Abstract
Animal studies have indicated that nitric oxide is a key signalling molecule involved in the tonic restraint of central sympathetic outflow from the brainstem. Extension of these findings to humans has been difficult because systemic infusion of nitric oxide synthase (NOS) inhibitors increases blood pressure due to inhibition of endothelial NOS, resulting in activation of the arterial baroreflex and subsequent inhibition of central sympathetic outflow. To overcome this confounding inhibitory influence of the baroreflex, in the current study we directly measured skin sympathetic nerve activity (SNA), which is not under baroreceptor control. Healthy, normotensive humans were studied before, during a 60 min intravenous infusion of the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 4 mg kg−1), and for 120 min following the infusion (i.e. 180 min total). Skin SNA and arterial blood pressure (BP) were continuously measured. BP was increased from baseline at the end of the l-NAME infusion (Δ14 ± 2 mmHg; P < 0.05) and remained significantly elevated for the remainder of the experiment (Δ18 ± 3 mmHg; P < 0.05). Similarly, systemic NOS inhibition produced time-dependent increases in skin SNA, such that skin SNA was elevated at the end of the l-NAME infusion (total activity, 200 ± 22% baseline; P= 0.08) and was further increased at the end of the study protocol (total activity, 350 ± 41% baseline; P < 0.05). Importantly, skin SNA remained unchanged during time and hypertensive (phenylephrine) control experiments. These findings indicate that pharmacological inhibition of NOS causes sympathetic activation and support a role of nitric oxide in central sympathetic control in humans.
Introduction
An estimated one billion people worldwide are hypertensive (Kearney et al. 2005), with greater than 90% of reported cases being classified as idiopathic hypertension (i.e. no identifiable cause) (Korner, 2007). While a multitude of factors have been implicated in the pathogenesis of hypertension, neurogenic mechanisms have been well documented. Indeed, increased sympathetic nerve activity (SNA) has been reported in both animal and human hypertension (Grassi, 2004; Fisher et al. 2009). However, the mechanism(s) driving sympathetic overactivity in hypertensive conditions remains to be elucidated.
It is widely recognized that nitric oxide (NO), produced via the oxidation of l-arginine by nitric oxide synthase (NOS) in the vasculature, is an important signalling molecule involved in the local control of blood flow (Moncada & Higgs, 1993). This classic pathway of endothelium-dependent vasodilatation and its impact on blood pressure has been well documented (Cardillo & Panza, 1998). Less appreciated is the emerging body of animal literature that has provided strong evidence that centrally derived NO is an important component of the signal transduction pathway that tonically restrains sympathetic outflow from the brainstem, placing a brake on α-adrenergic vasoconstriction and subsequent increases in blood pressure (Sander et al. 1995, 1997; Sander & Victor, 1999; Zucker & Liu, 2000; Thomas et al. 2001; Zucker et al. 2004). To date, cardiovascular studies in humans have primarily focused on the peripheral effects of NO with little regard for a role of NO in the central nervous system.
The conceptual framework establishing a major neurogenic role for NO in blood pressure control was derived from acute studies in anaesthetized animals, in which administration of NOS inhibitors into known sites of sympathetic regulation within the brainstem elicited increases in SNA and blood pressure (Zanzinger et al. 1995; Zhang & Patel, 1998). Further support for a central action of NO to restrain SNA was obtained in conscious animals, in which systemic NOS inhibition produced a biphasic SNA response: an initial transient decrease in SNA followed by a sustained increase in SNA (Sakuma et al. 1992; Augustyniak et al. 2006). Importantly, when the confounding influence of baroreflex activation was eliminated by sino-aortic baroreceptor denervation, intravenous NOS inhibition progressively increased SNA (Sakuma et al. 1992; Augustyniak et al. 2006). These data indicate that systemic infusion of NOS inhibitors causes central sympathoexcitation that can be offset by activation of the arterial baroreflex.
A key question is the extent to which these findings in experimental animals can be translated to humans. Although the results of a few human studies have suggested that pharmacologically induced hypertension caused by systemic NOS inhibition may be partially mediated by the sympathetic nervous system (Owlya et al. 1997; Lepori et al. 1998; Sander et al. 1999), results have been equivocal (Hansen et al. 1994; Spieker et al. 2000) and conclusions have been based on indirect indices of SNA (e.g. α-adrenergic blockade) or on direct measurements of SNA confounded by arterial baroreflex buffering. As such, a direct inhibitory action of NO on central sympathetic outflow has been difficult to demonstrate in humans.
The purpose of this study was to test the hypothesis that NO is involved in the tonic restraint of central sympathetic outflow in humans. This was accomplished by directly measuring skin SNA before, during and following systemic infusion of a NOS inhibitor. Cutaneous recordings of SNA were used because skin SNA is not influenced by the arterial baroreflex in normothermic conditions (Delius et al. 1972; Wallin et al. 1975; Wilson et al. 2001; Cui et al. 2004, 2006), whereas it is highly responsive to alterations in central sympathetic outflow (Vissing et al. 1991; Vongpatanasin et al. 1999; Cogliati et al. 2000; Menon et al. 2007). Thus, we hypothesized that an increase in skin SNA during NOS inhibition would provide direct, unequivocal evidence for modulation of central sympathetic outflow by NO in humans.
