Abstract
Interstitial cells of Cajal (ICC) are unique cells that generate electrical pacemaker activity in gastrointestinal (GI) muscles. Many previous studies have attempted to characterize the conductances responsible for pacemaker current and slow waves in the GI tract, but the precise mechanism of electrical rhythmicity is still debated. We used a new transgenic mouse with a bright green fluorescent protein (copGFP) constitutively expressed in ICC to facilitate study of these cells in mixed cell dispersions. We found that ICC express a specialized ‘slow wave’ current. Reversal of tail current analysis showed this current was due to a Cl− selective conductance. ICC express ANO1, a Ca2+-activated Cl− channel. Slow wave currents are not voltage dependent, but a secondary voltage-dependent process underlies activation of these currents. Removal of extracellular Ca2+, replacement of Ca2+ with Ba2+, or extracellular Ni2+ (30 μm) blocked the slow wave current. Single Ca2+-activated Cl− channels with a unitary conductance of 7.8 pS were resolved in excised patches of ICC. These are similar in conductance to ANO1 channels (8 pS) expressed in HEK293 cells. Slow wave current was blocked in a concentration-dependent manner by niflumic acid (IC50= 4.8 μm). Slow wave currents are associated with transient depolarizations of ICC in current clamp, and these events were blocked by niflumic acid. These findings demonstrate a role for a Ca2+-activated Cl− conductance in slow wave current in ICC and are consistent with the idea that ANO1 participates in pacemaker activity.
Introduction
Pacemaker activity in gastrointestinal (GI) muscles is generated by interstitial cells of Cajal (ICC; Langton et al. 1989; Ward et al. 1994; Huizinga et al. 1995; Dickens et al. 1999; see review by Sanders et al. 2006). ICC, identified by anti-Kit antibodies, retain a rhythmic phenotype in cell cultures and generate spontaneous pacemaker activity (Koh et al. 1998; Thomsen et al. 1998). These cells have been used extensively to study the mechanism of rhythmicity in ICC (Tokutomi et al. 1995; Ward et al. 2000; Huizinga et al. 2002; Kim et al. 2002; Koh et al. 2002), and models of pacemaker activity have been proposed from findings of these studies (Kim et al. 2005; Sanders et al. 2006; Faville et al. 2008). A caveat to the conclusions of experiments on cultured ICC is that depolarization of these cells fails to elicit slow waves, but it is well known that slow waves can be paced by application of current in intact muscles (Publicover & Sanders, 1986; Edwards et al. 1999; Kito et al. 2002). Thus, it is possible that an important conductance is lost from the pacemaker mechanism in cultured ICC.
Goto and colleagues (2004) studied freshly isolated ICC from the murine intestine and identified ICC by labelling with anti-Kit antibodies. Large inward currents, termed ‘autonomous’ currents, were activated by depolarization of these cells, and the authors concluded that the autonomous currents were due to a Ca2+-dependent, non-selective cation conductance. The ability to activate inward current with current injection is more consistent with what is known about the properties of slow waves in the GI tract. However, the preparation and identification of ICC are difficult, as described in this study, so the nature of the inward current has not been further elucidated.
Microarray analysis to characterize the expression phenotype of ICC in the small intestine was recently performed (Chen et al. 2007). Genes expressed by ICC were compared to expression levels in extracts of whole tissues, and genes highly expressed in ICC (>4 fold) were considered to be potentially important for the unique characteristics of ICC. One highly expressed gene identified by this screen was transmembrane protein 16A (Tmem16a), which encodes anoctamin 1 (ANO1; aka: TAOS2; ORAOV2 and FLJ10261; located on chromosome 7 in the mouse at 7F5). Tmem16a was about eightfold more highly expressed in ICC than in whole tissue extracts. At the time the genomic study was completed, the function of ANO1 was unknown. Gastrointestinal stromal tumours (GISTs) have also been shown to express this gene (referred to as FLJ2061 in these studies), and antibodies to the sequence of the encoded protein (called DOG1 for ‘discovered on GIST-1’) labelled up to 98% of GISTs with Kit mutations (West et al. 2004; Espinosa et al. 2008). Labelling of normal tissues with DOG1 antibody labelled cells with the same anatomical distribution as Kit-positive cells (Espinosa et al. 2008), and a recent study confirmed that ICC express ANO1 in the GI tract (Gomez-Pinilla et al. 2009). ANO1 was recently shown to function as a Ca2+-activated Cl− channel (Caputo et al. 2008; Schroeder et al. 2008; Yang et al. 2008), and loss or block of ANO1 blocks electrical slow waves in intact GI muscles (Hwang et al. 2009).
We have recently developed transgenic mice that express a bright form of green fluorescent protein (copGFP) in ICC constitutively. Dispersed ICC can be identified by fluorescence, and voltage-clamp of these cells confirms that large inward currents can be initiated by depolarization (as in Goto et al. 2004). In the present study we explored the properties of these currents in copGFP+ ICC. We found that ICC express ANO1 protein and Cl− channels with a unitary conductance consistent with that of ANO1 channels expressed in HEK293 cells (Yang et al. 2008). The reversal potential of the whole-cell inward current and blockade by low concentrations of niflumic acid are consistent with this current being due to a Cl− conductance. Spontaneous pacemaker activity in single ICC was also blocked by niflumic acid. Our findings support the participation of a novel Ca2+-activated Cl− conductance in the generation of slow waves in ICC.
Methods
Animals
A Kit targeting vector was prepared from the PAC library constructed from a female 129S6/SvEvTac mouse spleen genomic DNA (CHORI: Children's Hospital Oakland Research Institute, CA, USA). A copGFP gene amplified from a pFIV-copGFP reporter vector (System Biosciences, Mountain View, CA, USA) by PCR was inserted into the first exon of Kit along with a selection PGK-HPRT cassette (see online Supplemental Material, Supplementary Fig. 1). Targeted knock-in (KI) allele was confirmed by Southern blotting using two external probes. Since copGFP KI inactivates Kit allele, heterozygotes (Kit+/copGFP mice), 7–12 days of age, were used for studies of ICC. Mice were anaesthetized with isoflurane (Vetone, Meridian, ID, USA) prior to cervical dislocation. The institutional Animal Use and Care Committee at the University of Nevada approved all procedures used.
Preparation of dispersed cells and cell culture
Small strips of intestinal muscle were prepared and equilibrated in Ca2+-free Hanks’ solution for 20 min. Cells were dispersed from these strips, as described previously (Koh et al. 1998), with an enzyme solution containing (per ml): collagenase (Worthington Type II, 1.3 mg), bovine serum albumin (Sigma, St Louis, MO, USA, 2 mg), trypsin inhibitor (Sigma, 2 mg) and ATP (0.27 mg). Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 mg ml−1, BD Falcon, Franklin Lakes, NJ, USA) in 35 mm culture dishes. Giga seals were difficult to obtain and maintain in freshly dispersed copGFP+ cells with the dispersion techniques we used. Thus, the cells were allowed to stabilize overnight before experiments in culture medium at 37°C in a 95% O2–5% CO2 incubator in smooth muscle growth medium (Clonetics, San Diego, CA, USA) supplemented with 2% antibiotic–antimycotic (Gibco, Grand Island, NY, USA) and stem cell factor (5 ng ml−1, Sigma).
