side as the recorded neuron (arrows and grey electrodes; see text for details).
Unless stated otherwise, all values are quoted as means ±s.d. and statistical analysis was by Student's t test for paired data, using log-transformation where necessary to normalise data. Analysis was performed using Minitab software (Minitab Inc., State College, PA, USA).
Results
Neurons (dINs) providing excitation during swimming
The dIN neurons examined here form a continuous column extending from the hindbrain into the spinal cord of the young tadpole (Fig. 1; see Introduction) and have consistent anatomical and physiological features. They have dendrites from a multipolar soma and all are characterised by an ipsilateral descending axon projection. Some dINs, especially in the hindbrain, can also have an ascending axon. While other swimming circuit neurons fire at high frequency to positive current injection (Sautois et al. 2007), dINs typically fire a single, unusually long-duration spike to positive current injection (spike duration is 1.93 ± 0.43 ms at 0 mV, significantly longer than for other swimming circuit neurons, 0.69 ± 0.2 ms; data from Li et al. 2006). They can fire on rebound from hyperpolarising current injection provided they are held depolarised. Paired whole-cell recordings show that they make synaptic connections, where they corelease glutamate and acetylcholine, to directly excite all types of swimming circuit neurons on the same side of the spinal cord to activate nicotinic ACh, NMDA and AMPA receptors (Li et al. 2004, 2006). The rapid rise-time of EPSPs from dINs is suitable to evoke spikes at short latencies in postsynaptic neurons and this provides feed-forward excitation during swimming. We refer to all members of the column of dINs in the hindbrain and spinal cord collectively as dINs and treat them as a single population, as also suggested by van Mier (van Mier, 1988; van Mier & ten Donkelaar, 1989). In most of what follows, we make no a priori distinction between them based on longitudinal position. However, we use the term reticulospinal dIN when referring specifically to those in the hindbrain (< 0.85 mm from the midbrain–hindbrain boundary). The dINs form a part of the swimming circuit, but to allow us to distinguish them from other swimming circuit neurons in what follows, we use the term ‘non-dINs’ here to refer to neurons active during swimming that are not dINs.
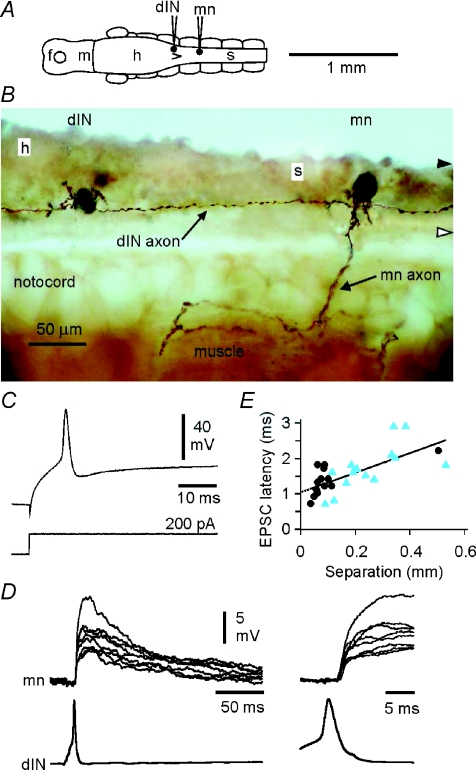
To test whether dINs drive firing in swimming circuit neurons during swimming, our aim was to interpret differences between dINs and non-dINs in the timing of their spikes and underlying excitation. However, we first examined the delays between pre- and postsynaptic activity at connections from dINs. This allowed us to estimate axonal conduction velocity and synaptic delays, to aid this interpretation. Delays were measured for 15 dIN–dIN pairs and 14 dIN–non-dIN neuron pairs where the start of the EPSP could be clearly resolved, the identification of pre- and postsynaptic neurons was clear (following neurobiotin injection into both neurons), and the longitudinal position of both neurons was established (Fig. 2A). In this sample, presynaptic dINs lay mainly within the hindbrain between 0.36 and 0.96 mm caudal to the midbrain. At a particular synapse, EPSPs followed presynaptic spikes with a latency that showed little variability (Fig. 2D; see also Li et al. 2006). Delays between the peak of the presynaptic spike and the onset of the EPSP lay between 0.7 and 2.9 ms for neuron separations of 0.04 and 0.53 mm. Delay correlated significantly with distance (Fig. 2E; Pearson correlation coefficient = 0.699, P < 0.001). The slope of the regression equation gave a mean spike conduction velocity of 0.36 m s−1 while the y-intercept (delay at zero separation) suggested a mean synaptic delay of ∼1.0 ms with a minimum of 0.7 ms.
Figure 2. Excitatory connections from dINs.
A, diagram of the CNS in dorsal view to show a recorded dIN and motoneuron (mn) (shown in B–D). B, photomicrograph of neurobiotin-filled neurons in lateral view where a rostral dIN with short dendrites has a descending axon that passes a more caudal motoneuron (mn) with mainly ventral dendrites and identified by its peripheral axon. C, the dIN in B shows a characteristic single, broad action potential in response to injected depolarising current. D, paired recording from the neurons in B (at two time scales; Mg2+ omitted from saline) shows that current-evoked spikes in the dIN produce long EPSPs in the motoneuron (left) at consistent, short latencies (right). E, latencies of EPSPs increase with separation between presynaptic dINs and their postsynaptic targets (dIN–dIN synapses, circles; dIN–non-dIN synapses, triangles). The regression equation is d = 1.04 + 2.79s where d is delay in ms and s is separation in mm.
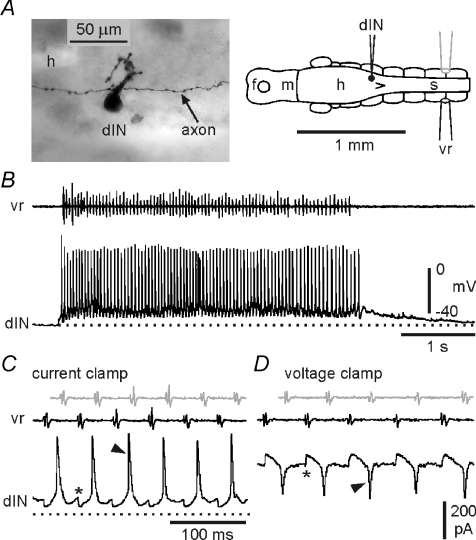
dINs fired reliably throughout swimming episodes (Fig. 3A and B). In only 4/30 recordings from dINs in which swimming activity occurred spontaneously or was evoked by stimulation of the skin were there occasional cycles towards the end of episodes where individual dINs failed to fire; for most dINs there were no failures. The basic activity of dINs during swimming was typical of other swimming circuit neurons described previously: a single spike per cycle alternating with mid-cycle reciprocal inhibition, superimposed on a ‘tonic’ background excitation (Fig. 3C; Roberts et al. 1997). The synaptic currents producing fast excitation underlying the spikes and mid-cycle inhibition are seen clearly under voltage clamp (Fig. 3D). The relationship between dIN spikes and these rhythmic, fast EPSCs forms a central component of this study and is considered in detail below.
Figure 3. Activity of a reticulospinal dIN during swimming.
A, anatomy of a recorded dIN with ascending and descending axons and mainly dorsal dendrites. The diagram shows the soma position and recording arrangement. The ventral root electrode (vr) was placed on the opposite side to the intracellular recording (black). B, single episode of swimming, monitored with the ventral root electrode (vr), showing the reliability of firing of a dIN. C, expanded swimming cycles from B to show the normal swimming pattern (*: IPSPs; arrowhead: spike). The grey vr trace shows the motor activity from the recorded, vr trace (black) adjusted to show the assumed timing of ipsilateral motor activity (as though recorded ipsilaterally by the grey electrode in diagram A). D, equivalent cycles of swimming recorded from the same dIN under voltage clamp reveal the underlying, rhythmic synaptic drive (*: IPSCs; arrowhead: fast EPSC). The grey vr trace is the assumed ipsilateral motor activity as in C.