Methods
General procedures
We studied 15 healthy subjects (10 men; age 25 ± 5 years; height, 172 ± 2 cm; weight, 69 ± 2 kg). All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the University of Missouri-Columbia Health Sciences Institutional Review Board and the Research and Development Committee at the Harry S. Truman Memorial Veterans’ Hospital. After receiving a detailed verbal and written explanation of the intended experimental protocol and measurements, each subject provided written informed consent. No subject had a history or symptoms of cardiovascular, pulmonary, metabolic, or neurological disease and none were taking medications. Subjects were requested to abstain from caffeinated beverages for 12 h and strenuous physical activity and alcohol for at least 24 h before experimental sessions. On experimental days, the subjects arrived at the laboratory a minimum of 2 h following a light meal.
Experimental measurements
All experiments were performed at a constant ambient room temperature of 23–24°C with the subject in the supine position. Heart rate (HR) was continuously monitored using a lead II electrocardiogram (Quinton Q710, Bothell, WA, USA). Arterial blood pressure was measured with an automated sphygmomanometer (Welch Allyn, Skaneatles Falls, NY, USA). Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position around the abdomen (Pneumotrace, UFI, Morro Bay, CA, USA). Multiunit recordings of postganglionic skin sympathetic nerve activity (SNA) were obtained by inserting unipolar tungsten microelectrodes percutaneously through the intact, unanaesthetized skin and positioned into skin nerve fascicles of the peroneal nerve near the fibular head. The nerve signal was processed by a pre-amplifier and an amplifier (Department of Bioengineering, University of Iowa, Iowa City, IA, USA), band pass filtered (bandwidth 700–2000 Hz), rectified and integrated (time constant, 0.1 s) to obtain a mean voltage neurogram. Recordings of skin SNA were considered acceptable when (1) stroking the skin elicited afferent impulses; (2) bursts of neural activity increased in response to arousal stimuli (loud noise), but not during end-expiratory breath-holds or Valsalva manoeuvres; (3) weak electrical stimulation through the microelectrode elicited paresthesias without muscle contraction; and (4) bursts of neural activity displayed no regular pulse synchronicity (Vallbo et al. 1979). Measurements were sampled at 1000 Hz (Powerlab, ADInstruments, Bella Vista, NSW, Australia) and stored for off-line analysis (Chart v5.2, ADInstruments).
Experimental protocols
Protocol 1: Skin SNA responses to systemic NOS inhibition
To examine the effect of systemic NOS inhibition on central sympathetic outflow, skin SNA, arterial blood pressure, heart rate and respiration were measured before, during and after intravenous infusion of NG-nitro-l-arginine methyl ester (l-NAME; 4 mg kg−1) over 60 min (Food and Drug Administration Investigational New Drug no. 75,339). Previous work has suggested an important time component of the sympathetic contribution to the hypertension induced by systemic NOS inhibition (Sander et al. 1997, 1999), and therefore the validity of the time course was verified in pilot studies (n= 5) in which skin SNA was measured for varying durations from 60 to 120 min following l-NAME infusion. These preliminary studies indicated that a total data collection time of 180 min would allow for a comprehensive assessment of the time course and magnitude of the skin SNA responses to systemic NOS inhibition (data not shown). Of the ten subjects in which recordings were made for 180 min, two subjects were not included in the data analysis due to an inability to maintain an acceptable nerve recording in one and because the study was prematurely terminated due to an excessive l-NAME induced increase in blood pressure in the other (ΔMAP of 45 mmHg).
Protocol 2: Time and hypertensive control experiments
Taking into account the length of these experiments and potential influence of arousal and/or discomfort stimuli on skin SNA, time control experiments were performed in a subset of subjects on a separate day (n= 6). In addition, to account for a potential blood pressure raising effect on skin SNA (Vongpatanasin et al. 1999), hypertensive control experiments were performed (n= 3). For these experiments, phenylephrine (0.5–1.5 μg kg−1 min−1) was used to raise arterial blood pressure to the same extent as that observed with l-NAME.
Data analysis
Mean arterial pressure (MAP) was calculated as diastolic pressure plus one-third pulse pressure. Skin SNA was evaluated from the integrated neurogram using a customized computer program (Chart v5.2, ADInstruments). The asymmetry of skin SNA bursts makes calculation of burst frequency difficult (i.e. multiple peaks within one burst) (Hagbarth et al. 1972; Cui et al. 2006; Young et al. 2009) and therefore skin SNA is reported as total integrated activity, which is the sum of the total area under all bursts detected in a given time period. Furthermore, findings from single unit recordings suggest that an increase in skin SNA is primarily governed by recruitment of additional neurons, rather than an increase in firing frequency of individual units, and therefore lends further support for the use of total integrated activity rather than frequency (Macefield & Wallin, 1999). This is particularly important for skin SNA, compared to muscle SNA, in which bursts are not limited by the cardiac cycle and thus recruitment of additional neurons likely contributes to the characteristic wide bursting pattern observed during sympathetic activation (Vallbo et al. 1979; Macefield & Wallin, 1999). A baseline segment of skin SNA was assigned a value of 100, and 5 min segments throughout the experimental protocol were used to compare l-NAME responses to baseline. Due to the intersubject variability in skin SNA, values are expressed as a percentage of the baseline value to provide an estimate of relative changes in integrated activity (Wilson et al. 2001, 2004, 2006).
