Abstract
Previous studies with adult humans and non-human animals revealed more rapid fear learning for spiders and snakes than for mushrooms and flowers. The current experiments tested whether 11-month-olds show a similar effect in learning associative pairings between facial emotions and fear-relevant and fear-irrelevant stimuli. Consistent with the greater incidence of snake and spider phobias in women, results show that female but not male infants learn rapidly to associate negative facial emotions with fear-relevant stimuli. No difference was found between the sexes for fear-irrelevant stimuli. The results are discussed in relation to fear learning, phobias, and a specialized evolved fear mechanism in humans.
The evolved function of fear is to organize responses when confronted with a particular kind of adaptive problem, namely, danger. Over evolutionary time, specific dangers were recurrent, and there would have been intense selection pressure for the emergence of psychological mechanisms that facilitate fear learning for them (Öhman & Mineka, 2001). Two recurrent dangers to humans from other species throughout human and primate evolution were spiders and snakes (Öhman & Mineka, 2003). Fear of these non-human animals is common in adults and children, and they elicit phobias in approximately 5.5% (snakes) and 3.5% (spiders) of the population (Fredrikson, Annas, Rischer, & Wik, 1996). Moreover, there is a consistent sex difference in the incidence of snake and spider phobias; women are four times more likely than men to have fears and phobias for these, but not other stimuli (e.g., injections, heights, flying) (Fredrikson et al., 2006; Marks, 1969). Is part of this greater incidence of snake and spider fear in women the result of a specialized evolved fear mechanism? This question was examined in the two experiments reported here.
It is particularly important for fear learning of specific threats to be facilitated by an evolved psychological mechanism because it is not adaptive to learn about the potentially dangerous nature of, for example, snakes and spiders by being bitten and killed. According to Öhman, Mineka, and colleagues (Öhman & Mineka 2001; Öhman, Flykt, & Esteves, 2001), human and non-human primates possess an evolved fear mechanism for fear-relevant stimuli such as snakes and spiders that is selectively responsive to, and is triggered by such stimuli. This fear mechanism predisposes children and adults to attend to snakes and spiders and prepares them rapidly learn to associate the appropriate emotional response – namely, fear—with such stimuli.
There is now considerable evidence from adults, children, infants, and non-human primates to support this view. For example, human adults show superior conditioning for images of snakes and spiders with a mid shock than for fear-irrelevant stimuli such flowers, mushrooms, or electric outlets (Öhman & Mineka, 2001). Human adults and children also more quickly detect snakes against a background of flowers or mushrooms than flowers or mushrooms against a background of snakes (Lobue & DeLoache, 2008; Öhman et al., 2001). There is also evidence that 7- to 18-month-old – both boys and girls - infants associate snakes with fear because they look longer at movies of snakes paired with a frightened human voice than movies of snakes paired with a happy human voice (DeLoache & Lobue, 2009). Furthermore, infants at 5 months of age look longer at a schematic image of a spider than a partly or completely scrambled image of a spider but do not do so for schematic and scrambled images of a flower (Rakison & Derringer, 2008). Finally, there is also evidence that rhesus monkeys reared in the lab associate snakes with a fearful response from another monkey more quickly that they associate flowers with a fearful response (Cook & Mineka, 1990).
According to Error Management Theory (EMT) (e.g., Haselton & Buss, 2000), many judgment and decision making adaptations are designed by natural selection to be biased to err in the direction of lower survival or reproductive cost. This view predicts that humans’ fear mechanism may be designed to be particularly sensitive to, or prepared for, pairings of negative emotions and specific recurrent threats because the fitness cost of not learning such pairings would be high (see also Nesse’s (2001) “Smoke detector principle” that defenses are often expressed too readily or too intensely). It is plausible that this may be the reason why so many individuals develop an irrational aversion, or phobia, of a recurrent threat, which is consistent with the fact that phobias tend to be related to evolutionarily relevant stimuli (e.g., snakes, spiders, heights, people).