Immunohistochemical identification of copGFP+ cells as ICC
The tunica muscularis was opened along the mesenteric border, pinned to the Sylgard floor of a dissecting dish, and stretched to 110% of the resting length and width. The mucosa was removed by sharp dissection fixing the tissue with paraformaledehyde (4% w/v for 30 min). Tissues were subsequently washed with 0.01 m phosphate buffered saline (PBS, pH 7.4) and incubated in bovine serum albumin (1% for 1 h) to reduce non-specific antibody binding. Intestines were then incubated with an antibody raised against Kit protein (goat anti-SCF (Stem cell factor) receptor/c-kit antiserum; 1 : 500 in PBS, R&D systems Minneapolis, MN, USA) at 4°C overnight, washed in PBS and incubated in Alexa fluor 594-coupled donkey anti-goat secondary antibody (1 : 1000 in PBS; 1 h at room temperature, Invitrogen, Carlsbad, CA, USA). Control tissues were prepared by omitting either the primary or secondary antibodies from the incubation solution.
Double labelling of Kit and ANO1
Double labelling immunohistochemistry was performed on whole mount preparations to determine whether Kit immunopositive ICC express ANO1 in the murine intestine. Whole mounts were prepared as described above but fixed in acetone (10 min, 4°C). Following fixation intestinal tissues were washed in PBS and double labelled with Kit and ANO1. Briefly, tissues were incubated with anti-cKit antibody (ACK2, 5 μg ml−1, Invitrogen) for 24 h, washed in PBS, and then incubated with anti-ANO1 (SP31, 1 : 1000; Abcam, Cambridge, MA, USA) for 24 h. After wash, immunoreactivity was detected via sequential incubation in Alexa fluor 488-coupled goat anti-rabbit and Alexa fluor 594-coupled donkey anti-goat secondary antibodies (Molecular Probes, Eugene, OR, USA; 1 : 500 in PBS; 1 h, room temperature). Control tissues were prepared by omitting either primary or secondary antibodies from the incubation solution.
Cells isolated from the small intestine that were copGFP+ (ICC) were also tested to be sure these specific cells express ANO1. After enzymatic dispersion cells were plated onto glass coverslips and fixed in paraformaldehyde (4% w/v for 10 min). After fixation, the cells were incubated with the anti-ANO1 antibody (1 : 1000) at 4°C overnight, washed in PBS and incubated in Alexa fluor 594-coupled goat anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA, USA; 1 : 1000 in PBS; 1 h at room temperature). Control cells were prepared by omitting either the primary or secondary antibodies from the incubation solution.
After incubation in secondary antibodies, tissues and cells were washed with PBS (overnight at 4°C) and mounted on glass slides using Aqua-Mount (Lerner Laboratories, Pittsburgh, PA, USA). Tissues and cells were examined with a Zeiss LSM 510 Meta confocal microscope (Zeiss, Germany) with an excitation wavelength appropriate for Alexa fluor 488 and Alexa fluor 594. Confocal micrographs are digital composites of Z-series scans of 10–40 optical sections through a depth of 5–40 μm. Final images were constructed using Zeiss LSM 5 Image Examiner software and converted to Tiff files for final processing in Adobe Photoshop 7.0 (Adobe Systems Inc., San Jose, CA, USA) and Corel Draw 12.0 (Corel Corp. Ontario, Canada).
Electrophysiological experiments
Interstitial cells of Cajal (ICC) were identified as green fluorescent protein positive cells under an inverted microscope with attached fluorescence set-up (see Fig. 1). The standard whole-cell patch clamp configuration was also employed to record membrane currents (voltage clamp) and potentials (current clamp, I= 0). For single channel recordings, the excised patch configuration was employed. Currents or potentials were amplified with an Axopatch 200B patch-clamp amplifier (Axon Instruments, Union City, CA, USA) and digitized with a 16-bit analog to digital converter (Digidata 1322A, Axon Instruments) and stored directly on-line using pCLAMP software (version 9.2, Axon Instruments). Data were sampled at 4 kHz and filtered at 2 kHz using an eight-pole Bessel filter for whole-cell experiments and 10 kHz sampling with 2 kHz filtration for single channel experiments. All data were analysed using clampfit (pCLAMP version, 9.2, Axon Instruments) and Graphpad Prism (version 3.0, Graphpad Software Inc., San Diego, CA, USA) software.
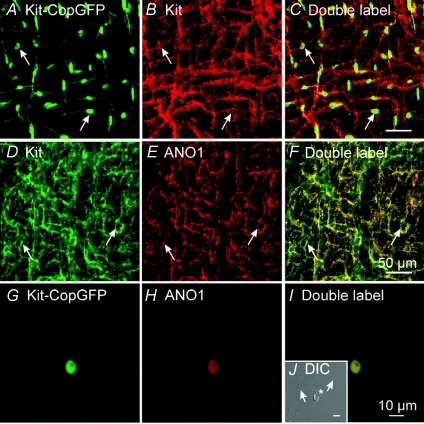
Figure 1. Expression of copGFP and ANO1 in ICC.
A, copGFP+ cells in the small intestine of Kit+/copGFP mice (green, arrows). copGFP fluorescence was primarily located within the perinuclear region of cells, cellular processes were labelled but to a lesser extent. Double labelling with Kit antibody (red, B) confirmed the copGFP+ cells were ICC (C, merged files). D, Kit labelling of ICC in the small intestine. E, labelling of cells with antibody to ANO1. ICC were Kit- and ANO1-immunopositive (F, merged file). Enzymatic dispersions of small intestinal muscles of Kit+/copGFP mice yielded ICC that were identified by copGFP (green cell; G). These cells were ANO1 immunopositive (red; H). I, a merged file of G and H. J, an insert in panel I, is a copGFP+ cell visualized with DIC. copGFP-negative cells are also present in nearly every field, and these cells were ANO1-negative (arrows). Scale bar in panel F applies to A–F. Scale bar in panel I applies to G–I. Scale bar in panel J is 10 μm.
The pipette tip resistance ranged between 3 and 6 MΩ for whole cell recordings and 5 and 10 MΩ for single channel recordings. Whole-cell and single channel experiments were conducted at 30°C with the use of a CL-100 bath heater (Warner Instruments, Hamden, CT, USA) and room temperature (20°C), respectively.