Statistical analysis
Univariate repeated measures ANOVA with one repeated factor (time) and when appropriate one grouping factor (l-NAME vs. control experiments) was used. Significant main effects were evaluated with Bonferroni's post hoc analysis when appropriate. Statistical significance was set at P < 0.05. Analyses were conducted using SigmaStat for Windows (Systat Software Inc., San Jose, CA, USA). Results are presented as means ± standard error of the mean (s.e.m.).
Results
Systemic NOS inhibition increases skin SNA
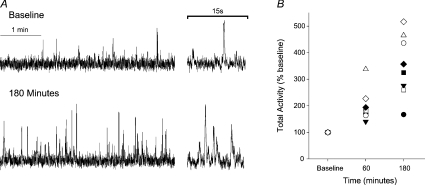
Systemic infusion of l-NAME produced time-dependent increases in skin SNA, such that skin SNA was increased above baseline values by the end of l-NAME infusion (i.e. 60 min; total activity, 200 ± 22% baseline; P= 0.08; Fig. 1B) and was progressively increased up to 3-fold higher at the end of the study protocol (i.e. 180 min; total activity, 350 ± 41% baseline; P < 0.05; Fig. 1A and B). Thirty minutes after the start of the l-NAME infusion, MAP was increased 7 ± 2 mmHg above baseline (P < 0.05), further increased by the end of l-NAME infusion (79 ± 1 vs. 93 ± 2 mmHg, P < 0.05), and remained significantly higher than baseline for the remainder of the experiment (Fig. 2B). Coincident with the rise in MAP, HR was decreased following l-NAME infusion and remained lower than baseline until the end of the study (Fig. 2C).
Figure 1. Systemic NOS inhibition with L-NAME increases skin SNA.
A, original records from one subject illustrating skin SNA at baseline and at the end of the study (i.e. 180 min). B, individual skin SNA responses at the end of l-NAME infusion (i.e. 60 min) and at the end of the study. n= 8.
Figure 2. Summary data showing the time course of skin SNA (A), mean arterial pressure (B) and heart rate (C) responses to time control experiments and l-NAME infusion.
n= 6. *P < 0.05 vs. baseline; #P < 0.05 vs. 30 min; †P < 0.05 vs. 60 min; ‡P < 0.05 vs. 90 min.
SNA remains unchanged during control experiments
In contrast to l-NAME administration, skin SNA remained unchanged during time control experiments (Figs 2 and 3). MAP and HR were also not different from baseline during time control trials (P > 0.05). During phenylephrine administration, in which MAP was increased to a similar extent as l-NAME (e.g. 95 ± 6 vs. 96 ± 2; l-NAME vs. phenylephrine at 120 min; P > 0.05), skin SNA was also unchanged from baseline (Fig. 3). HR was decreased to a comparable extent during these hypertensive control experiments when compared to the l-NAME trial across all time points (P > 0.05).
Figure 3. Skin SNA increases during systemic NOS inhibition, but remains unchanged during time and hypertensive control experiments.
A, original records from one subject illustrating skin SNA following l-NAME in comparison to time and phenylephrine control trials. B, summary data showing the time course of skin SNA responses to l-NAME, time and phenylephrine control trials. n= 3. *P < 0.05 vs. baseline; †P < 0.05 vs. 60 min.
Discussion
The major novel finding of the current study is that pharmacological NOS inhibition causes a robust and sustained increase in central sympathetic outflow in humans. Importantly, by using a baroreflex-independent measure of SNA, such as skin SNA, these results provide direct evidence for central sympatho-excitation following NOS inhibition in humans, clearly revealing the involvement of NO in the tonic restraint of sympathetic outflow from the central nervous system.
Systemic infusion of NOS inhibitors rapidly increases blood pressure due to the inhibition of endothelium-dependent vasodilatation, resulting in activation of the arterial baroreflex and buffering of centrally mediated increases in SNA (Hansen et al. 1994). To overcome this confounding influence of the arterial baroreflex, in the present study we utilized recordings of skin SNA as a method to examine a direct effect of NOS inhibition on central sympathetic outflow. Importantly, under thermoneutral conditions skin SNA, unlike muscle SNA, is not responsive to changes in arterial baroreceptor activity (Delius et al. 1972; Wallin et al. 1975; Wilson et al. 2001; Cui et al. 2004, 2006). We found that skin SNA was significantly elevated up to threefold above baseline after NOS inhibition. This robust increase in sympathetic outflow occurred despite sustained arterial baroreflex activation, as indicated by the significant increase in MAP and subsequent fall in heart rate. Furthermore, skin SNA remained unchanged during time and hypertensive control experiments indicating that the increase in skin SNA was specific to NOS inhibition and could not be attributed to non-specific temporal or blood pressure raising effects.
