Abstract
Activation of sympathetic efferent traffic is essential to maintaining adequate arterial pressures during reductions of central blood volume. Sympathetic baroreflex gain may be reduced, and muscle sympathetic firing characteristics altered with head-up tilt just before presyncope in humans. Volume redistributions with lower body negative pressure (LBNP) are similar to those that occur during haemorrhage, but limited data exist describing arterial pressure–muscle sympathetic nerve activity (MSNA) relationships during intense LBNP. Responses similar to those that occur in presyncopal subjects during head-up tilt may signal the beginnings of cardiovascular decompensation associated with haemorrhage. We therefore tested the hypotheses that intense LBNP disrupts MSNA firing characteristics and leads to a dissociation between arterial pressure and sympathetic traffic prior to presyncope. In 17 healthy volunteers (12 males and 5 females), we recorded ECG, finger photoplethysmographic arterial pressure and MSNA. Subjects were exposed to 5 min LBNP stages until the onset of presyncope. The LBNP level eliciting presyncope was denoted as 100% tolerance, and then data were assessed relative to this normalised maximal tolerance by expressing LBNP levels as 80, 60, 40, 20 and 0% (baseline) of maximal tolerance. Data were analysed in both time and frequency domains, and cross-spectral analyses were performed to determine the coherence, transfer function and phase angle between diastolic arterial pressure (DAP) and MSNA. DAP–MSNA coherence increased progressively and significantly up to 80% maximal tolerance. Transfer functions were unchanged, but phase angle shifted from positive to negative with application of LBNP. Sympathetic bursts fused in 10 subjects during high levels of LBNP (burst fusing may reflect modulation of central mechanisms, an artefact arising from our use of a 0.1 s time constant for integrating filtered nerve activity, or a combination of both). On average, arterial pressures and MSNA decreased significantly the final 20 s before presyncope (n= 17), but of this group, MSNA increased in seven subjects. No linear relationship was observed between the magnitude of DAP and MSNA changes before presyncope (r= 0.12). We report three primary findings: (1) progressive LBNP (and presumed progressive arterial baroreceptor unloading) increases cross-spectral coherence between arterial pressure and MSNA, but sympathetic baroreflex control is reduced before presyncope; (2) withdrawal of MSNA is not a prerequisite for presyncope despite significant decreases of arterial pressure; and (3) reductions of venous return, probably induced by intense LBNP, disrupt MSNA firing characteristics that manifest as fused integrated bursts before the onset of presyncope. Although fusing of integrated sympathetic bursts may reflect a true physiological compensation to severe reductions of venous return, duplication of this finding utilizing shorter time constants for integration of the nerve signal is required.
Introduction
Maintenance of arterial pressure under conditions of reduced central blood volume such as orthostasis or haemorrhage is accomplished in large part through sympathetic efferent traffic to the peripheral vasculature. Orthostatic intolerance with head-up tilt occurs when cerebral perfusion is insufficient, and is associated with reduced muscle sympathetic nerve activity (MSNA) resulting in systemic hypotension (Mano & Iwase, 2003). Withdrawal of MSNA has also been reported at presyncope during central blood volume reductions induced by application of lower body negative pressure (LBNP) (Cooke & Convertino, 2002; Khan et al. 2002). Both head-up tilt and LBNP reduce central volume and unload arterial baroreceptors, but splanchnic volume increases with head-up tilt and decreases with LBNP (Taneja et al. 2007). For this reason, LBNP is appropriate as a model to simulate haemorrhage (Cooke et al. 2004); as a consequence, understanding associations between arterial pressure and MSNA during LBNP assumes important clinical significance.
Arterial pressure is not a static entity, but rather a dynamic process with spontaneous oscillations occurring at low frequencies that are centred around 0.1 Hz in humans. Spontaneous low-frequency non-respiratory oscillations of arterial pressure were described by Mayer (1877); later, Guyton & Harris (1951) attributed these vasomotor waves to sympathetic baroreflex mechanisms. During spontaneous arterial pressure oscillations, rising pressures activate vagal and inhibit sympathetic baroreflex responses, and falling pressures activate sympathetic and inhibit vagal baroreflex responses. Guyton & Harris (1951) demonstrated that sympathetic denervation blunts arterial pressure oscillations, and concluded that it is the influence of sympathetic rather than vagal activation and withdrawal that primarily drives these low-frequency rhythms.
Arterial pressure oscillations increase with reductions in central blood volume induced by haemorrhage (Guyton & Harris, 1951), head-up tilt (Cooke et al. 1999) or LBNP (Convertino et al. 2004). These increased oscillations of pressure are associated with parallel increases of sympathetic traffic as measured directly in peripheral nerves with the microneurography technique (Iwase et al. 1987). MSNA increases as a linear function of reduced central blood volume (Iwase et al. 1987; Victor & Leimbach, 1987; Cooke et al. 1999, 2008; Convertino et al. 2004), and oscillates in conjunction (inversely) with arterial pressure (Furlan et al. 2000). It appears that the presence of marked low-frequency arterial pressure oscillations characterises compensation to central volume loss (Guyton & Harris, 1951), and reduced low-frequency arterial pressure oscillations have been associated with haemodynamic decompensation during tilt (Kamiya et al. 2005).
Muscle sympathetic burst prolongation and disruption of pulse synchrony have been induced by bilateral anaesthetic blocks of glossopharyngeal and vagus nerves in humans (Fagius et al. 1985). Altered burst duration and reflex latencies of MSNA have also been reported before presyncope induced with upright tilt (Iwase et al. 2002). Similar disruptions of normal MSNA burst characteristics might explain observations of reduced sympathetic baroreflex responsiveness documented before presyncope induced with LBNP (Ichinose et al. 2006). However, few studies report MSNA responses beyond about −45 to −50 mmHg (Khan et al. 2002; Convertino et al. 2004; Ichinose et al. 2006; Cooke et al. 2008). Subjects in those studies may experience presyncope, or they may not, depending on their individual tolerances to central blood volume reductions.
We therefore designed a study to test the hypotheses that intense LBNP to presyncope disrupts MSNA firing characteristics and leads to a dissociation between arterial pressure and sympathetic traffic.
