Abstract
Interstitial cells of Cajal (ICC) generate pacemaker activity (slow waves) in gastrointestinal (GI) smooth muscles, but the mechanism(s) of pacemaker activity are controversial. Several conductances, such as Ca2+-activated Cl− channels (CaCC) and non-selective cation channels (NSCC) have been suggested to be involved in slow wave depolarization. We investigated the expression and function of a new class of CaCC, anoctamin 1 (ANO1), encoded by Tmem16a, which was discovered to be highly expressed in ICC in a microarray screen. GI muscles express splice variants of the Tmem16a transcript in addition to other paralogues of the Tmem16a family. ANO1 protein is expressed abundantly and specifically in ICC in all regions of the murine, non-human primate (Macaca fascicularis) and human GI tracts. CaCC blocking drugs, niflumic acid and 4,4′-diisothiocyano-2,2′-stillbene-disulfonic acid (DIDS) reduced the frequency and blocked slow waves in murine, primate, human small intestine and stomach in a concentration-dependent manner. Unitary potentials, small stochastic membrane depolarizations thought to underlie slow waves, were insensitive to CaCC blockers. Slow waves failed to develop by birth in mice homozygous for a null allele of Tmem16a (Tmem16atm1Bdh/tm1Bdh) and did not develop subsequent to birth in organ culture, as in wildtype and heterozygous muscles. Loss of function of ANO1 did not inhibit the development of ICC networks that appeared structurally normal as indicated by Kit antibodies. These data demonstrate the fundamental role of ANO1 in the generation of slow waves in GI ICC.
Specialized cells, referred to as interstitial cells of Cajal (ICC), generate and actively propagate the spontaneous electrical activity known as slow waves in the gastrointestinal (GI) tract (Ward et al. 1994, Huizinga et al. 1995; Sanders, 1996). Slow waves time the phasic contractions of GI muscles and provide the underlying organization of excitability for gastric peristalsis and intestinal segmentation. ICC are also interposed between nerve terminals and smooth muscle cells and serve as sites of post-junctional transduction of responses to enteric motor neurotransmitters (see Burns et al. 1996; Ward et al. 2000a). There are at least two classes of ICC, those intermingled within muscle bundles of the circular (CM) and longitudinal muscle (LM) layers (ICC-IM), and those arranged in a dense network between the CM and LM (ICC-MY), at the submucosal surface of the CM (ICC-SM) and in the septa between bundles of smooth muscle cells (ICC-SEP). There have been many studies regarding the mechanisms of pacemaker activity and slow wave propagation, but the precise mechanisms of this behaviour remain controversial.
Studies of pacemaker activity in intact muscle strips and bundles have suggested the involvement of a Ca2+-dependent inward current because activity was reduced when the muscles were treated with membrane-permeable Ca2+ buffers (Edwards et al. 1999; Hirst et al. 2002; Kito & Suzuki, 2003). The Ca2+-dependent conductance has been thought to be a Cl− conductance, since a variety of Cl− channel blocking drugs reduced pacemaker activity in guinea-pig and murine muscles (see Hirst et al. 2002; Kito et al. 2002a; Kito & Suzuki, 2003). Similar conclusions were reached from studies of slow waves recorded directly from ICC-MY of the small intestine (e.g. Kito & Suzuki, 2003). In contrast, studies of isolated and cultured ICC have suggested that spontaneous activity is generated by activation of a non-selective cation conductance (Ward et al. 2000; Koh et al. 2002; Sanders et al. 2006), and the putative conductance was found to be inhibited by Ca2+ (Koh et al. 2002). Thus, pacemaker current may be initiated by a transient reduction in [Ca2+]i in a sub-compartment under the plasma membrane containing the non-selective cation conductance (Sanders et al. 2006). No Ca2+-activated inward currents were observed in cultured ICC, and the non-selective cation channels activated by reduced Ca2+ were inhibited by niflumic acid (Koh et al. 2002). Thus, use of Cl− channel antagonists does not necessarily indicate a role for Ca2+- activated Cl− channels in pacemaker activity.
A microarray genetic screen recently revealed that Tmem16a is expressed at far greater levels in ICC than in the rest of the muscularis (Chen et al. 2007). Tmem16a encodes ANO1, a Ca2+-activated Cl− channel (Caputo et al. 2008; Schroeder et al. 2008; Yang et al. 2008), and immunohistochemical studies have documented expression of ANO1 (also known as DOG1) protein by ICC (Espinosa et al. 2008; Gomez-Pinilla et al. 2009). Taken together these data suggest the hypothesis that expression and function of these channels may be important in pacemaker activity in the GI tract. Therefore, we have characterized expression of Tmem16a transcripts and ANO1 protein in the tunica muscularis of mouse, monkey (Macaca fascicularis) and human GI tracts using RT-PCR, amplicon sequencing and immunohistochemical techniques. We also evaluated the electrical activity of murine gastric and small intestine muscles, and tested the effects of Cl− channel-blocking drugs. Finally, we tested whether slow wave activity is affected in mice homozygous with null Tmem16a alleles (Tmem16atm1Bdh/tm1Bdh, see Rock et al. 2008). Our data show ubiquitous expression of ANO1 in ICC throughout the GI tract and inhibitory effects of Cl− channel blocking drugs on slow waves. Tmem16a−/− animals failed to generate slow waves, and pacemaker activity did not develop in organ culture after birth, as occurs in wildtype muscles. Together with voltage-clamp studies of isolated ICC (Zhu et al. 2009), our findings strongly support a role for ANO1 in the generation of slow wave currents of GI ICC and electrical slow waves in intact muscles. The model of pacemaker activity deduced from previous studies of cultured ICC (e.g. as detailed in Sanders et al. 2006) will require reconsideration in light of these new findings.
Methods
Mouse, monkey and human tissues
The gastric antrums and small intestines, obtained from C57BL/6 and W/WV mice (30–60 days old; Jackson Laboratory, Bar Harbor, MN, USA) and neonatal Tmem16a−/− (Tmem16atm1Bdh/tm1Bdh), Tmem16a+/+ or Tmem16a+/− (Tmem16a+/tm1Bdh) mice (generated by B. D. Harfe, University of Florida, Gainsville, USA; see Rock et al. 2008 for details on the production of these animals), were dissected after animals were exsanguinated following sedation with isoflurane and cervical dislocation. Tissues were placed in oxygenated cold (4°C) Krebs–Ringer buffer (KRB) for further preparation. Gastric antrum and intestinal tissues were also collected from six cynomolgus monkeys (Macaca fascicularis; Charles River Laboratories, Preclinical Services, Sparks, NV, USA) of either sex. Monkeys were initially sedated with Ketamine (10 mg kg−1), then administered 0.7 ml Beuthanasia-D solution (Schering-Plough AH, Kenilworth, NJ, USA) (pentobarbital sodium and phenytoin sodium) followed by exsanguination. Human small intestinal tissues were obtained as surgical waste from six patients undergoing gastric bypass surgery for morbid obesity at the University of California Davis. Protocols were approved by the Human Subjects Research Committees at the University of California, Davis and the University of Nevada, Reno Human Subjects Research Committees.
Animals used for these studies were maintained and the experiments performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Use and Care Committee at the University of Nevada approved all procedures used.
