Abstract
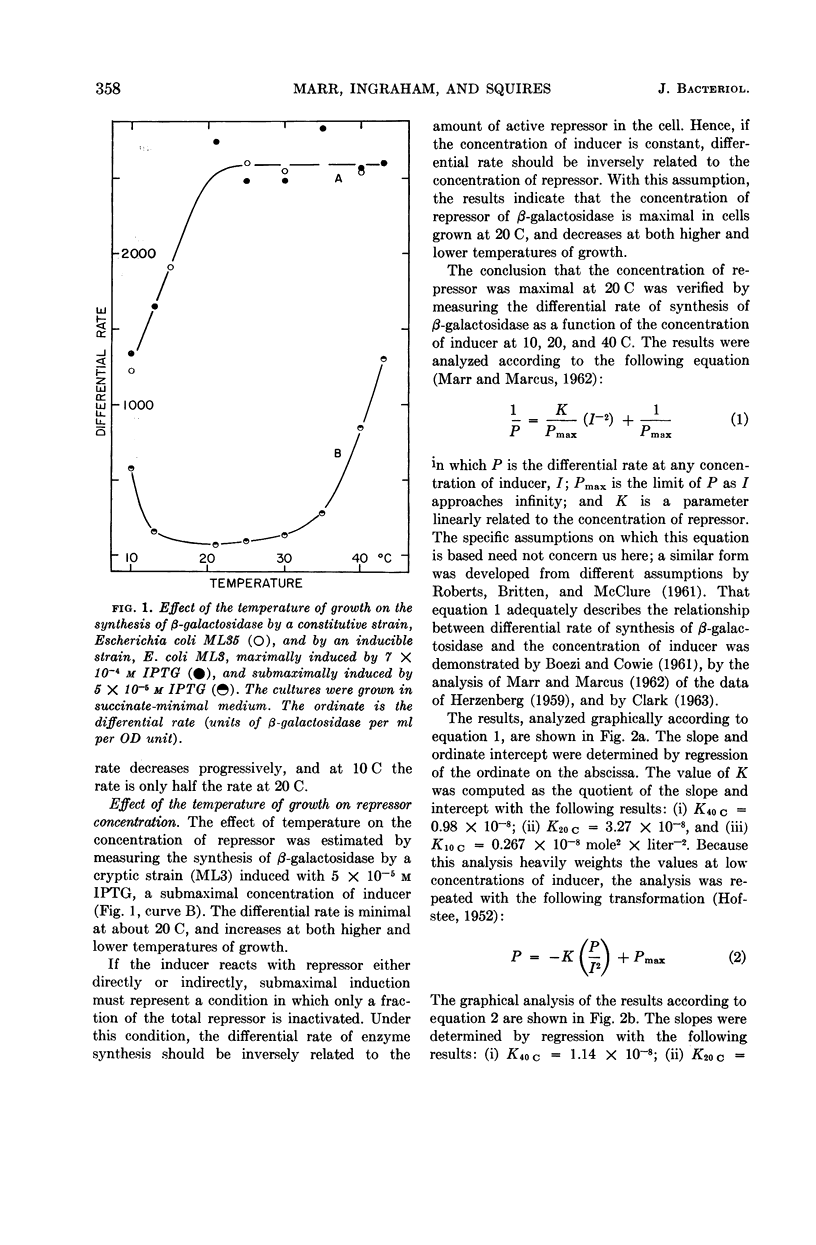
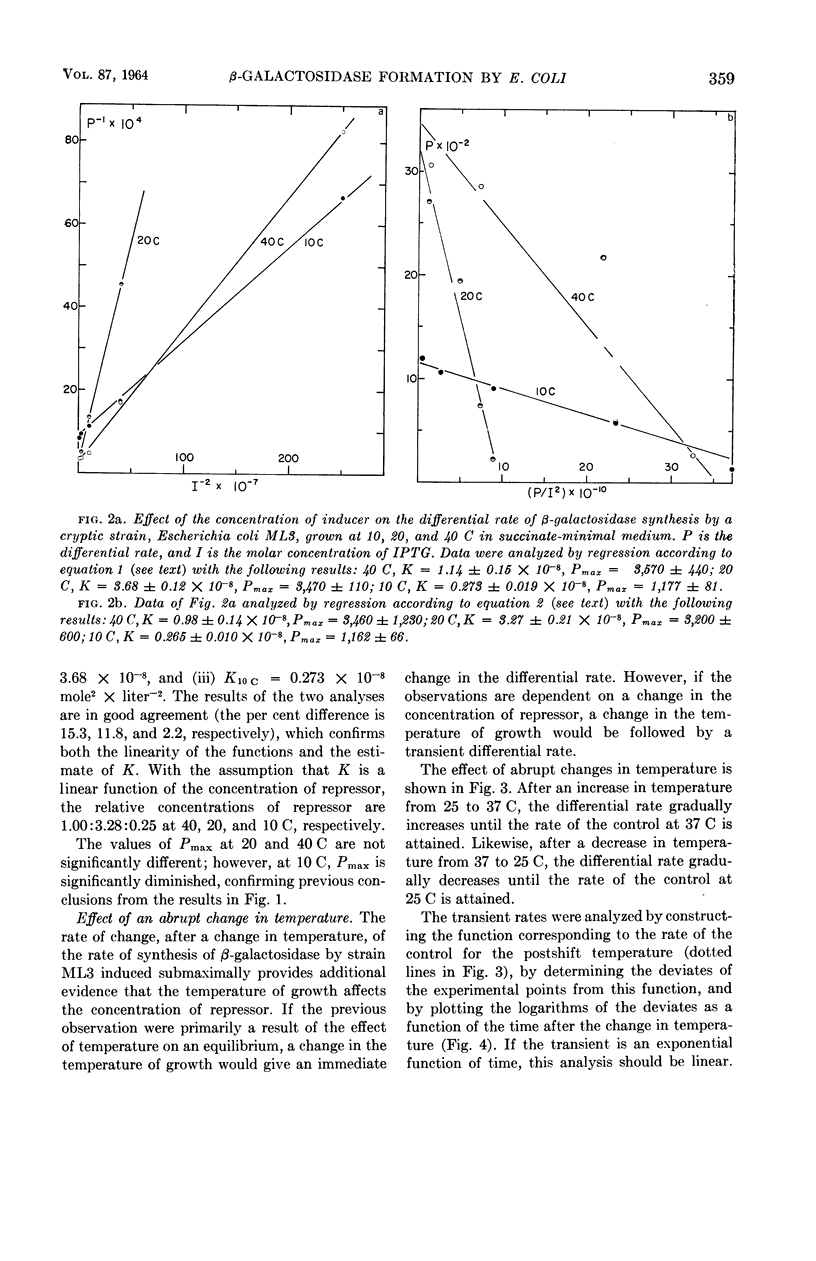
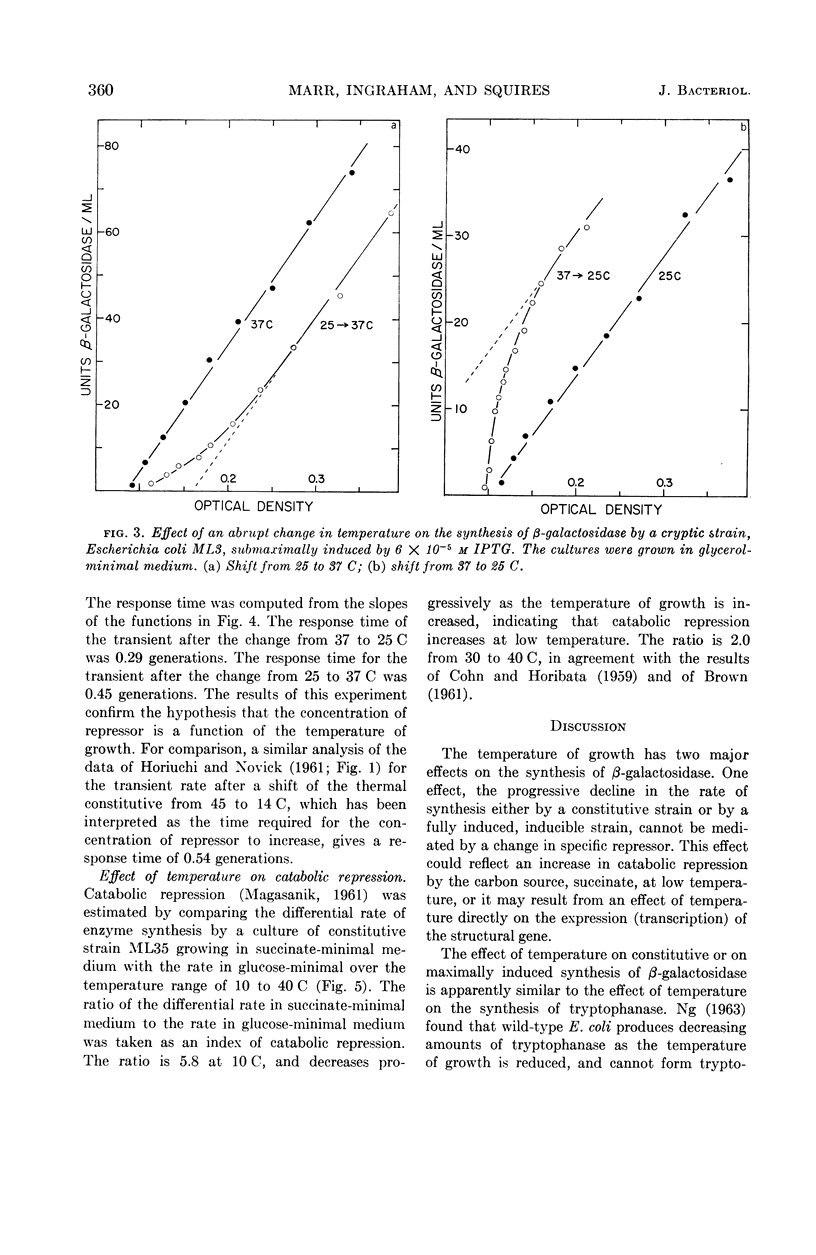
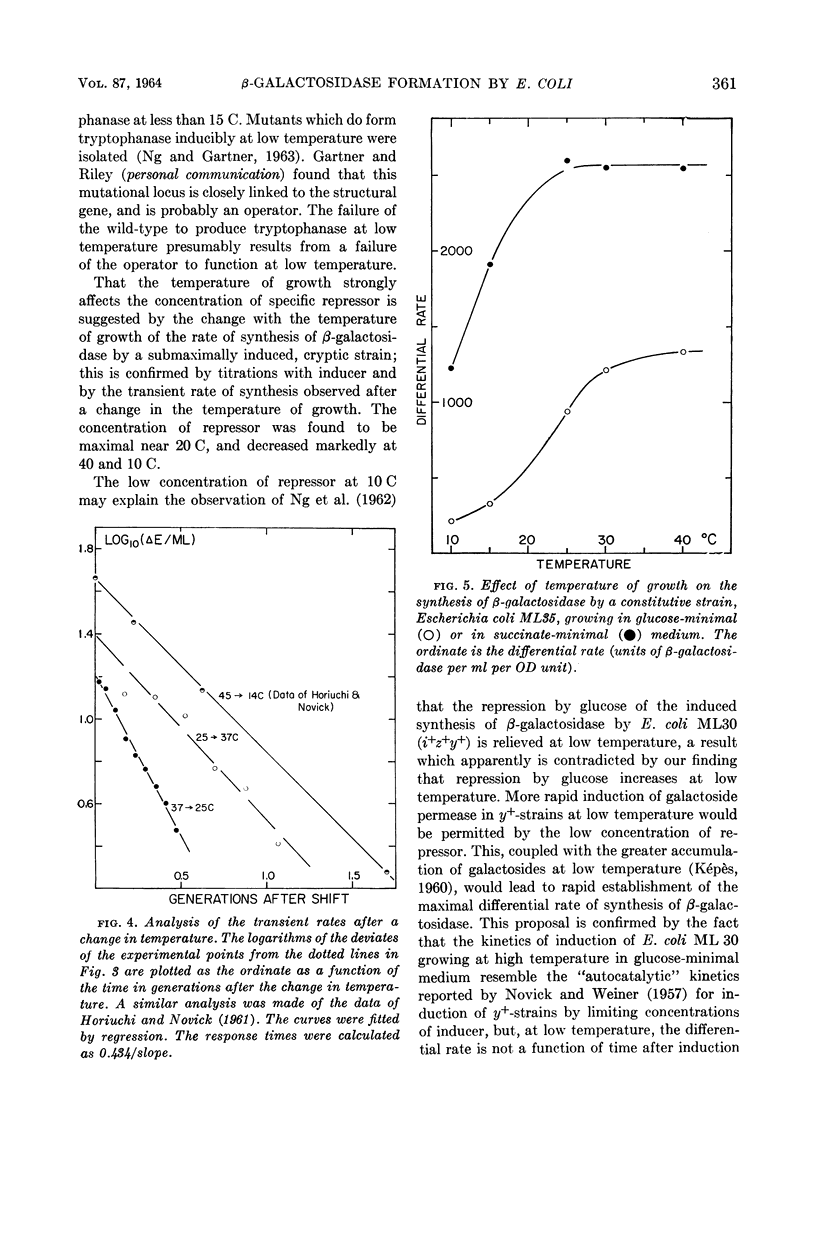
Marr, Allen G. (University of California, Davis), John L. Ingraham, and Craig L. Squires. Effect of the temperature of growth of Escherichia coli on the formation of β-galactosidase. J. Bacteriol. 87:356–362. 1964.—The synthesis of β-galactosidase was measured during exponential growth of Escherichia coli in a succinate-minimal medium over a temperature range of 10 to 43 C for the following: (i) a constitutive strain, and (ii) an inducible cryptic strain, induced maximally with isopropyl-thio-β-d-galactopyranoside (IPTG), or induced submaximally with IPTG. The differential rates of synthesis of β-galactosidase were identical for the constitutive strain and for the fully induced strain; the rates were constant from 20 to 43 C, and decreased progressively with a decrease in temperature below 20 C. Thus, in the absence of specific repression, the ability of E. coli to produce β-galactosidase decreases at low temperature. The differential rate of the submaximally induced culture was minimal between 