Methods
Subjects and ethical approval
From a total of 49 subjects in whom we were able to obtain adequate recordings of MSNA during LBNP, 17 subjects provided MSNA with stable baselines (ensuring that electrode position did not shift) at all levels of a progressive negative pressure protocol that was terminated as a result of presyncope. We therefore report data from these 17 healthy volunteers (12 males and 5 females; age 31 ± 2 years; height 173 ± 3 cm; weight 77 ± 3 kg; means ± standard error of the mean (s.e.m.)). All subjects received a verbal briefing and written descriptions of all procedures and risks associated with the study, and were made familiar with the laboratory, the protocol and procedures. To ensure subjects were free from previous or existing medical conditions that would preclude their participation, all subjects completed a medical history form and underwent a physical examination by a physician. Female subjects were not pregnant, as confirmed by a urine test before experimentation. Subjects were encouraged to ask questions of the investigators, and then they signed an informed consent form that had been approved by the Institutional Review Board for the protection of human subjects in research from Brooke Army Medical Center and The US Army Institute of Surgical Research, Fort Sam Houston, TX, USA. We have read the article by Drummond outlining standards and advice for The Journal of Physiology, and our experiment complies with the policies and regulations outlined (Drummond, 2008). This study was carried out in accordance with the standards set by the latest revision of the Declaration of Helsinki. All subjects maintained their normal sleep patterns, refrained from exercise, and abstained from caffeine and other autonomic stimulants including prescription or non-prescription drugs at least 24 h before the study.
Experimental protocol
Subjects were instrumented with a standard 4-lead ECG to record cardiac electrical potentials, and a finger cuff to record beat-by-beat finger arterial pressure (Finometer Blood Pressure Monitor, TNO-TPD Biomedical Instrumentation, Amsterdam, The Netherlands). MSNA was recorded directly from peroneal nerves with the microneurography technique (Hagbarth & Vallbo, 1968). Nerve signals were band-pass filtered (100–2000 Hz), and integrated (time constant, 0.1 s) to obtain mean voltage neurograms. Subjects were positioned supine within an airtight chamber that was sealed at the level of the iliac crest by way of a neoprene skirt. Each subject was taken to the point of presyncope using progressive LBNP. The LBNP protocol consisted of a 5 min control period (baseline) followed by 5 min of chamber decompression at −15, −30, −45 and −60 mmHg, and then additional increments of −10 mmHg every 5 min until the onset of presyncope, followed by a 10 min recovery period. Presyncope was identified in real time by the attending investigator by a precipitous fall in systolic pressure greater than 15 mmHg, progressive diminution of systolic pressure below 70 mmHg, bradycardia and/or voluntary subject termination due to discomfort from symptoms such as grey-out (loss of colour vision), tunnel vision, sweating, nausea or dizziness.
Data analysis
Data were sampled at 500 Hz and digitized to computer (WINDAQ, Dataq Instruments, Akron, OH, USA). Using commercially available data analysis software (WinCPRS, Abolute Aliens, Turku, Finland), R-waves from the ECG, as well as systolic and diastolic pressures generated from the Finometer, were detected. Stroke volumes were estimated from the Finometer using the pulse contour method (Jansen et al. 1990). The software also detected bursts of MSNA based on two primary criteria: (1) pulse synchronous spontaneous bursts with signal-to-noise ratios of about 3 : 1; and (2) reflex latencies from preceding R-waves of about 1.3 s (Fagius & Wallin, 1980). Computerized burst detection results were then checked manually by one experienced microneurographer. As the amplitude and area of sympathetic bursts varies among subjects due to electrode position, MSNA was normalised by dividing the integral of all bursts by the number of bursts occurring during the 5 min baseline period. Subsequent burst areas were then divided by this number (burst areas that were equal to the average baseline area were assigned a value of 1.0) and then multiplied by the number of bursts occurring during a given time period for calculation of total MSNA during LBNP.
Data were averaged over the entire 5 min for each LBNP stage. The last 5 min just prior to decompensation were also averaged for consistency despite variable times for the terminal LBNP stage. This resulted necessarily in some data overlap with the LBNP stage immediately preceding decompensation. Due to individual variabilities in the time to decompensation, we normalised time to decompensation for comparison between subjects. For example, if a subject experienced decompensation during the −70 mmHg stage, −70 mmHg was designated as 100%, or maximal LBNP tolerance, for that subject. LBNP stages were then designated as 80, 60, 40, 20 and 0% of maximal LBNP tolerance. Using this approach, absolute LBNP levels corresponded well to these ranges of percentages if subjects were able to continue at least to the −70 mmHg stage.
Oscillatory rhythms were quantified with a Fourier transform. Non-equidistant beat-to-beat data were interpolated linearly and resampled at a frequency of 5 Hz. Data then were passed through a low-pass impulse response filter with a cut-off frequency of 0.4 Hz. We used Fourier analysis to calculate the power spectrum over 5 min data sets. We then averaged the neighbouring spectral values using a sliding triangular weighting function. The width of the averaging window was 0.01 Hz (0.02 Hz for coherence) corresponding to 5 data points in the spectrum. The magnitude of R–R interval, arterial pressure and MSNA oscillations (individually identified burst areas) were quantified by calculating the power spectral density for the signal (total; 0.04–0.4 Hz). Signal areas were separated into high-frequency (HF; 0.15–0.4 Hz) and low-frequency (LF; 0.04–0.15 Hz) bands (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). As diastolic arterial pressure (DAP) correlates more closely with MSNA than systolic pressure (Sundlof & Wallin, 1978), we investigated rhythmic associations between DAP and MSNA. We calculated coherence by dividing cross-spectral densities by the product of the individual power spectral densities (de Boer et al. 1985, 1987), and averaged coherence within the low-frequency ranges of 0.04 to 0.15 Hz. In an effort to include small bursts that exceeded baseline noise but may have been missed using our burst detection algorithm, coherence was calculated between DAP and the integrated MSNA neurogram using the same resampling and interpolation described above. We considered a value of 0.5 to represent a strong association between the two signals (de Boer et al. 1985). Transfer function and phase angle were assessed with cross-spectral analysis of DAP and MSNA as described previously (Cooke et al. 1999). We tested the stationarity of signals by checking whether there were any large changes on the baseline of the signal. In this method, the signal was divided into sub-epochs where the mean value was calculated. The ratio of the standard deviation of sub-epoch means and the standard deviation of the whole data was taken as a measure of stationarity, termed StatAv (Palazzolo et al. 1998; Kuusela et al. 2002). To investigate haemodynamic and neural responses occurring just before the onset of presyncopal symptoms, we averaged data in bins from 40 s to 20 s, and then 20 s to 0 s before LBNP termination due to presyncope.
Statistical analysis
A one-way ANOVA for repeated measures was used for comparison of dependent variables across conditions (normalised levels of LBNP). Duncan's mean separation procedure was employed for levels of LBNP when the probabilities of LBNP main effects within levels were <0.05. All data are expressed as means ±s.e.m. unless specified otherwise. Mean values recorded during the 20 s before presyncope were subtracted from values recorded from 40 s to 20 s before presyncope, and DAP–MSNA associations were assessed using Pearson product–moment correlation.
