Abstract
Motor cortical plasticity induced by repetitive transcranial magnetic stimulation (rTMS) sometimes depends on the prior history of neuronal activity. These effects of preceding stimulation on subsequent rTMS-induced plasticity have been suggested to share a similar mechanism to that of metaplasticity, a homeostatic regulation of synaptic plasticity. To explore metaplasticity in humans, many investigations have used designs in which both priming and conditioning are applied over the primary motor cortex (M1), but the effects of priming stimulation over other motor-related cortical areas have not been well documented. Since the supplementary motor area (SMA) has anatomical and functional cortico-cortical connections with M1, here we studied the homeostatic effects of priming stimulation over the SMA on subsequent rTMS-induced plasticity of M1. For priming and subsequent conditioning, we employed a new rTMS protocol, quadripulse stimulation (QPS), which produces a broad range of motor cortical plasticity depending on the interval of the pulses within a burst. The plastic changes induced by QPS at various intervals were altered by priming stimulation over the SMA, which did not change motor-evoked potential sizes on its own but specifically modulated the excitatory I-wave circuits. The data support the view that the homeostatic changes are mediated via mechanisms of metaplasticity and highlight an important interplay between M1 and SMA regarding homeostatic plasticity in humans.
Introduction
Repetitive transcranial magnetic stimulation (rTMS) is a promising method to induce plastic changes in humans (Hallett, 2007). In some cases, the rTMS-induced plasticity is N-methyl-d-aspartate (NMDA) dependent, supporting the idea that changes in synaptic efficacy, such as long-term potentiation (LTP) and long-term depression (LTD), are implicated in rTMS-induced plasticity (Stefan et al. 2002; Huang et al. 2007). Several human studies have also shown effects of a prior history of neuronal activity on subsequent rTMS-induced plasticity (e.g. Siebner et al. 2004; Hamada et al. 2008a). These findings have been compared with metaplasticity, homeostatic regulation of synaptic plasticity in which the capacity of synapses to exhibit plasticity depends on prior levels of neuronal activity (Abraham & Bear, 1996; Ziemann & Siebner, 2008). This form of plasticity regulation might relate directly to the Bienenstock–Cooper–Munro (BCM) theory, a prevailing model for homeostatic mechanisms of synaptic plasticity (Bienenstock et al. 1982; Abraham, 2008).
The protocol for studying metaplasticity in an experimental context is to apply a period of priming stimulation (which on its own has little or no effect on synaptic plasticity) and then to test whether this changes the response to a second period of conditioning stimulation which produces LTP or LTD when given alone (Abraham, 2008). Many human studies have used designs in which both priming and conditioning are applied over the primary motor cortex (M1) (Iyer et al. 2003; Siebner et al. 2004; Lang et al. 2004; Müller et al. 2007; Hamada et al. 2008a). Others showed that metaplastic effects can be elicited by finger movements or voluntary muscle contraction, in place of priming stimulation, which might be associated with activity changes in cortical circuits within various motor-related areas (Ziemann et al. 2004; Gentner et al. 2008; Huang et al. 2008). More recently, Ragert et al. (2009) demonstrated the effects of priming stimulation over the right M1 on subsequent rTMS-induced plasticity of left M1 (Ragert et al. 2009). Of note is the fact that the effects of priming stimulation over motor-related areas other than ipsilateral or contralateral M1 have not been well documented, despite important anatomical and functional interplay between those areas and M1.
Among the motor-related areas, the lateral premotor cortex (PM) such as dorsal and ventral PM (PMd and PMv) has been extensively studied by means of TMS in humans to shed light on the interaction between M1 (see review by Reis et al. 2008). The supplementary motor area (SMA) also has dense cortico-cortical connections with M1 in animals (Dum & Strick, 1991; Luppino et al. 1993; Tokuno & Nambu, 2000) and plays a substantial role in higher motor control and learning (Tanji & Shima, 1994; Hikosaka et al. 1999; Nachev et al. 2008). However, as SMA is located in the interhemispheric fissure, it is a more difficult site to stimulate than the lateral PM (Reis et al. 2008). Thus, not much has been done in SMA as compared to PMd; two studies revealed that TMS over SMA can modulate the cortical excitability of M1 via cortico-cortical synaptic connections (Civardi et al. 2001; Matsunaga et al. 2005). In light of this accruing evidence, we aimed to explore the effects of preceding stimulation over SMA on subsequent rTMS-induced plasticity of the M1 in order to test the hypothesis that priming over the SMA modulates some cortical neurons within M1 via cortico-cortical connections, and that such prior history of neuronal activity alters subsequent rTMS-induced plasticity.
For priming and subsequent conditioning, we employed a new rTMS protocol, quadripulse stimulation (QPS) (Hamada et al. 2007, 2008a). QPS consists of repeated trains of four monophasic TMS pulses separated by inter-stimulus intervals (ISI) of 1.5–1250 ms, inducing bidirectional motor cortical plasticity in an ISI-dependent, non-linear form that are compatible with changes in synaptic plasticity. In addition, we showed that QPS interventions could interact in a metaplastic manner such that priming over M1 using QPS at short ISIs, which did not induce any plastic changes by itself, occluded subsequent LTP-like plasticity, whereas priming using QPS with long ISIs tended to do the reverse and increased the probability that facilitatory effects would be produced by a subsequent period of stimulation. The data support a BCM-like model of priming that shifts the crossover point at which the synaptic plasticity reverses from LTD to LTP. We proposed that such a broad range of after-effects produced by QPS facilitates detailed examinations of metaplasticity theories in humans (Hamada et al. 2008a). In the present study, we evaluated how LTD-like and LTP-like QPS-induced plasticity was altered by a preceding period of priming stimulation over SMA to understand metaplastic interplay between SMA and M1.
Methods
Subjects
Subjects were nine healthy volunteers (one woman, eight men; 27–45 years old, mean ±s.d., 34.5 ± 6.7 years) who gave their written informed consent to participate in the experiments. No subjects had neurological, psychiatric or other medical problems, or had any contraindication to TMS (Wassermann, 1998). All were right-handed according to the Oldfield handedness inventory (Oldfield, 1971). The protocol was approved by the Ethics Committee of the University of Tokyo and was carried out in accordance with the ethical standards of the Declaration of Helsinki. The procedures are in compliance with The Journal of Physiology's guidelines for experimentation on humans (Drummond, 2009).
Recordings
Subjects were seated on a comfortable chair. The electromyogram (EMG) activity was recorded from the right first dorsal interosseous muscle (FDI) and the right tibialis anterior muscle (TA) using a belly-tendon arrangement. Responses were input to an amplifier (Biotop; GE Marquette Medical Systems, Japan) through filters set at 100 Hz and 3 kHz; they were then digitized and stored on a computer for later offline analyses (TMS bistim tester; Medical Try System, Japan).
Stimulation
Focal TMS was given using a hand-held figure-of-eight coil (9 cm external diameter at each wing; The Magstim Co. Ltd, Whitland, Dyfed, UK). Single monophasic TMS pulses were delivered by a magnetic stimulator (Magstim 200; The Magstim Co. Ltd). Quadripulse stimuli were delivered by four magnetic stimulators (Magstim 2002; The Magstim Co. Ltd) connected with a specially designed combining module (The Magstim Co. Ltd). This device combines the outputs from four stimulators to allow a train of four monophasic magnetic pulses to be delivered through a single coil.
The optimal site for eliciting MEPs from the right FDI muscle (i.e. hot spot for FDI) was determined before each experiment and considered to be the primary motor cortex for FDI muscle (M1FDI). A figure-of-eight coil was placed tangentially over the scalp with the handle pointing backwards at about 45 deg laterally. We stimulated several positions in 1 cm increments in the anterio-posterior and medio-lateral direction from each other using the same intensity and determined M1FDI as the site at which the largest responses were elicited. This position was marked with a blue pen on the scalp for repositioning the coil. The resting motor threshold for FDI (RMTFDI) was defined as the lowest intensity that evoked a response of at least 50 μV in the relaxed FDI in at least 5 of 10 consecutive trials (Rossini et al. 1994). The active motor threshold for FDI (AMTFDI) was defined as the lowest intensity that evoked a small response (>100 μV) when the subjects maintained a slight contraction of the right FDI (∼10% of the maximum voluntary contraction), as observed using an oscilloscope monitor, in more than 5 of 10 consecutive trials. The stimulus intensity was changed in steps of 1% of the maximum stimulator output (MSO).
SMA stimulation was given with a coil centred at a point 3 cm anterior to the optimal site for eliciting MEPs in the right TA muscle (i.e. hot spot for TA) according to previous studies (Hikosaka et al. 1996; Lee et al. 1999; Terao et al. 2001; Matsunaga et al. 2005). The hot spot for TA was determined by moving the coil in 1 cm steps along the sagittal mid-line through the vertex (Cz) with the handle pointing to the right until we detected the position which evoked the largest MEP. This was considered to be the primary motor cortex for the TA muscle (M1TA). We then used this position to determine the AMT for TA (AMTTA). On average, the site of SMA stimulation (i.e. the point 3 cm anterior to M1TA) was 2–3 cm anterior to Cz, in line with data from previous studies (Hikosaka et al. 1996; Lee et al. 1999; Matsunaga et al. 2005).