Spikes in dINs precede those of other swimming circuit neurons during swimming
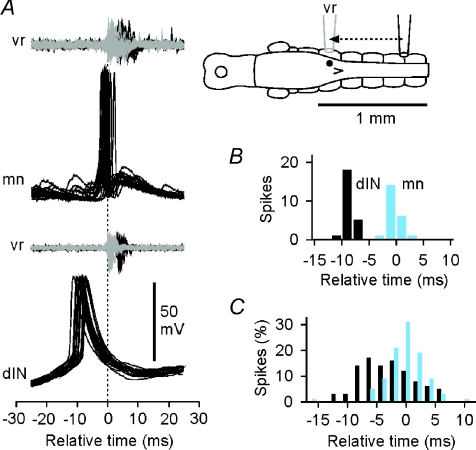
If the dINs are the neurons that excite other swimming circuit neurons on each cycle during swimming, we should expect their spikes to precede those of the neurons that they excite. This was first examined by measuring the timing of individual spikes in sequences of 14–27 consecutive swimming cycles in 16 dINs and 15 non-dINs in whole-cell recordings. The time of each spike peak was measured relative to the onset of an adjacent ventral root burst (for details, see Methods).
In general, spikes in dINs were early relative to those in non-dINs and ventral roots. Example recordings show a typical dIN and motoneuron (Fig. 4A) with quite different distributions of spike times (Fig. 4B). In group data, the distributions for timing of dIN and non-dIN spikes overlapped, but the dINs commonly fired earlier (Fig. 4C; difference in mean firing times 2.9 ms; dIN: −3.90 ± 4.14 ms, n= 16; non-dIN: −1.02 ± 1.55 ms, n= 15, two sample t test, P= 0.018).
Figure 4. Spikes in dINs are early relative to those in non-dINs and vrs during swimming.
A, overlapped spikes of a motoneuron (mn) and dIN aligned relative to the start of ‘adjacent’ ventral root (vr) bursts (dashed line) show that the dIN fires earlier. The inset diagram shows the typical recording arrangement (actual recording site and vr bursts, black; adjusted recording site and vr bursts, grey; see Fig. 1). B, distribution of spike peak timing for the two neurons in A. C, the relative timing of spike peaks for 16 dINs (dark bars) and 15 non-dINs (light bars), all adjusted to give timings relative to an adjacent ventral root. Distributions of spike timings for each neuron (e.g. B) were normalised (as % of the whole sample of spike times for each neuron, using 2 ms bins) and then combined to give overall spike time distributions for dINs and non-dINs.
Longitudinal differences in spike timing in dINs and non-dINs during swimming
Our prediction that dINs fire earlier than non-dINs on the same side of the CNS and drive their firing on each swimming cycle is supported by our initial measurements of spike timing. However, the possibility remained that dINs are themselves driven by other neurons. Our aim was to establish whether dINs are the primary excitatory drivers during swimming, rather than simply relaying excitation from yet earlier firing neurons. We therefore investigated the relative times of firing of neurons at different rostro-caudal locations. We used loose-patch recording to make sequential recordings from several neurons in each tadpole to minimise the effect of variability between animals and avoid any possibility that whole cell recording might subtly alter spike timing.
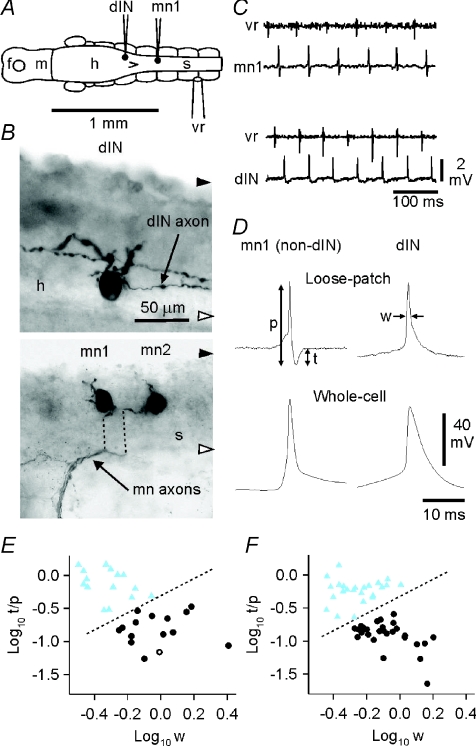
Our first requirement was to be able to identify whether dINs or non-dINs were being recorded in the loose patch recordings since it would not be possible to fill them all intracellularly with neurobiotin to confirm their identity anatomically, as would normally be the case. A reliable characteristic of dINs is that they have significantly broader impulses than non-dINs (Li et al. 2006). We therefore expected to be able to distinguish dIN and non-dIN extracellular spikes during swimming. We first made loose-patch recordings from a sample of 15 dINs and 15 non-dINs (including examples of all the non-dIN swimming circuit types: 3 aINs, 7 cINs and 5 mns) and recorded their spikes during swimming (Fig. 5A). Neuronal identity was confirmed anatomically and physiologically by subsequently attaining the whole-cell configuration and injecting neurobiotin (Fig. 5B). The recorded extracellular spikes were all biphasic with an initial positive peak followed by a more variable negative trough (Fig. 5C). We used two measurements to define the shape of these spikes: the width of the positive peak at half-amplitude (half-width) and the ratio of the trough to peak-to-peak amplitude (t/p; arrows: Fig. 5D) from averages of >20 consecutive spikes during swimming in each neuron. Cluster analysis (K mean; Fig. 5E) using these two characteristics confirmed the presence of two groups: one with broader initial peaks and a small trough (dINs; Fig. 5D) and one with narrower initial peaks and a more prominent (and narrower) trough (non-dINs; Fig. 5D). These differences in extracellular spikes reflected differences in the spike widths seen after attaining the whole cell configuration: broad spikes in dINs and narrow spikes in non-dINs (Fig. 5D, lower records; Li et al. 2006). We could therefore confidently distinguish between dINs and non-dINs active during swimming on the basis of the shapes of their extracellular spikes.
Figure 5. Distinguishing neurons by spike shape in loose patch recordings.
A, locations of illustrated neurons (from separate recordings; contralateral ventral root electrode). B, anatomical characterisation of a dIN (upper micrograph) with 2 large dorsal dendrites and a single descending axon (an additional axon is from a separate neuron) and one of a pair of motoneurons (lower micrograph; mn1; dotted lines indicate displacement of axons during processing). Black and white arrowheads indicate the dorsal and ventral limit of the CNS respectively (h: hindbrain, s: spinal cord). C, loose patch recordings of activity from a non-dIN neuron (mn1) and a dIN during swimming, monitored from a ventral root on the opposite side. D, distinguishing dINs from non-dINs by extracellular spike shape (upper records, normalised to the amplitude of the initial peak; p: peak-to-peak amplitude, t: trough amplitude, w: width at half initial peak amplitude). Lower records are corresponding spikes during swimming after attaining the whole-cell configuration (all averages of ∼20 spikes). E, cluster analysis distinguishes two groups of neurons that match a separation based on anatomical features (circles, dIN; triangles, non-dIN; open symbols are the illustrated neurons). F, cluster analysis on a larger group of neurons (n= 52) selected at random from those used to measure spike timing during swimming (see Fig. 6) again separates the neurons into two groups, equivalent to the dINs and non-dINs in E.