Results
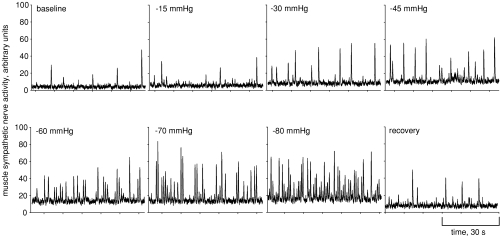
We recorded MSNA from 49 subjects, but abrupt baseline shifts in the MSNA recording were observed in 32 of these subjects during application of LBNP. Abrupt shifts in baseline nerve activity indicates to us that chamber pressure has either dislodged the microneurography electrode, or caused the electrode to shift within the nerve fascicle and move beyond the established recording region. Under these circumstances, comparison of nerve activity during LBNP to baseline activity is impossible. We therefore discarded all but 17 recordings that were maintained successfully until presyncope. Figure 1 shows progressive increases in MSNA for one subject to −80 mmHg and recovery. For this subject (and others), the gradual increase in baseline voltages probably represents increased sympathetic activity rather than movement of the recording electrode.
Figure 1.
Muscle sympathetic nerve activity is shown for one representative subject during progressive lower body negative pressure.
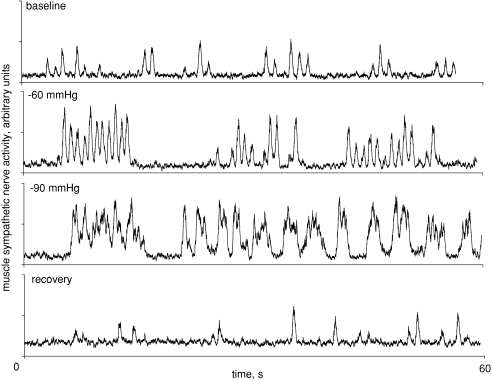
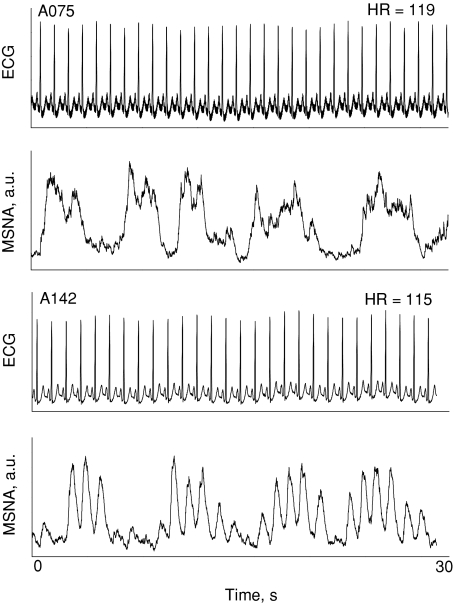
Figure 2 shows MSNA from a different subject who experienced coupling (at −60 mmHg LBNP) and then fusing of bursts during intense LBNP (–90 mmHg). Ten of our seventeen subjects responded to intense LBNP with similar bursting patterns. Fused bursts were not a result of integrating filtered nerve traffic with a time constant of 0.1 s, as Fig. 3 shows one subject with fused bursts (A075) and one with distinctly separate bursts (A142) despite both subjects demonstrating similar heart rates.
Figure 2.
Coupling (−60 mmHg) and then fusing (−90 mmHg) of muscle sympathetic nerve activity is shown for one representative subject during lower body negative pressure.
Figure 3. Muscle sympathetic activity and ECG tracings are shown for two different subjects.
Subject A075 (top panels) demonstrated burst fusing, and subject A142 (bottom panels) did not. Heart rates (HR) were similar between the two subjects (upper right hand corner of the ECG panels).
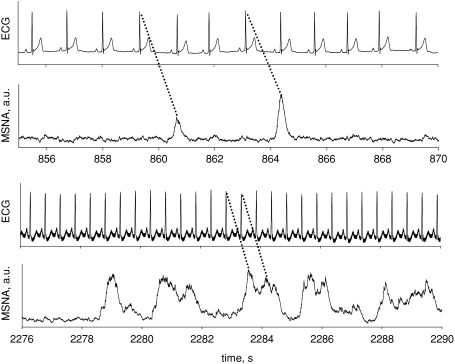
Such ‘burst fusions’ represent a challenge to traditional burst detection and quantification. We reasoned that pulse synchrony probably remains, but that in some subjects voltages between bursts cannot return to baseline values before an additional sympathetic volley is mounted. Figure 4 provides an example of how we identified individual bursts within a burst conglomerate.
Figure 4.
Example showing how individual bursts are detected in relation to preceding R-waves for single bursts (top panels) and fused bursts (bottom panels) of muscle sympathetic nerve activity.
Presyncope occurred in two subjects at −30, two at −60, six at −70, three at −80, three at −90, and one at −100 mmHg. Primary autonomic and haemodynamic responses to progressive LBNP are shown in Table 1 (for subjects experiencing presyncope at certain absolute levels of LBNP there was no corresponding percentage to assign at 20%, 40% and 80% presyncope; this accounts for unequal sample sizes presented in the tables). R–R intervals and arterial pressures were maintained fairly constant until late into the protocol (≥ 60% LBNP tolerance), and then they decreased moderately (with the exception of DAP) as compared with baseline. Pulse pressure and stroke volume decreased progressively and significantly. MSNA expressed as bursts per 100 heart beats increased early during LBNP (20% LBNP tolerance), and MSNA reflex latencies decreased significantly at the terminal LBNP stage.
Table 1.
Autonomic cardiac, haemodynamic and peripheral sympathetic neural responses to progressive central hypovolaemia
| Variable | LBNP 0 n= 17 | LBNP 20%n= 15 | LBNP 40%q= 13 | LBNP 60%n= 17 | LBNP 80%n= 15 | LBNP 100%n= 17 |
|---|---|---|---|---|---|---|
| RRI (ms) | 973 ± 51 | 975 ± 55 | 955 ± 62 | 845 ± 56* | 719 ± 50* | 614 ± 39* |
| SAP (mmHg) | 131 ± 4 | 131 ± 5 | 130 ± 5 | 125 ± 4* | 120 ± 4* | 111 ± 3* |
| DAP (mmHg) | 77 ± 2 | 77 ± 2 | 78 ± 3 | 79 ± 2 | 80 ± 3 | 77 ± 3 |
| MAP (mmHg) | 97 ± 3 | 97 ± 3 | 97 ± 3 | 95 ± 3 | 94 ± 3* | 90 ± 3* |
| PP (mmHg) | 54 ± 3 | 54 ± 4 | 52 ± 4 | 46 ± 3* | 40 ± 3* | 33 ± 3* |
| SV (ml) | 77 ± 2 | 75 ± 2 | 66 ± 3* | 60 ± 3* | 50 ± 3* | 42 ± 3* |
| MSNA (b min−1) | 15 ± 2 | 17 ± 3 | 23 ± 4* | 32 ± 5* | 42 ± 5* | 51 ± 6* |
| MSNA (b 100 hb−1) | 23 ± 3 | 29 ± 4* | 35 ± 3* | 37 ± 3* | 41 ± 3* | 42 ± 3* |
| MSNA (total) | 74 ± 10 | 31 ± 5 | 138 ± 28* | 177 ± 27* | 266 ± 31* | 302 ± 47* |
| Latency (s) | 1.3 ± .03 | 1.3 ± .03 | 1.31 ± .04 | 1.26 ± .01 | 1.11 ± .07 | 1.04 ± .07* |
Values are means ±s.e.m. Levels of lower body negative pressure (LBNP) are expressed as a percentage of decompensation, with LBNP 100% representing the level subjects experienced symptoms; RRI, R-R interval; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; PP, pulse pressure; SV, stroke volume; MSNA (b min−1), muscle sympathetic nerve activity expressed as bursts per minute; MSNA (b 100 hb−1), muscle sympathetic nerve activity expressed as bursts per 100 heart beats; MSNA (total), number of bursts multiplied by normalised burst areas; Latency, time between an R-wave and the peak of the next integrated nerve burst; * denotes LBNP stages where means were statistically distinguishable from baseline (LBNP 0) with a probability value ≤0.05.