Interestingly, skin SNA continued to progressively increase for up to 1 h after the infusion of l-NAME was completed and remained elevated for the duration of the protocol (i.e. 180 min). Presumably, this gradual increase reflects the time required for l-NAME to cross the blood brain barrier and inhibit NOS centrally (Dwyer et al. 1991; Iadecola et al. 1994; Salter et al. 1995; Traystman et al. 1995; Ayers et al. 1997). Indeed, previous studies have demonstrated that following systemic NOS inhibition with l-NAME, NOS activity in the brainstem and hypothalamic regions, known areas involved in the regulation of central sympathetic outflow, were time-dependently inhibited (Iadecola et al. 1994; Salter et al. 1995; Ayers et al. 1997). Moreover, Traystman et al. (1995) demonstrated a progressive decrease in brain NOS activity following systemic infusion of NOS inhibitors in a variety of animal species. Although, in these studies, an effect was noted at 30 min, near-complete inhibition of brain NOS did not occur until 120 min after infusion of l-NAME. The time required for NOS inhibitors to cross the blood–brain barrier corresponds with findings of previous studies suggesting that removal of endothelial NO-dependent vasodilatation is the primary mechanism underlying the initiation of the hypertensive response to NOS inhibition, but the sympathetic nervous system plays an important role in the full expression and maintenance of the blood pressure raising effect (Sander et al. 1995, 1997, 1999). The results of the current study utilizing direct recordings of SNA further support and extend this concept by demonstrating a delayed sympathetic response (∼60 min) followed by a progressive and sustained robust increase in SNA after l-NAME infusion. Moreover, by utilizing a baroreflex-independent measure of SNA we were, for the first time, able to demonstrate a clear increase in central sympathetic outflow without having to experimentally manipulate the arterial baroreflex to unmask a potential central effect of NOS inhibition.
Only a few previous studies in humans have been performed to investigate the potential role of NO in central sympathetic regulation, with evidence both for (Owlya et al. 1997; Lepori et al. 1998) and against (Hansen et al. 1994; Spieker et al. 2000) a sympathetic contribution to hypertension induced by NOS inhibition. Our study addresses a number of factors that likely contributed to these equivocal findings. First, as previously discussed, we chose to measure skin SNA to eliminate any confounding effects of the arterial baroreflex. In the previous studies, muscle SNA was measured and pharmacological (e.g. sodium nitroprusside infusion) or other experimental techniques were used to normalize arterial blood pressure after NOS inhibition (Hansen et al. 1994; Owlya et al. 1997; Lepori et al. 1998; Spieker et al. 2000). Although these studies were carefully performed, it is difficult to know if baroreflex activation was completely offset or whether residual inhibitory baroreflex activity could have obscured the full sympatho-excitatory effect of NOS inhibition. Second, we extended the time course of our experimental protocol to monitor SNA up to 120 min after the end of a 60 min l-NAME infusion in order to provide sufficient time for the NOS inhibitor to cross the blood–brain barrier and maximize the opportunity to detect an increase in central sympathetic outflow (Dwyer et al. 1991; Iadecola et al. 1994; Salter et al. 1995; Traystman et al. 1995; Ayers et al. 1997). We based this time course on studies in animals and humans showing that 60–90 min is the minimum time required to detect a sympathetic contribution to l-NAME-induced hypertension (Sander et al. 1997, 1999). In contrast, in the previous human studies SNA was measured only for 15–60 min (Hansen et al. 1994; Owlya et al. 1997; Lepori et al. 1998; Spieker et al. 2000), which may not have been long enough to detect an actual sympatho-excitatory response to NOS inhibition. Third, we used the NOS inhibitor l-NAME in our study in contrast to NG-monomethyl-l-arginine (l-NMMA), which was used in the previous studies (Hansen et al. 1994; Owlya et al. 1997; Lepori et al. 1998; Spieker et al. 2000). Although both arginine analogues are competitive inhibitors of NOS, l-NAME has been shown to be a more potent inhibitor than l-NMMA of NOS activity in the brain (Palacios et al. 1989; Heinzel et al. 1992).
The findings from the current study provide the first evidence in humans that NOS inhibition increases skin SNA. Although skin SNA contains vasoconstrictor, sudomotor, pilo erector and possibly active vasodilator fibres (Hagbarth et al. 1972), whereas muscle SNA reflects vasoconstrictor signals to skeletal muscle (Vallbo et al. 1979), previous work has demonstrated similar spectral oscillations in skin and muscle SNA suggesting that sympathetic outflow to these vascular beds may share common central mechanisms (Cogliati et al. 2000). Indeed, muscle SNA and skin SNA have been reported to increase similarly under various conditions (e.g. cigarette smoking), an effect attributed to central activation (Takeuchi et al. 1994; Narkiewicz et al. 1998). Although in these human studies we cannot localize the exact site or mechanism of action of NOS inhibition on central SNA, several central sympathetic nuclei may be involved. Studies have indicated that sympathetic pre-motor neurons in distinct brainstem regions, such as the rostral medullary raphe and the rostral ventrolateral medulla, are involved in the control of central sympathetic outflow to the cutaneous and skeletal muscle circulations (Barman & Gebber, 1997; Morrison, 2001). NOS containing neurons have been identified in both of these regions (Rodrigo et al. 1994) and therefore blockade of NOS within these regions, or areas projecting to these regions, such as the nucleus tractus solitarii or the hypothalamus (Dampney, 1994), could lead to the observed increases in SNA. In this regard, direct injection of NOS inhibitors into the rostral ventrolateral medulla elicits acute increases in sympathetic outflow (Zanzinger et al. 1995).