Measurement of motor cortical excitability
Motor cortical excitability was assessed by measuring the peak-to-peak amplitude of MEPs from the relaxed right FDI muscle elicited by single pulse TMS over the left M1FDI for all experiments. The stimulus intensity was adjusted to produce MEPs of about 0.5 mV in the right FDI muscle. During the experiments, EMG activity of the FDI was monitored with an oscilloscope monitor. Trials contaminated with voluntary EMG activities were discarded from analyses.
Quadripulse stimulation
The QPS protocol consisted of trains of TMS pulses with an inter-train interval (ITI) of 5 s (i.e. 0.2 Hz). Each train consisted of four magnetic pulses separated by inter-stimulus intervals (ISI) of 1.5, 5, 10, 30, 50 or 100 ms. These conditioning types were designated as QPS-1.5 ms to QPS-100 ms, respectively.
Experiment 1: Effects of SMA priming on subsequent QPS-induced plasticity
Six of nine subjects were enrolled. The experimental sessions were separated by 1 week or longer in the same subject. The order of the experiments was randomized and balanced among subjects.
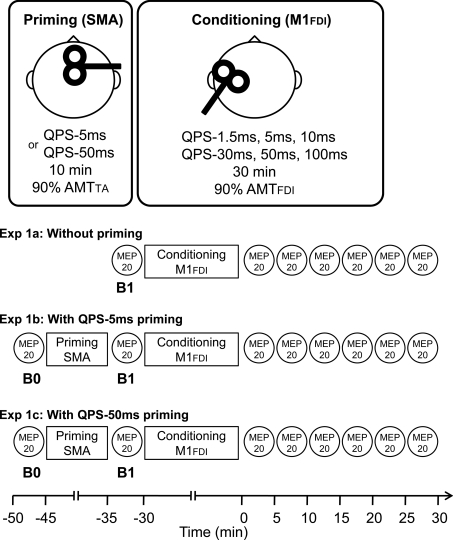
Experiment 1a: QPS-induced plasticity without priming over SMA (Fig. 1)
Figure 1.
Timelines of experiments (See Methods.)
To investigate QPS-induced plasticity without any priming, conditioning stimulation was applied over the left M1FDI using QPS at various ISIs (QPS-1.5 ms, QPS-5 ms, QPS-10 ms, QPS-30 ms, QPS-50 ms and QPS-100ms) for 30 min (Fig. 1). The stimulus intensity of each pulse for QPS conditioning was set at 90% AMTFDI (Hamada et al. 2008a). During the conditioning, no MEPs were observed.
Before QPS, 20 MEPs were obtained every 14.5–15.5 s using single-pulse TMS at a fixed intensity. The stimulus intensity was adjusted to produce MEPs of about 0.5 mV in the right FDI muscle at baseline (B1 in Fig. 1); the intensity was kept constant throughout the same experiment. After QPS conditioning, MEPs were measured every 5 min for 30 min. At each time point of the measurements, MEPs were collected in the same manner as baseline measurements.
Experiment 1b: QPS-induced plasticity with QPS-5 ms priming over SMA (Fig. 1)
Priming stimulation was applied over SMA using QPS-5 ms for 10 min (i.e. four pulses at an ISI of 5 ms with an ITI of 5 s for 10 min). Importantly, priming over SMA using QPS-5 ms for 10 min induces no substantial effects on MEP sizes (see Results). The aim of the experiment was to test whether this priming stimulation would have effects on subsequent QPS-induced plasticity according to previous animal studies, which showed that prior induction of LTP is not prerequisite for metaplasticity (Abraham & Tate, 1997; Abraham, 2008). The stimulus intensity of each pulse for priming was set at 90% AMTTA, which was identical to about 130% AMTFDI (i.e. 90% RMTFDI) (Table 1). The stimulus intensity used for priming over SMA was calculated relative to AMTTA in order to secure effective stimulation of SMA, given that its depth is almost the same as that of the M1 for foot muscles in the interhemispheric fissure. Additionally, 130% AMT of hand muscle over SMA has been considered not to spread to the PMd or M1 (Matsunaga et al. 2005).
Table 1.
Physiological parameters (mean ±s.d.)
| RMTFDI | AMTFDI | AMTTA | Stimulus intensify for SMA priming (relative to RMTFDI) | MEP size (mV), right FDI | |||
|---|---|---|---|---|---|---|---|
| Experiment 1a: | Baseline (B1) | B1 | |||||
| Without priming | |||||||
| QPS-1.5 ms | 57.1 ± 9.8% | 40.8 ± 6.9% | — | — | — | 0.46 ± 0.11 | |
| QPS-5 ms | 58.5 ± 9.5% | 43.6 ± 5.1% | — | — | — | 0.51 ± 0.13 | |
| QPS-10 ms | 61.8 ± 10.9% | 41.0 ± 6.6% | — | — | — | 0.48 ± 0.16 | |
| QPS-30 ms | 61.2 ± 11.6% | 40.6 ± 5.8% | — | — | — | 0.49 ± 0.09 | |
| QPS-50 ms | 60.5 ± 14.1% | 42.6 ± 5.4% | — | — | — | 0.51 ± 0.18 | |
| QPS-100 ms | 60.1 ± 12.1% | 40.8 ± 5.8% | — | — | — | 0.55 ± 0.06 | |
| Experiment 1b: With | Baseline (B1) | Mean ±s.d. (range) | B0 | B1 | |||
| QPS-5 ms priming | |||||||
| over SMA | |||||||
| QPS-1.5 ms | 61.8 ± 9.2% | 40.3 ± 1.9% | 60.2 ± 8.0% | 89 ± 8% (76–98%) | 0.51 ± 0.09 | 0.49 ± 0.14 | |
| QPS-5 ms | 59.8 ± 6.9% | 42.8 ± 5.0% | 60.8 ± 8.5% | 92 ± 5% (85–97%) | 0.43 ± 0.11 | 0.47 ± 0.28 | |
| QPS-10 ms | 60.0 ± 9.7% | 42.0 ± 4.9% | 61.1 ± 10.0% | 93 ± 4% (87–99%) | 0.48 ± 0.18 | 0.54 ± 0.14 | |
| QPS-30 ms | 58.3 ± 8.5% | 41.7 ± 5.4% | 60.8 ± 10.0% | 95 ± 6% (86–100%) | 0.51 ± 0.14 | 0.55 ± 0.18 | |
| QPS-50 ms | 61.8 ± 7.6% | 41.0 ± 5.1% | 63.6 ± 8.1% | 93 ± 5% (85–98%) | 0.53 ± 0.21 | 0.53 ± 0.20 | |
| QPS-100 ms | 60.8 ± 8.7% | 40.3 ± 5.0% | 61.5 ± 7.6% | 92 ± 4% (86–96%) | 0.53 ± 0.18 | 0.59 ± 0.21 | |
| Experiment 1c: With | Baseline (B1) | B0 | B1 | ||||
| QPS-50 ms priming | |||||||
| over SMA | |||||||
| QPS-5 ms | 61.6 ± 9.4% | 41.6 ± 5.3% | 62.6 ± 9.3% | 94 ± 3% (91–98%) | 0.50 ± 0.09 | 0.52 ± 0.08 | |
| QPS-10 ms | 60.6 ± 9.1% | 43.8 ± 5.9% | 63.5 ± 9.6% | 95 ± 2% (92–98%) | 0.57 ± 0.11 | 0.53 ± 0.21 | |
| QPS-30 ms | 58.1 ± 9.9% | 42.1 ± 4.8% | 62.2 ± 11.4% | 95 ± 5% (88–100%) | 0.53 ± 0.09 | 0.48 ± 0.04 | |
| QPS-100 ms | 60.5 ± 10.1% | 42.0 ± 6.4% | 65.2 ± 11.7% | 97 ± 3% (93–100%) | 0.52 ± 0.12 | 0.49 ± 0.16 | |
| Experiment 2 | Baseline (B0) | B0 | B1 | ||||
| Sham with priming | 61.8 ± 13.7% | 40.8 ± 7.8% | 61.5 ± 7.2% | 93 ± 4% (88–100%) | 0.46 ± 0.06 | 0.51 ± 0.14 | |
| QPS-10 ms with sham priming | 58.3 ± 7.4% | 40.5 ± 7.4% | — | — | 0.47 ± 0.12 | 0.48 ± 0.18 | |
| Experiment 3 | Baseline | Baseline | Post 1 | Post 2 | |||
| QPS-5 ms priming | 60.6 ± 11.0% | 42.0 ± 8.2% | 66.0 ± 7.7% | 95 ± 4% (88–100%) | 0.52 ± 0.24 | 0.54 ± 0.27 | 0.49 ± 0.26 |
| QPS-50 ms priming | 59.7 ± 10.1% | 41.5 ± 9.8% | 66.4 ± 8.3% | 95 ± 5% (88–100%) | 0.48 ± 0.14 | 0.53 ± 0.19 | 0.55 ± 0.11 |
| Experiment 4 | ISI 3 ms | ISI 6 ms | |||||
| 57.9 ± 9.6% | 43.3 ± 7.8% | 62.6 ± 9.4% | — | 0.59 ± 0.23 | 0.58 ± 0.27 | ||
Subsequent conditioning was applied over the left M1FDI using QPS at various ISIs (QPS-1.5 ms, QPS-5 ms, QPS-10 ms, QPS-30 ms, QPS-50 ms and QPS-100ms) for 30 min (i.e. 360 trains, 1440 pulses in total) (Fig. 1). The stimulus intensity of each pulse for this subsequent conditioning was set at 90% AMTFDI.