Arterial pressure and MSNA oscillations increased progressively with LBNP and remained elevated during the terminal LBNP stage. The average variance (StatAv) of diastolic pressures, an estimate of the degree of stationarity of the signal, was not statistically different until the terminal LBNP stage. These data are shown in Table 2.
Table 2.
Autonomic cardiac, haemodynamic and peripheral sympathetic neural oscillations during progressive lower body negative pressure
| Variable | LBNP 0 n= 17 | LBNP 20%n= 15 | LBNP 40%n= 13 | LBNP 60%n= 17 | LBNP 80%n= 15 | LBNP 100%n= 17 |
|---|---|---|---|---|---|---|
| DAPHF (mmHg2) | 0.8 ± 0.1 | 0.9 ± 0.2 | 3.9 ± 2.9 | 1.4 ± 0.4 | 1.8 ± 0.4 | 4.0 ± 1.1 |
| DAPLF (mmHg2) | 3.8 ± 0.4 | 5.5 ± 0.8 | 7.2 ± 1.3 | 11.0 ± 2.4* | 15.6 ± 2.5 | 18.2 ± 3.4 |
| MSNAHF (a.u.2) | 268 ± 106 | 401 ± 166 | 525 ± 166 | 578 ± 153 | 971 ± 282* | 789 ± 180 |
| MSNALF (a.u.2) | 437 ± 79 | 490 ± 83 | 679 ± 121 | 918 ± 128* | 1353 ± 291 | 1093 ± 214 |
| StatAv | 0.55 ± .02 | 0.50 ± .03 | 0.47 ± .03 | 0.46 ± .02 | 0.47 ± .04 | 0.71 ± .03* |
Values are means ±s.e.m. Levels of lower body negative pressure (LBNP) are expressed as a percentage of presyncope, with LBNP 100% representing the level subjects experienced symptoms; DAPHF and LF, diastolic arterial pressure spectral power at the high and low frequencies; MSNAHF and LF, muscle sympathetic nerve spectral power at the high and low frequencies (a.u. arbitrary units); StatAv, average diastolic pressure variance; * denotes the first stage of LBNP where means were statistically distinguishable from baseline (LBNP 0) with a probability value ≤0.05.
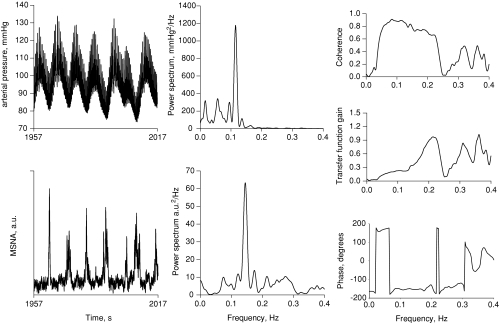
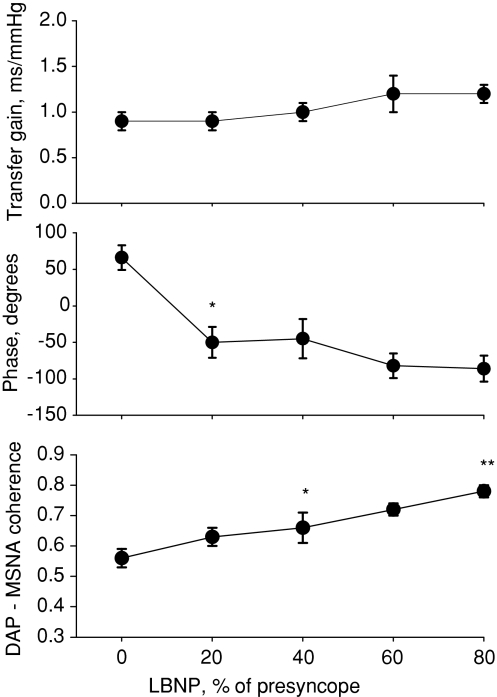
Figure 5 shows exaggerated arterial pressure oscillations, with sympathetic traffic increasing with decreases, and decreasing with increases in pressure (left panels). Fourier analysis of these time series reveals primary oscillatory components of both arterial pressure and MSNA in the centre panels, and cross-spectral analysis of DAP–MSNA shows the coherence, transfer gain and phase angle. Figure 6 shows transfer gain, phase and coherence for all subjects. Sympathetic baroreflex sensitivity as assessed with the transfer function did not change, but phase angle shifted from positive during baseline to negative with the application of LBNP. In the example shown in Fig. 5, taken from one subject during −60 mmHg LBNP, coherence at the low frequency was well above our criterion value of 0.5. At baseline, coherence averaged about 0.55 and increased progressively and significantly with negative pressure applied up to 80% of presyncope (Fig. 6).
Figure 5.
Arterial pressure and muscle sympathetic time series are shown in the left panels; conversion of time series to the frequency domain are shown in the middle panels; cross spectral associations are shown in the right panels for one representative subject during −60 mmHg lower body negative pressure (subject experienced presyncope at −90 mmHg).
Figure 6.
Diastolic pressure (DAP)-to-muscle sympathetic nerve activity (MSNA) transfer gain, phase and coherence are shown for the entire subject cohort as a function of normalised levels of lower body negative pressure (LBNP); *significant deviation from baseline; **significant deviation from 40%.
We chose to analyse 5 min periods in order to obtain enough data to adequately evaluate the relatively slow rhythms of the sympathetic baroreflex response (Guyton & Harris, 1951; de Boer et al. 1987). However, because the onset of decompensation can be rapid, we were unable to analyse coherence for the 100% LBNP level due to a loss in stationarity for DAP (see Table 2). Instead, we analysed our time-domain variables at 100% tolerance in bins from –40 to −20 s before presyncope, and then from –20 to 0 s before to presyncope. These results are shown in Table 3. Decompensation was associated with reductions in heart rate, arterial pressure and MSNA.