Solutions for patch clamp experiments
External solution for whole-cell recordings was a Ca2+-containing physiological salt solution (CaPSS) containing (mm): 5 KCl, 135 NaCl, 2 CaCl2, 10 glucose, 1.2 MgCl2, and 10 Hepes adjusted to pH 7.4 with Tris. In some experiments cells were perfused in Ca2+-free solution (omitting 2 mm CaCl2 from CaPSS). The pipette solutions used for these experiments are described in Table 1. The junction potential was digitally corrected during experiments. For single channel recordings, pipette solution and external solution was high N-methyl-d-glucamine (NMDG) solution containing (mm); 140 NMDGCl, 1 EGTA, 5 Hepes adjusted to pH7.2. Free Ca2+ concentrations were calculated by Maxchelator software (http://maxchelator.stanford.edu). The effects of niflumic acid (Sigma) and various concentration of cytosolic Ca2+ were tested by a fast bath perfusion system (Automate Scientific, Inc., Berkeley, CA, USA). Within 1 min perfusion, full changes of bath solution were successful.
Table 1.
The composition of pipette solutions
| Solutions (mm) | I | II | III | IV |
|---|---|---|---|---|
| KCl | 135 | 30 | 0 | 0 |
| CsCl | 0 | 0 | 135 | 30 |
| Potassium aspartate | 0 | 110 | 0 | 0 |
| Caesium aspartate | 0 | 0 | 0 | 110 |
| MgATP | 3 | 3 | 3 | 3 |
| NaGTP | 0.1 | 0.1 | 0.1 | 0.1 |
| Creatine phosphate disodium | 2.5 | 2.5 | 2.5 | 2.5 |
| EGTA | 0.1 | 0.1 | 0.1 | 0.1 |
| Hepes | 10 | 10 | 10 | 10 |
| Junction potential (mV) | 5.3 | 14.6 | 5.3 | 14.6 |
All pipette solutions were adjusted pH 7.2 with Tris.
Statistical analysis
Data were tabulated and presented as means ±s.e.m. The n values given represent the number of cells on which specific protocols were performed. Differences between data sets were determined with Student's paired t test and considered significant when P < 0.05.
Results
Expression of copGFP and ANO1 in ICC
Tunica muscularis of small intestine from Kit+/copGFP mice was labelled with Kit antibodies to verify that copGFP is expressed in ICC. Previous studies have shown that Kit-like immunoreactivity (Kit-LI) is a specific label for interstitial cells of Cajal (ICC) in the murine intestine (Ward et al. 1994). Examination of whole mount preparations revealed that all cells that expressed copGFP also expressed Kit-LI (Fig. 1A–C). Double labelling immunohistochemistry was also performed using anti-Kit and anti-ANO1 antibodies. We found that cells with Kit-LI also expressed ANO1-LI, as others have reported (Fig. 1D–F; Gomez-Pinilla et al. 2009; accompanying paper: Hwang et al. 2009). Dispersed copGFP+ cells (ICC) were also labelled with anti-ANO1 antibodies (Fig. 1G–I). Other cells in the mixed cell populations obtained by enzymatic digestion of intestinal muscles were ANO1-negative (Fig. 1J). Thus, expression of ANO1 protein persisted in copGFP+ cells after enzymatic dispersion.
Isolation of chloride-permeable ionic conductance in ICC
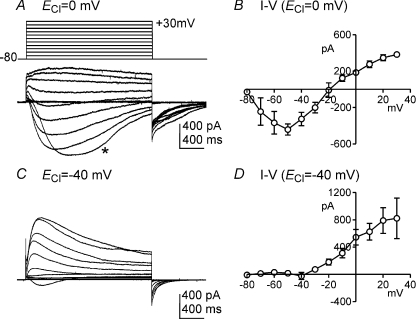
The conventional configuration of the patch clamp technique with cell dialysis was used for whole-cell experiments. External and pipette solutions were CaPSS and pipette solution I, respectively (ECl= 0 mV). The average capacitance of single ICC was 5.5 ± 0.5 pF under these conditions. Cells were held at −80 mV and depolarization steps from −80 to +30 mV in 10 mV increments yielded large transient membrane currents (Fig. 2A) averaging −442 ± 60 pA at maximum (−50 mV; n= 4). The currents evoked in ICC had the general characteristics of ‘autonomous currents’ observed in studies of ICC from the murine small intestine previously (Goto et al. 2004); however, as shown below, the conductance responsible for the large amplitude inward currents in ICC displayed different properties than previously reported. The evoked currents were inward at negative potentials and reversed at about −20 mV (Fig. 2B). We also examined the current–voltage (I–V) relationship using pipette solution II (ECl=−40 mV). Small net inward currents (average =−23 ± 52 pA; n= 4) were elicited by stepping voltage to −40 mV under these conditions, but outward currents were generated at all potentials positive to −40 mV (Fig. 2C and D). Large amplitude inward tail currents were observed upon returning to −80 mV at either value of ECl. These data suggest that a major component of whole-cell currents elicited by depolarization in ICC under physiological ionic gradients is carried by chloride ions. However, K+ conductances are likely to have contributed to the whole cell currents using pipette solutions I and II.
Figure 2. Whole cell currents of ICC dialysed with pipette solution I (K+-rich solution).
A and C, ICC was depolarized from −80 to +30 mV in 10 mV increments from a holding potential of −80 mV. When ECl of internal solution set at 0 mV (pipette solution I), inward currents were evoked at potentials negative to −20 mV (A). Current reversed at approximately −20 mV. Depolarization to −50 mV resulted in activation of inward current, but development of the current response (*) was delayed by 330 ms. Repolarization to −80 mV at the end of the depolarization steps yielded long-lasting tail currents. When ECl was adjusted to −40 mV (pipette solution II), little inward current was noted, and outward currents were observed at test potentials to −40 mV (C). B and D, summary current–voltage (I–V) relationships from experiments with pipette solutions I (n= 4) and II (n= 4), respectively.
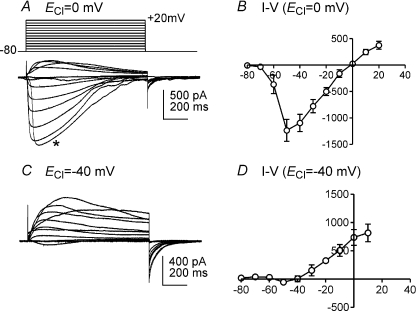
To eliminate contamination from K+ conductances, additional I–V protocols were performed with cells dialysed with pipette solution III (K+ concentration of pipette solution I was replaced with equimolar Cs+, ECl= 0 mV) to inhibit K+ currents. Under these conditions, inward currents were elicited at −60 mV, peaked at −50 mV (−1474 ± 382 pA, n= 4, Fig. 3A and B) and reversed near 0 mV (Fig. 3B). When ICC were dialysed with pipette solution IV (ECl=−40 mV; Cs+ replacement of intracellular K+), small inward currents were evoked at −50 mV but outward currents were elicited at potentials positive to −40 mV (Fig. 3C). The evoked currents reversed at about −40 mV (Fig. 3D). To confirm the reversal potential obtained during the I–V plots, additional experiments were performed to analyse the I–V relationship of tail currents.