20 and 30 C, and increased progressively with temperature both above 30 C and below 20 C. That the repressor concentration is maximal at 20 C was established by measuring the rate of induced synthesis of β-galactosidase as a function of the concentration of IPTG; the relative concentrations of repressor were 1.00:3.28:0.25 at 40, 20, and 10 C, respectively. After an abrupt change in temperature, the differential rate of a submaximally induced culture changed gradually to the rate of the steady state, which is in agreement with the proposal that the effect of temperature is on the concentration of repressor and not on the equilibrium between repressor and its site of action. The effect of temperature on catabolic repression was determined by comparing the differential rate of synthesis of β-galactosidase by a constitutive strain grown in succinate-minimal medium with the rate in glucose-minimal medium at various temperatures; the ratio of the rates in the two media decreased progressively and approached 2.0 as the temperature of growth was increased.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOEZI J. A., COWIE D. B. Kinetic studies of beta-galactosidase induction. Biophys J. 1961 Nov;1:639–647. doi: 10.1016/s0006-3495(61)86913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL L. L., Jr, WILLIAMS O. B. The effect of temperature on the nutritional requirements of facultative and obligate thermophilic bacteria. J Bacteriol. 1953 Feb;65(2):141–145. doi: 10.1128/jb.65.2.141-145.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M., HORIBATA K. Physiology of the inhibition by glucose of the induced synthesis of the beta-galactosideenzyme system of Escherichia coli. J Bacteriol. 1959 Nov;78:624–635. doi: 10.1128/jb.78.5.624-635.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLANT J. A. Thermal derepression of alkaline phosphatase synthesis. Biochem Biophys Res Commun. 1962 Aug 31;8:506–510. doi: 10.1016/0006-291x(62)90306-6. [DOI] [PubMed] [Google Scholar]
- HALPERN Y. S. Temperature-dependent inducer requirement for the synthesis of glutamic acid decarboxylase by Escherichia coli. Biochem Biophys Res Commun. 1961 Oct 23;6:33–37. doi: 10.1016/0006-291x(61)90180-2. [DOI] [PubMed] [Google Scholar]