Table 3.
Autonomic cardiac, haemodynamic and peripheral sympathetic neural activity before symptoms of presyncope during progressive lower body negative pressure
| Variable | −40 to −20 s before presyncope | −20 to 0 s before presyncope |
|---|---|---|
| RRI (ms) | 621 ± 45 | 683 ± 66 |
| HR (ms) | 104 ± 6 | 98 ± 7* |
| SAP (mmHg) | 98 ± 2 | 88 ± 3* |
| DAP (mmHg) | 69 ± 2 | 61 ± 3* |
| MAP (mmHg) | 79 ± 2 | 70 ± 3* |
| PP (mmHg) | 28 ± 2 | 26 ± 2* |
| MSNA (bursts) | 16 ± 2 | 11 ± 1* |
| MSNA (total) | 21 ± 4 | 16 ± 2* |
Values are means ±s.e.m. (n= 17). RRI, R-R interval; HR, heart rate; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; MAP, mean arterial pressure; PP, pulse pressure; MSNA (bursts), muscle sympathetic nerve activity expressed as absolute burst number; MSNA (total), total muscle sympathetic nerve activity as calculated by multiplying the absolute number of bursts by the average normalised burst areas; * denotes the means that were statistically distinguishable between the two time periods with a probability value ≤0.05.
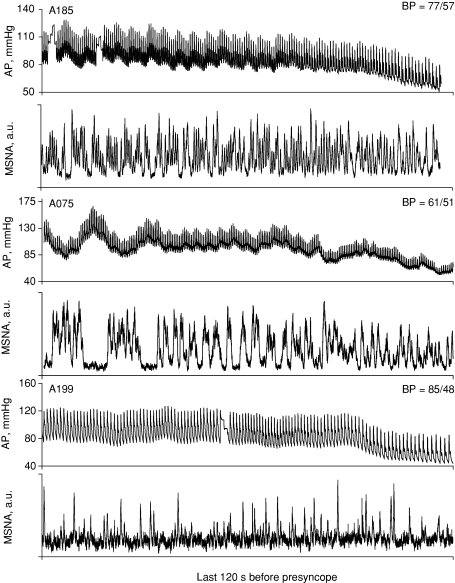
As a group, MSNA withdrew during the 20 s before presyncope (Table 3). However, when analysed individually, we observed remarkable variability in total MSNA during the terminal LBNP stage. Figure 7 shows original recordings from three different subjects who all experienced reductions in arterial pressure at presyncope. The lowest blood pressures recorded for each subject are shown in the upper right of each arterial pressure panel. MSNA was maintained at high levels for subject A185. Subject A075 displayed burst fusing and no apparent withdrawal of MSNA at presyncope. MSNA appeared to decrease at presyncope for subject A199.
Figure 7. Arterial pressure (AP) and muscle sympathetic nerve activity (MSNA) is shown for three representative subjects 2 min before the onset of presyncope.
The lowest arterial pressure (BP) recorded for each subject is shown in the upper right corner of the AP panel.
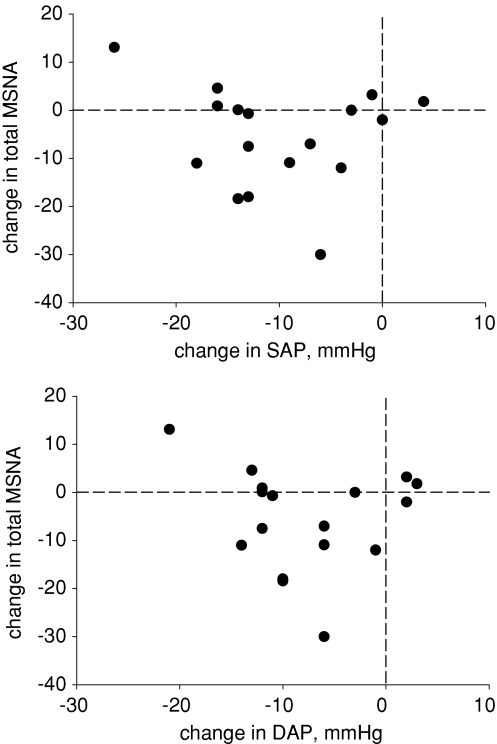
We subtracted average systolic and diastolic pressures and total MSNA from the last 20 s before presyncope from the last 40 to 20 s before presyncope and plotted individual responses in Fig. 8. Numerically, systolic pressure decreased in all but two, and diastolic pressure decreased in all but three subjects. Total MSNA increased numerically or did not change at all in seven, and decreased in 10 subjects. Regression analysis revealed no association between the magnitude of change in either systolic (r= 0.14) or diastolic (r= 0.12) pressure and the change in MSNA before presyncope.
Figure 8. Changes of muscle sympathetic nerve activity (MSNA) are plotted as a function of changes of diastolic (DAP) and systolic (SAP) pressure.
Changes were calculated by subtracting mean values recorded during the last 20 s before presyncope from mean values recorded during 40 s to 20 s before presyncope. Numbers of subjects deviating either above or below zero are separated by the horizontal (change in MSNA) and vertical (change in arterial pressure) dashed lines.
Discussion
We challenged arterial pressure control mechanisms by progressively decreasing central volume with LBNP until the point of presyncope to gain better insight into mechanisms of haemodynamic compensation and decompensation in humans. We report three primary findings: (1) progressive LBNP (and presumed progressive arterial baroreceptor unloading) increases cross-spectral coherence between arterial pressure and MSNA, but sympathetic baroreflex control is reduced before presyncope; (2) withdrawal of MSNA is not a prerequisite for presyncope despite significant decreases of arterial pressure; and (3) reductions of venous return, probably induced by intense LBNP, disrupt MSNA firing characteristics that manifest as fused integrated bursts before the onset of presyncope.
Central hypovolaemia and MSNA
Lower body negative pressure is commonly employed as a means to study cardiovascular reflex responses to central hypovolaemia (Wolthuis et al. 1974; Cooke et al. 2004). As fluid volumes are redistributed from the thorax to the lower body, unloading of various populations of baroreceptors (and perhaps other receptors such as muscle metaboreceptors and systemic chemoreceptors; Karemaker & Wesseling, 2008) results in increased efferent sympathetic traffic to the heart and peripheral vasculature (Wallin & Charkoudian, 2007). Muscle sympathetic nerve activity increases linearly with LBNP, but few studies have extended MSNA monitoring beyond −45 to −50 mmHg due to interference of the recording electrode by increasing negative chamber pressure. In fact, we are aware of only four previous studies that have presented MSNA responses to LBNP beyond −50 mmHg (Khan et al. 2002; Convertino et al. 2004; Ichinose et al. 2006; Cooke et al. 2008), and numbers of subjects in those studies are reduced at higher chamber pressures due to loss of the nerve signal. Out of an initial subject pool of 49, we were able to maintain stable nerve recordings throughout the LBNP protocol in 17 subjects. This is unique to the literature, as we are the first to present complete MSNA data in all subjects studied from baseline through presyncope without losing data due to loss of the nerve signal at high levels of negative pressure.