Figure 3. Whole cell currents in ICC dialysed with pipette solution III (Cs+-rich solution).
A and C, ICC was depolarized from −80 to +20 mV in 10 mV increments from a holding potential of −80 mV. When ECl was adjusted to 0 mV (pipette solution III) and outward currents were blocked by replacement of intracellular K+ with Cs+, inward currents were evoked at negative potentials. Current responses reversed at 0 mV (A). Depolarization to −60 mV resulted in activation of inward current, but development of the current response (*) was delayed by 95 ms. When ECl was adjusted to −40 mV (pipette solution IV), outward currents were elicited by potentials positive to −40 mV (C). B and D, summary current–voltage (I–V) relationships using pipette solutions III (n= 6) and IV (n= 4), respectively.
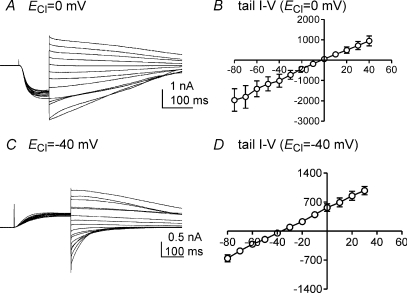
Tail currents were analysed by applying a pre-test step from −80 mV to −40 mV to maximally activate the inward current. The evoked current displayed inactivation, so short-duration (100 ms) pre-test steps were used. Instantaneous tail currents were recorded by stepping from −40 mV to potentials ranging from −80 mV to +40 mV (Fig. 4A). With pipette solution III (ECl= 0 mV), tail currents reversed at 0 mV (n= 4, Fig. 4B). Outward currents were evoked from a holding potential of −80 mV by a pre-test depolarization to 0 mV when cells were dialysed with pipette solution IV (ECl=−40 mV, Fig. 4C). The calculated reversal potential was −39 ± 4 mV with a linear regression plot (n= 4, Fig. 4D). These data suggest that the inward current evoked in ICC by depolarization is due to a Cl− selective conductance.
Figure 4. Reversal of tail currents in ICC.
A and C, reversal potentials were measured by analysis of instantaneous tail currents using CaPSS in the bath and pipette solution III and IV. In A the cell was stepped to −40 mV to activate inward current from a holding potential of −80 mV (pipette solution III, ECl= 0 mV). After full activation of inward currents (100 ms), the cell was stepped to various potentials from −80 to +40 mV in 10 mV increments. In C the cell was dialysed with pipette solution IV and stepped to 0 mV to activate outward current from a holding potential at −80 mV (ECl=−40 mV). After full activation of outward currents (200 ms), cells were stepped to various potentials from −80 to +40 mV in 10 mV increments. B and D, summaries of tail current I–V relationships from pipette solution III (n= 4) and IV (n= 4), respectively. The measured reversal potentials were 0 mV (B) and −39 mV (D) under these conditions.
Characteristics of chloride-permeable ionic conductance
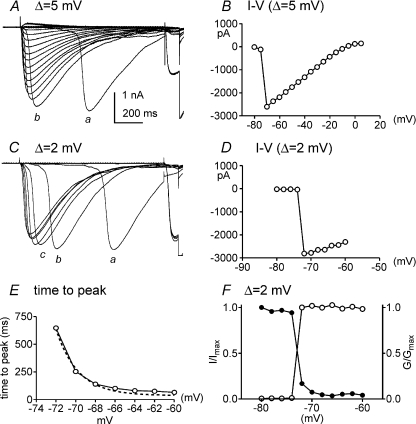
We further examined the kinetics, and voltage dependence of activation and inactivation of the chloride-permeable inward current using pipette solution III (ECl= 0 mV). In experiments like the one shown in Fig. 3A, we noted a very sharp increase in maximal current between −70 and −50 mV. At more positive potentials the amplitude of the current fell linearly with the driving force on Cl−. We also noted an odd delay in the activation of currents by steps to −60 mV (see trace denoted by asterisk in Fig. 3A), although peak current amplitude was activated at this potential. Thus, we explored the activation threshold for current and the voltage dependence and kinetics of the current using depolarization step intervals of 5 mV and 2 mV from a holding potential of −80 mV using pipette solution III (Fig. 5A and C).
Figure 5. Threshold and voltage dependence or activation and inactivation of inward currents.
The threshold for activation of inward currents was investigated using 5 mV and 2 mV increments. Cells were bathed in CaPSS, and pipette solution III (ECl= 0 mV) was used for these experiments. In A currents were elicited by voltage steps from −80 to +10 mV with 5 mV increments from a holding potential of −80 mV. A summary I–V relationship from 4 cells shows a sharp threshold for activation of current and a linear relationship between current and voltage positive to −65 mV (B). Note the delay, but eventual activation of full magnitude currents, with steps to −65 mV (a) and −60 mV (b). In C current were elicited in the same cell as in A using 2 mV increments between steps from −80 to −60 mV to measure times to peak current of each potential tested. Note the delays, but eventual activation of currents of full magnitude, with steps to −72 mV (a), −70 mV (b) and −68 mV (c). D, summary of the I–V relationship for 4 cells. Again, there was a sharp threshold at −72 mV and a linear relationship between current and voltage positive to −72 mV. Times-to-peak current responses are plotted in E from an experiment using 2 mV increments. The time-to-peak current slows at negative test potentials, suggesting a voltage-dependent mechanism may underlie activation of the inward currents. Voltage vs. activation and inactivation are plotted in F (data analysed from currents in C). Activation (normalized to maximum conductance, G/Gmax) and inactivation (normalized to maximum current, I/Imax) could not be fitted with Boltzmann equations suggesting the inward currents are not voltage dependent.
Using 5 mV step increments, the threshold potential for activation of the inward current was −70 mV (Fig. 5B). This was refined to −72 mV using 2 mV increments (Fig. 5D). The time to peak current (measured as the time from the application of the depolarization step to the peak of the evoked inward current) was dramatically dependent upon voltage over a very narrow range of potentials. For example, with 2 mV steps, the time to peak current was 651 ms at −72 mV and 90 ms at −64 mV (Fig. 5E). We also noted that the current amplitude was not affected by voltage other than linear dependence upon the driving force for Cl−. Once the conductance was activated, currents of maximal amplitude developed (and the maximal current amplitude was only affected by the change in driving force for Cl− ions; Fig. 5B). The same sharp activation threshold was noted in cells with low current density.
We also analysed the voltage dependence of activation and inactivation of chloride-permeable inward currents using 2 mV voltage-step increments. The activation and inactivation properties were poorly fitted with Boltzmann equations, suggesting that the Cl−-permeable conductance responsible for the inward current activated by depolarization is not itself voltage dependent (Fig. 5F). A secondary voltage-dependent process (e.g. Ca2+ entry or Ca2+-induced Ca2+ release) may be responsible for activating the inward current (see Fig. 5C).