In addition to a direct effect on central sympathetic nuclei, the potential influence of NOS inhibition on ganglionic transmission cannot be completely excluded as NOS is also present in sympathetic preganglionic neurons (Blottner & Baumgarten, 1992; Dun et al. 1992, 1993). In this regard, investigations utilizing spinal administration of NOS inhibitors have provided evidence for a role of NO in sympathetic ganglion transmission. Interestingly, studies have suggested that NO may provide both an excitatory and an inhibitory influence at the ganglion (Wu & Dun, 1995, 1996; Wu et al. 1997). Indeed, Hakim et al. (1995) demonstrated that intrathecal injection of l-NAME in anaesthetized rabbits decreased renal SNA, whereas other investigations in rodent models demonstrated a dose-dependent increase in blood pressure with intrathecal administration of l-NAME (del Carmen Garcia et al. 1997; Koga et al. 1999; Lu et al. 1999; Chen & Shyr, 2005), a response that was eliminated by α-adrenergic blockade (Koga et al. 1999). Although the reason for the divergent responses evoked by l-NAME in the ganglion is unclear, these results clearly suggest a potential role for NO in modulating SNA within ganglionic synaptic areas. Adding to the complexity of NO as a signalling molecule, recent data suggest that NO may also play an important role in modulating ganglionic transmission within cholinergic neurons of the parasympathetic nervous system (Rafalzik et al. 2008; Herring & Paterson, 2009) as well as afferent neuronal pools (e.g. arterial baroreflex) (Fong et al. 2000). Thus, although the present results clearly demonstrate an increase in sympathetic outflow during systemic NOS inhibition, determining the precise mechanism(s) responsible (i.e. central processing, ganglionic transmission, etc.) will require further studies including more invasive animal investigations. Overall, an abundance of animal studies have identified a prominent role for NO in the central control of SNA and the results from the present study using skin SNA, together with previous work using α-adrenergic blockade or short-term muscle SNA recordings, support this contention in humans.
Several points should be considered when interpreting the findings of the current study. First, due to the inherent limitations of human experimentation, we cannot determine the actual percentage of central NOS that was inhibited with systemic l-NAME administration. Previous investigations in experimental animals have reported 50–100% inhibition of brain NOS activity following systemic l-NAME infusions ranging from 10 to 50 mg kg−1 (Dwyer et al. 1991; Iadecola et al. 1994; Salter et al. 1995; Traystman et al. 1995; Ayers et al. 1997); however, potential species differences make comparisons difficult. The dosage in the current study was chosen based on previous work demonstrating a robust increase in MAP of approximately 20 mmHg in young, healthy subjects (Sander et al. 1999). Thus, in order to minimize potential deleterious side effects, we felt that this was the highest possible dose we could safely infuse systemically. Second, we cannot completely rule out that systemic NOS inhibition influenced body temperature and this led to direct and confounding effects on skin SNA. However, it should be noted that the role of NO in body temperature regulation appears complex with systemic infusion of l-NAME in rodents reducing core body temperature (Scammell et al. 1996; Branco et al. 1997; Steiner et al. 1998), whereas intracerebroventricular administration of l-NAME increases core body temperature (Branco et al. 1997; Steiner et al. 1998). Moreover, we are unaware of any studies that have examined this question in humans, and given the differences in thermoregulation between rodents and humans this remains an important question. Importantly, for our studies, great care was taken to ensure subject comfort during these experiments and no subject reported feeling cold or hot during the course of the experiments. Lastly, although our findings of no difference between the bradycardic response to l-NAME and phenylephrine is in line with the findings of others (Owlya et al. 1997; Lepori et al. 1998), caution should be used in interpreting these results given that an influence of NO on cardiac baroreflex sensitivity (Chowdhary et al. 2000; Spieker et al. 2000) was not a primary focus of our study and the phenylephrine infusions were performed in only a subset of subjects.
In summary, systemic NOS inhibition elicited robust and sustained increases in central sympathetic outflow, a response that was not evident during time or hypertensive control trials. Importantly, by using skin SNA measures that are not under baroreflex control in thermoneutral conditions, these results provide direct evidence for central sympatho-excitation following NOS inhibition in humans. Overall, our findings with skin SNA, together with previous findings with muscle SNA, provide compelling evidence that nitric oxide is involved in the regulation of sympathetic nerve activity in humans, extending a wealth of data from animal studies supporting a prominent role of nitric oxide in central sympathetic control.
Acknowledgments
The time and effort expended by all of the volunteer subjects is greatly appreciated. This research is the result of work supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO and by National Institute of Health Grant DK076636 to P.J.F.
Glossary
Abbreviations
- BP
blood pressure
- HR
heart rate
- l-NAME
NG-nitro-l-arginine methyl ester
- NOS
nitric oxide synthase
- SNA
sympathetic nerve activity
Author contributions
C.N.Y. contributed to data acquisition, data analysis, data interpretation and wrote the first draft of the manuscript. J.P.F. contributed to data acquisition, data interpretation and critical review of the manuscript. K.M.G., A. W-C. and K.C. provided clinical support and contributed to data acquisition. R.G.V. contributed to study design and data interpretation. G.D.T. contributed to study design, data interpretation and critical review of the manuscript. P.J.F. contributed to study design, data acquisition, data analysis, data interpretation and critical review of the manuscript. All authors approved the final version of the manuscript. The experiments of this study were conducted in the Harry S. Truman Memorial Veterans Hospital at the University of Missouri, Columbia Mo.