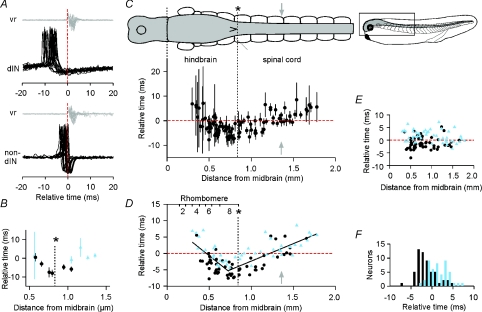
To examine the timing of spikes during swimming, we then made loose-patch recordings from 5–16 neurons in each of 10 tadpoles at positions between ∼0.3 and 1.8 mm caudal to the midbrain–hindbrain boundary (Fig. 6C; the obex, a conventional boundary between the hindbrain and spinal cord, is indicated by the asterisk). Cluster analysis of spike shapes from a sample of 52 of these neurons again reliably divided them into two groups (Fig. 5F) corresponding to the two previously identified as dINs and non-dINs (Fig. 5E).
Figure 6. Timing of neuron spikes during swimming.
A, timing of spikes in a dIN (upper) and non-dIN (lower) recorded in the same animals with loose patch, aligned relative to the start of vr bursts adjusted to the 5th post-otic cleft. The vertical dashed line indicates vr burst onset. B, the timing of spikes (±s.d.) in dIN (circles) and non-dIN (triangles) neurons recorded at different longitudinal positions in a single animal. C, the timing of spikes (±s.d.) at different longitudinal positions for the whole data set (103 neurons). The dorsal view of the CNS and swimming muscles (labelled in Fig. 1B) is shown at the same scale and horizontal alignment as the graph; the small lateral view of the tadpole shows the region illustrated (*: obex; grey arrow: 5th post-otic cleft used as a timing reference; horizontal dashed line: 0 ms). D, the timing of spikes as in C showing neuron identification (dIN, circles; non-dIN, triangles) and contiguous regression lines (0–0.725 mm: y= 9.575 – 20.605x, where y is in ms and x is in mm, R2= 0.51; from 0.725 mm: y=–13.013 + 10.659x; R2= 0.63). Approximate rhombomere boundaries and obex (*) are indicated. E, deviation of timing measurements from the regression lines in D (slope: 0.05 ms mm−1, R2= 0.00). F, mean firing times of dINs (dark bars) and non-dINs (light bars), transformed to remove the effect of longitudinal position as in part E.
The next step was to measure the timing of spikes during swimming. Measurements were made on 13–181 spikes (median 65) from individual swimming episodes for each of 103 neurons recorded in 10 tadpoles. Timing of positive spike peaks from extracellular, loose-patch recordings was again measured relative to the start of adjacent ventral root bursts (Fig. 6A). The relationship between intracellular and extracellular spike features is unclear but it is likely that the extracellular positive peak corresponds to the fastest part of the intracellular spike upswing, i.e. near the start of the spike. In most cases, the ventral root recording was at the 5th post-otic cleft (∼1.36 mm from the midbrain); in the remaining cases, timing measurements were again adjusted (for details, see Methods) according to the distance of the ventral root recording site from the 5th post-otic cleft, once more assuming a rostro-caudal delay of 3.5 mm ms−1 (Fig. 6A). To provide a consistent reference, timing measurements were then all expressed relative to ventral root burst onset at the 5th post-otic cleft (t= 0 ms). The longitudinal positions of each recorded neuron and the ventral root electrode in each tadpole were measured in situ during the experiment.
Examples of the spike timing values for a series of recordings from dINs and non-dINs in a single tadpole are shown in Fig. 6B. It shows the mean ±s.d. of spike timings for 11 neurons plotted according to their longitudinal location (distance from the midbrain). There is a clear rostro-caudal pattern in the spike timing that is reflected in the combined measurements for all 103 neurons. The earliest firing was seen in neurons in the caudal hindbrain (Fig. 6C; asterisk indicates obex), ∼0.6–0.8 mm from the midbrain. In the spinal cord caudal to this, firing was progressively later. Surprisingly, firing was also progressively later rostral to this region in the hindbrain. Using the mean firing time for each neuron, this pattern was well described by two contiguous linear regression lines with a changeover 0.725 mm from the midbrain (Fig. 6D). Distinguishing the spike timing of dINs from non-dINs revealed that firing of dINs was generally earlier. This was particularly clear in the caudal hindbrain (corresponding to rhombomeres 7 and 8; Fig. 6D) and rostral spinal cord, where the earliest firing neurons were all dINs. The difference became less pronounced more caudally in the spinal cord.
Because measurements of timing within a single animal were made relative to the same ventral root recording, no assumptions were necessary to compare timing between neurons (Fig. 6B). However, when comparing between animals, timing measurements were normalised relative to ventral root burst onset at the 5th post-otic cleft by assuming a rostro-caudal, ventral root burst delay of 3.5 ms mm−1 (see above). To test that this assumption did not jeopardise our conclusions, the analysis was repeated assuming the rostro-caudal delay was the fastest (2.0 ms mm−1) or the slowest (5.0 ms mm−1) value observed experimentally (Tunstall & Roberts, 1991). Neither assumption markedly changed the shape of the relationship seen in Fig. 6D or the conclusions based on it (see the online Supplemental Material).
To separate differences in firing time between dINs and non-dINs from differences due to longitudinal position, timing values for each neuron were re-calculated as their deviation from the fitted regression lines (Fig. 6E). This removed all correlation of firing time with longitudinal position (regression slope: 0.05 ms mm−1, r2= 0.00). Using these transformed data, mean firing times for dIN neurons were overall 2.7 ms earlier than for non-dINs (Fig. 6F; dIN: −1.31 ± 2.24 ms, n= 56; non-dIN: 1.43 ± 2.3 ms, n= 47; two sample t test: P < 0.001). This difference in firing times was the same as estimated from intracellular impulses in the different, smaller sample of neurons described earlier (Fig. 4). In the caudal hindbrain and rostral spinal cord (0.5–1.0 mm from the midbrain, this difference was greater (3.0 ms, n= 53; P < 0.001); more caudally in the spinal cord (>1.2 mm from the midbrain), the difference was small and no longer significant (0.4 ms, n= 25; P= 0.76).
Firing in some of the most rostral neurons, in the mid-hindbrain, showed an unusually loose rhythm (as indicated by their large standard deviations, Fig. 6C); they could fire at almost any phase of the swimming cycle. Their brief spikes were typical of non-dINs rather than dINs and their loose timing showed they could not be a source of the rhythmic excitatory drive during swimming, which is very tightly phase-locked.
These results indicate that dINs fire earlier than non-dINs throughout the hindbrain and rostral spinal cord 0.3 to 1.2 mm caudal to the midbrain. Importantly, they also indicate that the earliest of all neurons to fire on each cycle during swimming are reticulospinal dINs in the caudal part of the hindbrain. This region has previously been highlighted as playing a key role in generation of sustained episodes of swimming (Li et al. 2006).