Why, then, should women be more likely than men to develop phobias for snakes and spiders? There are a number of plausible explanations for this sex difference. One possibility is that social transmission of fears and phobias is more common or promoted among women than men (Fredrikson, Annas, & Wik, 1997). Alternatively, women’s fear mechanism may be more sensitive to snakes and spiders than males’ fear mechanism because they were more exposed to them over evolutionary time (e.g., during child-care, while foraging and gathering food). It is also feasible, as predicted by EMT, that a fear of snakes and spider was particularly important in women because it protects both their child and themselves. In other words, the fitness costs of being bitten by a snake or spider would have been greater for women than for men because infants and young children, historically, rarely survived a mother’s death (Buss, 2008). Finally, because of the higher reproductive variance for men, evolution would have selected against males with overly powerful fears because it could have inhibited risk taking involved in, for example, large game hunting.
The current experiments were designed to establish whether indeed the basis for adult females’ greater incidence of fear and phobias for snakes and spiders is rooted in an evolutionary mechanism that is present in infancy. According to EMT, the nature of this evolved mechanism would lead female infants to show an advantage over male infants in learning associations between snakes and spiders and a negative facial emotion but would not do so for fear-irrelevant stimuli. Alternatively, work by DeLoache and Lobue (2009) in which no sex-differences were found suggests that both boys and girls should show an advantage for learning associations between recurrent threats and a negative facial emotion relative to associations between non-threats and a negative facial expression. It is also plausible that an evolved fear mechanism is agnostic about the emotion to be paired with a recurrent threat, which suggests that boys and girls should show an advantage for learning associations between recurrent threats and any facial emotion relative to learning associations between non-threats and a facial expression. Note that although any of these findings would provide evidence for the presence of an evolved fear mechanism in infants, they would not necessarily rule out any of the evolutionary explanations presented above for why women are more likely than males to develop fears and phobias for recurrent threats. Finally, if fear learning is unrelated to a specialized evolved mechanism and is underpinned by more general learning mechanisms, infants should not show any difference in learning associations between facial emotions and threats or non-threats.
Experiment 1
In this experiment, 11-month-old infants were tested in the visual habituation paradigm with an adaptation of the Switch design. Infants were habituated to a single color photo of a spider or a snake paired with either a happy or fearful schematic face. In the test phase, infants were presented with a novel spider or snake paired with a different face (e.g., happy if habituated to a fearful face) as well as a mushroom or flower paired with the same novel face. As outlined above, if infants possess a specialized evolved fear mechanism then girls but not boys, or both girls and boys, who were habituated to the fearful face paired with a snake or spider should look longer at the novel pairing between the snake or spider and a positive facial emotion relative to the novel pairing between the flower or mushroom and a positive facial emotion. In contrast, if phobias for snakes and spiders are grounded in general learning mechanisms and not evolved specialized learning mechanisms then boys and girls would be expected to look longer at the novel test trial with the snake or spider regardless of the facial emotion presented during (i.e., fearful or happy face).
Method
Participants
Participants were 20 healthy full-term infants with a mean age of 11 months 9 days (range: 10 months, 13 days to 11 months, 22). There were an equal number of boys and girls. An additional eight infants were tested but not included in the final analysis because of failure to habituate (5), experimenter error (1), or looking more than 2 SD beyond the condition mean (2).