References
- Augustyniak RA, Victor RG, Morgan DA, Zhang W. L-NAME- and ADMA-induced sympathetic neural activation in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R726–732. doi: 10.1152/ajpregu.00768.2004. [DOI] [PubMed] [Google Scholar]
- Ayers NA, Kapas L, Krueger JM. The inhibitory effects of Nω-nitro-L-arginine methyl ester on nitric oxide synthase activity vary among brain regions in vivo but not in vitro. Neurochem Res. 1997;22:81–86. doi: 10.1023/a:1027385522859. [DOI] [PubMed] [Google Scholar]
- Barman SM, Gebber GL. Subgroups of rostral ventrolateral medullary and caudal medullary raphe neurons based on patterns of relationship to sympathetic nerve discharge and axonal projections. J Neurophysiol. 1997;77:65–75. doi: 10.1152/jn.1997.77.1.65. [DOI] [PubMed] [Google Scholar]
- Blottner D, Baumgarten HG. Nitric oxide synthetase (NOS)-containing sympathoadrenal cholinergic neurons of the rat IML-cell column: evidence from histochemistry, immunohistochemistry, and retrograde labelling. J Comp Neurol. 1992;316:45–55. doi: 10.1002/cne.903160105. [DOI] [PubMed] [Google Scholar]
- Branco LG, Carnio EC, Barros RC. Role of the nitric oxide pathway in hypoxia-induced hypothermia of rats. Am J Physiol Regul Integr Comp Physiol. 1997;273:R967–971. doi: 10.1152/ajpregu.1997.273.3.R967. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Panza JA. Impaired endothelial regulation of vascular tone in patients with systemic arterial hypertension. Vasc Med. 1998;3:138–144. doi: 10.1177/1358836X9800300208. [DOI] [PubMed] [Google Scholar]
- Chen CH, Shyr MH. Blockade of spinal nitric oxide synthase on blood pressure variability and hepatic microcirculation. Acta Anaesthesiol Taiwan. 2005;43:67–72. [PubMed] [Google Scholar]
- Chowdhary S, Vaile JC, Fletcher J, Ross HF, Coote JH, Townend JN. Nitric oxide and cardiac autonomic control in humans. Hypertension. 2000;36:264–269. doi: 10.1161/01.hyp.36.2.264. [DOI] [PubMed] [Google Scholar]
- Cogliati C, Magatelli R, Montano N, Narkiewicz K, Somers VK. Detection of low- and high-frequency rhythms in the variability of skin sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2000;278:H1256–1260. doi: 10.1152/ajpheart.2000.278.4.H1256. [DOI] [PubMed] [Google Scholar]
- Cui J, Sathishkumar M, Wilson TE, Shibasaki M, Davis SL, Crandall CG. Spectral characteristics of skin sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2006;290:H1601–1609. doi: 10.1152/ajpheart.00025.2005. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci. 2004;116:54–61. doi: 10.1016/j.autneu.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- del Carmen Garcia M, Celuch SM, Adler-Graschinsky E. Possible participation of spinal nitric oxide in the control of the blood pressure in anaesthetized rats. Brain Res. 1997;764:67–74. doi: 10.1016/s0006-8993(97)00421-6. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiologica Scandinavica. 1972;84:177–186. doi: 10.1111/j.1748-1716.1972.tb05168.x. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Forstermann U, Tseng LF. Nitric oxide synthase immunoreactivity in rat spinal cord. Neurosci Lett. 1992;147:217–220. doi: 10.1016/0304-3940(92)90599-3. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Wu SY, Forstermann U, Schmidt HH, Tseng LF. Nitric oxide synthase immunoreactivity in the rat, mouse, cat and squirrel monkey spinal cord. Neuroscience. 1993;54:845–857. doi: 10.1016/0306-4522(93)90579-5. [DOI] [PubMed] [Google Scholar]
- Dwyer MA, Bredt DS, Snyder SH. Nitric oxide synthase: irreversible inhibition by L-NG-nitroarginine in brain in vitro and in vivo. Biochem Biophys Res Commun. 1991;176:1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci. 2009;148:5–15. doi: 10.1016/j.autneu.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong AY, Talman WT, Lawrence AJ. Axonal transport of NADPH-diaphorase and [3H]nitro-L-arginine binding, but not [3H]cGMP binding, by the rat vagus nerve. Brain Res. 2000;878:240–246. doi: 10.1016/s0006-8993(00)02789-x. [DOI] [PubMed] [Google Scholar]
- Grassi G. Counteracting the sympathetic nervous system in essential hypertension. Curr Opin Nephrol Hypertens. 2004;13:513–519. doi: 10.1097/00041552-200409000-00006. [DOI] [PubMed] [Google Scholar]
- Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiologica Scandinavica. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hakim MA, Hirooka Y, Coleman MJ, Bennett MR, Dampney RA. Evidence for a critical role of nitric oxide in the tonic excitation of rabbit renal sympathetic preganglionic neurones. J Physiol. 1995;482:401–407. doi: 10.1113/jphysiol.1995.sp020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Jacobsen TN, Victor RG. Is nitric oxide involved in the tonic inhibition of central sympathetic outflow in humans? Hypertension. 1994;24:439–444. doi: 10.1161/01.hyp.24.4.439. [DOI] [PubMed] [Google Scholar]