Spikes in rostral dINs can precede their underlying EPSCs
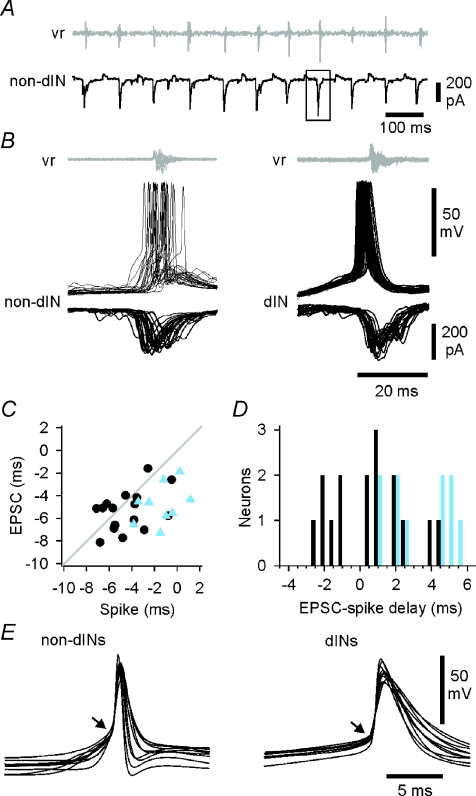
Our results show that the earliest recorded neurons to fire on each swimming cycle are a subgroup of dINs, lying in the caudal hindbrain. As outlined above, firing in neurons that are active during swimming is typically driven by phasic excitation (Fig. 3D). However, we are suggesting that by firing first these caudal hindbrain reticulospinal dINs provide the primary, rhythmic excitatory drive to the other neurons during swimming. If so, then their spikes should precede this underlying phasic synaptic excitation. To examine the timing of phasic excitation underlying spiking during swimming, we analysed voltage clamp recordings from 26 neurons held at ∼−50 mV (16 dINs and 10 non-dINs) in 16 tadpoles (Fig. 7A). These neurons lay in the caudal hindbrain and rostral spinal cord (0.39–1.00 mm from the midbrain). As before, the onset time of individual EPSCs was measured relative to the start of adjacent ventral root bursts. To allow direct comparison of EPSCs and spikes, we also measured the timing of spikes under current clamp in the same neurons (Fig. 7B). Spike timing was measured here as the time of crossing a –15 mV threshold, again relative to burst onset at the ventral root. We plotted the timing of spikes and EPSCs against each other for the same neurons (Fig. 7C; grey line indicates equal timing). Spikes in non-dINs were reliably later than the start of their underlying EPSCs, consistent with their spikes being driven by the EPSCs. This was also true for some of the dINs, but for 6/16 dINs, the spikes started consistently sooner than the underlying EPSCs, meaning that they could not be initiated by the EPSCs (Fig. 7D). In a further two dINs, the spikes had reached −15 mV (the threshold for measurement) within 1 ms of the start of the EPSC. All eight of these dINs lay in the caudal hindbrain between 0.45 and 0.85 mm from the midbrain.
Figure 7. The relative timing of spikes and EPSCs during swimming.
A, recording from a non-dIN neuron under voltage clamp during swimming showing EPSCs for timing measurement (e.g. box). Swimming was monitored from a ventral root on the opposite side (vr: shown adjusted as though recorded from the same side). B, superimposed, spikes and vr bursts from consecutive swimming cycles in a non-dIN and a dIN. Below each are overlapped EPSCs from separate swimming sequences in the same neurons, also aligned to the starts of vr bursts (adjusted as in A). C, the timing of spikes and EPSCs (median values from individual neurons) measured relative to vr burst onset (dIN, circles; non-dIN, triangles; line indicates equality). D, the delay between EPSCs and spikes in the same neuron, plotted for individual dINs (dark bars) and non-dINs (light bars). E, overlapped averages of spikes recorded from different neurons during swimming to show pre-potentials (= EPSPs) in non-dINs and immediate take-offs in dINs (arrows).
When current clamp recordings of later and early firing neurons were then compared during swimming, we found a consistent difference. In later firing non-dINs, a pre-potential occurred before spikes, presumably the underlying EPSP (arrow, Fig. 7E, left). No such pre-potential was seen in the earlier firing dINs (arrow, Fig. 7E, right).
The contrast in the relative timing of spikes and EPSCs in dINs and non-dINs, illustrated in Fig. 7D, was analysed statistically (using a general linear model with Tukey's pairwise comparisons).There was no significant difference (P= 0.99) between the onset times of EPSCs in dINs and non-dINs; this was as expected if the EPSCs come from the same pre-synaptic source (dINs). Non-dIN spikes were significantly later (3.6 ± 1.8 ms; P < 0.001) than their underlying EPSCs (timing of spikes and EPSCs −1.03 ± 1.93 and −4.58 ± 1.78 respectively). This delay would give ample time for the spikes to have been driven by the underlying EPSCs. Overall, spikes in dINs were also slightly later than their underlying EPSCs (timing of spikes and EPSCs: −4.44 ± 2.02 and −5.34 ± 1.77 respectively). However, in this case the difference (0.9 ± 2.0 ms) was not significant (P= 0.53).
We then used the distributions of timings of dIN spikes and EPSCs in our sample to model the expected timing of the earliest spikes within the whole hindbrain dIN population and also the earliest EPSCs, assuming both are normally distributed, which is the case in our samples.
For a modest dIN population size of 50 neurons, the model predicted that the earliest dIN spikes would significantly precede the earliest EPSCs by ∼1 ms (see the online Supplemental Material). This difference was also the mean value for synaptic delay measured in paired whole-cell recordings (see above). It is therefore quite plausible that the earliest firing dINs are the source of even the earliest EPSCs seen in other dINs as well as in non-dIN swimming circuit neurons. Our results also show that the phasic EPSCs cannot be the direct cause of firing in the earliest firing dINs.
Post-inhibitory rebound firing in dINs
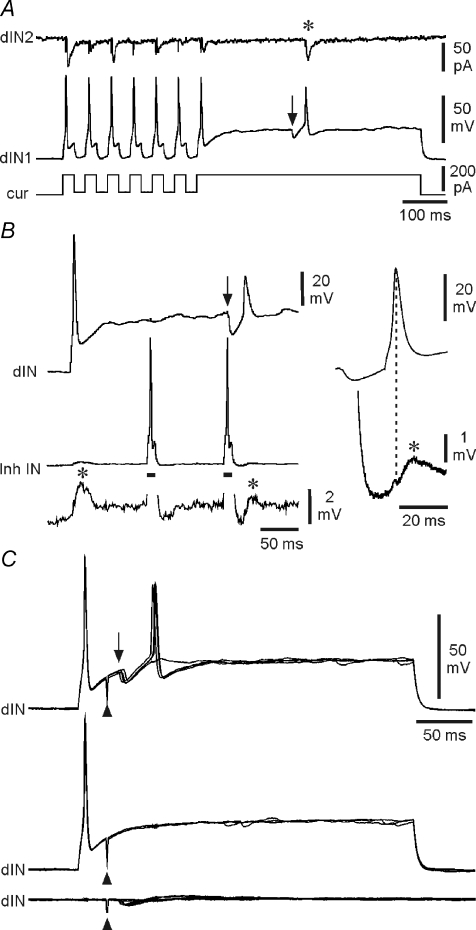
Our results show that firing of at least a proportion of the dINs in the caudal hindbrain precedes, and is therefore not directly driven by, the fast excitation that drives rhythmic firing in other swimming circuit neurons. What else might control the firing in these dINs, which our results suggest are the first neurons to fire on a particular side of the CNS at the start of each swimming cycle and therefore play a central role in maintaining the swimming rhythm from cycle-to-cycle? There is currently no evidence that dINs show pacemaker activity under normal physiological conditions: they only fire a single impulse even when the membrane potential is stepped substantially above spike threshold by current injection (Fig. 2C; Li et al. 2006). An early proposal was that post inhibitory rebound from mid-cycle, reciprocal inhibition plays a key role in swimming rhythm generation (Roberts & Tunstall, 1990). Rebound firing like this could allow dINs to ‘restart’ swimming on each cycle (Sautois et al. 2007). As has been shown previously (Li et al. 2006), dINs will fire on recovery from brief hyperpolarising pulses (‘anode break’), provided these pulses are superimposed on a super-threshold sustained depolarisation (Fig. 8A). We have now sought direct evidence that dINs will fire on rebound from IPSPs.
Figure 8. Post-inhibitory rebound in dINs.
A, rebound spikes to current pulses (dIN1), then a rebound spike following a spontaneous IPSP (at arrow), producing an EPSC (*) in a postsynaptic neuron (dIN2). B, when held just above spike threshold, a dIN fires on rebound following an IPSP (at arrow) elicited by the second of two spikes evoked in an inhibitory interneuron (Inh IN) by depolarising current (bars). Evoked and rebound spikes in the dIN both evoke EPSPs (*) in the inhibitory interneuron. Right, averages of 5 rebound spikes in the dIN and resulting EPSPs in the inhibitory interneuron. C, rebound in a dIN, depolarised just above spike threshold, following IPSPs (arrow) evoked by stimulation (at arrowhead) of the contralateral side of the spinal cord (upper overlapped traces). No rebound occurs where IPSPs fail (middle traces) or when the dIN is at rest (lower traces).