RT-PCR
Total RNA was isolated from gastric antrum and intestinal tunica muscularis of C57BL/6 mice and cynomolgus monkey using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The RNA was treated with 1 U μl−1 DNase I (Promega Corp., Madison, WI, USA) and cDNA was prepared using Oligo dT(12-18) primer and SuperScript II reverse transcriptase (Invitrogen). PCR was performed with specific primers for each murine and monkey Tmem16 paralogue using AmpliTaq Gold PCR mix (Applied Biosystems, Foster City, CA, USA). The following GenBank accession numbers for each murine and monkey Tmem16 paralogue were used to design specific PCR primers: Tmem16a (mouse NM_178642; monkey XR_012484); Tmem16b (mouse NM_153589; monkey XM_001118212), Tmem16c (mouse NM_001081556; monkey XM_001091004); Tmem16d (mouse NM_178773; monkey XM_001090523); Tmem16e (mouse NM_177694, 167 bp; monkey XR_011243); Tmem16f (mouse NM_175344; monkey XM_001092876); Tmem16g (mouse NM_207031; monkey XR_011041); Tmem16h (mouse XM_889480; human NM_020959); Tmem16j (mouse NM_178381; monkey DV_769801); and Tmem16k (mouse NM_133979; monkey XM_001115031). All primers were designed to span intronic sequences to eliminate amplification of contaminating genomic DNA in the source RNA. For the analysis of Tmem16a transcript variants PCR primers were designed to amplify across alternative exons that were previously reported for the human TMEM16A transcript (Caputo et al. 2008). Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH, accession no. NM_008084, amplicon 170 bp) was used as a control for cDNA integrity. No-template PCR reactions served as controls for primer contamination. PCR fragments were sequenced at the Nevada Genomics Centre.
Genotype analysis of Tmem16atm1Bdh/tm1Bdh mice
Tmem16atm1Bdh/tm1Bdh mice were generated by replacing exon 12 of Tmem16a with a phosphoglycerate kinase-neomycin cassette by homologous recombination in embryonic stem cells (Rock et al. 2008). Genomic DNA was isolated from transgenic mice tails using standard procedures. DNA (0.5 μl) was amplified in each PCR reaction to determine the genotypes of the transgenic mice. A 393 bp PCR fragment was amplified from the Tmem16a+/+ allele with primers that bind within and spanning exon 12. The Tmem16a−/− allele (350 bp) was amplified with primers that bind to the PGK–neomycin cassette.
Immunohistochemical studies
To examine the cellular localization of ANO1/TMEM16A within the gastric antrum and intestines of mouse, monkey and human tunica muscularis, immunohistochemical experiments were performed on whole mount preparations and cryostat sections. Single and double labelling immunohistochemical experiments were performed with antibodies raised against ANO1 and Kit to determine whether ANO1 was localized to specific populations of ICC which express Kit (Burns et al. 1997). Three antibodies against ANO1 were used: rabbit monoclonal SP31 (1 : 1000; Abcam, Cambridge, MA, USA), chicken polyclonal ab16293 (1 : 500; Abcam) and a rabbit polyclonal (1 : 1000; made for B. Harfe by Open Biosystems, Huntsville, AL, USA). All three antibodies labelled cells with a similar structure. For immunohistochemical studies on whole mount preparations, tissues were pinned to the Sylgard elastomer (Dow Corning Corp., Midland, MI, USA) base of a dissecting dish containing fresh KRB with the circular muscle layer facing upward, and the mucosa was removed by sharp dissection. The remaining strips of tunica muscularis were stretched to 110% of the resting length and width before being immersed in fixative solution. Tissues were fixed in acetone (4°C; 10 min for mouse tissues and 30 min for monkey and human tissues). Following fixation, tissues were washed overnight in phosphate-buffered saline (PBS; 0.01 m, pH 7.2) and rewashed with fresh PBS the following day for 4 h with a change of PBS every hour. Tissues were subsequently incubated in bovine serum albumin (BSA; 1%; 1 h at room temperature) to reduce non-specific antibody binding. For whole mount preparations, some acetone-fixed tissues were incubated singularly or sequentially in a combination of primary antibodies. The first incubation was carried out for 48 h at 4°C. The tissues were subsequently washed in PBS before being incubated in the second primary antibody for an additional 48 h at 4°C. The combination of antibodies used was a goat monoclonal antibody (hSCF-R; diluted 1 : 500 in 0.5% Triton-X 100; R&D Systems Inc., Minneapolis, MN, USA) to identify Kit-positive cells and a rabbit monoclonal antibody (SP31; 1 : 1000 in 0.5% Triton-X 100) to identify ANO1-postive cells. Following incubation in primary antibodies, immunoreactivity was detected via sequential incubation in Alexa fluor 488-coupled goat anti-rabbit and Alexa fluor 594-coupled donkey anti-goat secondary antibodies (Molecular Probes, Eugene, OR, USA; 1 : 500 in PBS; 1 h, room temperature). Control tissues were prepared by omitting either primary or secondary antibodies from the incubation solution.
For cryostat sections, tissues were fixed in acetone as described above prior to being dehydrated in graded sucrose solutions (5–20% w/v in PBS, 15 min each for 5–15% and overnight in 20%), embedded in a 50% mixture of 20% sucrose in PBS and Tissue Tek (Miles, IL, USA), and rapidly frozen in liquid nitrogen. Cryostat sections (10 μm) were cut using a Leica CM3050 cryostat and collected on Vectabond-coated (Vector Laboratories, Burlingame, CA, USA) dry glass slides. Tissues sections were allowed to dry at room temperature for at least 1 h before being washed with PBS and then incubated in BSA (room temperature, 1 h). Sections were then sequentially incubated in primary (4°C, overnight) and secondary antibodies (room temperature, 1 h) in a manner similar to that described for whole mounts. The SP31 antibody was diluted 1 : 1600 in 0.5% Triton-X for labelling of cryostat sections.
As monkey antrum tissues were too thick to be examined as whole mounts, they were fixed, dehydrated and frozen as described above for cryostat sections. Thick (100 μm), flat sections were then cut throughout the entire muscle layer using a Leica CM3050 cryostat. Each thick tissue section was placed in an individual well of a 24-well plate containing PBS. These sections were then processed in a manner similar to that described for whole mount tissues.
After incubation in secondary antibodies, tissues and cryostat sections were washed with PBS (overnight at 4°C) and mounted on glass slides using Aqua-Mount (Lerner Laboratories, Pittsburgh, PA, USA). Tissues and sections were examined with a Zeiss LSM 510 Meta confocal microscope (Zeiss, Germany) with an excitation wavelength appropriate for Alexa fluor 488 and Alexa fluor 594. Confocal micrographs are digital composites of Z-series scans of 10–100 optical sections through a depth of 4–100 μm. Final images were constructed using Zeiss LSM 5 Image Examiner software and converted to Tiff files for final processing in Adobe Photoshop 7.0 (Adobe Co., Mountain View, CA, USA) and Corel Draw X3 (Corel Corp., Ontario, Canada).