- Heinzel B, John M, Klatt P, Bohme E, Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281:627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring N, Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol. 2009;94:46–53. doi: 10.1113/expphysiol.2008.044776. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Xu X, Zhang F, Hu J, el-Fakahany EE. Prolonged inhibition of brain nitric oxide synthase by short-term systemic administration of nitro-L-arginine methyl ester. Neurochem Res. 1994;19:501–505. doi: 10.1007/BF00967330. [DOI] [PubMed] [Google Scholar]
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Koga N, Takano Y, Honda K, Saito R, Kamiya H. Roles of nitric oxide in the spinal cord in cardiovascular regulation in rats. Neurosci Lett. 1999;267:173–176. doi: 10.1016/s0304-3940(99)00358-4. [DOI] [PubMed] [Google Scholar]
- Korner PI. Essential Hypertension and its Causes: Neural and Non-Neural Mechanisms. New York: Oxford University Press; 2007. [Google Scholar]
- Lepori M, Sartori C, Trueb L, Owlya R, Nicod P, Scherrer U. Haemodynamic and sympathetic effects of inhibition of nitric oxide synthase by systemic infusion of NG-monomethyl-L-arginine into humans are dose dependent. J Hypertens. 1998;16:519–523. doi: 10.1097/00004872-199816040-00013. [DOI] [PubMed] [Google Scholar]
- Lu PP, Shee JJ, Chen HM, Lin CC, Shyr MH. Spinal nitric oxide participates in the control of the blood pressure during graded hemorrhage in the conscious rat. Shock. 1999;12:222–226. doi: 10.1097/00024382-199909000-00009. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Wallin BG. Respiratory and cardiac modulation of single sympathetic vasoconstrictor and sudomotor neurones to human skin. J Physiol. 1999;516:303–314. doi: 10.1111/j.1469-7793.1999.303aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon DV, Wang Z, Fadel PJ, Arbique D, Leonard D, Li JL, Victor RG, Vongpatanasin W. Central sympatholysis as a novel countermeasure for cocaine-induced sympathetic activation and vasoconstriction in humans. J Am College Cardiol. 2007;50:626–633. doi: 10.1016/j.jacc.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol. 2001;281:R683–698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Hausberg M, Cooley RL, Winniford MD, Davison DE, Somers VK. Cigarette smoking increases sympathetic outflow in humans. Circulation. 1998;98:528–534. doi: 10.1161/01.cir.98.6.528. [DOI] [PubMed] [Google Scholar]
- Owlya R, Vollenweider L, Trueb L, Sartori C, Lepori M, Nicod P, Scherrer U. Cardiovascular and sympathetic effects of nitric oxide inhibition at rest and during static exercise in humans. Circulation. 1997;96:3897–3903. doi: 10.1161/01.cir.96.11.3897. [DOI] [PubMed] [Google Scholar]
- Palacios M, Knowles RG, Palmer RM, Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989;165:802–809. doi: 10.1016/s0006-291x(89)80037-3. [DOI] [PubMed] [Google Scholar]
- Rafalzik S, Pehl U, Ott D, Strotmann J, Wolff M, Gerstberger R. Cholinergic signal transduction in the mouse sphenopalatine ganglion. Brain Res. 2008;1241:42–55. doi: 10.1016/j.brainres.2008.08.095. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Springall DR, Uttenthal O, Bentura ML, Abadia-Molina F, Riveros-Moreno V, Martinez-Murillo R, Polak JM, Moncada S. Localization of nitric oxide synthase in the adult rat brain. Philos Trans R Soc Lond B Biol Sci. 1994;345:175–221. doi: 10.1098/rstb.1994.0096. [DOI] [PubMed] [Google Scholar]
- Sakuma I, Togashi H, Yoshioka M, Saito H, Yanagida M, Tamura M, Kobayashi T, Yasuda H, Gross SS, Levi R. NG-methyl-L-arginine, an inhibitor of L-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo. A role for nitric oxide in the central regulation of sympathetic tone? Circ Res. 1992;70:607–611. doi: 10.1161/01.res.70.3.607. [DOI] [PubMed] [Google Scholar]
- Salter M, Duffy C, Garthwaite J, Strijbos PJ. Substantial regional and hemispheric differences in brain nitric oxide synthase (NOS) inhibition following intracerebroventricular administration of Nω-nitro-L-arginine (L-NA) and its methyl ester (L-NAME) Neuropharmacology. 1995;34:639–649. doi: 10.1016/0028-3908(95)00036-6. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Victor RG. A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension. 1999;33:937–942. doi: 10.1161/01.hyp.33.4.937. [DOI] [PubMed] [Google Scholar]
- Sander M, Hansen J, Victor RG. The sympathetic nervous system is involved in the maintenance but not initiation of the hypertension induced by Nω-nitro-L-arginine methyl ester. Hypertension. 1997;30:64–70. doi: 10.1161/01.hyp.30.1.64. [DOI] [PubMed] [Google Scholar]
- Sander M, Hansen PG, Victor RG. Sympathetically mediated hypertension caused by chronic inhibition of nitric oxide. Hypertension. 1995;26:691–695. doi: 10.1161/01.hyp.26.4.691. [DOI] [PubMed] [Google Scholar]