Compensation to central hypovolaemia and mechanisms of low-frequency rhythms
Although linear increases of sympathetic traffic are expected as a normal compensatory response to progressive unloading of arterial baroreceptors, the rhythmic firing of sympathetic nerves and consequent modulation of arterial pressure rhythms may represent a more fundamental issue – that is, the appropriate coupling of naturally occurring cardiovascular oscillators. Maintenance of stable arterial pressures with progressive LBNP (Table 1) suggests that increases in coherence between DAP and MSNA represent appropriate compensatory responses (Fig. 6).
At least two primary hypotheses have been advanced to explain low-frequency arterial pressure rhythms in humans. The central oscillator theory ascribes the origin of low-frequency rhythms to a pacemaker somewhere in the central nervous system (Preiss & Polosa, 1974). Barman et al. (2003) mathematically removed linear associations between MSNA and the ECG with partial autospectral analysis and revealed residual cardiac-related power that probably derived from a non-linear central oscillator. In addition, patients with spinal cord injury display spontaneous bursts of MSNA, but activity is significantly lower than that of neurologically intact patients, and is probably too low to account for low-frequency oscillations occurring around 0.1 Hz (Stjernberg et al. 1986). We averaged DAP–MSNA phase angles over the entire low-frequency range (0.4 to 0.15 Hz) and did not quantify centre frequency. We therefore cannot estimate with any certainty the time latencies in seconds between DAP and MSNA. However, phase angles shifted from positive at baseline to negative with application of LBNP. With DAP as the first and MSNA as the second signal input into our cross-spectral analyses, phase angles suggest that at rest MSNA oscillations lead those of DAP, but that as arterial baroreceptors are unloaded with LBNP, oscillations of DAP lead those of MSNA. Bursts of MSNA occur as inverse functions of arterial (primarily diastolic) pressures (Wallin & Nerhed, 1982) but can also occur spontaneously without pressure stimuli, especially when heart rates are low (Kienbaum et al. 2001). The positive phase angles between DAP and MSNA (Fig. 6) we demonstrate before application of LBNP suggests that a central oscillator controlling MSNA may be operative at baseline. With application of LBNP and probable reduction in arterial dimensions (Taylor et al. 1995), phase angles shift such that the presumed central oscillator is then overridden by arterial baroreflex disinhibition, with reductions of DAP then directly triggering bursts of MSNA.
The second hypothesis, the resonance theory, ascribes low-frequency arterial pressure oscillations to arterial baroreflex modulation of sympathetic activity (Guyton & Harris, 1951; de Boer et al. 1987). With this construct, sympathetic activation manifests in effector responses both at the heart and peripheral vasculature with a total time period of about 10 s (Wallin & Nerhed, 1982) or 0.1 Hz. In the present study, progressive increases of negative chamber pressure caused progressive increases in oscillations of both arterial pressure and MSNA. Coherence between arterial pressure (diastolic) and MSNA increased with increasing chamber pressure (Fig. 6) as calculated from cross-spectral analysis. These results are consistent with those of Furlan et al. (2000), who showed that head-up tilt is also associated with an increased association between low-frequency MSNA and arterial pressure oscillations. Our observations and those of Furlan et al. (2000) are consistent with predictions based on the resonance theory, but cannot rule out potential contributions from a central oscillator.
Sympathetic baroreflex responsiveness and MSNA firing characteristics
Several studies have documented withdrawal of MSNA at the point of haemodynamic decompensation in humans (Wallin & Sundlof, 1982; Sanders & Ferguson, 1989; Converse et al. 1992; Morillo et al. 1997; Cooke & Convertino, 2002; Kamiya et al. 2005). Such responses are consistent with classic vaso-vagal physiology with no active baroreflex control. Using LBNP as a model to induce central hypovolaemia, Ichinose et al. (2006) found that sympathetic baroreflex sensitivity as assessed from linear regression analysis between DAP and MSNA was shifted up during LBNP, and was then reduced significantly 1 to 2 min prior to decompensation. They concluded that LBNP-induced hypotension is preceded by a reduced baroreflex control over sympathetic neural activity, and that this reduced baroreflex sensitivity is associated with syncope.
We reasoned that if sympathetic baroreflexes are operative just before presyncope, a direct linear relationship between the magnitude of arterial pressure reductions and increase of MSNA should be obtained. Linear regression analysis revealed very weak relationships between arterial pressure and MSNA changes before presyncope (Fig. 8), suggesting either reduced sensitivity (Ichinose et al. 2006) or complete removal (Fagius et al. 1985) of sympathetic baroreflex influences. These observations are consistent with the contention of Karemaker & Wesseling (2008), that irrespective of arterial baroreflexes, central sympathetic drive is disinhibited when arterial pressures are dropping. More specifically, the normal pattern of sympathetic efferent traffic that may be modulated by baroreceptor afferent traffic could be lost as a consequence of severe reductions in stroke volume and pulse pressure (indicative of reduced venous return; Table 1).
Arterial pressure changes are effectively buffered by spontaneous sympathetic activity driven by classic negative feedback mechanisms. Sympathetic nerves fire in response to reductions of arterial dimensions (resulting in close associations with diastolic pressure) and are silenced with the consequent systolic pressure up-stroke resulting in integrated bursts with steep slopes, sharp peaks and almost constant latencies from preceding R-waves (Sundlof & Wallin, 1978; Fagius & Wallin, 1980). However, characteristic firing patterns of MSNA including burst reflex latencies and burst durations were altered in subjects susceptible to presyncope during routine upright tilt tests (Iwase et al. 2002). Latencies were variable, and burst durations were prolonged up to five times normal levels resulting in broad muscle sympathetic bursts that appeared to take on characteristics of skin nerves (Hagbarth et al. 1972). Prolonged, or fused bursts that lose their pulse synchrony were observed after bilateral blocks of glossopharyngeal and vagus nerves, suggesting that appearance of fused bursts may represent sympathetic baroreflex deafferentation (Fagius et al. 1985). We report in the present study similar changes in burst characteristics induced by intense LBNP (Figs 2–4, and 7). This new finding may have particular importance, because fluid shifts induced by LBNP are similar to haemorrhage, whereas shifts induced by head-up tilt are not (Taneja et al. 2007). Strongly forced LBNP decreases splanchnic volume, and probably attenuates pressor responsiveness of the vascular bed to sympathetic stimulation. This speculation could manifest as reduced arterial pressure–MSNA associations and drive continuous sympathetic firing in humans. Continuous sympathetic firing has been observed in animals; fused bursts of renal sympathetic nerve activity appeared in sheep during severe haemorrhage, followed by complete sympathetic withdrawal and cardiovascular decompensation (Batchinsky et al. 2007).