Image analysis
To compare the percentage area occupied by Kit-like immunoreactivity (Kit-LI) ICC-MY in neonatal Tmem16a−/− (Tmem16atm1Bdh/tm1Bdh), Tmem16a+/+ or Tmem16a+/− (Tmem16a+/tm1Bdh) mice, confocal Z-series were captured using a 63× objective and 0.25 μm steps. The confocal aperture was set at 1 Airy unit and laser power and gain settings were kept the same between specimens. Stacks of images were deconvolved (AutoQuantX, MediaCybernetics, MD, USA) then imported into custom software (Volumetry G6b, GWH). Since ICC-MY are in a discrete layer in postnatal day 0 (P0) animals, the density of Kit-immunopositive cells was calculated from a maximum intensity projection from each Z-series. The surface area of ICC-MY was expressed as a percentage of the field of view. A frequency–intensity histogram was calculated from the maximum intensity projection of each Z-series and the threshold intensity which outlined Kit-immunoreactive cells was set at 800 (intensity range 0–10 000; 16-bit scale). The number of pixels greater than the threshold was calculated and converted to a percentage of the field of view.
Electrophysiological measurements
For electrophysiological measurements, gastric antrums and small intestines of C57BL/6, W/WV mice and Tmem16a−/− and +/+ or +/− siblings and cynomolgus monkeys and human intestine were prepared by first removing the mucosa by sharp dissection. Cross sections through the entire tunica muscularis of monkey antrum and intestine and human intestine were prepared with parallel scalpel blades. Murine antrum and ileal muscle strips (10 × 5 mm) were cut and pinned to the Sylgard elastomer (Dow Corning Corp, Midland, MI, USA) floor of a recording chamber with the longitudinal (antrum) or circular (jejunum) muscle facing upward. In some experiments murine antral circular muscle bundles were prepared as previously described (Suzuki et al. 2003). Muscles were restrained using fine diameter (80 μm) tungsten wire. Circular muscle cells were impaled with glass microelectrodes filled with 3 m KCl and having resistances between 70 and 100 MΩ. Transmembrane potentials were measured using a high input impedance amplifier (Axon Instruments/Molecular Devices Corp., Sunnyvale, CA, USA) and outputs displayed on a digital oscilloscope. Electrical signals were digitized using an analog-to-digital converter (Digidata 1300 series; Axon Instruments), recorded and stored on a computer running Axoscope 9.0 software. Electrical recordings were made in the presence of nifedipine (1 μm) to reduce muscle contraction and maintain cellular impalements.
Organotypic culture
After removal of the mucosa, intestinal tissues from P0 animals were cultured for 6–7 days as previously described (Ward et al. 1997). Briefly, tissues were pinned with the mucosal side of the circular muscle up to the Sylgard elastomer-coated base of sterile 35 mm polypropylene dishes (Corning Glass Works, Corning, NY, USA). The muscles were washed with sterile culture medium at least five times and incubated at 37°C in a humidified atmosphere (90%) of 95% O2–5% CO2 in M199 medium (Sigma) supplemented with an antibiotic–antimycotic mixture (penicillin G sodium, 200 i.u. ml−1; streptomycin sulfate, 200 μg ml−1; amphotericin B, 0.5 μg ml−1; Gibco BRL) plus l-glutamine (2 mm; Gibco BRL). Culture media were changed every second day.
Solutions and drugs
The electrophysiological bath chamber was constantly perfused with oxygenated Krebs–Ringer buffer (KRB) of the following composition (mm): NaCl 118.5; KCl 4.5; MgCl2 1.2; NaHCO3 23.8; KH2PO4 1.2; dextrose 11.0; CaCl2 2.4. The pH of the KRB was 7.3–7.4 when bubbled with 97% O2–3% CO2 at 37 ± 0.5°C. Muscles were left to equilibrate for at least 1 h before experiments were begun. For electrophysiological experiments nifedipine (Sigma; St Louis, MO, USA) was dissolved in ethanol at a stock concentration of 100 μm before being added to the perfusion solution at a final concentration of 1 μm to inhibit contractile activity. It has been previously reported that nifedipine (1 μm) does not change antral or small intestinal slow wave activity (Ward et al. 1994; Suzuki & Hirst, 1999). Niflumic acid and disodium DIDS was dissolved in dH2O and further diluted in KRB at the final concentration.
Data analysis
Data are expressed as means ± standard errors of the mean (s.e.m.). Student's t test was used where appropriate to evaluate differences in the data. P values less than 0.05 were taken as a statistically significant difference. The ‘n’ values reported in the text refer to the number of animals used for each experimental protocol. Several electrical parameters were analysed: (i) resting membrane potential (RMP), (ii) slow wave amplitude, (iii) frequency and (iv) duration (calculated at the half-maximal amplitude of slow waves). Figures displayed were made from digitized data using Adobe Photoshop 7.0 (Adobe Co., San Jose, CA, USA) and Corel Draw X3 (Corel Corp. Ontario, Canada).
Results
Expression of transcript variants of Tmem16a
Previous gene array studies detected transcripts of Tmem16a in the murine GI tract and transcripts were many-fold higher in ICC than in the rest of the tissue (Chen et al. 2007). The veracity of the array data was tested by RT-PCR and amplicons were sequenced to determine the structures of Tmem16a transcripts from the tunica muscularis. In human, TMEM16A has been reported to exhibit at least four alternatively spliced exons, named a, b, c and d (Caputo et al. 2008), resulting in proteins between 712 and 1006 amino acids. We examined whether similar Tmem16a transcript splice variants were present in mouse. We performed RT-PCR on extracts of tunica muscularis from the stomach and small bowel with primers that amplify across corresponding alternative exons in the mouse Tmem16a homologue. Figure 1A is a representative gel of the major splice variants of murine Tmem16a we identified in GI muscles. Subsequent sequence analysis confirmed that we had identified transcript variants that were not previously reported for mouse Tmem16a. These variants are the result of an additional 66 bp, 12 bp or 78 bp region that are homologous to the b, c and d exon variants of human TMEM16A reported by Caputo et al. (2008; Fig. 1A). All Tmem16a expressed in murine gastrointestinal muscles include the 12 bp ‘c’ variant. In addition, Tmem16a transcripts are present that either include or exclude the alternative 66 bp ‘b’ and 78 bp ‘d’ exon variants.
Figure 1. RT-PCR analysis of Tmem16a transcript variants and Tmem16 paralogues in mouse and cynomolgus monkey gastrointestinal tracts.
A, RT-PCR primer sets were designed to amplify regions of murine Tmem16a that exhibit alternative exon splicing (variants b, c and d) in human TMEM16a. PCR products were resolved on 2% agarose gels alongside 100 bp markers. Gel and sequence analysis indicate that transcript variants b, c and d are present in antrum and small intestine. Note the differences in molecular size of PCR products for variants b and d of 66 bp and 78 bp, respectively. B–E show RT-PCR products that were generated from mouse and monkey through the use of gene-specific primers for each Tmem16 family member (a–h, j–k) as indicated. The amplicon sizes for murine and primate paralogues are as follows: Tmem16a (mouse 196 bp; monkey 202 bp); Tmem16b (mouse 183 bp; monkey 257 bp); Tmem16c (mouse 190 bp; monkey 261 bp); Tmem16d (mouse 173 bp; monkey 263 bp); Tmem16e (mouse 167 bp; monkey 249 bp); Tmem16f (mouse 181 bp; monkey 246 bp); Tmem16h (mouse 195 bp; monkey162 bp); Tmem16j (mouse 150 bp; monkey 252 bp) and Tmem16k (mouse 193 bp; monkey 257 bp). Tmem16g transcripts were absent in all these preparations.