- Sander M, Victor RG. Neural mechanisms in nitric oxide-deficient hypertension. Curr Opin Nephrol Hypertens. 1999;8:61–73. doi: 10.1097/00041552-199901000-00011. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Elmquist JK, Saper CB. Inhibition of nitric oxide synthase produces hypothermia and depresses lipopolysaccharide fever. Am J Physiol Regul Integr Comp Physiol. 1996;271:R333–338. doi: 10.1152/ajpregu.1996.271.2.R333. [DOI] [PubMed] [Google Scholar]
- Spieker LE, Corti R, Binggeli C, Luscher TF, Noll G. Baroreceptor dysfunction induced by nitric oxide synthase inhibition in humans. J Am College Cardiol. 2000;36:213–218. doi: 10.1016/s0735-1097(00)00674-4. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Carnio EC, Antunes-Rodrigues J, Branco LG. Role of nitric oxide in systemic vasopressin-induced hypothermia. Am J Physiol Regul Integr Comp Physiol. 1998;275:R937–941. doi: 10.1152/ajpregu.1998.275.4.R937. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Iwase S, Mano T, Okada H, Sugiyama Y, Watanabe T. Sleep-related changes in human muscle and skin sympathetic nerve activities. J Auton Nerv Syst. 1994;47:121–129. doi: 10.1016/0165-1838(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Zhang W, Victor RG. Nitric oxide deficiency as a cause of clinical hypertension: promising new drug targets for refractory hypertension. JAMA. 2001;285:2055–2057. doi: 10.1001/jama.285.16.2055. [DOI] [PubMed] [Google Scholar]
- Traystman RJ, Moore LE, Helfaer MA, Davis S, Banasiak K, Williams M, Hurn PD. Nitro-L-arginine analogues. Dose- and time-related nitric oxide synthase inhibition in brain. Stroke. 1995;26:864–869. doi: 10.1161/01.str.26.5.864. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Vissing SF, Scherrer U, Victor RG. Stimulation of skin sympathetic nerve discharge by central command. Differential control of sympathetic outflow to skin and skeletal muscle during static exercise. Circ Res. 1991;69:228–238. doi: 10.1161/01.res.69.1.228. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W, Mansour Y, Chavoshan B, Arbique D, Victor RG. Cocaine stimulates the human cardiovascular system via a central mechanism of action. Circulation. 1999;100:497–502. doi: 10.1161/01.cir.100.5.497. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlof G, Delius W. The effect of carotid sinus nerve stimulation on muscle and skin nerve sympathetic activity in man. Pflugers Arch. 1975;358:101–110. doi: 10.1007/BF00583921. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole-body heating in humans. J Physiol. 2001;536:615–623. doi: 10.1111/j.1469-7793.2001.0615c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Dyckman DJ, Ray CA. Determinants of skin sympathetic nerve responses to isometric exercise. J Appl Physiol. 2006;100:1043–1048. doi: 10.1152/japplphysiol.00579.2005. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Kuipers NT, McHugh EA, Ray CA. Vestibular activation does not influence skin sympathetic nerve responses during whole body heating. J Appl Physiol. 2004;97:540–544. doi: 10.1152/japplphysiol.00174.2004. [DOI] [PubMed] [Google Scholar]
- Wu SY, Dun NJ. Calcium-activated release of nitric oxide potentiates excitatory synaptic potentials in immature rat sympathetic preganglionic neurons. J Neurophysiol. 1995;74:2600–2603. doi: 10.1152/jn.1995.74.6.2600. [DOI] [PubMed] [Google Scholar]
- Wu SY, Dun NJ. Potentiation of IPSCs by nitric oxide in immature rat sympathetic preganglionic neurones in vitro. J Physiol. 1996;495:479–490. doi: 10.1113/jphysiol.1996.sp021608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Dun SL, Forstermann U, Dun NJ. Nitric oxide and excitatory postsynaptic currents in immature rat sympathetic preganglionic neurons in vitro. Neuroscience. 1997;79:237–245. doi: 10.1016/s0306-4522(96)00612-4. [DOI] [PubMed] [Google Scholar]
- Young CN, Keller DM, Crandall CG, Fadel PJ. Comparing resting skin sympathetic nerve activity between groups: caution needed. J Appl Physiol. 2009;106:1751–1752. doi: 10.1152/japplphysiol.91538.2008. author reply 1753. [DOI] [PubMed] [Google Scholar]
- Zanzinger J, Czachurski J, Seller H. Inhibition of basal and reflex-mediated sympathetic activity in the RVLM by nitric oxide. Am J Physiol Regul Integr Comp Physiol. 1995;268:R958–962. doi: 10.1152/ajpregu.1995.268.4.R958. [DOI] [PubMed] [Google Scholar]
- Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol. 1998;275:R728–734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Liu JL. Angiotensin II–nitric oxide interactions in the control of sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:27–43. doi: 10.1023/A:1009894007055. [DOI] [PubMed] [Google Scholar]
- Zucker IH, Schultz HD, Li YF, Wang Y, Wang W, Patel KP. The origin of sympathetic outflow in heart failure: the roles of angiotensin II and nitric oxide. Progr Biophys Mol Biol. 2004;84:217–232. doi: 10.1016/j.pbiomolbio.2003.11.010. [DOI] [PubMed] [Google Scholar]