Ca2+ dependence of chloride-permeable conductance and channels
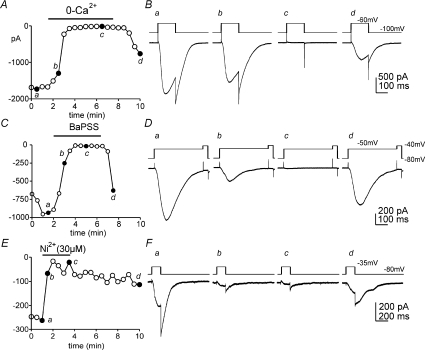
We next examined the Ca2+ dependence of the chloride-permeable currents in ICC. For these experiments we used Ca2+-free solution (i.e. Ca2+ removed from the external buffer). The chloride-permeable inward currents were completely abolished by Ca2+-free solution (Fig. 6A and B) and reversed upon restoration of [Ca2+]o in the CaPSS (see protocol Fig. 6B inset). We also examined the effects of replacing [Ca2+]o with Ba2+ (i.e. BaPSS in which 2 mm Ca2+ was replaced with equimolar Ba2+) on chloride-permeable inward currents. BaPSS also inhibited inward currents (Fig. 6C and D), and the currents were restored by restoration of [Ca2+]o. These data suggest that the chloride-permeable currents are Ca2+ dependent or may depend upon the entry of Ca2+ for initiation of the current. We also tested of Ni2+, a blocker of low-threshold voltage-dependent Ca2+ channels. The Cl− current was blocked by Ni2+ (30 μm; n= 5; Fig. 6E and F).
Figure 6. Effects of external Ca2+ on inward currents in ICC.
In A peak currents were recorded repetitively by stepping cells from −100 mV to −60 mV every 30 s (pipette solution III; ECl= 0 mV). External CaPSS was replaced with Ca2+-free (0-Ca2+) solution (black bar). Reduction in external Ca2+ caused inhibition of inward current, and this effect was reversible upon restoration of CaPSS. B, representative traces (a–d) before, during and after removal of Ca2+ at time points indicated in A. C, peak currents were recorded by stepping repetitively from −80 mV to −50 mV every 30 s. External Ca2+ (CaPSS) was replaced with equimolar Ba2+ (2 mm; BaPSS; black bar). Peak currents were inhibited by BaPSS, and this effect was reversible when CaPSS was restored. Representative traces from the time points indicated in C are shown in D (a–d). E, peak currents were recorded by stepping repetitively from −80 mV to −35 mV every 30 s. Ni2+ (30 μm, black bar) added to the CaPSS inhibited the inward current. The effects of Ni2+ were slow to wash out. Representative traces from the time points indicated in E are shown in F (a–d).
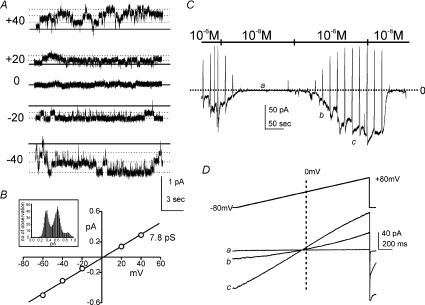
We performed experiments using excised patches from ICC and symmetrical gradients of NMDGCl (140/140 mm) to determine whether Ca2+-dependent Cl− channels are expressed by ICC (as immunohistochemical studies implied). Under the conditions of these experiments only Cl− channels could be detected. Channel openings with a single channel conductance of 7.8 ± 0.2 pS (n= 4) were observed, and currents reversed at 0 mV (Fig. 7A and B). Ramp depolarization of patches (i.e. −80 mV to +80 mV) elicited currents that were greatly increased in amplitude when the cytoplasmic surface of the patches was exposed to high Ca2+ (10−6m) vs. low Ca2+ (10−8m). The low concentration of Ca2+ (10−8m) decreased the open probability of 7.8 pS channels (Fig. 7C and D). Reversal potential (0 mV) was unchanged by differences in [Ca2+]i. These experiments demonstrate that the 7.8 pS Cl− channels in ICC are activated by Ca2+. These channels are potentially responsible for the Ca2+-activated Cl− currents identified in whole-cell experiments.
Figure 7. Single channel currents in ICC.
Patches were excised from ICC to study single channel conductances that might contribute to inward currents. A, the external and internal solutions were symmetrical NMDGCl (140/140 mm). Under these conditions, only Cl− could be recorded. The calculated free Ca2+ concentration was 10−6m. The representative traces at various holding potentials show channel openings (dotted lines to denote unitary current levels; the 0 current level is denoted by the continuous line in each trace). An all-points histogram describing currents at −40 mV is shown as an inset in B. The single channel currents reversed at 0 mV (continuous line). B, a plot of the average current–voltage relationships from 5 patches (error bars contained within symbols). The calculated single channel conductance by linear regression was 7.8 pS. C, cytoplasmic [Ca2+] was changed from 10−6 to 10−8m. The low [Ca2+] reduced or abolished channel openings. Ramp protocol from −80 mV to +80 mV from a holding potential of −80 mV were applied to the patches. Low [Ca2+] (10−8m) completely abolished currents. These experiments were repeatable. The 0 current level is denoted by the dotted line. D, representative current traces elicited by ramp protocols applied at the points noted in C ([Ca2+]= 10−6m, a; intermediate value during exchange, b; and Ca2+= 10−8m, c).
Niflumic acid sensitivity of Ca2+-activated chloride currents and its functional role on pacemaker potentials
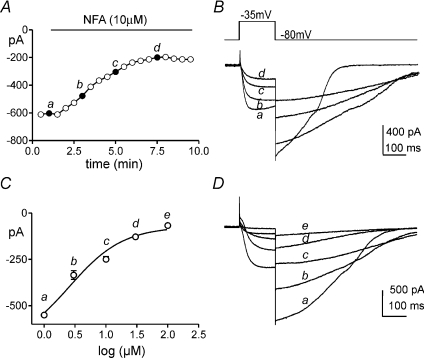
We tested the effect of niflumic acid on inward current elicited in ICC by depolarization. Using pipette solution III, cells were stepped to −35 mV every 30 s from a holding potential of −80 mV. The peak current was plotted as a function of time. Niflumic acid (10 μm) decreased the inward current from −761 ± 75 pA to −162 ± 33 pA (n= 5; P < 0.01, Fig. 8A and B). Various concentrations of niflumic acid (1–100 μm) were also tested to determine the concentration–response relationship for block of the inward current. An IC50 of 4.8 μm (Hill slope = 0.34) was calculated from these experiments. It should be noted that although niflumic acid reduced the amplitude of the inward current, the kinetics of deactivation of the current were slowed by this compound (Fig. 8B).
Figure 8. Inhibition of inward currents by niflumic acid.