We can only speculate on the mechanisms responsible for reductions of reflex latencies and fusing of integrated bursts. Reductions of reflex latencies were observed during prolonged LBNP, and occurred in conjunction with increases in burst amplitude (Wallin et al. 1994). Wallin et al. (1994) attributed reduced latencies to recruitment of additional postganglionic fibres with increasingly rapid conduction velocities. In addition to reduced reflex latencies, several of our subjects responded to intense LBNP with continuous sympathetic firing that resulted in fusing of integrated bursts. We considered that perhaps during intense LBNP, heart rates are too fast for voltages recorded from the nerve to return to baseline before a second volley of activity is mounted. This simple explanation seems unlikely given the two representative subjects with similar heart rates presented in Fig. 3. However, although data presented in Fig. 3 suggest an ability to differentiate classic bursting activity from burst fusing, we cannot account for the possibility that our use of a set time constant of 0.1 s to integrate filtered nerve activity resulted in artefactual bursting characteristics. Beat-to-beat separation of sympathetic bursts theoretically becomes unclear as heart rate increases and it may therefore be desirable in future experiments to incorporate shorter time constants for integration of the nerve signal (for example, 0.04 s). It should also be noted that the shape of our fused bursts are remarkably similar to those predicted by a mathematical simulation showing a direct linear relationship between the number of action potentials contributing to a burst and the resulting burst amplitude (Tang et al. 2003). We did not record activity from single fibres, and so we cannot confirm or refute this notion. Regardless of underlying neural mechanisms responsible for fused bursts, it seems reasonable that continuous sympathetic firing may be driven by severe reductions of venous return, and represents in some subjects their maximal sympathetic reserve capacity (Engelki et al. 1996; Fu et al. 2004).
Summary
Neurally mediated haemodynamic decompensation is surprisingly common, can be debilitating, and exact causes can be difficult to determine (Mosqueda-Garcia et al. 2000). The autonomic nervous system plays a critical role in maintaining haemodynamic stability during conditions of severe central hypovolaemia, with sympathetic activation being a cornerstone of compensation. Haemodynamic instability leading to decompensation has been associated with abrupt sympathetic neural withdrawal (Wallin & Sundlof, 1982; Cooke & Convertino, 2002), reduced low-frequency sympathetic oscillations (Kamiya et al. 2005), and reduced sympathetic baroreflex sensitivity (Ichinose et al. 2006). Our current data indicate that abrupt sympathetic neural withdrawal cannot explain decompensation, since 40% of our subjects experienced presyncope without reductions in MSNA. The apparent dissociation between sympathetic nerve activity and arterial pressure observed immediately before presyncope suggests a disinhibition of central sympathetic drive (Karemaker & Wesseling, 2008), resulting in continuous sympathetic firing to counter severe reductions of venous return. Although fusing of integrated sympathetic bursts may reflect a true physiological compensation to severe reductions of venous return, duplication of this finding utilizing shorter time constants for integration of the nerve signal is required.
Acknowledgments
We thank the research volunteers for their cheerful participation and Mr Gary Muniz for his excellent laboratory assistance. This study was funded by the United States Department of Defense, Medical Research and Materiel Command.
Glossary
Abbreviations
- AP
arterial pressure
- DAP
diastolic arterial pressure
- LBNP
lower body negative pressure
- MSNA
muscle sympathetic nerve activity
Author contributions
All authors contributed to: (1) Conception and design, or analysis and interpretation of data; (2) Drafting the article or revising it critically for important intellectual content; (3) Final approval of the version to be published.
References
- Barman SM, Fadel PJ, Vongpatanasin W, Victor RG, Gebber GL. Basis for the cardiac-related rhythm in muscle sympathetic nerve activity of humans. Am J Physiol Heart Circ Physiol. 2003;284:H584–H597. doi: 10.1152/ajpheart.00602.2002. [DOI] [PubMed] [Google Scholar]
- Batchinsky AI, Cooke WH, Kuusela TA, Jordan BS, Wang JJ, Cancio LC. Sympathetic nerve activity and heart rate variability during severe hemorrhagic shock in sheep. Auton Neurosci. 2007;136:43–51. doi: 10.1016/j.autneu.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Converse RL, Jacobsen TN, Jost CM, Toto RD, Grayburn PA, Obregon TM, Fouad-Tarazi F, Victor RG. Paradoxical withdrawal of reflex vasoconstriction as a cause of hemodialysis-induced hypotension. J Clin Invest. 1992;90:1657–1665. doi: 10.1172/JCI116037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convertino VA, Ludwig DA, Cooke WH. Stroke volume and sympathetic responses to lower-body negative pressure reveal new insight into circulatory shock in humans. Auton Neurosci. 2004;111:127–134. doi: 10.1016/j.autneu.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Convertino VA. Association between vasovagal hypotension and low sympathetic neural activity during presyncope. Clin Auton Res. 2002;12:483–486. doi: 10.1007/s10286-002-0057-3. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. J Physiol. 1999;517:617–628. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke WH, Rickards CA, Ryan KL, Convertino VA. Autonomic compensation to simulated hemorrhage monitored with heart period variability. Crit Care Med. 2008;36:1892–1899. doi: 10.1097/CCM.0b013e3181760d0c. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol. 2004;96:1249–1261. doi: 10.1152/japplphysiol.01155.2003. [DOI] [PubMed] [Google Scholar]
- de Boer RW, Karemaker JM, Strackee J. Relationships between short-term blood-pressure fluctuations and heart-rate variability in resting subjects 1: a spectral analysis approach. Med Biol Eng Comput. 1985;23:352–358. doi: 10.1007/BF02441589. [DOI] [PubMed] [Google Scholar]
- de Boer RW, Karemaker JM, Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am J Physiol Heart Circ Physiol. 1987;253:H680–H689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2008;587:713–716. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelki KA, Doerr DF, Convertino VA. Application of acute maximal exercise to protect orthostatic tolerance after simulated microgravity. Am J Physiol Regul Integr Comp Physiol. 1996;271:R837–R847. doi: 10.1152/ajpregu.1996.271.4.R837. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Sympathetic reflex latencies and conduction velocities in normal man. J Neurol Sci. 1980;47:433–448. doi: 10.1016/0022-510x(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin G, Sundlof G, Nerhed C, Englesson S. Sympathetic outflow in man after anaesthesia of the glossopharyngeal and vagus nerves. Brain. 1985;108:423–438. doi: 10.1093/brain/108.2.423. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004;110:2931–2937. doi: 10.1161/01.CIR.0000146384.91715.B5. [DOI] [PubMed] [Google Scholar]
