Abstract
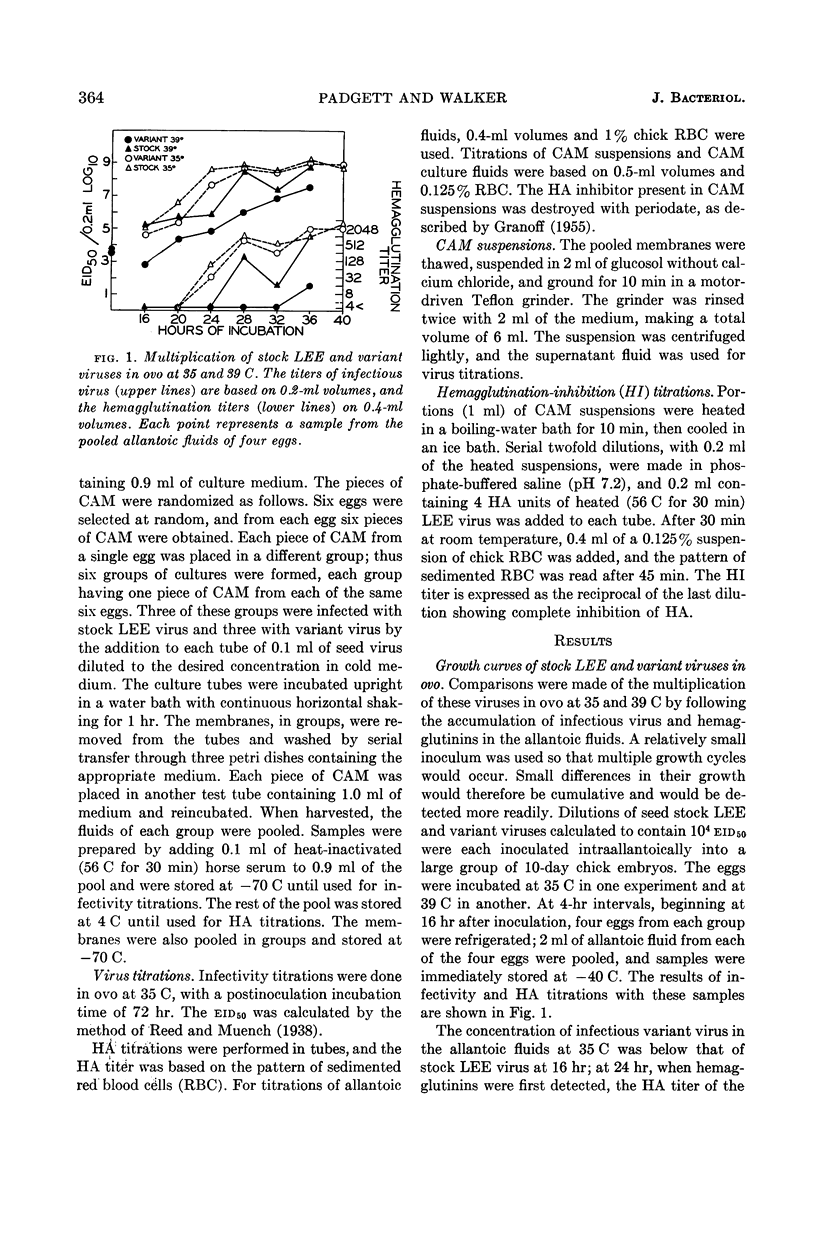
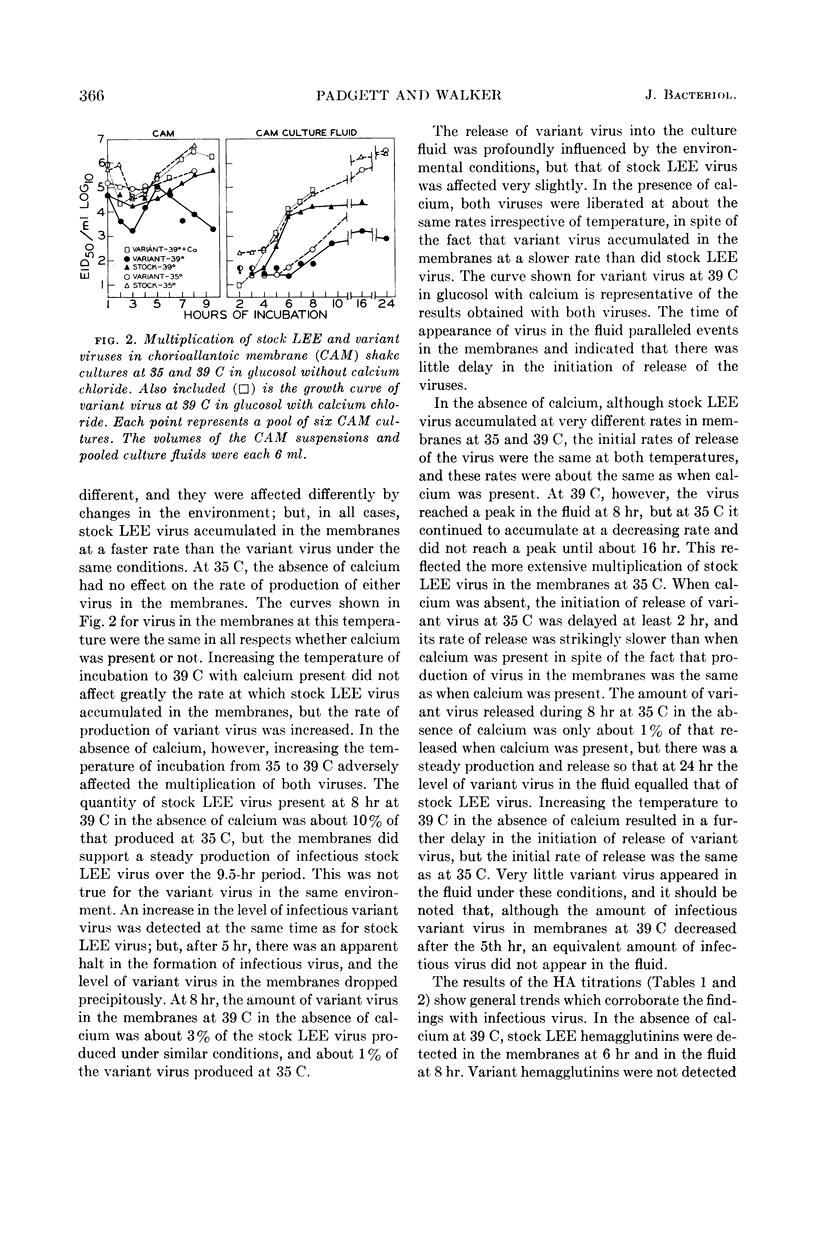
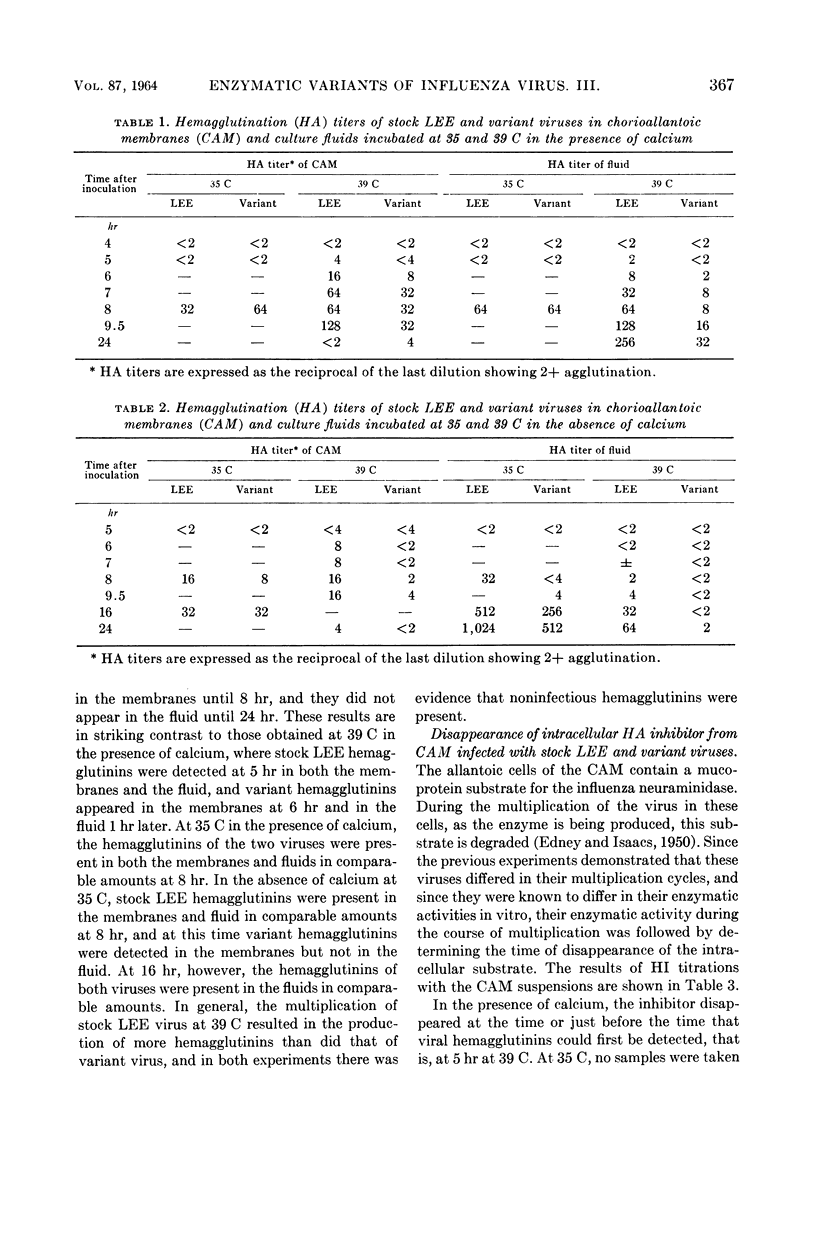
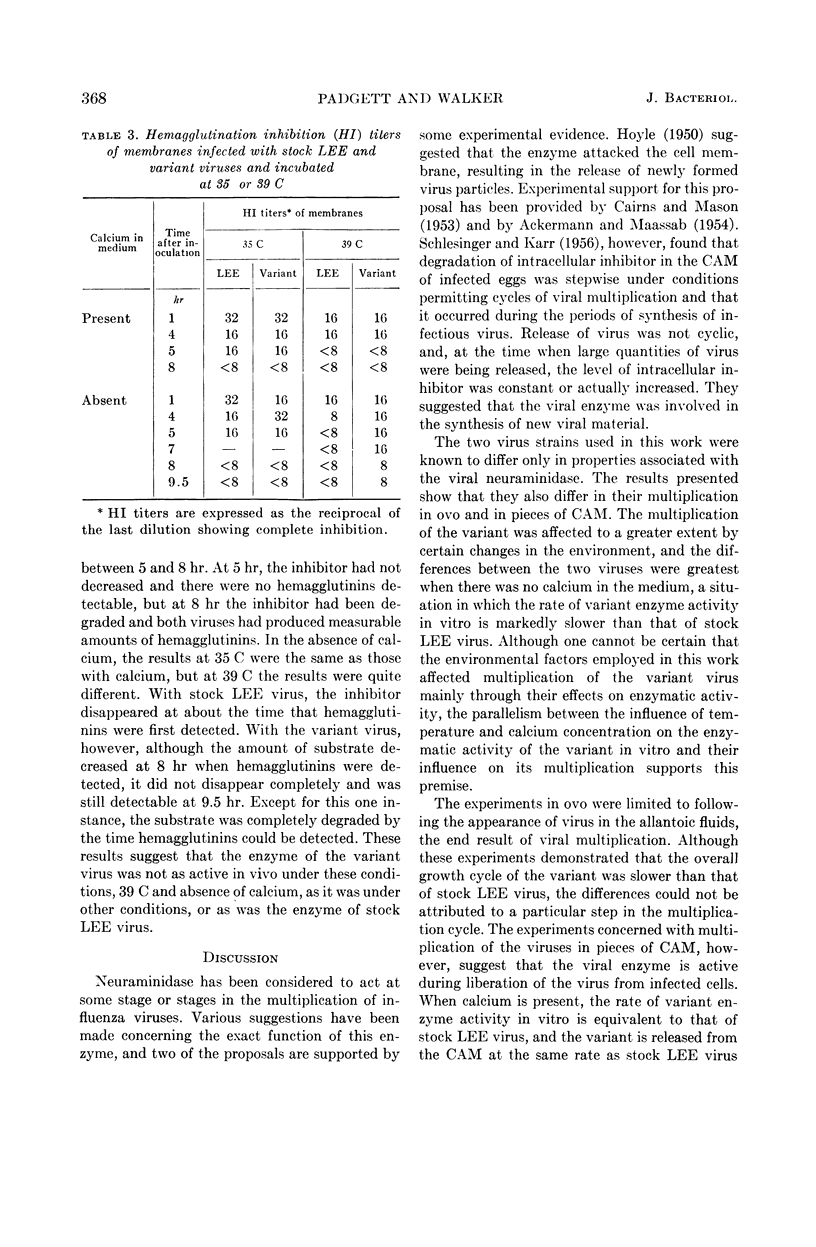
Padgett, Billie L. (University of Wisconsin, Madison), and Duard L. Walker. Enzymatic variants of influenza virus. III. Function of neuraminidase in the viral growth cycle. J. Bacteriol. 87:363–369. 1964.—Multiplication of a slowly reacting enzymatic variant of influenza B virus, strain LEE, was compared with that of parent virus in ovo. At 35 C, although their final yields were equal, variant virus reached its peak concentration in the allantoic fluids later than parent virus. At 39 C, multiplication of both viruses was slower, parent virus requiring 4 hr and variant virus 8 hr longer to reach infectivity levels comparable with those at 24 hr at 35 C. Variant enzyme activity in vitro can be controlled by altering the temperature and calcium concentration. Growth curves of these viruses in pieces of chorioallantoic membrane (CAM) in culture under conditions in which the variant should be as active as the parent revealed only minor differences between them. Under conditions in which variant enzyme activity would be much slower than the parent, the release of variant virus from the CAM was delayed and the rate of release was slower. Under the most adverse conditions, 39 C and no calcium, formation of infectious variant virus ceased after 5 hr, and the hemagglutination inhibitor in the cells was not degraded although hemagglutinins were produced. These findings are discussed in relation to the function of neuraminidase during viral multiplication.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERMANN W. W., MAASSAB H. F. Growth characteristics of influenza virus; biochemical differentiation of stages of development. J Exp Med. 1954 Oct 1;100(4):329–339. doi: 10.1084/jem.100.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS H. J., MASON P. J. Production of influenza A virus in the cells of the allantois. J Immunol. 1953 Jul;71(1):38–40. [PubMed] [Google Scholar]
- EDNEY M., ISAACS A. Interference between inactive and active influenza viruses in the chick embryo. III. Inhibitor of virus haemagglutination in the chorioallantoic membrane. Aust J Exp Biol Med Sci. 1950 Nov;28(6):603–612. doi: 10.1038/icb.1950.62. [DOI] [PubMed] [Google Scholar]
- FULTON F., ARMITAGE P. Surviving tissue suspensions for influenza virus titration. J Hyg (Lond) 1951 Jun-Sep;49(2-3):247–262. doi: 10.1017/s0022172400044132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANOFF A. Noninfectious forms of Newcastle disease and influenza viruses; studies on noninfectious virus occurring within cells that are producing fully infectious virus. Virology. 1955 Dec;1(5):516–532. doi: 10.1016/0042-6822(55)90040-4. [DOI] [PubMed] [Google Scholar]
- PADGETT B. L., WALKER D. L. Enzymatic variants of influenza virus. I. Isolation and characterization of slowly reacting enzymatic variants of influenza B virus. J Exp Med. 1957 Jul 1;106(1):53–68. doi: 10.1084/jem.106.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADGETT B. L., WALKER D. L. Enzymatic variants of influenza virus. II. Effect of environmental factors on enzymatic characteristics of a variant of influenza B virus. J Exp Med. 1958 Nov 1;108(5):651–664. doi: 10.1084/jem.108.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLESINGER R. W., KARR H. V. Influenza virus and its mucoprotein substrate in the chorioallantoic membrane of the chick embryo. II. Stepwise inactivation of substrate and its relation to the mode of viral multiplication. J Exp Med. 1956 Mar 1;103(3):333–349. doi: 10.1084/jem.103.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM I., FOLKERS K., HORSFALL F. L., Jr Inhibition of influenza virus multiplication by alkyl derivatives of benzimidazole. II. Measurement of inhibitory activity by hemagglutination titrations. J Exp Med. 1953 Sep;98(3):229–243. doi: 10.1084/jem.98.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]