Materials and design
During the pretest, infants were shown two stimuli (snake or spider and mushroom or flower) one at a time to determine any a-priori preferences. They were then habituated to events in which the target stimulus (the snake or spider shown during the pretest) was presented on the left side of the screen for 2 s after which the schematic face (either happy or fearful) appeared on the right side of the screen. Both images remained motionless on the screen for another 7 s. The kind of face (happy or fearful) paired with the stimuli in habituation was counterbalanced across infants. A blue occluding screen lowered and rose between each event (lasting .5 s each). There were two trials in the test phase. In the fear-relevant trial, infants were shown a novel snake or spider stimulus with a different face from that seen during habituation. For example, if infants were habituated to a snake paired with a fearful face in the test trial they would see a new snake paired with the happy face. In the fear-irrelevant trial, infants were shown a novel mushroom or flower paired with a different face from that seen during habituation. For example, if infants were habituated to a snake paired with a fearful face in the fear-irrelevant test trial they would see a mushroom paired with the happy face. The rationale for this design is that if infants during habituation associated the snake or spider with the face stimulus, they should look longer at the snake or spider paired with a different face than at a novel stimulus (flower or mushroom) paired with a novel face.
The stimuli were four color photographs of spiders and four color photographs of snakes (see Figure 1). There were also two different drawings of faces, one depicting a happy emotion and one depicting a fearful emotion (see Figure 1). The habituation, test, and control stimuli, as well as the pairing between face-type and stimulus, were counterbalanced across infants.
Figure 1.

Stimuli used in Experiment 1 and 2. The upper part of the figure shows the two schematic faces used during habituation and the test trials. The lower part of the figure shows the four snakes, spiders, flowers, and mushrooms used in the pretest, the habituation, and test trials.
Procedure
Each infant sat on their caretakers’ lap in front of a computer screen (size: 14 in. × 24 in.; distance: 24 in.). The pretest stimuli appeared on the monitor for a maximum of 20 s or until the infant looked away from the monitor for 2 s. During the habituation phase, each event was presented until the infant visually fixated away from the monitor for over 1 s or until 20 s of uninterrupted looking had elapsed. The habituation phase ended when an infant’s looking time for a block of three trials decreased to 50% of that recorded during the first three trials. The test trials were presented until the infant looked away for over 1 s or after 20 s of continuous looking. A green expanding and contracting circle on a black background with a synchronous bell sound was presented prior to the first habituation trial and between each habituation and test trial. The primary experimenter coded the looking time behavior online by pressing and releasing a preset keyboard key. A second judge who was blind to the hypothesis and which trial was presented recoded the looking times from a videotape of the session. Interrater reliability in all of the experiments reported here was >97%.
Results and Discussion
The first preliminary analysis examined looking times to the two pretest items with a mixed-design analysis of variance (ANOVA) with sex (male vs. female) as a between-subjects factor and stimulus (fear-relevant: snake/spider vs. fear-irrelevant: mushroom/flower) as a within-subjects factor. The analysis revealed no significant main effect for stimulus, F(1,18)=0.01, p>.9, ηp2 = 0.00, or sex, F(1,18)=1.18, p>.2, ηp2 = 0.06, and no significant interaction between the variables, F(1,18)=0.25, p>.6, ηp2 = 0.01. Thus, there were no a-priori preferences among the girls or boys for the two kinds of stimuli.
A second set of preliminary analyses compared the number of habituation trials and the total looking time during habituation for the girls and boys. The analyses revealed that girls (M = 10.00; SD = 2.98) and boys (M = 10.40; SD = 3.98) required a comparable number of trials to habituate, t(18)=0.25, p>.8, and that girls (M = 106.37; SD = 61.33) and boys (M = 112.42; SD = 59.62) looked equally long overall during habituation, t(18)=0.22, p>.8.