Expression of Tmem16 paralogues in gastric antrum and small intestine tunica muscularis
In mammals, the TMEM16 family of genes consists of 10 paralogues (TMEM16a–h and j–k; Rock & Harfe, 2008) that encode highly conserved membrane-spanning proteins with N- and C-termini located in the cytosol. The expression of Tmem16 paralogues was identified by RT-PCR of isolated tunica muscularis of gastric antrum and jejunum from mouse and monkey. PCR products were generated using specific primers for each Tmem16 paralogue that were designed to be non-homologous to other members of the Tmem16 family of genes. Species-specific (Mus musculus and Macaca mulatta) nucleotide sequences for Tmem16 genes were determined from BLAST searches on the NCBI GenBank database. No non-human primate sequence was available for Tmem16h, so primers were designed using human Tmem16h. Detectable amplicons of the expected molecular size for Tmem16a, b, c, d, e, f, h, j and k were observed in all mouse and monkey gastrointestinal muscle preparations (Fig. 1B–E), Tmem16g was not detected. RT-PCR performed on spleen RNA, did result in Tmem16g amplification product of the expected size (data not shown) indicating that these primers were functional and we had not observed a false negative in our gastrointestinal preparations.
Cellular localization of ANO1 in the stomach
We next examined the cellular localization of ANO1 in situ using confocal reconstructions from whole mounts and cryostat sections. ANO1-like immunoreactivity (ANO1-LI) was observed in cells with specific morphologies in the circular (CM) and longitudinal muscle (LM) layers and in the intramuscular plane between the muscle layers in the region occupied by the myenteric plexus. In the gastric antrum two distinct populations of ANO1-LI cells were present. The first population lay within the CM and possessed a spindle-shaped morphology (Fig. 2A–C, and G–I). The second population of cells lay within the intermuscular plane between the LM and CM. These cells had numerous branching processes extending from a rounded perinuclear region. The processes contacted adjacent cell processes and cell bodies of cells with ANO1-LI, forming a dense network (Fig. 2A–C (mouse) and G–I (monkey)). We also tested tissues of five W/WV mice in the gastric antrum where ICC-IM are absent but ICC-MY are present in reduced numbers. Labelling of W/WV tissues with ANO1 antibodies was similar to results with Kit antibodies: ICC-IM with ANO1-LI were absent in gastric tissues but ICC-MY were present in the antrum (online Supplemental Fig. 1).
Figure 2. ANO1/TMEM16A expression in interstitial cells of Cajal of murine, monkey and human gastrointestinal tracts.
A–C show Kit-LI (A; red) and ANO1 (B; green) ICC-IM within the circular layer (arrows) and ICC-MY (arrowheads) of the murine gastric antrum. Co-localization of Kit-LI and ANO1-LI in both ICC populations is shown in C (yellow). D–F show Kit-LI (D, red) and ANO1-LI (E, green) co-localized in ICC-DMP (arrows) and ICC-MY (arrowheads) in the murine small intestine. Co-localization of Kit-LI and TMTM16A-LI is shown in F (yellow). G–I show Kit-LI (G; red) ICC-IM within the circular layer (arrows) and ICC-MY (arrowheads) in the monkey antrum. Both populations of ICC were ANO1-LI (H, green). Merged image in panel l shows cellular co-localization of Kit-LI and ANO1-LI (yellow). J–L show similar co-localization of Kit-LI ICC and ANO1-LI in the monkey small intestine. Both intramuscular ICC at the level of the deep muscular plexus (ICC-DMP; J, arrows) and ICC-MY (arrowheads) were Kit-LI (J; red) and ANO1-LI (K; green) in the small intestine. Panel L shows cellular co-localization of Kit-LI and ANO1-LI (yellow) in ICC-DMP and ICC-MY. Panels M–O show Kit-LI (red; arrows in M) intramuscular ICC within the circular muscle layer of the human small intestine (ICC-DMP) were ANO1-LI (green, arrows in N). Merged image is shown in yellow in panel O (arrows). P–R show Kit-LI (red; arrowheads; P) and ANO1-LI (green; arrowheads; Q) in ICC at the level of the myenteric plexus (ICC-MY). Merged image showing cellular co-localization is shown in R (yellow). Scale bar in A applies to all panels.
Cellular localization of ANO1 in the small intestine
Cells of the small intestine labelled with ANO1-LI were a discrete population of spindle-shaped cells within the CM and branching cells within the intermuscular plane between the CM and LM. In the mouse small intestine spindle-shaped cells with ANO1-LI were located within the deep muscular plexus (DMP) (Fig. 2D–F). The second population of cells with ANO1-LI was located at the level of the myenteric plexus. These cells had rounded cell bodies and multiple branching processes and formed an anastomosing network with the processes of other cells with ANO1-LI (Fig. 2D–F). In monkey and human tissues, the spindle-shaped cells were interspersed through the CM and were concentrated at the level of the DMP near the submucosal surface of the CM (Fig. 2J–L (monkey) and M–O (human)). A dense anastomosing network of ANO1-LI cells was also localized at the level of the myenteric plexus (Fig. 2J–L (monkey) and P–R (human)). We also examined the distribution of ICC in W/WV small intestines with ANO1 antibodies. ICC-MY were greatly reduced or absent, but ICC-DMP were labelled with ANO1 antibodies. This is the same distribution of ICC labelled with Kit antibodies in the W/WV small intestine (Supplemental Fig. 1).
Double labelling of ANO1 and Kit
The anatomical localization and arrangement of cells with ANO1-LI into networks in the tunica muscularis of the gastric antrum and small intestines of all three species suggested that the labelled cells could be interstitial cells of Cajal (ICC). ICC generate pacemaker activity and mediate excitatory and inhibitory enteric motor neurotransmission in the GI tract (Sanders 1996; Sanders et al. 2006). We investigated the identity of the cells with ANO1-LI by performing double-labelling experiments using antibodies raised against ANO1 and Kit which, when coupled with cell morphology, is a diagnostic marker for ICC in the GI tract (see Ward et al. 1994; Torihashi et al. 1995). It should be noted that Kit also labels mast cells that populate the tunica muscularis, particularly of human and primate muscles (S. M. Ward, unpublished observation), however, these cells are small round cells that are clearly distinct from the spindle-shaped and branching structures of ICC. Stomach and small intestinal tissues from mouse, monkey and human co-labelled with ANO1 and Kit, demonstrating that each class of ICC (intramuscular ICC (ICC-IM in stomach and ICC-DMP in the small intestine) and ICC in the region of the myenteric plexus (ICC-MY)) expresses ANO1-LI (Fig. 2).
Effects of chloride channel blockers on slow wave activity
TMEM16A transcripts were recently shown to have properties of Ca2+-activated Cl− channels (e.g. Schroeder et al. 2008), and channels of this type have been suggested as participants in GI electrical activity. We tested whether this conductance might contribute to the generation of spontaneous electrical rhythmicity in ICC by testing the effects of chloride channel-blocking drugs, niflumic acid and DIDS, on slow wave activity recorded with intracellular microelectrodes.