Before the priming stimulation (B0 in Fig. 1), 20 MEPs were collected every 14.5–15.5 s using single-pulse TMS at a fixed intensity, which was adjusted to elicit MEPs of about 0.5 mV in the right FDI muscle at B0 and kept constant throughout the experiment. After priming over SMA, 20 MEPs were again obtained in the same manner as measurements at B0 (B1 in Fig. 1). Following this measurement at B1 (i.e. immediately after priming), QPS conditioning of various types over the left M1FDI was performed. After each QPS, MEPs were measured every 5 min for 30 min (Fig. 1).
Experiment 1c: QPS-induced plasticity with QPS-50 ms priming over SMA (Fig. 1)
QPS-50 ms for 10 min (i.e. four pulses at an ISI of 50 ms with an ITI of 5 s for 10 min) was selected as another priming stimulation over SMA to reveal the opposite priming effects to QPS-5 ms priming. The stimulus intensity for each pulse was set at 90% AMTTA. Subsequent conditioning types after QPS-50 ms priming over SMA were QPS-5 ms, QPS-10 ms, QPS-30 ms and QPS-100 ms over the left M1FDI for 30 min. We have previously shown that QPS-50 ms priming over M1 produced substantial changes in subsequent QPS-induced plasticity at ISIs of 10, 30 and 100 ms (Hamada et al. 2008a). Since it would be of value to compare those results and SMA priming effects in the present study to understand the difference in priming stimulation site (i.e. M1 versus SMA), we selected the four ISIs used in the previous paper for Experiment 1c. The stimulus intensity of each pulse of QPS conditioning was set at 90% AMTFDI. MEP measurements were exactly the same as those of Experiment 1b.
Experiment 2: Control experiments
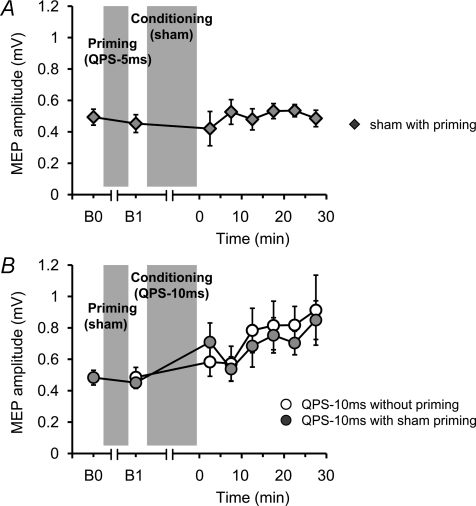
In the first control experiment (Fig. 6A), priming stimulation using QPS-5 ms over SMA for 10 min was followed by sham conditioning stimulation (i.e. sham with priming) to examine whether the priming alone affects motor cortical excitability. In the second control experiment (Fig. 6B), sham priming stimulation was followed by QPS-10 ms over M1 to confirm that sham priming did not affect the QPS-induced plastic changes (QPS-10 ms with sham priming). QPS-10 ms was chosen as conditioning because this protocol induced mild facilitatory after-effects (see Results) which we thought might make it more susceptible to any bidirectional effects of sham priming.
Figure 6. Control experiments.
A, sham conditioning with real priming did not modify motor cortical excitability. B, the after-effects of QPS-10 ms without priming (open circles) were not different from those of QPS-10 ms with sham priming (grey circles).
The sham stimulation procedure used for these control experiments was identical to those described in our previous reports (Okabe et al. 2003; Hamada et al. 2008b). In brief, four electric pulses (each electric pulse was of 0.2 ms duration with intensity of twice the sensory threshold) were given to the scalp at 0.2 Hz at an ISI of 10 ms for sham conditioning (first control experiment) and at 5 ms for sham priming (second control experiment) with a conventional electric peripheral nerve stimulator to mimic the skin sensation of TMS. Electric pulses were applied through the electrodes placed over the left M1FDI or SMA and the vertex. A coil, which was disconnected from the stimulator, was placed over the left M1FDI or SMA to mimic real TMS. Another coil, which was connected to a combining module with four stimulators, was held off the scalp but placed near the subject. This coil was discharged simultaneously with the scalp electrical stimulation to produce a similar sound to that associated with real QPS.
Experiment 3: Effects of priming over SMA on intracortical circuits of M1
Nine subjects participated in this experiment. To explore the effects of SMA priming alone on either excitatory or inhibitory circuits of M1, short-interval intracortical inhibition (SICI), intracortical facilitation (ICF) (Kujirai et al. 1993), short-interval intracortical facilitation (SICF) (Tokimura et al. 1996; Ziemann et al. 1998; Hanajima et al. 2002), and long-interval intracortical inhibition (LICI) (Valls-Sole et al. 1992; Wassermann et al. 1996) were measured using the paired-pulse technique before and after QPS-5 ms or QPS-50 ms priming over SMA (i.e. four pulses at an ISI of 5 ms or 50 ms with an ITI of 5 s for 10 min; the stimulus intensity of each pulse, 90% AMTTA).
SICI was examined at an ISI of 3 ms using a conditioning stimulus (CS) intensity of 80% AMTFDI. ICF was measured at an ISI of 10 ms with a CS intensity of 90% AMTFDI. SICF was measured at an ISI of 1.5 ms. The intensity of the second stimulus (S2) was set at 10% below AMTFDI. LICI was measured at an ISI of 100 ms with a CS intensity of 110% RMTFDI. The intensity of the test stimulus (TS) (i.e. the first stimulus (S1) for SICF) was adjusted to elicit MEPs of 0.4–0.5 mV from relaxed FDI at baseline. Twelve trials were recorded for each condition and randomly intermixed with 18 trials of TS alone with an ITI of 5.5–6.5 s (about 6 min in total). The SICI, ICF, SICF and LICI were all studied simultaneously in one session using the four magnetic stimulators (i.e. three stimulators produced the different CS and the other one gave TS). Measurements of these values were performed in blocks immediately before (baseline) and just after QPS-5 ms priming (post 1) as well as 20 to 26 min (post 2). Conditioning intensities and test intensity were not changed after the priming because the test MEP sizes were not altered by SMA priming (see Results and Table 1).
Experiment 4: Supplementary experiments
The aim of these experiments was to test whether the conditioning stimulus over SMA spreads to M1 or PMd (Experiment 4a), or whether it exerts a direct effect on the spinal motor neurons (Experiment 4b). Eight subjects participated in this series of experiments.
Experiment 4a: Effects of a conditioning stimulus over SMA on MEP
We investigated whether the stimulus over SMA spreads to other cortical areas using a paired-pulse technique. The test response in the right relaxed FDI elicited by single pulse TMS over the left M1FDI was conditioned by single pulse TMS over SMA at an ISI of 3 ms or 6 ms. The intensity of the TS was adjusted to elicit MEPs of about 0.5 mV in the relaxed FDI when given alone. The stimulus intensities for conditioning over SMA were set at 70, 90 and 110% AMTTA. At each ISI (i.e. 3 or 6 ms), 12 trials were recorded for each condition (70, 90 and 110% AMTTA) and randomly intermixed with 18 trials of TS alone with an ITI of 5.5–6.5 s in a single block. Thus, two blocks of measurements at each ISI were performed. The order of blocks was randomized. As the inhibitory interneurons of the M1FDI might have a lower threshold than the intrinsic I-wave circuits (Reis et al. 2008), we argued that, if the current over SMA spread to the M1FDI, then some inhibitory effects on the test response would be observed at an ISI of 3 ms in a conditioning intensity-dependent manner. Indeed, it is known that conditioning over the PMd at an ISI of 6 ms has either inhibitory or facilitatory effects on MEP sizes depending on the conditioning intensity (Civardi et al. 2001). Thus, we argued that, if the current over SMA spread to PMd, then some inhibitory or facilitatory effects on the test response would be observed in an intensity-dependent manner.
Experiment 4b: Effects of a stimulus over SMA on MEP in active condition
To test whether the current over SMA spreads to the M1FDI directly and whether direct stimulation of SMA activates some neurons in SMA projecting to the spinal motor neurons directly (Dum & Strick, 1991, 1996), single pulse TMS was applied over SMA during contraction of the right FDI muscle (20% maximum voluntary contraction). If the current over SMA spread to M1FDI directly or if the current stimulated some SMA neurons enough to produce any descending volley, then small MEPs would be elicited during voluntary contraction in an intensity-dependent manner. Ten stimuli were applied every 5 s at an intensity of 100% AMTTA. The stimulus intensity was then increased by 10% of AMTTA and another 10 stimuli were applied. This process was repeated until the intensity reached 190% AMTTA or 100% MSO.
Data analyses
Experiment 1a
The after-effects of different conditioning types were analysed with absolute MEP amplitudes using two-way repeated-measures analysis of variance (ANOVA) (within-subject factors, CONDITION (QPS-1.5 ms, QPS-5 ms, ···, QPS-100ms), and TIME (B1 and following six time points)). If the factors CONDITION and TIME showed a significant interaction, post hoc paired t tests (two-tailed) with Bonferroni's corrections for multiple comparisons were used for further analyses. The Greenhouse–Geisser correction was used if necessary to correct for non-sphericity; P values less than 0.05 were considered significant.