Sometimes, small spontaneous IPSPs were recorded during super-threshold depolarisation of dINs produced by current injection. Rather infrequently (5/60 cases examined), these presumed unitary IPSPs were relatively large. In these cases, dINs fired a single spike on rebound (Fig. 8A). Simultaneous recording with a postsynaptic neuron showed that these (usually smaller) rebound spikes were functional and produced a postsynaptic EPSC. A single example of rebound from evoked, unitary IPSPs was also obtained from a paired recording between a dIN and an ipsilateral inhibitory neuron. Depolarising the dIN evoked a single impulse and an EPSP in the inhibitory neuron (Fig. 8B). Current evoked impulses in the inhibitory neuron produced unreliable IPSPs in the dIN. When these unitary IPSPs were sufficiently large, they each produced a rebound spike in the dIN, which was again functional and produced an EPSP in the inhibitory neuron.
Lastly, we examined rebound firing more directly by using extracellular electrical stimulation to evoke spikes in a small group of inhibitory neurones (cINs) on one side of the spinal cord. The cINs mediate reciprocal inhibition between the two sides of the spinal cord and produce the mid-cycle inhibition which precedes firing in dINs during swimming (Fig. 3C and D). Experiments were carried out in preparations where excitatory synaptic connections were blocked pharmacologically (with the glutamate antagonists NBQX and d-AP5 and the nicotinic ACh antagonist dihydro-β-erythroidine (DHβE)). Stimulation produced IPSPs in dINs in the caudal hindbrain (n= 7 examples). This form of stimulation allowed us to provide a more reliable and stronger source of IPSPs and to examine rebound at a time after a preceding spike approximating to a single cycle of swimming. Depolarisation of the dINs by long injected current pulses again produced only single spikes but contralateral cord stimulation, shortly after the spike, evoked an IPSP and reliable rebound spike (Fig. 8C). There was no rebound firing if the IPSP failed or if it was evoked when the dIN was at rest (Fig. 8C).
Taken together, these lines of evidence confirm that one mechanism that could allow some rostral dINs to fire in the absence of (or ahead of) an underlying fast EPSP is rebound from a preceding reciprocal inhibitory IPSP, provided the dIN is already depolarised (see Discussion).
Discussion
In this study we test the proposal that a group of characterised reticulospinal neurons provide the primary, cycle-by-cycle excitatory drive for the swimming rhythm in hatchling Xenopus tadpoles. These neurons are the rostral members of a population of excitatory neurons with ipsilateral descending axons (dINs) that extends from the mid-hindbrain along the spinal cord. The dINs directly excite other members of the swimming network via their descending axons, and we have previously argued that mutual excitation within the rostral part of the dIN population provides sustained excitation to maintain the swimming rhythm (Li et al. 2006). We have now presented direct evidence that the dINs are not simply acting to pass on excitation originating in other neurons, perhaps in higher brain centres. Instead, the reticulospinal members of the dIN population in the caudal hindbrain are the primary source of the rhythmic excitation that drives swimming from cycle-to-cycle, and are an integral part of the rhythm generating circuitry. Specifically we showed that dINs in this region are the first to fire at the start of each swimming cycle on one side and that this firing can precede the rhythmic synaptic excitation that underlies firing in all other swimming circuit neurons.
Our finding that spikes in caudal hindbrain reticulospinal dINs can precede any underlying on-cycle EPSCs means that these dINs are not driven by synaptic excitation from other, earlier firing excitatory neurons elsewhere. We suggest that on each swimming cycle a proportion of the reticulospinal dINs fire first (probably not the same individual dINs on each cycle) and drive the rest of the dIN population, together with the other swimming circuit neurons, via the EPSCs seen in these neurons. Synapses are made onto the short (< 50 μm) dendrites of the young tadpole neurons, which are electrically compact (Wolf et al. 1998). It is therefore very unlikely that the whole-cell recordings made here from neuron somata failed to reveal additional sources of rhythmic synaptic excitation. Rhythmic firing in the earliest reticulospinal dINs to spike on each cycle must therefore result from the properties of the neurons themselves and their other synaptic interactions. The mechanism highlighted here is rebound firing following reciprocal inhibition. We already demonstrated rebound firing in dINs following hyperpolarising current pulses and conditional on their being depolarised above rest (Li et al. 2006). Critically, we have now demonstrated that the reticulospinal dINs can fire on rebound following the same IPSPs they receive during swimming: those mediated by reciprocal inhibitory cINs. During swimming, the background depolarisation in dINs, needed for rebound, results from summation of the long NMDAR-mediated components of their underlying rhythmic excitation. These results finally provide direct support for post-inhibitory rebound, which is a central element of our long-standing hypothesis on swimming rhythm generation (Roberts & Tunstall, 1990). According to this, the excitatory premotor dIN interneurons drive swimming because of their own connections and properties: their mutual excitation, summing from cycle-to-cycle to hold them in a depolarised state (Dale & Roberts, 1985); their cellular properties of firing only a single spike when depolarised or on rebound from IPSPs; and finally the crossed, ascending, reciprocal inhibition that permits rebound firing to restart successive cycles. Clearly in this scheme for swimming rhythm generation, the reciprocal inhibitory neurones also play a key role in maintaining swimming by providing a basis for rebound firing. However, the crucial excitatory event at the start of each cycle is the early firing of a proportion of the reticulospinal dINs, which then drive other swimming circuit neurons on the same side.
Establishing this major mechanism of rhythm generation in the tadpole does not, of course, rule out additional possibilities suggested by earlier experiments showing rhythm generation within an isolated single half of the CNS (Soffe, 1989; see also: Cangiano & Grillner, 2005), including some form of intrinsic membrane bistability (Prime et al. 1999). There is also evidence that electrical coupling between dINs contributes to the reliability of swimming rhythm generation in tadpoles (Li et al. 2009).
A consequence of our results is that we need to look again at the nature of the brainstem commands to spinal locomotor systems. The classic hypothesis is that unpatterned, descending excitation drives the rhythmic activity of spinal locomotor circuits (in the manner of Brown, 1911). The general acceptance of this hypothesis is partly a consequence of the widespread use of neuro-active substances, in particular NMDA, to provide sustained excitation which elicits patterned motor activity during experiments on spinal circuits of many vertebrates (Cohen & Wallen, 1980; Barry & O’Donovan, 1987; Kudo & Yamada, 1987; Hernandez et al. 1991; Wheatley & Stein, 1992; Douglas et al. 1993; McDearmid & Drapeau, 2006; Gabriel et al. 2008). Emphasis for maintenance of locomotion has therefore been on unpatterned descending excitation, whose strength determines the nature of the locomotor patterns generated by spinal central pattern generators (CPGs). This is despite the finding that descending reticulospinal activity is typically modulated in time with locomotor rhythms (Drew et al. 1986; Kasicki & Grillner, 1986; Kasicki et al. 1989; Zelenin, 2005; and see Orlovsky et al. 1999), for example by ascending ‘efference copy’ signals from the spinal circuits (Dubuc & Grillner, 1989; Vinay & Grillner, 1992; Einum & Buchanan, 2004, 2005; Antri et al. 2009). The picture from the young tadpole is now rather different. The reticulospinal dINs in the caudal hindbrain are not only responsible for maintaining swimming, but at the same time their descending excitation imposes the basic swimming rhythm on the spinal circuitry. Rather than simply supplying a tonic drive that is converted to a rhythmic pattern within the spinal cord, these reticulospinal neurons are an integral part of the rhythm generating circuitry. It may be that the use of artificial excitants in many other preparations has been misleading, and that the rhythmic component of descending reticulospinal commands is more important in controlling spinal circuits than previously appreciated.