In the murine antrum, resting membrane potential (RMP) averaged −62 ± 1.0 mV and slow waves 26 ± 0.3 mV in amplitude occurred at a frequency of 3.2 ± 0.2 cycles min−1 (n= 7). In the monkey antrum, RMP averaged −63 ± 1.0 mV and slow waves, 36 ± 4.5 mV in amplitude and 2.5 ± 0.3 s in duration, occurred at 3.0 ± 0.3 cycles min−1 (n= 6). Niflumic acid reduced slow wave frequency in mouse and monkey antrums in a concentration-dependent manner (IC50= 5.4 ± 0.3 and 3.6 ± 1.4 μm, respectively). In the murine antrum slow wave frequency was significantly reduced at 5 μm (P= 0.006 compared to control; Fig. 3), and slow waves were abolished at 30 μm (n= 7). The reduction in slow wave frequency in the monkey antrum was significant at 5 μm (P= 0.03 compared to control) and slow waves were completely inhibited at 100 μm (Fig. 4; n= 6). The reduction in slow wave frequency was not associated with a significant change in RMP in either species (P= 0.35 and 0.33 and for mouse and monkey, respectively). The effect of niflumic acid (5 μm) on slow wave duration and the rate of rise was not significant in mouse or monkey tissues (P= 0.8 and 0.6, respectively). A summary of the effects of niflumic acid on murine and monkey gastric antrums is shown in Figs 3C and D, and 4C and D.
Figure 3. Dose-dependent effects of niflumic acid on murine gastric antrum slow waves.
A shows dose-dependent effects on slow wave frequency. Concentrations of 1 μm or greater caused a dose-dependent reduction in slow wave frequency (IC50= 5.42 ± 0.3). Slow waves were abolished at 30 μm or greater. B shows individual slow waves at a faster sweep speed at each concentration of niflumic acid. C and D show summaries (n= 7) of the dose-dependent effects of niflumic on antral slow wave frequency and duration. Filled circles represent recording under control conditions.
Figure 4. Dose-dependent effects of niflumic acid on monkey gastric antrum slow waves.
A shows cumulative doses (1–100 μm) on antral slow waves. Concentrations of 1 μm and above caused a dose-dependent decrease in slow wave frequency (IC50= 3.59 ± 1.4 μm). Slow wave activity was abolished at 100 μm. B shows slow waves at an expanded time scale at each concentration of niflumic acid. C and D show summaries (n= 6) of the dose-dependent effects of niflumic on antral slow wave frequency and duration. Filled circles represent recording under control conditions.
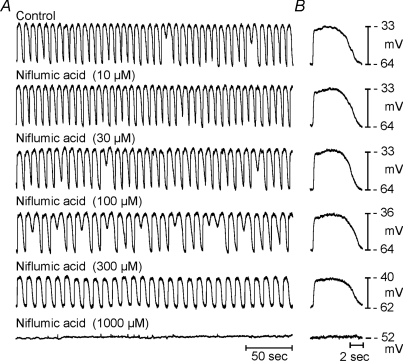
Niflumic acid also significantly reduced the amplitude and frequency of slow waves in the murine and monkey small intestine. Under control conditions RMP of the murine small intestine averaged −65 ± 1.0 mV and slow waves, 25 ± 1.2 mV in amplitude and 0.85 ± 0.04 s−1 in duration, occurred at a frequency of 32 ± 1.3 cycles min−1 (n= 11; Fig. 5). The RMP of monkey small intestine averaged −57 ± 1.0 mV, and slow waves, 14.2 ± 1.6 mV in amplitude and 2.8 ± 0.2 s in duration, occurred at a frequency of 11.8 ± 0.2 cycles min−1 (n= 6; Fig. 6). Similar to the gastric antrum, slow waves in murine and monkey intestines were inhibited by niflumic acid. However, intestinal slow waves were significantly more resistant to niflumic acid than the slow waves of the antrum, and a higher concentration was required to inhibit pacemaker activity (IC50 for murine and monkey intestinal slow waves was 150 ± 4 and 160 ± 2 μm, respectively; P= 0.000002 and 0.004). Summaries of the effects of niflumic acid on murine and monkey slow wave frequency and duration are shown in Figs 5C and D, and 6C and D, respectively. Two additional experiments were performed on human small intestine. RMP of human small intestine averaged −64 mV, and slow waves, 28.4 mV in amplitude and 5.8 s in duration, occurred at a frequency of 7.5 cycles min−1 (n= 2). Niflumic acid inhibited slow waves in human small intestine (Fig. 7).
Figure 5. Effects of niflumic acid on slow waves from the murine small intestine.
A shows cumulative doses (1–300 μm) on jejunal slow waves. Slow waves were abolished at concentrations greater than 300 μm. B shows the effects of different concentrations of niflumic acid on slow waves at a faster sweep speed. C and D show summaries (n= 11) of the dose-dependent effects of niflumic on intestinal slow wave frequency and duration, respectively. Filled circles represent recording under control conditions.
Figure 6. Effects of niflumic acid on slow waves from the monkey small intestine.
A shows cumulative doses (1–300 μm) on jejunal slow waves. Slow waves were nearly blocked at concentrations greater than 300 μm. B shows the effects of niflumic acid on individual slow waves at a faster sweep speed. C and D show summaries (n= 6) of the dose-dependent effects of niflumic on intestinal slow wave frequency and duration. Filled circles represent recording under control conditions.
Figure 7. Dose-dependent effects of niflumic acid on slow waves from the human small intestine.
Niflumic acid reduced slow wave amplitude and decreased frequency. Intestinal slow waves were inhibited at a concentration of 1000 μm.
DIDS also inhibited slow wave activity in antral and intestinal muscles. As with responses to niflumic acid, antral slow waves were more sensitive to DIDS than small intestinal slow waves (Fig. 8). DIDS had little effect on frequency in the small intestine but reduced the amplitude of slow waves. The IC50 for DIDS on antral slow waves was 150 ± 3 μm compared with 1368 ± 2 μm in the small intestine. The effects of DIDS on antral and small intestinal slow waves are shown in Fig. 8A–D and E–H, respectively.
Figure 8. Effect of DIDS on slow waves of the murine antrum and small intestine.
A shows dose-dependent effects of DIDS on antral slow waves (100 and 300 μm). DIDS at concentrations above 10 μm significantly reduced slow wave frequency and at a concentration of 300 μm greatly reduced the amplitude of slow waves. B shows the effects of DIDS on individual slow waves at a faster sweep speed. C and D show summaries (n= 5) of the dose-dependent effects of DIDS on antral slow wave frequency and duration. E shows effects of DIDS at (1 and 3 mm) on jejunal slow waves. Slow waves were abolished at concentrations greater than 3 mm. F shows the effects of DIDS at 1 and 3 mm on slow waves at a faster sweep speed. G and H show summaries (n= 6) of the dose-dependent effects of DIDS on intestinal slow wave amplitude and duration, respectively. Filled circles represent recording under control conditions.