Experiment 1b and 1c
Absolute values of MEPs at B0 and B1 (Fig. 1) were compared using paired t tests in each experiment. To evaluate the priming effects on subsequent QPS-induced plasticity, the absolute amplitudes of MEPs collected in Experiment 1a (i.e. without priming) and Experiment 1b (i.e. with QPS-5 ms priming over the SMA) or Experiment 1c (i.e. with QPS-50 ms priming over the SMA) were entered in three-way repeated-measures ANOVA with PRIMING (with and without priming), CONDITION ((QPS-1.5 ms, QPS-5 ms, ···, and QPS-100ms) for Experiment 1b, and (QPS-5 ms, QPS-10 ms, QPS-30 ms and QPS-100ms) for Experiment 1c), and TIME (B1, and following six time points) as within-subject factors to match the measurement time points relative to QPS conditioning among experiments. Additionally, it might be valid to evaluate the effect of priming stimulation on subsequent QPS-induced plasticity using these values because the absolute amplitudes obtained at B0 and B1 were not significantly different (see Results). If the factors PRIMING, CONDITION and TIME showed a significant interaction, post hoc paired t tests (two-tailed) with Bonferroni's corrections for multiple comparisons were used.
Experiment 2
The time course of after-effects for the first control experiment (i.e. sham conditioning with real priming) on absolute MEP sizes was analysed using one-way repeated measures ANOVA (within-subject factor, TIME (B1 and following six points)). For the second control experiment, the after-effects of QPS-10 ms with sham priming were compared with those of QPS-10 ms without priming using two-way repeated-measures ANOVA (within-subject factors, CONDITION (QPS-10 ms with sham priming, QPS-10 ms without priming) and TIME (B1 and following six points)).
Experiment 3
The ratio of the mean amplitude of the conditioned response to that of the control response was calculated for each condition in each subject. These individual mean ratios were then averaged to give a grand mean ratio. The time course of after-effects was analysed using three-way repeated measures ANOVA (within-subject factors, PRIMING (QPS-5 ms and QPS-50ms), BISTIM (SICI, ICF, SICF and LICI) and TIME (baseline, post 1 and post 2)). If the factors PRIMING, BISTIM and TIME showed a significant interaction, Dunnett's post hoc test in each condition was used for further analyses.
Experiment 4a
The ratio of the mean amplitude of the conditioned response to that of the control response was calculated for each condition in each subject. These individual mean ratios were then averaged to give a grand mean ratio. The ratios were entered in one-way repeated measures ANOVA (within-subject factor, INTENSITY of conditioning stimulation). Paired t tests (two-tailed) were used for further analyses.
Data were analysed with the commercialized software (SPSS version 17.0 for Windows; SPSS Inc.). All figures depict the group data.
Results
None of the subjects reported any adverse effects during or after any of the experiments. Baseline physiological data did not differ significantly among different experiments (Table 1). The stimulus intensities for SMA priming were all below RMTFDI (Table 1).
Experiment 1: Effects of SMA priming on subsequent QPS-induced plasticity
Experiment 1a: QPS-induced plasticity without priming over SMA
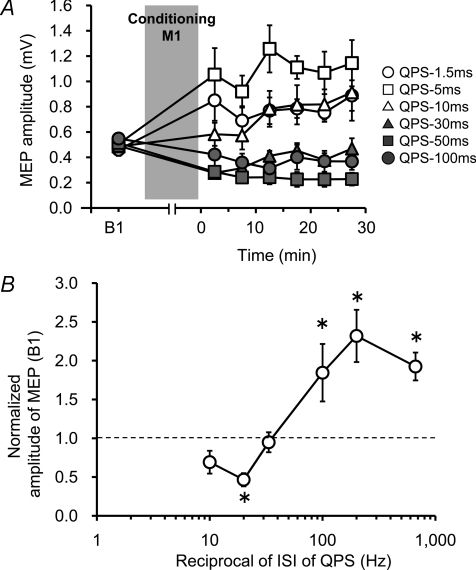
In line with our previous report (Hamada et al. 2008a), QPS at short ISIs (QPS-1.5 ms, QPS-5 ms and QPS-10ms) produced an increase in the MEP amplitude, whereas QPS at long ISIs (QPS-30 ms, QPS-50 ms and QPS-100ms) suppressed MEPs (Fig. 2A). Two-way repeated measures ANOVA revealed a significant CONDITION (QPS-1.5 ms, QPS-5 ms, ···, and QPS-100ms) × TIME interaction (F(2.941,14.705)= 4.384, P < 0.001). Figure 2B presents the MEP amplitude normalized to the baseline MEP at 30 min after QPS as a function of the reciprocal of the ISI used in each QPS burst. There was a non-linear relation between MEP excitability changes and ISI indicating the presence of threshold for inducing LTP-like plasticity. Post hoc analysis revealed that QPS-1.5 ms, QPS-5 ms and QPS-50ms were significantly different from QPS-30 ms.
Figure 2. QPS-induced plasticity without priming over SMA.
A, time courses of MEP amplitude following QPS at various ISIs without priming (mean ±s.e.m.). B, stimulus–response function of QPS-induced plasticity. Normalized amplitudes of MEP measured at 30 min after QPS as a function of the reciprocal of ISI of QPS: mean (±s.e.m.) of baseline. Note that the x-axis is logarithmic. Asterisks denote significant difference from QPS-30 ms (*P < 0.05).
Experiment1b: QPS-induced plasticity with QPS-5 ms priming over SMA
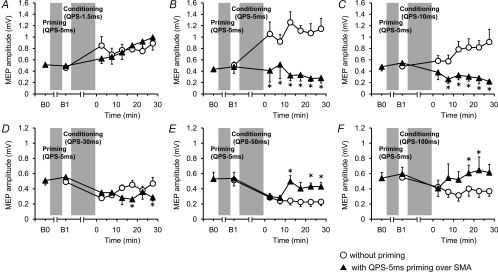
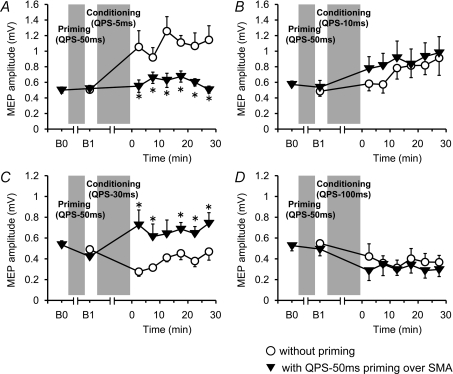
Figure 3 shows the time courses of MEP amplitude following QPS at various ISIs with and without QPS-5 ms priming over SMA. No difference was found in MEP amplitudes at B0 and B1 in any conditions (paired t test, P > 0.5). Although SMA priming with QPS-5 ms did not occlude MEP facilitation by QPS-1.5 ms, it produced lasting MEP suppression after QPS-5 ms, QPS-10 ms and QPS-30 ms (Fig. 3B–D). By contrast, MEP suppression induced by QPS-50 ms and QPS-100 ms was not enhanced, but its duration was shortened by SMA priming (Fig. 3E and F). Three-way repeated measures ANOVA revealed a significant PRIMING × CONDITION × TIME interaction (F(3.464,17.319)= 3.826, P= 0.025). Post hoc paired t tests revealed a significant effect of SMA priming on the after-effects of QPS-5 ms, QPS-10 ms, QPS-30 ms, QPS-50 ms and QPS-100 ms (Fig. 3A–F).
Figure 3. Effects of QPS-5 ms priming over SMA on QPS-induced plasticity.
Time courses of MEP amplitude following QPS at various ISIs with (▴) and without (○) QPS-5 ms priming over SMA (mean ±s.e.m.). A, SMA priming did not change subsequent LTP-like plasticity induced by QPS-1.5 ms. B and C, priming reversed MEP sizes induced by QPS-5 ms (B) or QPS-10 ms (C). D, priming enhanced suppression of MEP by QPS-30 ms. E and F, MEP suppression induced by QPS-50 ms (E) and QPS-100 ms (F) were not enhanced, but shortened with SMA priming. Asterisks denote significant difference of MEP sizes with priming from those without priming at each time point (P < 0.05 by post hoc paired t tests).
Experiment 1c: QPS-induced plasticity with QPS-50 ms priming over SMA
Figure 4 shows the change in MEP amplitude following QPS at various ISIs with and without QPS-50 ms priming over SMA. No difference in MEP amplitude was found at B0 and B1 in any conditions (paired t test, P > 0.5). QPS-50 ms priming over SMA did not enhance MEP facilitation by QPS-5 ms. It produced slight enhancement of MEP facilitation by QPS-10 ms (Fig. 4A and B). In contrast, transient MEP suppression induced by QPS-30 ms turned to facilitation after SMA priming, but there was no change in the after-effects of QPS-100 ms (Fig. 4C and D). Three-way repeated measures ANOVA revealed a significant PRIMING × CONDITION interaction (F(2.593,9.948)= 5.612, P= 0.023), but revealed no significant PRIMING × CONDITION × TIME interaction (F(3.363,16.810)= 3.079, P= 0.051). The results reveal that priming stimulation affected subsequent QPS-induced plasticity, irrespective of the time after QPS conditioning. Post hoc paired t tests revealed a significant effect of SMA priming with QPS-50 ms on the after-effects of QPS-5 ms and QPS-30 ms (Fig. 4A and C).