As in other vertebrate locomotor systems, the isolated tadpole spinal cord can be activated by excitants like NMDA to produce an alternating swimming rhythm (Dale & Roberts, 1984; Soffe & Roberts, 1989; Soffe, 1996). Does this imply that there are tonically active neurons releasing glutamate and activating NMDARs? During sensory evoked swimming we have no evidence for tonic firing neurons in the tadpole brainstem or spinal cord. Swimming continues to be sustained following transection at the mid-hindbrain level (Li et al. 2006). A very large number of random whole-cell (n > 500) and extracellular (n > 200) recordings of hindbrain neurons made caudal to this level during swimming have revealed none that fire tonically at significantly above the swimming frequency (>50 Hz) and only a very few (∼2%) that fire sporadically, in a way that is weakly phase-locked to the swimming cycle (W.-C. Li, unpublished observations). It is therefore highly unlikely that tonic or sporadic-firing neurons contribute a significant sustained drive to activate the spinal swimming circuit. Instead, we suggest that the spinal swimming circuit neurons are primarily driven cycle-by-cycle by descending excitation from rhythmic firing dINs.
Our results may also give some insight into the origin of hindbrain reticulospinal neurons and their relationship to spinal neurons. A broad picture is emerging that the transcription factors that are expressed during development of the neuraxis can be used in all vertebrates to define a limited number of different types of spinal neurons which form longitudinal columns at characteristic dorso-ventral positions (Jessell, 2000; Goulding et al. 2002; Lewis, 2006). In simpler vertebrates, like the tadpole and the zebrafish, the available evidence, from the expression patterns of transcription factors (Cepeda-Nieto et al. 2005; Kimura et al. 2006, 2008; A. Roberts, unpublished observations) and inhibitory neurotransmitters (Roberts et al. 1987, 1988), indicates that columns of ‘spinal’ interneurons extend well into the hindbrain. This supports the view that the excitatory dIN interneurons that we have been studying mainly in the hindbrain (see also Li et al. 2006) are actually part of a longitudinal column of similar neurons extending into the spinal cord. The first hypothesis on the role of these excitatory premotor dIN interneurons in providing feedback excitation to sustain tadpole swimming was actually based on recordings from the spinal cord (Dale & Roberts, 1985). Furthermore, all the neuronal elements of a model network that was able to generate swimming are present in the spinal cord (Roberts & Tunstall, 1990). However, while the isolated tadpole spinal cord can generate swimming-like activity when excitants like NMDA are bath applied, like other vertebrates (see above), lesion experiments have more recently shown that reliable, self-sustained swimming following a brief sensory stimulus requires the presence of at least some of the caudal hindbrain and the excitatory dINs within it (Li et al. 2006). Our conclusion is therefore that the reticulospinal dINs that play a critical role in driving swimming are just the rostral members of a dIN population that extends into the spinal cord. The spinal cord members of this population are the premotor excitatory interneurons active during locomotion which have proven so elusive in the mammal spinal cord (Butt et al. 2005; Wilson et al. 2005; Al-Mosawie et al. 2007). They are the analogues, and possibly homologues, of the excitatory premotor alx (Chx10) expressing CiD interneurons in zebrafish (Kimura et al. 2006).
The view that excitatory dINs form a continuous column of related neurons extending from the hindbrain into the spinal cord raises problems in naming them. The same population includes both hindbrain reticulospinal neurons and spinal interneurons. We suggest that what we describe in the hatchling tadpole is likely to be an early developmental stage of a specialization process within the locomotor system during which reticulospinal driver neurons will become distinct from spinal locomotor central pattern generator neurons. However, progressive specialisation of reticulospinal neurons does not mean that the rhythmic component of the descending drive they supply will become of less central importance than it is in the young tadpole.
Working on a very young and simple vertebrate, where little subtle movement control has yet developed, has allowed us to explore the fundamental organisation and role of reticulospinal neurons and their spinal targets. This simple situation contrasts with the sophisticated descending control even in an animal like the lamprey where reticulospinal neurons directly excite spinal neurons (Buchanan & Cohen, 1982; Ohta & Grillner, 1989) but have complex properties (see review: Dubuc et al. 2008) and heterogeneous roles in coordinating swimming (Zelenin et al. 2001; Zelenin, 2005). This is far less tractable than the situation in the hatchling tadpole, where there is a very direct relationship between reticulospinal dINs and the swimming neurons that they excite. The organisation in the tadpole is more comparable to the highly specialised reticulospinal control of electric organs in adult fish, where distinct relay neurons carry the descending output from a pacemaker nucleus to the motoneurons controlling the electric organ (Dye & Meyer, 1986). However, even in the tadpole, other distinct groups of reticulospinal neurons have been defined. A group of GABAergic reticulospinal neurons confined to the hindbrain can terminate swimming (Perrins et al. 2002) and control excitability through tonic inhibition of swimming neurons (Lambert et al. 2004). A further group of excitatory reticulospinal–spinal neurons with a population extending into the spinal cord are recruited when continuous skin stimulation evokes the stronger struggling motor pattern (Soffe, 1993). These reticulospinal repetitive-firing dINs (dINrs) are anatomically very similar to other dINs except that none possess ascending axons. However, they are physiologically quite distinct from other dINs, firing repetitive, short duration spikes to injected current (Li et al. 2007). The dINrs rarely fire during swimming so would not have been recorded in this study. In a similar way, dINs fire only weakly during struggling. If dINs and dINrs share a common origin as suggested by their similar anatomy and glutamatergic phenotype, then they already represent a diversification of excitatory reticulospinal neurons which we expect to increase during subsequent development.
We have now been able to anatomically and physiologically characterise a group of excitatory reticulospinal neurons (dINs) with a specific function in controlling locomotion. Substantial evidence has now been amassed to show that these reticulospinal dINs provide the primary rhythmic drive for swimming locomotion, exciting neurons in the caudal brainstem and spinal cord that comprise the swimming circuit. Rebound firing in dINs, following reciprocal inhibition from the other, active side of the nervous system, together with their mutual excitatory connections, provides a mechanism that can plausibly sustain cycle-by-cycle firing. The excitatory reticulospinal neurons we have characterised in the tadpole are the rostral members of a longitudinal column of neurons sharing common properties, which extends from the spinal cord into the hindbrain. We suggest that the organisation seen in this rather simple vertebrate system reveals something of the origin of reticulospinal control: a progressive specialisation and diversification in the rostral members of initially homogeneous populations of neurons distributed widely along the neuraxis.
Acknowledgments
We should like to thank Erin Anderson, Tim Colborn, Emma Hanmore, and Jenny Maxwell for technical help, Dr Lisa James for advice on rhombomere spacings, and the Wellcome Trust for financial support. W.-C.L. is a Royal Society University Research Fellow.
Glossary
Abbreviations
- aIN
ascending interneuron
- cIN
commissural interneuron
- dIN
descending interneuron
- dINr
repetitive-firing descending interneuron
Author contributions
All three authors contributed to the conception and design, data interpretation, and revision of the manuscript. The experiments were carried out in the University of Bristol by W.-C.L. S.R.S. carried out the main data analysis and was the main writer.
Author's present address
W.-C. Li: School of Biology, University of St Andrews, Bute Building, St Andrews, Fife KY16 9TS, UK.