Effects of niflumic acid on unitary potentials
Small bundles of CM from guinea-pig and murine gastric muscles generate noisy electrical fluctuations termed unitary potentials (Burns et al. 1996; Suzuki & Hirst, 1999; van Helden et al. 2000). These events can summate and are thought to be the basic pacemaker activity that generates slow waves in ICC (Edwards et al. 1999; Sanders et al. 2006). We tested the effects of niflumic acid and DIDS on unitary potentials in short segments of murine gastric antrum bundles from wildtype animals and compared this to recordings from W/WV mutant animals that lack ICC-IM (Burns et al. 1996). In five experiments, a concentration of 30 μm had no effect on unitary potential activity (Fig. 9). Niflumic acid was further tested at a concentration of 100 μm. This concentration also had little effect on RMP and unitary potentials in antral bundles from wildtype animals (n= 4). In the gastric antrums of W/WV mutants unitary potentials were greatly attenuated or absent as previously reported (Suzuki et al. 2003). Niflumic acid had little or no effect on RMP in these muscles (n= 2).
Figure 9. Niflumic acid did not affect unitary potential discharge in the murine antrum.
Aa–e show control recordings from isolated antral circular muscle bundles from 5 different animals. Ba–e show recordings after 15 min in a concentration of niflumic acid (30 μm) that inhibited slow waves in intact tissues. Four of five of the recordings after niflumic acid addition were performed in the same cell as control recordings were made from.
Loss of slow wave activity in Tmem16a−/− (Tmem16atm1Bdh/tm1Bdh) mice
Experiments were also performed on Tmem16a−/−, Tmem16a+/+ and Tmem16a+/− mice (Rock et al. 2008) to determine the role of these channels in slow wave activity. Approximately 80% of animals homozygous for the null Tmem16a allele die within 1 week of birth. Only two to three Tmem16a−/− offspring were expected per litter (approximately 25%), so neonatal mice were killed at birth (P0) and the electrical activity of muscles was monitored in fresh muscles and after 6 days in organotypic cultures, where slow waves continue to develop for many days after birth (Ward et al. 1997). No differences in activity were observed in Tmem16a+/+ and Tmem16a+/− mice, so data from these animals were grouped. At P0, RMP averaged −64 ± 0.4 mV in small intestinal muscles of Tmem16a+/+ and Tmem16a+/− mice. Spontaneous slow waves 11.1 ± 0.5 mV in amplitude and 0.95 ± 0.04 s in duration occurred at a frequency of 19.7 ± 0.4 cycles min−1 (n= 41) in the muscles of these animals. RMP averaged −59 ± 0.7 mV in muscles of Tmem16a−/− mice and spontaneous slow waves were absent in these animals (n= 18; 8 litters; Fig. 10B). After 6 days in organotypic culture, RMPs from Tmem16a+/+ and Tmem16a+/− mice averaged −67.6 ± 0.7 mV (n= 32) and slow waves 28.1 ± 1.2 mV in amplitude and 0.95 ± 0.06 s in duration occurred at a frequency of 15.2 ± 0.6 cycles min−1 (Fig. 10C). Slow waves failed to develop in muscles of Tmem16a−/− mice after 6 days in culture, as occurs in wildtype mice during the first week after birth in organ culture (Ward et al. 1997). The muscles from Tmem16a−/− mice had RMPs averaging −60.0 ± 1.6 mV, demonstrating the viability of the muscles in the absence of slow waves (n= 13; Fig. 10C). Geneotyping of tissues from each animal was performed blindly to determine the absence or presence of functional Tmem16a (Fig. 10A).
Figure 10. Slow waves are absent in the small intestines of Tmem16a−/− neonatal mice.
A shows genotyping results from the same litter that electrical recordings were performed on in B and C. The wildtype allele was absent in animals 6, 9 and 11 demonstrating that these mice were Tmem16a−/− mutants. Electrical recordings were not performed on animal number 1. B shows intracellular electrical recordings from the small intestines of 10 P0 sibling mice. Electrical slow waves were present in all animals except animals 6, 9 and 11. In C, intestinal tissues from animals in A were placed in organotypic culture and electrical recordings made after 6–7 days. Slow waves became more robust in amplitude and were similar to activity recorded from the intestines of adult animals, except animals 6, 9 and 11, where slow waves were absent. D and E show the presence of ICC-MY networks (arrows) in the small intestines of P0 animals from Tmem16a+/+ and Tmem16a−/− mice, respectively. At P0 ICC-DMP does not form a discrete network in intestinal tissues. Scale bar in E applies to both panels.
Lack of slow wave in muscles of Tmem16a−/− mice does not fully establish the importance of this protein in pacemaker activity, as ANO1 may be important for the development and/or maintenance of functional ICC. Developmental defects in the trachea have been observed (Rock et al. 2008). Previously studies have demonstrated loss of slow wave activity in muscles lacking ICC (Ward et al. 1994; Huizinga et al. 1995). Therefore, we performed Kit immunohistochemistry to verify that ICC networks develop normally in Tmem16a−/− mice. There was no statistical difference in the area of Kit-immunoreactive ICC-MY in jejunums from Tmem16a−/−versus Tmem16a+/+ mice. The percentage of ICC-MY in Tmem16a−/− mice averaged 17.36 ± 2.72% (n= 4) compared to 23.57 ± 2.18% for Tmem16a+/+ mice (n= 4; P= 0.15; Fig. 10D and E).
In spite of the high mortality of Tmem16a−/− mice (Rock et al. 2008), two litters were allowed to develop for up to P23. Three Tmem16a−/−mutants survived and electrical recordings from antral and intestinal muscles were made from these adult animals (Fig. 11). Slow waves did not develop in the stomachs or small intestines of the Tmem16a−/−mutants. In the circular muscle layer of gastric antrums of a Tmem16a+/+ and Tmem16a+/− mouse RMP averaged −69.0 mV, and slow waves 34 mV in amplitude and 4.25 s in duration occurred at a frequency of 2.3 cycles min−1. In the gastric antrums of the Tmem16a−/− mutants RMP averaged −64.0 ± 4.0 mV and slow waves were not recorded. In the small intestines of Tmem16a+/+ and Tmem16a+/− mice RMP averaged −68.2 mV, and slow waves 29.0 mV in amplitude and 0.7 s in duration occurred at a frequency of 33.0 cycles min−1. In the small intestines of Tmem16a−/−mutants RMP averaged −53.0 ± 1.0 mV and slow waves were not observed. These data demonstrate that loss of slow wave activity in Tmem16a−/− mice is not simply a feature of delayed development.
Figure 11. Slow waves are absent in adult gastric and intestinal muscles of Tmem16a−/− mice.
A shows genotyping of mice of two litters. The wildtype allele was absent in animals 1–3, demonstrating that these mice were Tmem16a−/−. Animals 4 and 5 were a heterozygote and a wildtype, homozygote, respectively. B and C show intracellular electrical recordings from antral and jejunal muscles of each of the 5 animals. Note absence of slow waves in gastric and intestinal muscles of Tmem16a−/− mice. Slow waves of normal characteristics were recorded from the heterozygote Tmem16a+/− mouse.