Figure 4. Effects of QPS-50 ms priming over SMA on QPS-induced plasticity.
Time courses of MEP amplitude following QPS at various ISIs with (▾) and without (^) QPS-50 ms priming over SMA (mean ±s.e.m.). A, SMA priming occluded subsequent LTP-like plasticity induced by QPS-5 ms. B, priming did not change plasticity induced by QPS-10 ms. C, priming reversed suppression of MEP by QPS-30 ms. D, MEP suppression induced by QPS-100 ms was not altered with SMA priming. Asterisks denote significant difference of MEP sizes with priming from those without priming at each time point (P < 0.05 by post hoc paired t tests).
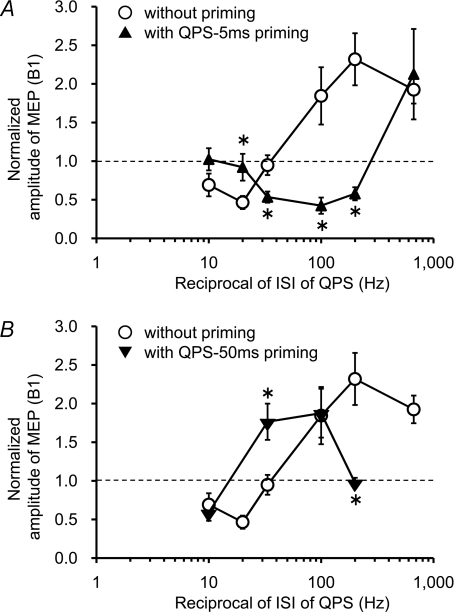
Stimulus–response function with priming over SMA
The normalized MEP amplitudes at 30 min post conditioning are plotted as a function of the reciprocal of the ISI used in each QPS with and without priming over SMA (Fig. 5). The crossover point from MEP suppression to facilitation appears to shift in either direction along the x-axis according to which priming stimulation was employed. Post hoc analysis revealed that QPS-5 ms priming over SMA significantly reduced MEP sizes after QPS-5 ms, QPS-10 ms and QPS-30 ms, but occluded MEP suppression by QPS-50 ms. QPS-50 ms priming over SMA inhibited MEP sizes after QPS-5 ms, whereas it facilitated MEP sizes after QPS-30 ms (Fig. 5).
Figure 5. Priming-induced shifts in the stimulus–response function.
The normalized amplitudes of MEP at 30 min post conditioning as a function of the reciprocal of ISI of QPS with and without priming over SMA (○). A, QPS-5 ms priming (▴). B, QPS-50 ms priming (▾). Note that the x-axis is logarithmic axis. *P < 0.05 by post hoc paired t tests.
Experiment 2: Control experiments
First, the after-effects of sham conditioning with real priming were monitored to examine whether priming alone (i.e. QPS-5 ms over SMA for 10 min) affects motor cortical excitability. Figure 6A shows the time course of the MEP amplitude following sham conditioning with real priming. No difference was found in MEP amplitudes at B0 and B1 (paired t test, P > 0.5). Sham conditioning with real priming did not change the MEP amplitude for at least 30 min after conditioning (one-way repeated measures ANOVA: effect of TIME, F(6,30)= 0.410, P= 0.866). Second, the after-effect of real conditioning (QPS-10ms) with sham priming was compared to that of real conditioning without priming to confirm that sham priming did not affect motor cortical plasticity induced by real conditioning. Figure 6B shows the absolute amplitude of MEPs following real conditioning (QPS-10ms) without priming and with sham priming. No difference was found between MEPs at B0 and B1 (paired t test, P > 0.5). Furthermore, MEP amplitudes following QPS-10 ms with sham priming were not different from those without priming (two-way repeated measures ANOVA: effect of CONDITION (QPS-10 ms with sham priming, QPS-10 ms without priming), F(1,5)= 0.095, P= 0.770; CONDITION × TIME interaction, F(6,30)= 0.410, P= 0.866).
Experiment 3: Effects of priming over SMA on intracortical circuits of M1
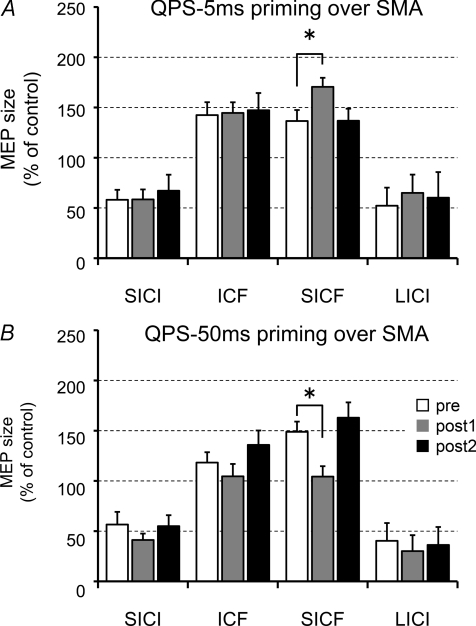
Figure 7 shows the effects of priming over SMA on SICI, ICF, SICF and LICI. Three-way repeated measures ANOVA revealed significant PRIMING × BISTIM × TIME interaction (F(6,48)= 2.498, P= 0.035). Post hoc tests revealed that only SICF was modulated after SMA priming; the effects on SICF were transient, being significant only at post 1 (Fig. 7A and B).
Figure 7. Effects of priming over SMA on intracortical circuits of M1.
A, SICF was enhanced after QPS-5 ms. SICI, ICF nor LICI were altered by QPS-5 ms priming over SMA alone. B, SICF was suppressed after QPS-50 ms over SMA, whereas others were not altered. Baseline (white bars); post 1 (grey bars), 0–6 min after QPS; post 2 (black bars), 20–26 min after QPS over SMA. *P < 0.05 by post hoc Dunnett's test.
Experiment 4: Supplementary experiments
Experiment 4a: Effects of a conditioning stimulus over the SMA on MEP
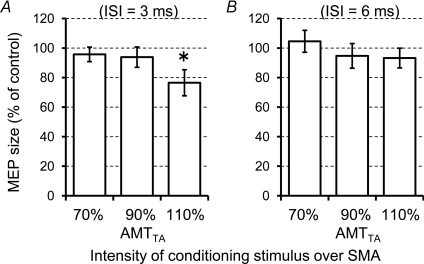
Using the paired-pulse technique at an ISI of 3 ms, we investigated whether the stimulus over SMA spreads to M1FDI. Figure 8A shows the changes of conditioned MEP relative to the unconditioned MEP in each block using three different conditioning intensities. One-way repeated measures ANOVA revealed a significant main effect of INTENSITY (F(2,14)= 3.904, P= 0.045). Post hoc paired t tests revealed that the test MEPs were significantly inhibited by conditioning stimulus at 110% AMTTA (t= 2.39, P= 0.048), whereas no significant inhibition was found at 70% or 90% AMTTA.
Figure 8. Effects of a conditioning stimulus over SMA on MEP.
The test MEPs evoked by the left M1FDI was conditioned by stimulation of SMA at an ISI of 3 ms (A) or at an ISI of 6 ms (B). Asterisks denote a significant change relative to unconditioned response.
Figure 8B shows the changes of conditioned MEP relative to the unconditioned MEP using the paired-pulse technique at an ISI of 6 ms. No significant effect of conditioning intensity over SMA at an ISI of 6 ms was found (one-way repeated measures ANOVA: effect of INTENSITY, F(2,14)= 0.679, P= 0.523).
Experiment 4b: effects of a stimulus over the SMA on MEP in active condition
To test whether the current induced by TMS over the SMA directly spreads to the M1FDI, single pulse TMS was applied over SMA during contraction of the right FDI muscle (20% maximum). Although the stimulus intensity reached 190% AMTTA or 100% MSO, no MEPs were recorded in any condition.
Discussion
We showed that LTD-like and LTP-like QPS-induced plasticity was altered by a preceding period of priming stimulation over SMA. QPS at short ISIs produced an increase in the MEP amplitude, whereas QPS at long ISIs suppressed MEPs (Experiment 1a, Fig. 2A). QPS-5 ms priming over SMA occluded MEP facilitation after QPS-5 ms and QPS-10 ms. Furthermore, it enhanced suppression of MEP after QPS-30 ms, but occluded MEP suppression by QPS-50 ms (Experiments 1b, Fig. 5). By contrast, QPS-50 ms priming over SMA inhibited MEP sizes after QPS-5 ms, whereas it facilitated MEP sizes after QPS-30 ms (Experiments 1c, Fig. 5). QPS-5 ms or QPS-50 ms priming over SMA did not change MEP sizes, SICI, ICF and LICI but altered SICF; QPS-5 ms priming enhanced SICF, whereas QPS-50 ms priming erased SICF (Experiment 3, Fig. 7). Finally, a single conditioning TMS over SMA at 110% AMTTA with an ISI of 3 ms significantly inhibited test MEP sizes, whereas no effect was found at a conditioning intensity lower than 110% AMTTA. In addition, no significant effects were found at any conditioning intensity using an ISI of 6 ms (Experiment 4, Fig. 8). We will argue that the present findings provide strong support for the hypothesis that priming over the SMA transiently altered the synaptic efficiencies of excitatory circuits within M1, and that such prior history of neuronal activity alters subsequent LTD-like and LTP-like QPS-induced plasticity through the metaplastic interplay between SMA and M1.