Supplemental material
References
- Al-Mosawie A, Wilson JM, Brownstone RM. Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur J Neurosci. 2007;26:3003–3015. doi: 10.1111/j.1460-9568.2007.05907.x. [DOI] [PubMed] [Google Scholar]
- Antri M, Fenelon K, Dubuc R. The contribution of synaptic inputs to sustained depolarizations in reticulospinal neurons. J Neurosci. 2009;29:1140–1151. doi: 10.1523/JNEUROSCI.3073-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN. Cerebellum and Rhythmical Movements. New York: Springer; 1986. [Google Scholar]
- Barry MJ, O’Donovan MJ. The effects of excitatory amino acids and their antagonists on the generation of motor activity in the isolated chick spinal cord. Brain Res. 1987;433:271–276. doi: 10.1016/0165-3806(87)90030-7. [DOI] [PubMed] [Google Scholar]
- Brown TG. The intrinsic factor in the act of progression of the mammal. Proc R Soc Lond B Biol Sci. 1911;84:308–319. [Google Scholar]
- Buchanan JT, Cohen AH. Activities of identified interneurons, motoneurons, and muscle fibres during fictive swimming in the lamprey and effects of reticulospinal and dorsal cell stimulation. J Neurophysiol. 1982;47:948–960. doi: 10.1152/jn.1982.47.5.948. [DOI] [PubMed] [Google Scholar]
- Butt SJB, Lundfald L, Kiehn O. EphA4 defines a class of excitatory locomotor-related interneurons. Proc Natl Acad Sci U S A. 2005;102:14098–14103. doi: 10.1073/pnas.0503317102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano L, Grillner S. Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: the lamprey hemicord. J Neurosci. 2005;25:923–935. doi: 10.1523/JNEUROSCI.2301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda-Nieto AC, Pfaff SL, Varela-Echavarria A. Homeodomain transcription factors in the development of subsets of hindbrain reticulospinal neurons. Mol Cell Neurosci. 2005;28:30–41. doi: 10.1016/j.mcn.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Clarke JDW, Hayes BP, Hunt SP, Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AH, Wallen P. The neuronal correlate of locomotion in fish. ‘Fictive swimming’ induced in an in vitro preparation of the lamprey spinal cord. Exp Brain Res. 1980;41:11–18. doi: 10.1007/BF00236674. [DOI] [PubMed] [Google Scholar]
- Dale N, Ottersen OP, Roberts A, Storm-Mathisen J. Inhibitory neurones of a motor pattern generator in Xenopus revealed by antibodies to glycine. Nature. 1986;324:255–257. doi: 10.1038/324255a0. [DOI] [PubMed] [Google Scholar]
- Dale N, Roberts A. Excitatory amino acid receptors in Xenopus embryo spinal cord and their role in the activation of swimming. J Physiol. 1984;348:527–543. doi: 10.1113/jphysiol.1984.sp015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Roberts A. Dual component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Fagerstedt P, Grillner S, Orlovsky GN. Activity of reticulospinal neurons during locomotion in the freely behaving lamprey. J Neurophysiol. 2000;83:853–863. doi: 10.1152/jn.2000.83.2.853. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Orlovsky GN. Encoding and decoding of reticulospinal commands. Brain Res Rev. 2002;40:166–177. doi: 10.1016/s0165-0173(02)00199-6. [DOI] [PubMed] [Google Scholar]
- Douglas JR, Noga BR, Dai X, Jordan LM. The effects of intrathecal administration of excitatory amino acid agonists and antagonists on the initiation of locomotion in the adult cat. J Neurosci. 1993;13:990–1000. doi: 10.1523/JNEUROSCI.13-03-00990.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T, Dubuc R, Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol. 1986;55:375–401. doi: 10.1152/jn.1986.55.2.375. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Brocard F, Antri M, Fenelon K, Gariepy JF, Smetana R, Menard A, Le Ray D, Viana Di Prisco G, Pearlstein E, Sirota MG, Derjean D, St-Pierre M, Zielinski B, Auclair F, Veilleux D. Initiation of locomotion in lampreys. Brain Res Rev. 2008;57:172–182. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Grillner S. The role of spinal cord inputs in modulating the activity of reticulospinal neurons during fictive locomotion in the lamprey. Brain Res. 1989;483:196–200. doi: 10.1016/0006-8993(89)90055-3. [DOI] [PubMed] [Google Scholar]
- Dye JC, Meyer JH. Central control of the electric organ discharge in weakly electric fish. In: Bullock TH, Heiligenberg W, editors. Electroreception. New York: Wiley & Sons; 1986. pp. 71–102. [Google Scholar]
- Einum JF, Buchanan JT. Reticulospinal neurons receive direct spinobulbar inputs during locomotor activity in lamprey. J Neurophysiol. 2004;92:1384–1390. doi: 10.1152/jn.00625.2003. [DOI] [PubMed] [Google Scholar]
- Einum JF, Buchanan JT. Membrane potential oscillations in reticulospinal and spinobulbar neurons during locomotor activity. J Neurophysiol. 2005;94:273–281. doi: 10.1152/jn.00695.2004. [DOI] [PubMed] [Google Scholar]
- Gabriel JP, Mahmood R, Walter AM, Kyriakatos A, Hauptmann G, Calabrese RL, El Manira A. Locomotor pattern in the adult zebrafish spinal cord in vitro. J Neurophysiol. 2008;99:37–48. doi: 10.1152/jn.00785.2007. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M, Lanuza G, Sapir T, Narayan S. The formation of sensorimotor circuits. Curr Opin Neurobiol. 2002;12:508–515. doi: 10.1016/s0959-4388(02)00371-9. [DOI] [PubMed] [Google Scholar]
- Grillner S, Georgopoulos AP, Jordan LM. Selection and initiation of motor behaviour. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, Networks and Motor Behavior. Cambridge, MA: MIT Press; 1997. pp. 1–19. [Google Scholar]
- Grillner S, Wallen P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates: an overview. Brain Res Rev. 2008;57:2–12. doi: 10.1016/j.brainresrev.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Hernandez P, Elbert K, Droge MH. Spontaneous and NMDA evoked motor rhythms in the neonatal mouse spinal cord: an in vitro study with comparisons to in situ activity. Exp Brain Res. 1991;85:66–74. doi: 10.1007/BF00229987. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kahn JA, Roberts A. The central nervous origin of the swimming motor pattern in embryos of Xenopus laevis. J Exp Biol. 1982;99:185–196. doi: 10.1242/jeb.99.1.185. [DOI] [PubMed] [Google Scholar]
- Kasicki S, Grillner S. Muller cells and other reticulospinal neurones are phasically active during fictive locomotion in the isolated nervous system of the lamprey. Neurosci Lett. 1986;69:239–243. doi: 10.1016/0304-3940(86)90486-6. [DOI] [PubMed] [Google Scholar]
- Kasicki S, Grillner S, Ohta Y, Dubuc R, Brodin L. Phasic modulation of reticulospinal neurones during fictive locomotion and other types of spinal motor activity in lamprey. Brain Res. 1989;484:203–216. doi: 10.1016/0006-8993(89)90363-6. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Satou C, Higashijima S-i. V2a and V2b neurons are generated by the final divisions of pair-producing progenitors in the zebrafish spinal cord. Development. 2008;135:3001–3005. doi: 10.1242/dev.024802. [DOI] [PubMed] [Google Scholar]
- Kudo N, Yamada T. N-methyl-D,L-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett. 1987;75:43–48. doi: 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- Lambert TD, Li WC, Soffe SR, Roberts A. Brainstem control of activity and responsiveness in resting frog tadpoles: tonic inhibition. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:331–342. doi: 10.1007/s00359-004-0505-8. [DOI] [PubMed] [Google Scholar]