A, currents were elicited by stepping from −80 to −35 mV every 30 s. Peak currents are plotted as a function of time. Niflumic acid (NFA, 10 μm) decreased the amplitude of the inward currents. Representative traces taken at the time points noted in A are shown in B (a–d). Note the slowing of the inactivation kinetics in response to NFA. The concentration-response relationship for the inhibitory effects of niflumic acid (1–100 μm) are shown in C and fit with a sigmoidal function (Hill equation). The calculated IC50 was 5 μm (Hill slope = 1.38, n= 5). Representative currents elicited by voltage steps from −80 to 0 mV show the effects of 1 μm (a), 5 μm (b), 10 μm (c), 30 μm (d) and 100 μm (e) niflumic acid in one experiment (Panel D).
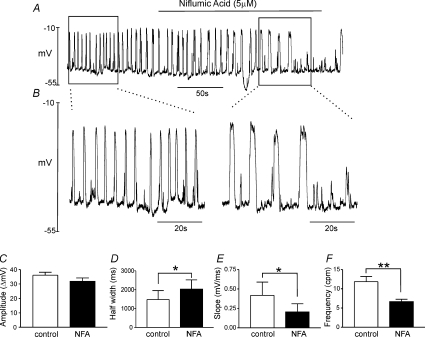
Current clamp experiments were also performed. We have previously shown that ICC discharge small amplitude, unitary potentials in current clamp conditions (Kim et al. 2002). Thus, a spontaneous pacemaker mechanism exists in ICC that can depolarize cells to the threshold for the generation of the large inward current measured under voltage-clamp. Switching to current clamp (I= 0), we observed large-amplitude, spontaneous oscillations in membrane potential in ICC dialysed with pipette solution I (Fig. 9). The resting membrane potentials (RMP) of cells (maximum potential between voltage oscillations) averaged −51 ± 3 mV (n= 6). The amplitude of the spontaneous oscillatory membrane potentials was 36 ± 2 mV and these events occurred at a frequency of 11.8 ± 1.4 cycles min−1. The half-duration of the oscillations was (1478 ± 477 ms) and maximal upstroke velocity was (0.42 ± 0.17 mV ms−1; measured from 10% of RMP to 90% of peak amplitude). Niflumic acid (5 μm) did not significantly affect RMP (−47 ± 2 mV) and the amplitude of spontaneous oscillatory membrane potentials (32 ± 2 mV), but increased the half-duration of the transient depolarizations to 2029 ± 489 ms (P < 0.05) and decreased maximal rising slope (0.21 ± 0.10 mV ms−1, P < 0.05) and frequency (6.67 ± 0.61 cycles min−1, P < 0.01) significantly (n= 6). The increase in half-duration of the membrane potential oscillations is consistent with the observation that niflumic acid slowed the kinetics of the deactivation of the inward current. Higher concentrations of niflumic acid (>10 μm) blocked the large amplitude spontaneous membrane potential oscillations, but did not block the underlying small unitary potential activity. These data suggest that Ca2+-activated Cl− currents are involved in spontaneous slow waves in ICC.
Figure 9. Effects of niflumic acid on membrane potential in ICC under current clamp.
Spontaneous membrane potential oscillations were recorded in single ICC under current clamp (I= 0). CaPSS is the external solution and pipette solution I was used for the data recorded in A. Application of niflumic acid (NFA, 5 μm) decreased the frequency of spontaneous oscillations without affecting resting membrane potentials (most negative potential between oscillations). When regular oscillations were inhibited by niflumic acid, smaller amplitude noisy transient depolarization potentials were still present. The inhibitory effects of niflumic acid were reversible upon washout. B, data during the periods denoted in A by the boxes before and during NFA are shown at higher sweep speed. C–F, the effects of niflumic acid on amplitude (C), half-width (D), maximum slope (E) and frequency (F) from 6 cells. *P < 0.05, **P < 0.01.
Discussion
In the present study we measured large amplitude inward currents in ICC, identified by constitutive expression of copGFP, with some characteristics similar to the ‘autonomous’ currents previously reported by Goto and coworkers (2004). The inward currents in ICC were activated by depolarization, but the conductance underlying these currents does not appear to be intrinsically voltage dependent, as a very sharp threshold voltage was detected for activation and inactivation, and, once exceeded, full activation and inactivation processes occurred. Furthermore, the activation and inactivation properties of the inward current were poorly fit by Boltzmann functions. It appears that another voltage-dependent process underlies depolarization-induced initiation of the large inward current in ICC. At potentials close to the threshold for activation, we also noted dramatic and atypical time-dependent characteristics in which the activation of the current was delayed for periods of time that decreased as a function of voltage over a narrow range of potentials near threshold. When whole cell currents were free from contamination by K+ currents, the reversal potential for the current closely followed ECl. Reversal of tail currents demonstrated that the large inward currents in ICC were due to activation of a Cl− conductance, in contrast to the conclusions of Goto and coworkers (2004). We found that the inward current was blocked by low concentrations of niflumic acid, and this compound blocked the spontaneous, slow wave-like depolarizations in ICC under conditions of current clamp. Slow waves were also blocked by niflumic acid in intact GI muscles (Hwang et al. 2009); however the block of spontaneous depolarizations in single ICC occurred at lower concentrations than block of slow waves in intact muscles. In single channel experiments we found Ca2+-activated Cl− channels with a unitary conductance of 7.8 pS in excised membranes of ICC. This conductance is equivalent to the single channel conductance of ANO1 channels expressed in HEK cells (i.e. 8 pS; Yang et al. 2008). Ca2+-activated Cl− channels of this magnitude have also been recorded in endothelial cells (Nilius et al. 1997) and hepatocytes (Koumi et al. 1994); however the molecular identity of these channels has not yet be determined.
Discovery and initial characterization of an inward current activated by depolarization in freshly dispersed Kit-positive cells of the murine small intestine was an important contribution to understanding the pacemaker activity of ICC (Goto et al. 2004). The currents recorded previously (Goto et al. 2004) were termed ‘autonomous’ because once activated, the current persists in spite of repolarization, as evidenced by persistence of long-duration tail currents upon repolarization (as in Fig. 4). The inward current we observed in ICC is not ‘autonomous’ in the strict sense of the word, and its kinetics are more likely a function of the dynamics of intracellular Ca2+. The contribution of this conductance to slow waves in ICC and in intact muscles (Hwang et al. 2009) suggests that ‘slow wave current’ is a better descriptive term for this current. The channels responsible for and/or the mechanism of voltage-dependent activation of inward current are lost in cultured cells within a few days (as are other features fundamental to the ICC phenotype, such as Kit expression; K. M. Sanders and S. Ro, unpublished observation). We failed to resolve this conductance in previous characterizations of pacemaker activity in cultured ICC (e.g. Koh et al. 2002). Thus, models that we, and others, have proposed in the past to describe pacemaker activity, which are based on periodic activation of non-selective cation currents, are incomplete (e.g. Sanders et al. 2006; Youm et al. 2006; Faville et al. 2008; Faville et al. 2009). Non-selective cation conductances may still be responsible for the spontaneous transient inward currents that underlie unitary potentials in ICC, because Cl− channel blocking drugs did not inhibit these events in intact muscles (Hwang et al. 2009) and in isolated ICC (this study). Constitutive labelling of ICC by expression of copGFP greatly facilitates identification of ICC in mixed cell populations. This animal model will enable future studies of excitability and the pacemaker mechanism in ICC.