Effects of TMS over SMA
Given that there is now good evidence that TMS can stimulate SMA neurons (Civardi et al. 2001; Terao et al. 2001; Serrien et al. 2002; Verwey et al. 2002; Matsunaga et al. 2005; Hamada et al. 2008b), our results are compatible with the idea that rTMS can produce lasting changes in the excitability of these circuits. We argue that such mechanisms underpin the present findings, although we cannot be completely certain about the precise site of our SMA stimulus. According to previous studies, the optimal site of SMA stimulation has been shown to be between 2 and 4 cm anterior to Cz (Terao et al. 2001; Serrien et al. 2002; Verwey et al. 2002); neuroimaging methods also locate the hand area of the SMA proper some 2 to 3 cm anterior to Cz (Hikosaka et al. 1996; Lee et al. 1999). It is thereby conceivable that our TMS stimulus mainly activates SMA neurons.
A second question is whether currents induced by stimulation over SMA spread to other motor-related areas. The data suggest that this was unlikely with the intensities of stimulation that we used. Experiment 4a showed that a conditioning pulse over SMA at 110% AMTTA with an ISI of 3 ms significantly inhibited test MEP sizes, which corresponds to the timing of SICI (Kujirai et al. 1993), whereas no effect was found at a conditioning intensity of either 70% AMTTA or 90% AMTTA. These findings suggest that the current induced by placing the coil over SMA spreads to M1FDI only when the stimulus intensity is higher than 110% AMTTA in line with a preceding report (Matsunaga et al. 2005). In addition, no significant changes in the size of test responses were found at any conditioning intensity using an ISI of 6 ms, which corresponds to the optimal ISI to produce effects in the pathway from PMd to M1 (Civardi et al. 2001). Finally, Experiment 4b revealed that no MEPs were elicited during voluntary contraction with single pulse TMS over SMA at very high stimulus intensities up to 100% MSO, indicating no direct activation of excitatory interneurons or cortical output neurons within M1. We cannot completely exclude the possibility that various subliminally stimulated cortical areas including SMA, PMd, PMv, M1 and others regions were implicated in the effects of SMA priming. However, the present findings lead us to conjecture that the effects of priming over SMA can be mainly ascribed to stimulation of the cortex beneath the coil, namely SMA.
Effects of priming over SMA alone
We did not evaluate possible effects of sham priming on subsequent QPS-induced plasticity elicited by all kinds of QPS protocols used in the present paper. In Experiment 2, QPS-10 ms was chosen as a representative of all QPS protocols because it induced mild facilitatory after-effects rendering it more susceptible to possible effects of sham priming. In fact, two control experiments revealed that neither priming alone nor cutaneous sensation had any lasting effect on MEPs.
The results of Experiment 3 show that QPS-5 ms or QPS-50 ms priming over SMA did not change MEP sizes, SICI, ICF and LICI. Its only effect was a transient modulation of SICF, which did not persist as long as the priming effects on QPS. We cannot exclude the possibility that subtle changes in inhibitory circuits were missed because paired-pulse measurements addressing intracortical excitability were assessed only at a single ISI with a single conditioning intensity. Nonetheless, the long period for measurements using various ISIs with multiple conditioning intensities might in turn miss the effects of priming owing to transient effects of priming on SICF.
The present results raise the question why such selective modulation of SICF was produced by SMA priming. Although this selectivity seems puzzling, it is not a unique observation since we have shown that QPS priming over M1 produced transient modulation of SICF without affecting MEP sizes, SICI, ICF and LICI (Hamada et al. 2008a). It has been also reported that selective modulation of SICI can be produced by low-intensity theta burst stimulation (TBS) without any changes in MEP (McAllister et al. 2009). Also, 5 Hz rTMS over PMd reduced paired-pulse excitability at an ISI of 7 ms without any changes in MEP amplitude (Rizzo et al. 2004). Thus, it is possible to modulate inhibitory or excitatory circuits selectively, which is not accompanied by lasting changes in MEP sizes under certain specified conditions.
What is the mechanism behind the modulation of SICF by SMA priming? At first, an interaction of I-wave inputs is thought to be the cause of SICF presumably by the summation of excitatory postsynaptic potentials (EPSPs) elicited by the first suprathreshold TMS stimulus with subliminal depolarization of interneurons that is produced by the second subthreshold TMS in cortical interneurons (Hanajima et al. 2002). Thus, modulation of SICF by SMA priming might therefore be consistent with the idea that SMA priming alters synaptic efficiency at which I-waves summate during paired-pulse TMS.
Then why are such changes in excitatory circuits of M1 produced by SMA priming?Matsunaga et al. (2005) proposed that there are at least two possible explanations for modulation of motor cortical excitability produced by rTMS over SMA. One possibility is that rTMS over SMA stimulates mainly cortico-cortical projections from SMA to M1 (Dum & Strick, 1991; Tokuno & Nambu, 2000; Nachev et al. 2008). The other is that rTMS over SMA alters local balance of excitability within SMA, leading to alteration of activity of cortico-cortical projections from SMA to M1. They conclude that the latter might pertain to their findings since the intensity of stimulation was low (Matsunaga et al. 2005). The present study as with the previous study was not designed to test these hypotheses, thus we cannot comment on this issue further. Besides, it is also possible that functional modulation of basal ganglia via cortico-subcortical connections might contribute to the effects of priming over SMA (Inase et al. 1999; Kaneda et al. 2002; Akkal et al. 2007). Several studies also suggest that SMA proper sends direct projections to the spinal cord (Dum & Strick, 1991, 1996). Thus, SMA priming could produce some excitability changes in spinal motor neurons. However, it was impossible to evoke any MEPs during voluntary contraction of hand muscle using TMS over SMA at any intensities from 100% AMTTA to 190% AMTTA (i.e. 100% MSO), indicating that a higher intensity (above 100% MSO) is needed to stimulate neurons in SMA that project directly to the spinal cord. We cannot exclude the fact that repetitive stimulation of SMA with QPS, even at low intensities, might produce subthreshold temporal facilitation at spinal interneurons. In any case, we showed that QPS over SMA for 10 min produced bidirectional changes in SICF. Based on the fact that lasting motor cortical plasticity was induced by QPS over M1 for 30 min (Hamada et al. 2008a), the present result raises a new question as to whether QPS over SMA for 30 min produces plastic changes in bilateral M1. The issue should be addressed in future studies.
QPS-induced plasticity and synaptic plasticity
QPS is a newly developed protocol for inducing bidirectional (i.e. either facilitatory or inhibitory) motor cortical plasticity in humans (Hamada et al. 2007, 2008a). QPS at short intervals facilitated MEPs for more than 75 min, whereas QPS at long intervals suppressed MEPs for more than 75 min. The QPS-induced plasticity appears to be rather synapse-specific, as suggested by the following in the previous study (Hamada et al. 2008a); motor thresholds, which are dependent on ion channel conductivity and might reflect membrane excitability (Mavroudakis et al. 1994, 1997; Ziemann et al. 1996; Chen et al. 1997), were unchanged after QPS; SICI, which is considered to reflect γ-aminobutyric acid (GABA)-ergic inhibitory function of M1 (Kujirai et al. 1993), remained unchanged; SICF and ICF were enhanced by QPS-5 ms whereas reduced by QPS-50 ms (Hamada et al. 2008a). Based on the hypothesis of the mechanism of SICF (Hanajima et al. 2002), it is possible that QPS changes the quantity of EPSPs in excitatory circuits responsible for SICF (Hamada et al. 2008a). These results led us to surmise that the mechanism of QPS-induced plasticity involves synaptic plasticity in the excitatory circuits of M1 with features of non-linear dependence on the ISI of QPS, suggesting the presence of threshold for LTP- and LTD-like plasticity induction in line with previous findings of synaptic plasticity (Dudek & Bear, 1992).
Possible mechanisms of effects of priming over SMA on QPS-induced plasticity
The present results suggest that SMA priming influenced subsequent induction of synaptic plasticity in the excitatory circuits of M1. Priming over SMA elicited bidirectional shifts of the crossover point of the stimulus–response function of motor cortical plasticity (Fig. 5). The priming effects depended on the precise parameters of priming stimulation which specifically and transiently altered SICF without significant changes in MEP. We note that at least three possible mechanisms might account for the present findings: metaplasticity (Abraham & Bear, 1996), a gating mechanism (Ziemann & Siebner, 2008) and state-dependent effects (Fujiwara & Rothwell, 2004; Huang et al. 2008).
SMA priming-induced metaplasticity
First, it might be possible to interpret these findings within the framework of metaplasticity, or the plasticity of synaptic plasticity, in which neural activity at one point in time can change cells or synapses such that their ability to exhibit LTP or LTD after a later bout of activity is altered (Abraham, 2008). Metaplasticity is best documented by showing that an experimental manipulation which itself causes no persistent synaptic plasticity can nevertheless produce a shift in the crossover point of the frequency–response function of synaptic plasticity induced by a period of conditioning stimulation (Abraham, 2008). More specifically, if a manipulation reduces LTP then this should reflect elevation of the induction threshold for LTP; thus, the frequency of the conditioning stimulation required to produce LTP becomes higher, compatible with a shift in the crossover point of LTP and LTD in the conditioning frequency–response curve (Wang & Wagner, 1999; Zhang et al. 2005).