- Lewis KE. How do genes regulate simple behaviours? Understanding how different neurons in the vertebrate spinal cord are genetically specified. Philos Trans R Soc B Biol Sci. 2006;361:45–56. doi: 10.1098/rstb.2005.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-C, Roberts A, Soffe SR. Locomotor rhythm maintenance: electrical coupling among premotor excitatory interneurons in the brainstem and spinal cord of young Xenopus tadpoles. J Physiol. 2009;587:1677–1693. doi: 10.1113/jphysiol.2008.166942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-C, Sautois B, Roberts A, Soffe SR. Reconfiguration of a vertebrate motor network: specific neuron recruitment and context-dependent synaptic plasticity. J Neurosci. 2007;27:12267–12276. doi: 10.1523/JNEUROSCI.3694-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-C, Soffe SR, Roberts A. Spinal inhibitory neurons that modulate cutaneous sensory pathways during locomotion in a simple vertebrate. J Neurosci. 2002;22:10924–10934. doi: 10.1523/JNEUROSCI.22-24-10924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-C, Soffe SR, Roberts A. Glutamate and acetylcholine corelease at developing synapses. Proc Natl Acad Sci U S A. 2004;101:15488–15493. doi: 10.1073/pnas.0404864101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-C, Soffe SR, Wolf E, Roberts A. Persistent responses to brief stimuli: feedback excitation among brainstem neurons. J Neurosci. 2006;26:4026–4035. doi: 10.1523/JNEUROSCI.4727-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan AD, Grillner S. Activation of ‘fictive swimming’ by electrical microstimulation of brainstem locomotor regions in an in vitro preparation of the lamprey central nervous system. Brain Res. 1984;300:357–361. doi: 10.1016/0006-8993(84)90846-1. [DOI] [PubMed] [Google Scholar]
- McDearmid JR, Drapeau P. Rhythmic motor activity evoked by NMDA in the spinal zebrafish larva. J Neurophysiol. 2006;95:401–417. doi: 10.1152/jn.00844.2005. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Tables of Xenopus laevis (Daudin) Amsterdam, North Holland: 1956. [Google Scholar]
- Ohta Y, Grillner S. Monosynaptic excitatory amino acid transmission from the posterior rhombencephalic reticular nucleus to spinal neurons involved in the control of locomotion in lamprey. J Neurophysiol. 1989;62:1079–1089. doi: 10.1152/jn.1989.62.5.1079. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion. Oxford: Oxford University Press; 1999. [Google Scholar]
- Perrins R, Walford A, Roberts A. Sensory activation and role of inhibitory reticulospinal neurons that stop swimming in hatchling frog tadpoles. J Neurosci. 2002;22:4229–4240. doi: 10.1523/JNEUROSCI.22-10-04229.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prime L, Pichon Y, Moore LE. N-Methyl-D-aspartate-induced oscillations in whole cell clamped neurons from the isolated spinal cord of Xenopus laevis embryos. J Neurophysiol. 1999;82:1069–1073. doi: 10.1152/jn.1999.82.2.1069. [DOI] [PubMed] [Google Scholar]
- Roberts A. Early functional organization of spinal neurons in developing lower vertebrates. Brain Res Bull. 2000;53:585–593. doi: 10.1016/s0361-9230(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Roberts A, Alford ST. Descending projections and excitation during fictive swimming in Xenopus embryos: neuroanatomy and lesion experiments. J Comp Neurol. 1986;250:253–261. doi: 10.1002/cne.902500212. [DOI] [PubMed] [Google Scholar]
- Roberts A, Dale N, Ottersen OP, Storm-Mathisen J. The early development of neurons with GABA immunoreactivity in the CNS of Xenopus laevis embryos. J Comp Neurol. 1987;261:435–449. doi: 10.1002/cne.902610308. [DOI] [PubMed] [Google Scholar]
- Roberts A, Dale N, Ottersen OP, Storm-Mathisen J. Development and characterization of commissural interneurones in the spinal cord of Xenopus laevis embryos revealed by antibodies to glycine. Development. 1988;103:447–461. doi: 10.1242/dev.103.3.447. [DOI] [PubMed] [Google Scholar]
- Roberts A, Soffe S, Perrins R. Spinal networks controlling swimming in hatchling Xenopus tadpoles. In: Stein P, Grillner S, Selverston A, Stuart D, editors. Neurons, Networks and Motor Behavior. Cambridge, MA: MIT Press; 1997. pp. 83–89. [Google Scholar]
- Roberts A, Tunstall MJ. Mutual re-excitation with post-inhibitory rebound: a simulation study on the mechanisms for locomotor rhythm generation in the spinal cord of Xenopus embryos. Eur J Neurosci. 1990;2:11–23. doi: 10.1111/j.1460-9568.1990.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Roberts A, Walford A, Soffe SR, Yoshida M. Motoneurons of the axial swimming muscles in hatchling Xenopus tadpoles: features, distribution, and central synapses. J Comp Neurol. 1999;411:472–486. doi: 10.1002/(sici)1096-9861(19990830)411:3<472::aid-cne9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Sautois B, Soffe SR, Li W-C, Roberts A. Role of type-specific neuron properties in a spinal cord motor network. J Comput Neurosci. 2007;23:59–77. doi: 10.1007/s10827-006-0019-1. [DOI] [PubMed] [Google Scholar]
- Soffe SR. Roles of glycinergic inhibition and N-methyl-D-aspartate receptor mediated excitation in the locomotor rhythmicity of one half of the Xenopus embryo central nervous system. Eur J Neurosci. 1989;1:561–571. doi: 10.1111/j.1460-9568.1989.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Soffe SR. Two distinct rhythmic motor patterns are driven by common premotor and motor neurons in a simple vertebrate spinal cord. J Neurosci. 1993;13:4456–4469. doi: 10.1523/JNEUROSCI.13-10-04456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffe SR. Motor patterns for two distinct rhythmic behaviours evoked by excitatory amino acid agonists in the Xenopus embryo spinal cord. J Neurophysiol. 1996;75:1815–1825. doi: 10.1152/jn.1996.75.5.1815. [DOI] [PubMed] [Google Scholar]
- Soffe SR, Roberts A. The influence of magnesium ions on the NMDA mediated responses of ventral rhythmic neurons in the spinal cord of Xenopus embryos. Eur J Neurosci. 1989;1:507–515. doi: 10.1111/j.1460-9568.1989.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Tunstall MJ, Roberts A. Longitudinal coordination of motor output during swimming in Xenopus embryos. Proc R Soc Lond B Biol Sci. 1991;244:27–32. doi: 10.1098/rspb.1991.0046. [DOI] [PubMed] [Google Scholar]
- van Mier P. Reticulospinal neurons, locomotor control and the development of tail swimming in Xenopus. Acta Biol Hung. 1988;39:161–177. [PubMed] [Google Scholar]
- van Mier P, ten Donkelaar HJ. Structural and functional properties of reticulospinal neurons in the early-swimming stage Xenopus embryo. J Neurosci. 1989;9:25–37. doi: 10.1523/JNEUROSCI.09-01-00025.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana Di Prisco G, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science. 1997;278:1122–1125. doi: 10.1126/science.278.5340.1122. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Pearlstein E, Le Ray D, Robitaille R, Dubuc R. A cellular mechanism for the transformation of a sensory input into a motor command. J Neurosci. 2000;20:8169–8176. doi: 10.1523/JNEUROSCI.20-21-08169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay L, Grillner S. Spino-bulbar neurons convey information to the brainstem about different phases of the locomotor cycle in the lamprey. Brain Res. 1992;582:134–138. doi: 10.1016/0006-8993(92)90327-6. [DOI] [PubMed] [Google Scholar]
- Wannier T, Deliagina TG, Orlovsky GN, Grillner S. Differential effects of the reticulospinal system on locomotion in lamprey. J Neurophysiol. 1998;80:103–112. doi: 10.1152/jn.1998.80.1.103. [DOI] [PubMed] [Google Scholar]
- Wheatley M, Stein RB. An in vitro preparation of the mudpuppy for simultaneous intracellular and electromyographic recording during locomotion. J Neurosci Methods. 1992;42:129–137. doi: 10.1016/0165-0270(92)90143-2. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessell TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci. 2005;25:5710–5719. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E, Zhao FY, Roberts A. Non-linear summation of excitatory synaptic inputs to small neurones: a case study in spinal motoneurones of the young Xenopus tadpole. J Physiol. 1998;511:871–886. doi: 10.1111/j.1469-7793.1998.871bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin PV. Activity of individual reticulospinal neurons during different forms of locomotion in the lamprey. Eur J Neurosci. 2005;22:2271–2282. doi: 10.1111/j.1460-9568.2005.04395.x. [DOI] [PubMed] [Google Scholar]
- Zelenin PV, Grillner S, Orlovsky GN, Deliagina TG. Heterogeneity of the population of command neurons in the lamprey. J Neurosci. 2001;21:7793–7803. doi: 10.1523/JNEUROSCI.21-19-07793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.