The current study identified several important new properties of the large inward current in ICC. Of primary importance, the conductance responsible for depolarization-activated inward currents is Cl− selective, as determined by the reversal of currents and tail currents at ECl. The properties of the current suggested that the Cl− conductance itself is not voltage dependent, but rather it is activated by an underlying voltage-dependent process. Threshold for activation of the inward current under voltage clamp was quite negative (approximately −70 mV), and near-threshold depolarization resulted in delayed activation of full-amplitude currents. Significant delays between application of a stimulus and activation of a slow wave (termed by the authors –‘regenerative potentials’) have also been observed in response to depolarizations of small muscle bundles that slightly exceeded threshold (Hirst et al. 2002). The latency of activation decreased as a function of the intensity of the depolarizing current. Although latencies were longer in intact muscles than in isolated cells, in both cases the latencies between depolarization and activation exceeded the normal time constants for voltage-dependent activation of ion channels (millisecond range) by orders of magnitude. Causes for this latency have been suggested to include activation of an underlying voltage-dependent current (possibly a Ca2+ conductance; Kim et al. 2002) that might result in (i) entry of Ca2+ and Ca2+-induced release of Ca2+ from intracellular stores or (ii) voltage-dependent activation of phospholipase C, increased IP3 production and release of Ca2+ from intracellular stores (Ganitkevich & Isenberg, 1993; Hirst et al. 2002). Either process might be capable of entraining Ca2+ release and activation of a Ca2+-activated Cl− conductance. Our data favour the former mechanism of activation, however, because low concentrations of Ni2+ also blocked depolarization-induced activation of inward current.
The large inward current activated by depolarization in ICC appears to be Ca2+ dependent. We found that the current was reduced within 1 min by removal of extracellular Ca2+ or its replacement with Ba2+. Thus, entry of Ca2+ and possibly recruitment of Ca2+ release from intracellular stores might be the signal that activates the large inward current. Previous studies have suggested that voltage-dependent activation of low-threshold Ca2+ channels is required for entraining slow waves (Kim et al. 2002). In single channel studies in excised patches, we resolved Ca2+-dependent Cl− channels in ICC, and these channels had the same unitary conductance (7.8 pS) as ANO1 channels expressed in HEK cells (Yang et al. 2008). Genomic and immunohistochemical evidence of high levels of ANO1 expression in ICC and loss of slow waves in muscles of mice with inactivated Tmem16a (Hwang et al. 2009) support the idea that this class of Ca2+-activated Cl− channels is responsible for the Ca2+-dependent inward currents in ICC. Experiments on ICC from Tmem16a−/− animals would tighten this correlation further, but Tmem16a−/− mice, which have limited post-natal viability, with copGFP-labelled ICC have not yet been produced.
By superficial comparison, the time course and kinetics of inward current in ICC and the current–voltage relationship for this current do not closely resemble Ca2+-activated Cl− currents carried by ANO1 expressed in HEK293 cells (see Hartzell et al. 2009). However, the differences in characteristics are likely to be due to the dynamics of cytoplasmic Ca2+ concentration ([Ca2+]c), which may change in ICC during the initiation and time course of the inward current. Subcompartments in ICC may also influence the [Ca2+] available to interact with channels. Pure ANO1 currents are outwardly rectifying and carry little inward current at submaximal [Ca2+]c, but as [Ca2+]c rises the current–voltage relationship becomes linear (Schroeder et al. 2008). The conductance responsible for inward current in ICC carries significant inward current at negative potentials and is relatively linear (see Figs 4 and 5). Thus, activation of inward current may be due to entry and/or liberation of Ca2+ in ICC, raising [Ca2+] near the plasma membrane to levels that facilitate inward current (i.e. >1 μm). The time course and kinetics of the inward current in ICC are also likely to be influenced by the dynamics of [Ca2+]. In experiments on expressed ANO1 in axolotl oocytes, brief laser pulses (250 ms) to uncage IP3 resulted in currents and membrane potential changes lasting for several seconds (Schroeder et al. 2008). The kinetics of these currents presumably followed the time course of [Ca2+]c that was elevated in oocytes for a period that far exceeded the duration of the stimulus. Thus, ANO1 currents can be initiated by brief stimuli and then follow the time course of intracellular Ca2+ dynamics and possibly other regulatory mechanisms. This is analogous to the inward current we measured in ICC, which can be initiated by depolarization, but once threshold is achieved, intracellular regulatory mechanisms (presumably changes in [Ca2+]) determine the magnitude and time course of the current.
Previous studies have reported a variety of conductances in ICC, including (i) voltage-dependent Ca2+ channels (Kim et al. 2002); (ii) ether-à-go-go-related K+ channels (Zhu et al. 2003; McKay et al. 2006); (iii) ATP-sensitive K+ channels (Choi et al. 2006), (iv) Ca2+-activated K+ channels (Fujita et al. 2001; Zhu & Huizinga, 2008); (v) delayed rectifier (KV1.1) channels (Hatton et al. 2001); and (vi) inwardly rectifying chloride channels (Zhu et al. 2005). From these studies a variety of mechanisms have been proposed to explain the spontaneous voltage transients in GI muscles, known as slow waves, but no previous studies have described the Ca2+-activated Cl− conductance activated by depolarization that we observed in individually indentified ICC. Others have proposed a role for the Cl− conductances in ICC pacemaking (Huizinga et al. 2002; Zhu et al. 2005); however the conductances described were not Ca2+ sensitive and no mechanism was proposed for spontaneous and periodic activation of these conductances as must occur in slow wave activity. It must be recognized that in previous studies each cell tested was not definitively identified as an ICC. Instead, cells were selected because they displayed morphology similar to the cells labelled by Kit antibody (e.g. Koh et al. 2002). There are numerous cell phenotypes present in dispersions of GI muscle tissues, and it is abundantly clear that the phenotypes and morphologies of cells change dramatically in cell cultures and explants. Compounding this problem, ICC, smooth muscle cells and fibroblast-like cells form gap junctions with each other, and therefore, unless a cell is physically isolated from others, it is not possible to be certain that conductances measured are the exclusive properties of the cell with which the giga seal is formed. In cell dispersions from the mouse intestine, cells with copGFP fluorescence were small (5 pF cell capacitance) and round, some displayed short processes (see Fig. 1). Enzymatic dispersion may break off or limit the extent of cell processes. These cells were clearly isolated from other cells as demonstrated by the low capacitance and cell access. Our data and initial observations from studies of ICC that are constitutively labelled suggest that many of the published properties of ICC need to be carefully re-examined in light of the problems of identification of ICC and phenotypic changes that develop in various culture conditions (e.g. single cell cultures, explants, and influence of substrate).