In this context, QPS-5 ms priming over SMA transiently enhances SICF, and this prior history of cortical activity elevated the threshold for inducing LTP-like plasticity. This is confirmed by the observation that QPS-5 ms priming over SMA did not occlude LTP-like plasticity by QPS-1.5 ms, whereas the same priming interfered with LTP-like plasticity induced by QPS-5 ms and QPS-10 ms, which produced LTD-like plasticity instead. These results indicate elevation of the threshold of LTP-like plasticity induction. In fact, QPS-5 ms priming over SMA shifted the crossover point of the stimulus–response function to the right along the x-axis (Fig. 5).
Theoretically, enhancement of prior neuronal activity should promote LTD (Bienenstock et al. 1982). This is confirmed by the fact that QPS-30 ms with QPS-5 ms priming produced a lasting decrease of MEP sizes as compared to QPS-30 ms without priming (Fig. 3D). However, LTD-like plasticity induced by QPS-50 ms and QPS-100 ms were not enhanced although the duration of suppression was shortened by QPS-5 ms priming over SMA. The disparity may be partly explained by previous studies of metaplasticity which revealed that the balance of NMDA receptor subunits (i.e. NR2A and NR2B) is one important factor in modulating the threshold for synaptic plasticity (Philpot et al. 2003, 2007). Some forms of metaplasticity are NMDA receptor dependent (Huang et al. 1992; Christie & Abraham, 1992; Abraham & Huggett, 1997; Zhang et al. 2005), and the ratio of NR2A/2B, which is increased by prior cortical activity, elevates the thresholds for both LTP and LTD induction (Yashiro & Philpot, 2008). Perhaps a similar mechanism is implicated in our present observation that QPS-5 ms priming over SMA elevates the thresholds for both LTP- and LTD-like plasticity.
In contrast, after QPS-50 ms priming over SMA, which transiently reduced SICF, LTP-like plasticity was induced by QPS-30 ms, which had produced transient MEP suppression when given alone. Indeed our results are compatible with the view that metaplastic changes can best be recognized by using a near-threshold stimulation regimen (i.e. near the crossover point) (Christie et al. 1995) since QPS-50 ms priming over SMA did not interfere with LTP- or LTD-like plasticity induced by QPS-10 ms or QPS-100 ms (Fig. 5). One result is apparently at odds with this explanation though; QPS-50 ms priming over SMA erased LTP-like plasticity usually seen after the QPS-5 ms protocol. According to the BCM rule, LTP-like plasticity should be enhanced after QPS-50 ms priming since it transiently reduced cortical activity. One possible explanation comes from animal studies of metaplasticity which suggest an inverted U-shaped relation between the amount of TBS and the degree of LTP (Christie et al. 1995; Abraham & Huggett, 1997). Such time-dependent LTP reversal process or overstimulation effect has been believed to be a normal feature of LTP induction, probably caused by a depotentiation mechanism during the massed presentation of tetanic stimulation (Christie et al. 1995; Abraham & Huggett, 1997). These authors found that only two trains of TBS were required to induce LTP but eight trains of TBS, which induce LTP on its own, were unable to induce LTP with low-frequency priming stimulation. This blocking of LTP has been suggested to reflect the overstimulation effect (Christie et al. 1995). We suggest that similarly, the present results might reflect comparable overstimulation effects with priming stimulation.
One conspicuous point of our results is that the SICF was modulated transiently by the priming stimulation alone. Thus, the effects of SMA priming on SICF could have returned to baseline by the time QPS was delivered following the collection of 20 MEPs at B1. This is consistent with our previous study showing transient modulation of SICF with priming over M1 (Hamada et al. 2008a). We favour the view that this transient modulation of SICF reflects prior history of cortical activity and that such prior activity substantially influences subsequent QPS-induced plasticity in a metaplastic manner (Hamada et al. 2008a). However, it is still possible that such transient modulation of SICF has a weak association with the priming effects because the time course of the influence on SICF did not parallel that of the priming effect.
Previous human studies demonstrated the effects of priming stimulation on subsequent rTMS-induced plasticity, indicating metaplasticity (Iyer et al. 2003; Lang et al. 2004; Siebner et al. 2004; Müller et al. 2007; Ragert et al. 2009). We have also shown metaplastic changes of QPS-induced plasticity by priming stimulation (Hamada et al. 2008a). In the previous reports, both priming and conditioning were applied over M1 using exactly the same stimulus intensity, suggesting that they modulated the same synaptic connections (Hamada et al. 2008a). According to animal experiments showing homosynaptic and heterosynaptic metaplasticity (Wang & Wagner, 1999; Abraham et al. 2001), our previous results indicate that the homeostatic changes were mediated via homosynaptic mechanism of metaplasticity. In contrast to the previous studies, in the present work priming was applied over SMA, leading to bidirectional modulation of SICF, whereas the subsequent conditioning was applied over M1. It is reasonable to believe that the synapses in M1 that are altered by the SMA priming are those involved in a SMA–M1 projection, and that these are likely to be partially different from those modulated by conditioning over M1. This raises the intriguing possibility that the effects of priming can be ascribed to a mixture of homosynaptic and heterosynaptic metaplasticity. Another implication from this paper is that SMA priming would have metaplastic effects on subsequent rTMS-induced plasticity of M1 which can be produced by any rTMS protocols that are capable of inducing LTP- and LTD-like changes.
Gating mechanism and state-dependent effect
Although we favour a metaplasticity theory to account for the present findings, other mechanisms might be involved. One possibility is a gating mechanism (Ziemann & Siebner, 2008); if QPS-5 ms priming had increased intracortical inhibition, then it might have rendered subsequent QPS conditioning less effective in exciting cortical output neurons trans-synaptically. In such a case, each burst of QPS would have produced a smaller amount of calcium influx into the neurons, resulting in induction of LTD-like plasticity. However, neither SICI nor LICI was altered by priming over SMA, thus leading to the tentative conclusion that it is much less likely that the priming effects arise as a consequence of the alteration of intracortical inhibitory circuits.
Another possible explanation is related to several human studies in which voluntary contraction influences rTMS-induced plasticity (Fujiwara & Rothwell, 2004; Gentner et al. 2008; Huang et al. 2008). The after-effects of rTMS depend on the state of the cortex at the time the stimulation is applied. Thus, the effect of 5 Hz rTMS on SICI was reversed by muscle contraction during rTMS (Fujiwara & Rothwell, 2004). More recently, voluntary contraction during TBS abolished TBS-induced plasticity (Huang et al. 2008). Huang et al. (2008) proposed two possibilities to account for such state-dependent effects. First, the voluntary contraction perhaps changes the membrane potential or Ca2+ concentration of pyramidal neurons, directly affecting TBS-induced plasticity. ‘Busy line’ effects are another possible explanation; given that synapses stimulated by TBS are the same as those activated by voluntary contraction, extra activation of those synapses by TBS is negligible (Huang et al. 2008). Yet another study showed that voluntary contraction of sufficient duration changes the direction of TBS-induced plasticity; the authors contrastingly interpret their findings within the framework of metaplasticity theory (Gentner et al. 2008). Perhaps it is still an open question regarding the mechanism of effects of voluntary contraction on TBS-induced plasticity. In any case, SMA priming which produced changes in SICF might be a sign of some sort of baseline difference in the state of motor cortex independent of metaplasticity, resulting in alteration of subsequent QPS-induced plasticity.
SMA–M1 interplay and metaplasticity
Experimental observations of metaplasticity are considered to represent a major form of homeostatic mechanism of synaptic plasticity that prevents neuronal circuits from becoming destabilized and that maintains them within a dynamic range of modifiability (Abbott & Nelson, 2000; Abraham, 2008). Our findings might therefore highlight a homeostatic (or metaplastic) regulation of synaptic plasticity within excitatory circuits of M1 by input from SMA. Since SMA is implicated in higher motor control and the learning process (Luppino et al. 1993; Tanji & Shima, 1994; Tanji, 1996; Hikosaka et al. 1999; Nachev et al. 2008), the present results further raise the intriguing possibility that a preceding period of learning which entails a change in neuronal activity of SMA may regulate subsequent learning that is handled by neuronal circuits of M1 (Hikosaka et al. 1999; Sane & Donoghue, 2000). Since this study was not designed to test this hypothesis, future studies would be needed to shed light on possible metaplastic interplay between SMA and M1 during motor learning.
Finally, the shortcoming of the present study is that the lack of direct recording of synaptic responses in conscious humans renders any hypothesis about the precise neuronal mechanisms underlying QPS-induced plasticity or metaplasticity speculative (Cooke & Bliss, 2006). However, although the interpretation of the present data is inferential, the present study does suggest strongly that there may be important interactions between M1 and SMA in terms of metaplasticity.
Conclusions
Preceding stimulation over SMA elicited bidirectional shifts of the crossover point of the stimulus–response function of subsequent motor cortical plasticity. SMA priming transiently altered the synaptic efficiencies of excitatory circuits within M1. The data support the view that the homeostatic changes are mediated via mechanisms of metaplasticity. These findings highlight an important interplay between M1 and SMA regarding metaplasticity which might underpin learning and memory processes.