Discussion
In the present study we demonstrated expression of Tmem16a, which encodes Ca2+-activated Cl− channels (ANO1), in the GI tracts of mouse, monkey and humans. ANO1-LI was observed in specialized populations of cells with the morphologies of all classes of ICC. Expression of ANO1 (also referred to as DOG1) was previously reported in cells consistent with the distribution of ICC in human small intestine (Espinosa et al. 2008), and a recent study demonstrated expression of this protein in murine ICC (Gomez-Pinilla et al. 2009). Co-labelling of cells with ANO1 and Kit antibodies in our study confirms that ANO1 is expressed with high specificity in ICC. These data are consistent with the genomic studies that initiated the hypothesis of the present study showing that Tmem16a is 6- to 8−fold more highly expressed in ICC of the murine small intestine than in whole tissue (Chen et al. 2007). We found that Cl− channel-blocking drugs inhibited slow waves in murine and monkey gastric and small intestinal muscles and in human intestine. Antral and small intestinal muscles of Tmem16a−/− mice failed to develop slow wave activity in spite of ICC networks that appeared normal. The loss of slow waves in Tmem16a−/− mice was not simply a feature of delayed development, because we observed no slow wave activity in antral and small intestinal muscles of adult (P23) animals. These data suggest a novel role for ANO1 Cl− channels in the generation of slow waves in GI muscles.
Our studies confirmed, as others have shown previously, that ANO1 is a highly specific biomarker for ICC in normal muscles of three species, and ANO1 was also shown previously to be a sensitive and selective label for many gastrointestinal stromal tumours (GISTs; West et al. 2004; Espinosa et al. 2008). In contrast to Kit labelling, we observed no cross-labelling of mast cells (West et al. 2004; Gomez-Pinilla et al. 2009) with the ANO1 antibodies tested. Cells other than ICC were not resolved in the tunica muscularis with ANO1 antibodies. Thus, antibodies for this protein provide even more selective labelling of ICC than Kit antibodies (Ward et al. 1994; Burns et al. 1997). Several GI motility disorders are associated with loss of ICC (for review see Farrugia, 2008). ANO1 is a surface protein in ICC, so if destruction of ICC networks in GI disease is accompanied by programmed cell death, then appearance of ANO1 in the circulation might be a useful biomarker to assess the status of ICC non-invasively. It is tempting to suggest that selective labelling of ICC and a high percentage of Kit-positive GISTs with ANO1 antibodies provides another indication that GISTs arise from ICC. However, it should be noted that up to 78% of GISTs positive for platelet-derived growth factor receptor α (PDGFRα) mutations, but weak or negative for Kit, also expressed ANO1 (Espinosa et al. 2008), and there is no evidence that tumours of this genotype arise from ICC. This is an interesting observation since it is clear from the specificity of co-localization of Kit and ANO1 immuoreactivity that cells in normal tissues expressing PDGFRα (Iino et al. 2009) do not express ANO1. Thus, ANO1 expression may develop after transformation of PDGFRα-positive cells to GISTs or it may be expressed in a common GIST progenitor or stem cell.
It has been suggested that ANO1 channels might be therapeutic targets for GISTs (Espinosa et al. 2008). The function of these channels in GIST cells is unknown, but our findings suggest that blockers of ANO1 channels might have adverse side effects on GI motility, because electrical rhythmicity, which drives phasic contractions and peristalsis in GI organs, might be negatively impacted by these drugs. Mutations in Kit result in animals with reduced ICC and motility defects (see Sanders & Ward, 2007), demonstrating the importance of pacemaker activity in normal GI physiology. As an extreme example of the consequences of blocking ANO1, Tmem16a knockouts do not gain weight normally and have abnormal GI tracts at necropsy with some animals displaying grossly distended abdomens and air-filled stomachs, and small intestines, suggesting possible pseudo-obstruction of GI organs (Rock et al. 2008).
Tmem16a was recently shown to encode Ca2+-activated Cl− channels (Yang et al. 2008; Caputo et al. 2008; Schroeder et al. 2008). There are 10 paralogues of Tmem16a in humans, so collectively these channels potentially represent a novel family of Ca2+-activated Cl− channels. Murine Tmem16a cDNA, amplified from retina, consists of an open reading frame of 2880 nucleotides, and encodes a protein with 960 amino acids and 91% sequence homology with the human orthologue (Yang et al. 2008). It is likely that the antibodies we used, which were raised against the human sequence, recognized ANO1 in murine cells due to the high degree of sequence homology between species; however, we noted that labelling with these antibodies was less robust in mouse than in human and monkey samples. It should be noted that the gene expression screen identified two additional Tmem16 family paralogues in ICC (i.e. Tmem16 d and f; Chen et al. 2007). Expression of Tmem16d and f did not exceed 2-fold greater expression in ICC than in whole tissues, so neither is considered a key protein for the unique pacemaker properties of ICC. Our PCR performed in the present study identified nine members of the Tmem16 family in gastrointestinal muscles (but some of these genes may be expressed by other cell types). It is possible that other members of the Tmem16 family could contribute to inward currents in ICC, if these transcripts also function as Cl− channels. Loss of slow waves in Tmem16a−/− mice suggests that this conductance is fundamental for slow waves in ICC, and this observation is consistent with our genomic study that found Tmem16a is the most abundant family member expressed in ICC (Chen et al. 2007).
Unitary potentials, which are small irregular noisy fluctuations in membrane potential and may be the primary pacemaker activity that underlies slow waves, can be resolved in intracellular recordings from small bundles of GI muscles (Burns et al. 1996; Edwards et al. 1999; van Helden et al. 2000) or recordings from ICC (Kito et al. 2002b). Others have suggested that unitary potentials are due to discharge of Ca2+-activated Cl− currents (Kito et al. 2002b; Suzuki et al. 2003); however, in the present study these events were insensitive to concentrations of niflumic acid that blocked slow waves. This is consistent with our findings that spontaneous transient inward currents from gastric (Takeda et al. 2008) and small intestinal ICC (Jin et al. 2009), which are the membrane current events likely to be responsible for unitary potentials, are due to non-selective cation channels.
Two confusing issues regarding the effects of Ca2+-activated Cl− channel blockers on electrical slow waves are the difference in concentration–response relationships in gastric versus small intestine and the relative lack of inhibition of slow wave amplitude and duration until slow waves were nearly blocked. Concentration–response data from ANO1 channels expressed in HEK cells suggest more than 90% inhibition of currents by 10 μm niflumic acid and DIDS (Yang et al. 2008). Although never matching this level of sensitivity in intact muscles, murine gastric slow waves were far more sensitive to niflumic acid (IC50= 5.4 μm) and DIDS (IC50= 150 μm) than small intestinal slow waves (IC50s= 150 μm and 1368 μm for niflumic acid and DIDS, respectively). ANO1/TMEM16A was prominently expressed in ICC in both muscles, but from the discrepancies in concentration–response data, it is tempting to speculate that small intestinal slow waves are less dependent upon a Ca2+-activated Cl− conductance than gastric muscles. Two other observations argue against this point, however: (i) spontaneous activity (current clamp) and slow wave currents (voltage clamp) of isolated ICC from small intestine were inhibited by <10 μm niflumic acid (Zhu et al. 2009), directly demonstrating the potency of these inhibitors on Ca2+-activated Cl− currents in intestinal ICC; and (ii) slow waves were absent in the small bowels of Tmem16a−/− mice, demonstrating the importance of this conductance in generation and/or propagation of slow waves. At the present time the cause for the differences in the sensitivity to niflumic acid in the two regions of the GI tract are unknown. The present study also identified that Tmem16a splice variants are present in gastric and small intestine muscles. In a recent study it was found that that human TMEM6A splice variants (a, ac, abc and abcd) produced Ca2+-activated Cl− currents when expressed in HEK cells (Caputo et al. 2008). Suggestions for future studies to understand the variable pharmacology of Cl− channel-blocking drugs may be to determine specific expression of ANO1 splice variants and/or paralogues in ICC and to determine regulation of channels by subunits in different ICC.