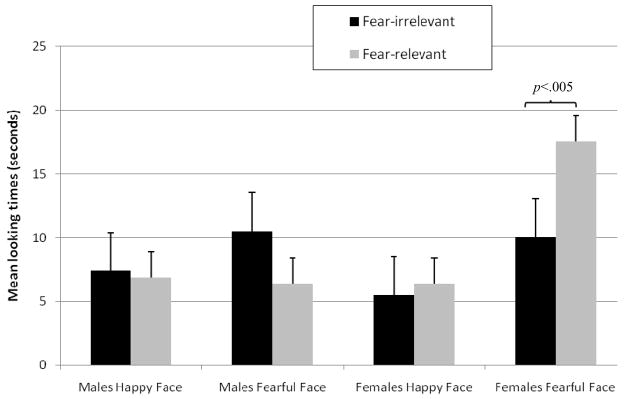
The main analysis used a mixed-design ANOVA with test trial (fear-relevant vs. fear-irrelevant) as the within-subjects factor and habituation face (happy vs. fearful) and sex (male vs. female) as between-subjects factors. Infants’ looking times are presented in Figure 2. The analysis revealed a significant interaction between the sex of the infant and test trial, F(1,16)=8.40, p<.01, ηp2 = 0.34, which was moderated by a significant interaction between habituation face, sex of the infant, and test trial, F(1,16)=5.17, p<.05, ηp2 = 0.24. There were no other significant effects, all p>.2. Planned comparisons indicated that girls who were habituated to the fearful face paired with the spider or snake looked significantly longer at the fear-relevant test trial (M = 17.54; SD = 3.38) than at the fear-irrelevant test trial (M = 10.02; SD = 5.08), F(1,4)=31.24, p<.005, ηp2 = 0.88. In contrast, girls who were habituated to the happy face paired with the spider or snake looked equally long at the fear-relevant test trial (M = 6.42; SD = 5.96) and fear-irrelevant test trial (M = 5.50; SD = 4.96), F(1,4)=0.44, p>.5, ηp2 = 0.09. The analyses also revealed that boys looked equally long at both trials regardless of the habituation stimuli, all p>.4.
Figure 2.
Infant looking times for male and female infants in the two test trials in Experiment 1. Error bars represent standard error.
Discussion
These data show that girls, but not boys, who were habituated to a fearful face and a recurrent threat looked significantly longer when a novel snake or spider was paired with a different facial emotion relative to when a mushroom or flower was paired with the same facial emotion. This suggests that female infants associated the snake or spider seen during habituation with the fearful facial emotion and generalized it to the novel snake or spider in the test trials. The same effect was neither found for male infants who were habituated to the fearful face nor for male or female infants who were habituated to a pairing of a happy face with a recurrent threat.
Experiment 2
An alternative explanation for girls’ looking pattern in Experiment 1 is that they are highly attuned to learn the pairing between a negative emotion and any stimulus. If this were the case, the results of the first experiment would be unrelated to learning about the pairing of a fearful emotion with snakes or spiders per se. To test this explanation, Experiment 2 was designed to be identical to the first experiment except that infants were habituated to a single color photo of a fear-irrelevant stimulus (i.e., mushroom or flower) paired with either a happy or a fearful schematic face. As in Experiment 1, infants were then tested with a novel mushroom or flower, as well as a novel spider or snake, paired with a different facial emotion to that seen during habituation.
Participants
Participants were 20 healthy full-term infants with a mean age of 11 months 4 days (range: 10 months, 11 days to 11 months, 18). There were an equal number of boys and girls. An additional seven infants were tested but not included in the final analysis because of failure to habituate (1), experimenter error (3), fussiness (2), or looking more than 2 SD beyond the condition mean (1).
Materials and procedure
The stimuli in the experiment were identical to those in Experiment 1; however, pictures of mushrooms and flowers served as the habituation stimuli. All other aspects of the design and procedure were the same as Experiment 1.
Results and Discussion
Analysis of the pretest trials revealed no differences in looking times for the spider/snake and mushroom/flower across all the infants, F(1,18)=0.05, p>.8, ηp2 = 0.01, or for male and female infants, F(1,18)=0.31, p>.5, ηp2 = 0.02. There were also no differences between the males and females in the number of habituation trials, t(18)=0.33, p>.7, and the total looking time during habituation, t(18)=1.20, p>.2.