- HERZENBERG L. A. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., NOVICK A. A thermolabile repression system. Cold Spring Harb Symp Quant Biol. 1961;26:247–248. doi: 10.1101/sqb.1961.026.01.030. [DOI] [PubMed] [Google Scholar]
- KEPES A. [Kinetic studies on galactoside permease of Escherichia coli]. Biochim Biophys Acta. 1960 May 6;40:70–84. doi: 10.1016/0006-3002(60)91316-0. [DOI] [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- NG H., GARTNER T. K. Selection of mutants of Escherichia coli constitutive for tryptophanase. J Bacteriol. 1963 Jan;85:245–246. doi: 10.1128/jb.85.1.245-246.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NG H., INGRAHAM J. L., MARR A. G. Damage and derepression in Escherichia coli resulting from growth at low temperatures. J Bacteriol. 1962 Aug;84:331–339. doi: 10.1128/jb.84.2.331-339.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A., Weiner M. ENZYME INDUCTION AS AN ALL-OR-NONE PHENOMENON. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):553–566. doi: 10.1073/pnas.43.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS R. B., BRITTEN R. J., McCLURE F. T. A model for the mechanism of enzyme induction. Biophys J. 1961 Nov;1:649–656. doi: 10.1016/s0006-3495(61)86914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]