Acknowledgments
This project was supported by a research grants from NIDDK, DK41315 and DK40569. S.M.W. was also supported by DK57236. Images were collected using a Zeiss LSM510 confocal microscope obtained with support from NIH1 S10 RR16871. The authors would also like to acknowledge Yulia R. Bayguinov and Nancy Horowitz for excellent technical assistance and Huili Zheng for help with breeding and maintenance of the transgenic animals.
Glossary
Abbreviations
- ANO1
anoctamin 1
- BaPSS
2 mm Ca2+ in CaPSS replaced with equimolar Ba2+
- CaPSS
Ca2+-containing physiological salt solution
- ECl
equilibrium potential of chloride
- GFP
green fluorescent protein
- GI
gastrointestinal
- GISTs
gastrointestinal stromal tumours
- ICC
interstitial cells of Cajal
- KI
knock-in
- Kit-LI
Kit-like immunoreactivity
- NMDG
N-methyl-d-glucamine
- RMP
resting membrane potentials
- Tmem16a
transmembrane protein 16A
Author contributions
M.H.Z., T.W.K. and S.D.K. performed patch-clamp experiments. S.R. and W.Y. generated the copGFP knock-in mice used to isolate ICC. S.M.W. performed immunohistochemistry to verify that copGFP-expressing cells were ICC. S.D.K. and K.M.S. planned experiments, analysed data and wrote the manuscript.
Supplemental material
References
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Chen H, Ordög T, Chen J, Young DL, Bardsley MR, Redelman D, Ward SM, Sanders KM. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics. 2007;31:492–509. doi: 10.1152/physiolgenomics.00113.2007. [DOI] [PubMed] [Google Scholar]
- Choi S, Park CG, Kim MY, Lim GH, Kim JH, Yeum CH, Yoon PJ, So I, Kim KW, Jun JY. Action of imipramine on activated ATP-sensitive K+ channels in interstitial cells of Cajal from murine small intestine. Life Sci. 2006;78:2322–2328. doi: 10.1016/j.lfs.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, Varma S, Corless CL, Heinrich MC, Smith KS, Wang Z, Rubin B, Nielsen TO, Seitz RS, Ross DT, West RB, Cleary ML, van de Rijn M. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. J Physiol. 1999;519:235–250. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faville RA, Pullan AJ, Sanders KM, Smith NP. A biophysically based mathematical model of unitary potential activity in interstitial cells of Cajal. Biophys J. 2008;95:88–104. doi: 10.1529/biophysj.107.122507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faville RA, Pullan AJ, Sanders KM, Koh SD, Lloyd CM, Smith NP. Biophysically based mathematical modelling of interstitial cells of Cajal slow wave activity generated from a discrete unitary potential basis. Biophys J. 2009;96:4834–4852. doi: 10.1016/j.bpj.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Takeuchi T, Saitoh N, Hanai J, Hata F. Expression of Ca2+-activated K+ channels, SK3, in the interstitial cells of Cajal in the gastrointestinal tract. Am J Physiol Cell Physiol. 2001;281:C1727–C1733. doi: 10.1152/ajpcell.2001.281.5.C1727. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphae-mediated Ca2+ transients in guinea-pig coronary myocytes. J Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T, Farrugia G. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1370–G1381. doi: 10.1152/ajpgi.00074.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol. 2004;559:411–422. doi: 10.1113/jphysiol.2004.063875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol. 2009;587:2127–2139. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton WJ, Mason HS, Carl A, Doherty P, Latten MJ, Kenyon JL, Sanders KM, Horowitz B. Functional and molecular expression of a voltage-dependent K+ channel (Kv1.1) in interstitial cells of Cajal. J Physiol. 2001;533:315–327. doi: 10.1111/j.1469-7793.2001.0315a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in [Ca2+]i and Cl− channels. J Physiol. 2002;540:907–919. doi: 10.1113/jphysiol.2001.014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterol. 2002;123:1627–1636. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJA, Britton FC, O’Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM, Ward SM. Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol. 2009;587:4887–4904. doi: 10.1113/jphysiol.2009.176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, So I, Stanfield PR, Kim KW. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129:1504–1517. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 2002;541:797–810. doi: 10.1113/jphysiol.2002.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Yamamoto Y, Suzuki H. Excitation of smooth muscles isolated from the guinea-pig gastric antrum in response to depolarization. J Physiol. 2002;543:155–167. doi: 10.1113/jphysiol.2002.020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Jun JY, Kim TW, Sanders KM. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803–814. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumi S, Sato R, Aramaki T. Characterization of the calcium-activated chloride channel in isolated guinea-pig hepatocytes. J Gen Physiol. 1994;104:357–373. doi: 10.1085/jgp.104.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci U S A. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CM, Ye J, Huizinga JD. Characterization of depolarization-evoked ERG K currents in interstitial cells of Cajal. Neurogastroenterol Motil. 2006;18:324–333. doi: 10.1111/j.1365-2982.2006.00764.x. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Szücs G, Wei L, Tanzi F, Voets T, Droogmans G. Calcium-activated chloride channels in bovine pulmonary artery endothelial cells. J Physiol. 1997;498:381–396. doi: 10.1113/jphysiol.1997.sp021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover NG, Sanders KM. Effects of frequency on the waveform of propagated slow waves in canine gastric antral muscle. J Physiol. 1986;371:179–189. doi: 10.1113/jphysiol.1986.sp015967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen L, Robinson TL, Lee JCF, Farraway LA, Hughes MJG, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nature Med. 1998;4:848–851. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- Tokutomi N, Maeda H, Tokutomi Y, Sato D, Sugita M, Nishikawa S, Nishikawa S, Nakao J, Imamura T, Nishi K. Rhythmic Cl− current and physiological roles of the intestinal c-kit positive cells. Pflugers Arch. 1995;431:169–177. doi: 10.1007/BF00410188. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R, Goldblum JR, Brown PO, Heinrich MC, van de Rijn M. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- Youm JB, Kim N, Han J, Kim E, Joo H, Leem CH, Goto G, Noma A, Earm YE. A mathematical model of pacemaker activity recorded from mouse small intestine. Philos Transact A Math Phys Eng Sci. 2006;364:1135–1154. doi: 10.1098/rsta.2006.1759. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Huizinga JD. Nitric oxide decreases the excitability of interstitial cells of Cajal through activation of the BK channel. J Cell Mol Med. 2008;12:1718–1727. doi: 10.1111/j.1582-4934.2008.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Golden CM, Ye J, Wang XY, Akbarali HI, Huizinga JD. ERG K+ currents regulate pacemaker activity in ICC. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1249–G1258. doi: 10.1152/ajpgi.00149.2003. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Mucci A, Huizinga JD. Inwardly rectifying chloride channel activity in intestinal pacemaker cells. Am J Physiol Gastrointest Liver Physiol. 2005;288:G809–G821. doi: 10.1152/ajpgi.00301.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.