Acknowledgments
M.H. is a research fellow of the Japan Society for the Promotion of Science, Tokyo, Japan. We are grateful to Professor John Rothwell for his constructive comments and English editing. We also thank Mr Mikio Asano and Mr Tsukasa Kajiwara for technical assistance. Part of this work was supported by Research Project Grants-in-aid for Scientific Research No. 18590928 (Y.T.) and No. 17590865 (R.H.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Research Committee on rTMS Treatment of Movement Disorders from the Ministry of Health, Labour and Welfare of Japan (17231401), the Research Committee on Dystonia, the Ministry of Health, Labour and Welfare of Japan, the Committee of the Study of Human Exposure to Electromagnetic Fields, Ministry of Internal Affairs and Communications, the Life Science Foundation of Japan, and the Magnetic Health Science Foundation.
Glossary
Abbreviations
- CS
conditioning stimulus
- FDI
first dorsal interosseous muscle
- ICF
intracortical facilitation
- ISI
inter-stimulus intervals
- ITT
inter-train interval
- LICI
long-interval intracortical inhibition
- LTD
long-term depression
- LTP
long-term potentiation
- M1
primary motor cortex
- MEP
motor-evoked potential
- PMd and PMv
dorsal and ventral premotor cortex
- QPS
quadripulse stimulation
- rTMS
repetitive transcranial magnetic stimulation
- SICF
short-interval intracortical facilitation
- SICI
short-interval intracortical inhibition
- SMA
supplementary motor area
- TA
right tibialis anterior muscle
- TS
test stimulus
Author contributions
M.H. and Y.U. contributed to the conception and design of the experiments. M.H. performed the experiments and analysed the data. All authors participated in data interpretation. M.H., Y.T. and Y.U. wrote the manuscript. All authors revised the manuscript and approved the final manuscript. The experiments were performed at Department of Neurology, the University of Tokyo, Japan.
References
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387–399. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Huggett A. Induction and reversal of long-term potentiation by repeated high-frequency stimulation in rat hippocampal slices. Hippocampus. 1997;7:137–145. doi: 10.1002/(SICI)1098-1063(1997)7:2<137::AID-HIPO3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Mason-Parker SE, Bear MF, Webb S, Tate WP. Heterosynaptic metaplasticity in the hippocampus in vivo: a BCM-like modifiable threshold for LTP. Proc Natl Acad Sci U S A. 2001;98:10924–10929. doi: 10.1073/pnas.181342098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: Orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Samii A, Canos M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology. 1997;49:881–883. doi: 10.1212/wnl.49.3.881. [DOI] [PubMed] [Google Scholar]
- Christie BR, Abraham WC. Priming of associative long-term depression by θ frequency synaptic activity. Neuron. 1992;9:79–84. doi: 10.1016/0896-6273(92)90222-y. [DOI] [PubMed] [Google Scholar]
- Christie BR, Stellwagen D, Abraham WC. Reduction of the threshold for long-term potentiation by prior theta-frequency synaptic activity. Hippocampus. 1995;5:52–59. doi: 10.1002/hipo.450050107. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss VP. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and the effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6531–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Rothwell JC. The after effects of motor cortex rTMS depend on the state of contraction when rTMS is applied. Clin Neurophysiol. 2004;115:1514–1518. doi: 10.1016/j.clinph.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008;18:2046–2053. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: A primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hamada M, Hanajima R, Terao Y, Arai N, Furubayashi T, Inomata-Terada S, et al. Quadro-pulse stimulation is more effective than paired-pulse stimulation for plasticity induction of the human motor cortex. Clin Neurophysiol. 2007;118:2672–2682. doi: 10.1016/j.clinph.2007.09.062. [DOI] [PubMed] [Google Scholar]
- Hamada M, Terao Y, Hanajima R, Shirota Y, Nakatani-Enomoto S, Furubayashi T, Matsumoto H, Ugawa Y. Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J Physiol. 2008a;586:3927–3947. doi: 10.1113/jphysiol.2008.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Ugawa Y, Tsuji S, Effectiveness of rTMS on Parkinson's Disease Study Group, Japan High-frequency rTMS over the supplementary motor area for treatment of Parkinson's disease. Mov Disord. 2008b;23:1524–1531. doi: 10.1002/mds.22168. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, et al. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. J Physiol. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning sequential procedures. Trends Neurosci. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Putz B. Activation of human presupplementary motor area in learning of sequential procedures: a functional MRI study. J Neurophysiol. 1996;76:617–621. doi: 10.1152/jn.1996.76.1.617. [DOI] [PubMed] [Google Scholar]
- Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Chen RS, Rothwell JC, Wen HY. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.clinph.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Inase M, Tokuno H, Nambu A, Akazawa T, Takada M. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain Res. 1999;833:191–201. doi: 10.1016/s0006-8993(99)01531-0. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:10867–10872. doi: 10.1523/JNEUROSCI.23-34-10867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda K, Nambu A, Tokuno H, Takada M. Differential processing patterns of motor information via striatopallidal and striatonigral projections. J Neurophysiol. 2002;88:1420–1432. doi: 10.1152/jn.2002.88.3.1420. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Woe O, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004;56:634–639. doi: 10.1016/j.biopsych.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lee KM, Chang KH, Roh JK. Subregions within the supplementary motor area activated at different stages of movement preparation and execution. Neuroimage. 1999;9:117–123. doi: 10.1006/nimg.1998.0393. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- McAllister SMA, Rothwell JC, Ridding MC. Selective modulation of intracortical inhibition by low-intensity theta burst stimulation. Clin Neurophysiol. 2009;120:820–826. doi: 10.1016/j.clinph.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Maruyama A, Fujiwara T, Nakanishi R, Tsuji S, Rothwell JC. Increased corticospinal excitability after 5 Hz rTMS over the supplementary motor area. J Physiol. 2005;562:295–306. doi: 10.1113/jphysiol.2004.070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Effects of diphenylhydantoin on motor potentials evoked with magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1994;93:428–433. doi: 10.1016/0168-5597(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Effects of vigabatrin on motor potentials with magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:124–127. doi: 10.1016/s0924-980x(96)96607-2. [DOI] [PubMed] [Google Scholar]
- Müller JF, Orekhov Y, Liu Y, Ziemann U. Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation. Eur J Neurosci. 2007;25:3461–3468. doi: 10.1111/j.1460-9568.2007.05603.x. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Okabe S, Ugawa Y, Kanazawa I, Effectiveness of rTMS on Parkinson's Disease Study Group 0.2-Hz repetitive transcranial magnetic stimulation has no add-on effects as compared to a realistic sham stimulation in Parkinson's disease. Mov Disord. 2003;18:382–388. doi: 10.1002/mds.10370. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Cho KK, Bear MF. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis of metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Camus M, Dimyan MA, Vandermeeren Y, Cohen LG. Modulation of iTBS effects applied over primary motor cortex (M1) by conditioning stimulation of the opposite M1. J Neurophysiol. 2009;102:766–773. doi: 10.1152/jn.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo V, Siebner HR, Modugno N, Pesenti A, Munchau A, Gerschlager W, Webb RM, Rothwell JC. Shaping the excitability of the human motor cortex with premotor rTMS. J Physiol. 2004;554:483–495. doi: 10.1113/jphysiol.2003.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application.Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Sane JN, Donoghue Plasticity and primary motor cortex. Ann Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Strens LH, Oliviero A, Brown P. Repetitive transcranial magnetic stimulation of the supplementary motor area (SMA) degrades bimanual movement control in humans. Neurosci Lett. 2002;328:89–92. doi: 10.1016/s0304-3940(02)00499-8. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: Evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J. New concepts of the supplementary motor area. Curr Opin Neurobiol. 1996;6:782–787. doi: 10.1016/s0959-4388(96)80028-6. [DOI] [PubMed] [Google Scholar]
- Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Enomoto H, Furubayashi T, Shiio Y, Machii K, et al. Hemispheric lateralization in the cortical motor preparation for human vocalization. J Neurosci. 2001;21:1600–1609. doi: 10.1523/JNEUROSCI.21-05-01600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Nambu A. Organization of nonprimary motor cortical inputs on pyramidal and nonpyramidal tract neurons of primary motor cortex: An electrophysiological study in the macaque monkey. Cereb Cortex. 2000;10:58–68. doi: 10.1093/cercor/10.1.58. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Verwey W, Lammens R, van Honk J. On the role of the SMA in the discrete sequence production task: a TMS study. Neuropsychologia. 2002;40:1268–1276. doi: 10.1016/s0028-3932(01)00221-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Wagner JJ. Priming-induced shift in synaptic plasticity in the rat hippocampus. J Neurophysiol. 1999;82:2024–2028. doi: 10.1152/jn.1999.82.4.2024. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacol. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kirschstein T, Sommerberg B, Merkens M, Manahan-Vaughan D, Elpersma Y, Beck H. Hippocampal synaptic metaplasticity requires inhibitory autophosphorylation of Ca2+/calmodulin-dependent kinase II. J Neurosci. 2005;25:7697–7707. doi: 10.1523/JNEUROSCI.2086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Iliac TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci. 2004;24:1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Siebner HR. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stim. 2008;1:60–66.. doi: 10.1016/j.brs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I-wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]