- Furlan R, Porta A, Costa F, Tank J, Baker L, Schiavi R, Robertson D, Malliani A, Mosqueda-Garcia R. Oscillatory patterns in sympathetic neural discharge and cardiovascular variables during orthostatic stimulus. Circulation. 2000;101:886–892. doi: 10.1161/01.cir.101.8.886. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Harris JW. Pressoreceptor-autonomic oscillation: a probable cause of vasomotor waves. Am J Physiol. 1951;165:158–166. doi: 10.1152/ajplegacy.1951.165.1.158. [DOI] [PubMed] [Google Scholar]
- Hagbarth K-E, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972;84:164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hagbarth K-E, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle nerves. Acta Physiol Scand. 1968;74:96–108. doi: 10.1111/j.1748-1716.1968.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Fujii N, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during severe orthostatic stress. J Physiol. 2006;576:947–958. doi: 10.1113/jphysiol.2006.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase S, Mano T, Kamiya A, Niimi Y, Fu Q, Suzumura A. Syncopal attack alters the burst properties of muscle sympathetic nerve activity in humans. Auton Neurosci. 2002;95:141–145. doi: 10.1016/s1566-0702(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Iwase S, Mano T, Saito M. Effects of graded head-up tilting on muscle sympathetic activities in man. Physiologist. 1987;30:S62–S63. [PubMed] [Google Scholar]
- Jansen JR, Wesseling KH, Settles JJ, Schreuder JJ. Continuous cardiac output monitoring by pulse contour during cardiac surgery. Eur Heart J. 1990;11:26–32. doi: 10.1093/eurheartj/11.suppl_i.26. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Hayano J, Kawada T, Michikami D, Yamamoto K, Ariumi H, Shimizu S, Uemura K, Miyamoto T, Aiba T, Sunagawa K, Sugimachi M. Low-frequency oscillation of sympathetic nerve activity decreases during development of tilt-induced syncope preceding sympathetic withdrawal and bradycardia. Am J Physiol Heart Circ Physiol. 2005;289:H1758–H1769. doi: 10.1152/ajpheart.01027.2004. [DOI] [PubMed] [Google Scholar]
- Karemaker JM, Wesseling KH. Variability in cardiovascular control: the baroreflex reconsidered. Cardiovasc Eng. 2008;8:23–29. doi: 10.1007/s10558-007-9046-4. [DOI] [PubMed] [Google Scholar]
- Khan MH, Kunselman AR, Leuenberger UA, Davidson WR, Ray CA, Cray KS, Hogeman CS, Sinoway LI. Attenuated sympathetic nerve responses after 24 hours of bed rest. Am J Physiol Heart Circ Physiol. 2002;282:H2210–H2215. doi: 10.1152/ajpheart.00862.2001. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela TA, Jartti TT, Tahvanainen KUO, Kaila TJ. Nonlinear methods of biosignal analysis in assessing terbutaline-induced heart rate and blood pressure changes. Am J Physiol Heart Circ Physiol. 2002;282:H773–H783. doi: 10.1152/ajpheart.00559.2001. [DOI] [PubMed] [Google Scholar]
- Mano T, Iwase S. Sympathetic nerve activity in hypotension and orthostatic intolerance. Acta Physiol Scand. 2003;177:359–365. doi: 10.1046/j.1365-201X.2003.01081.x. [DOI] [PubMed] [Google Scholar]
- Mayer S. Studien zur Physiologie des Herzens und der Blutgefasse V.Uber spontane Blutdruckschwankungen. Sitzungsberichte der Kaiserlichen Akademie der Wissenshaften. Mathematisch-Naturwissenschaftliche Classe. 1877;74:281–307. [Google Scholar]
- Morillo CA, Eckberg DL, Ellenbogen A, Beightol LA, Hoag JB, Tahvanainen KUO, Kuusela TA, Diedrich AM. Vagal and sympathetic mechanisms in patients with orthostatic vasovagal syncope. Circulation. 1997;96:2509–2513. doi: 10.1161/01.cir.96.8.2509. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R, Furlan R, Tank J, Fernandez-Violante R. The elusive pathophysiology of neurally mediated syncope. Circulation. 2000;102:2898–2906. doi: 10.1161/01.cir.102.23.2898. [DOI] [PubMed] [Google Scholar]
- Palazzolo JA, Estafanous FG, Murray PA. Entropy measures of heart rate variation in conscious dogs. Am J Physiol Heart Circ Physiol. 1998;274:H1099–H1105. doi: 10.1152/ajpheart.1998.274.4.H1099. [DOI] [PubMed] [Google Scholar]
- Preiss G, Polosa C. Patterns of sympathetic neuron activity associated with Mayer waves. Am J Physiol. 1974;226:724–730. doi: 10.1152/ajplegacy.1974.226.3.724. [DOI] [PubMed] [Google Scholar]
- Sanders JS, Ferguson DW. Profound sympathoinhibition complicating hypovolemia in humans. Ann Intern Med. 1989;111:439–441. doi: 10.7326/0003-4819-111-5-439. [DOI] [PubMed] [Google Scholar]
- Stjernberg L, Blumberg H, Wallin BG. Sympathetic activity in man after spinal cord injury. Outflow to muscle below the lesion. Brain. 1986;109:695–715. doi: 10.1093/brain/109.4.695. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja I, Moran C, Medow MS, Glover JL, Montgomery LD, Stewart JM. Differential effects of lower body negative pressure and upright tilt on splanchnic blood volume. Am J Physiol Heart Circ Physiol. 2007;292:H1420–H1426. doi: 10.1152/ajpheart.01096.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Chander AR, Schramm LP. Sympathetic activity and the underlying action potentials in sympathetic nerves: a simulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1504–R1513. doi: 10.1152/ajpregu.00339.2003. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Taylor JA, Halliwill JR, Brown TE, Hayano J, Eckberg DL. ‘Non-hypotensive’ hypovolaemia reduces ascending aortic dimensions in humans. J Physiol. 1995;483:289–298. doi: 10.1113/jphysiol.1995.sp020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Leimbach N. Effects of lower body negative pressure on sympathetic discharge to leg muscles in humans. J Appl Physiol. 1987;63:2558–2562. doi: 10.1152/jappl.1987.63.6.2558. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Burke D, Gandevia S. Coupling between variations in strength and baroreflex latency of sympathetic discharges in human muscle nerves. J Physiol. 1994;474:331–337. doi: 10.1113/jphysiol.1994.sp020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Nerhed C. Relationship between spontaneous variations of muscle sympathetic activity and succeeding changes of blood pressure in man. J Auton Nerv Syst. 1982;6:293–302. doi: 10.1016/0165-1838(82)90002-9. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Sundlof G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287–291. doi: 10.1016/0165-1838(82)90001-7. [DOI] [PubMed] [Google Scholar]
- Wolthuis RA, Bergman SA, Nicogossian AE. Physiological effects of locally applied reduced pressure in man. Physiol Rev. 1974;54:566–595. doi: 10.1152/physrev.1974.54.3.566. [DOI] [PubMed] [Google Scholar]