The characteristics of the inhibition by niflumic acid differed in ICC versus whole muscle. Niflumic acid caused a concentration-dependent reduction in the amplitude of slow wave currents in ICC and reduced the frequency, upstroke velocity and duration of slow waves under current clamp (Zhu et al. 2009). In intact muscles, where intracellular recordings were made from smooth muscle cells that are electrically coupled to the ICC-MY network, niflumic acid reduced the frequency of slow waves without substantial effects on the waveforms of these events until close to the level of total inhibition (see Figs 3–7). These data suggest that ANO1 may be important in the initiation of slow waves in intact muscles, but additional currents (i.e. voltage-dependent currents summing with ANO1 currents during cell-to-cell propagation), contribute to the waveforms of slow waves recorded from smooth muscle cells. Further studies will be needed to sort out the discrepancies in the pharmacology of pacemaker activity in ICC and whole muscles.
We previously formulated a model for pacemaker activity in ICC that was based on electrical recordings from cultured ICC (see Sanders et al. 2006). This model did not include contributions from a Ca2+-activated Cl− conductance to pacemaker activity. Although such a conductance had been suggested by previous investigations of intact muscles (e.g. Hirst et al. 2002; Kito & Suzuki, 2003) and cultured ICC (Tokutomi et al. 1995), we, and others, had previously failed to identify a Ca2+-activated Cl− conductance in ICC (e.g. Thomsen et al. 1998; Koh et al. 2002; Goto et al. 2004; Park et al. 2005; Parsons & Sanders, 2008). In our studies, cultured ICC displayed a prominent Ca2+-inhibited, non-selective cation conductance, and this conductance was inhibited by Cl− channel-blocking drugs, making it difficult to deduce a role for Cl− conductances based on pharmacological studies alone. Lack of a measureable current in cultured cells and dependence of pacemaker currents upon Ca2+ release from intracellular stores (see Suzuki et al. 2000; Ward et al. 2000) and mitochondrial uptake of Ca2+ (Ward et al. 2000) was the basis of the model for pacemaker activity proposed (e.g. Sanders et al. 2006). In the present study we have demonstrated robust expression of a protein transcript that functions as a Ca2+-activated Cl− conductance (ANO1) and loss of slow waves in animals homozygous for null alleles of Tmem16a (i.e. lacking functional ANO1), making it more credible to suggest that Cl− channel-blocking drugs affect slow waves via block of Cl− conductances. Data from the present study and voltage-clamp studies of isolated ICC (Zhu et al. 2009) will require reformulation of the pacemaker model as it becomes clear what phase of Ca2+ transients in ICC are responsible for activation of inward currents (e.g. the present study suggests that a rise in Ca2+ would activate Cl− efflux from ICC) and what additional conductances/cellular mechanisms are responsible for voltage-dependent active propagation.
Acknowledgments
This project was supported by a research grants from National Institute of Diabetes and Digestive and Kidney Diseases, DK41315 and DK40569. S.M.W. was also supported by DK57236. Images were collected using a Zeiss LSM510 confocal microscope obtained with support from National Institutes of Health S10 RR16871. The authors would also like to acknowledge Neal W. Fleming for providing human tissues and Emily Fee and Lauren E. Peri for excellent technical assistance.
Supplemental material
References
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Chen H, Ordög T, Chen J, Young DL, Bardsley MR, Redelman D, Ward SM, Sanders KM. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics. 2007;31:492–509. doi: 10.1152/physiolgenomics.00113.2007. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. J Physiol. 1999;519:235–250. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20:54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J. Physiol. 2004;559:411–422. doi: 10.1113/jphysiol.2004.063875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in [Ca2+]i and Cl− channels. J Physiol. 2002;540:907–919. doi: 10.1113/jphysiol.2001.014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- Jin NG, Koh SD, Sanders KM. Caffeine inhibits non-selective cationic currents in interstitial cells of Cajal from the murine jejunum. Am J Physiol Cell Physiol. 2009 doi: 10.1152/ajpcell.00155.2009. 2009 Jul 22 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflugers Arch. 2002a;445:202–217. doi: 10.1007/s00424-002-0884-z. [DOI] [PubMed] [Google Scholar]
- Kito Y, Suzuki H. Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol. 2003;553:803–818. doi: 10.1113/jphysiol.2003.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Suzuki H, Edwards FR. Properties of unitary potentials recorded from myenteric interstitial cells of Cajal distributed in the guinea-pig gastric antrum. J Smooth Muscle Res. 2002b;38:165–179. doi: 10.1540/jsmr.38.165. [DOI] [PubMed] [Google Scholar]
- Koh SD, Jun JY, Kim TW, Sanders KM. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803–814. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Mckay CM, Zhu Y, Huizinga JD. Volume-activated chloride currents in interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2005;289:G791–G797. doi: 10.1152/ajpgi.00050.2005. [DOI] [PubMed] [Google Scholar]
- Parsons SP, Sanders KM. An outwardly rectifying and deactivating chloride channel expressed by interstitial cells of cajal from the murine small intestine. J Membr Biol. 2008;221:123–132. doi: 10.1007/s00232-007-9084-2. [DOI] [PubMed] [Google Scholar]
- Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321:141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Rock JR, Harfe BD. Expression of TMEM16 paralogs during murine embryogenesis. Dev Dyn. 2008;237:2566–2574. doi: 10.1002/dvdy.21676. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Hirst GD. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J Physiol. 1999;517:563–573. doi: 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GD. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751–763. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Koh SD, Sanders KM, Ward SM. Differential expression of ionic conductances in interstitial cells of Cajal in the murine gastric antrum. J Physiol. 2008;586:859–873. doi: 10.1113/jphysiol.2007.140293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848–851. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- Tokutomi N, Maeda H, Tokutomi Y, Sato D, Sugita M, Nishikawa S, et al. Rhythmic Cl− current and physiological roles of the intestinal c-Kit positive cells. Pflugers Arch. 1995;431:169–177. doi: 10.1007/BF00410188. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Nishikawa S-I, Nishi K, Kobayashi S, Sanders KM. c-Kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tiss Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- van Helden DF, Imtiaz MS, Nurgaliyeva K, von der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Beckett EAH, Wang X-Y, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000a;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-Kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Harney SC, Bayguinov JR, McLaren GJ, Sanders KM. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J Physiol. 1997;505:241–258. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000b;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- Zhu M, Kim TW, Ward SM, Koh SD, Sanders KM. A Ca2+-activated conductance in interstitial cells of Canal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.