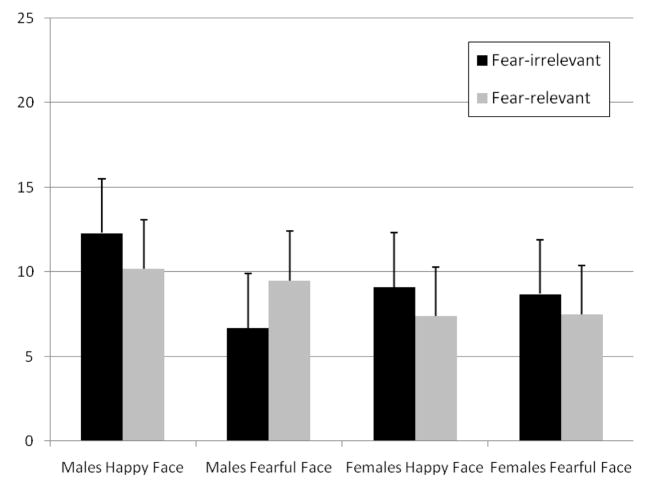
As in Experiment 1, the looking times on the two test trials (see Figure 3) were entered into a mixed-design ANOVA with test trial (fear-relevant vs. fear-irrelevant) as the within-subjects factor and habituation face (happy vs. fearful) and sex (male vs. female) as between-subjects factors. The analysis revealed no significant main effect for test trial, sex of the infant, or habituation face, all p’s>.1. Crucially, however, there was no significant interaction between sex of the infant and test trial, F(1,16)=0.65, p>.4, ηp2 = 0.04, and no significant interaction between habituation face, sex of the infant, and test trial, F(1,16)=0.41, p>.5, ηp2 = 0.03.
Figure 3.
Infant looking times for male and female infants in the two test trials in Experiment 2. Error bars represent standard error.
These results show that male and female 11-month-olds did not learn the relation between either a positive or a negative facial emotion and a mushroom or flower. This implies that the different behavior of female infants relative to male infants in Experiment 1 was not due to a general advantage in encoding a pairing between a negative facial emotion and a second stimulus but was specific to associative learning for spiders and snakes.
General Discussion
The two experiments reported here show that female 11-month-olds—but not males of the same age—learn the relation between a negative facial expression and fear-relevant stimuli such as snakes and spiders. Importantly, the same effect was not found for paired associate learning between facial emotions and fear-irrelevant stimuli such as mushrooms and flowers. As such, the current data support the hypothesis that women may be more predisposed than men to learn to the appropriate emotion for non-human animals that were recurrent threats over evolutionary time. Note, however, that the results should not be interpreted to mean that males are unable to learn relations between facial emotions and fear-relevant stimuli. Rather, the data suggest that female infants are able to do so after a relatively brief experience and that male infants may require a longer period of exposure to such stimuli.
The results of the present experiments are consistent with a large body of work that has shown a differential response by humans and primate to fear-relevant and fear-irrelevant stimuli. Work with captive monkeys, for example, showed that they can rapidly be taught to fear snakes but not flowers through social referencing (Cook & Mineka, 1990). Similarly, research with human adults revealed that conditioned fear is more resistant to extinction with fear-relevant (e.g., snakes) than with fear-irrelevant stimuli (e.g., flowers) (Öhman & Mineka, 2001). Although infants in the current experiments were not taught fear for specific stimuli, these data are the first to show a differential response by female infants to such stimuli. Previous research with infants on this issue has failed to detect such a sex-difference, perhaps because of limited sample sizes (e.g., DeLoache & Lobue, 2009). The current experiments indicate that humans possess a specialized evolved fear mechanism, that it is operational in the first year of life, and that it is particularly sensitive in females. The results also are consistent with the idea that infants possess a perceptual template that specifies the structure of snakes as well as spiders (Rakison & Derringer, 2008). It is this template that “prepares” infants, particularly female infants, to attend to fear-relevant stimuli and learn the appropriate negative emotional response for them.
The finding that female infants—but not male infants—learn associations between fearful facial emotions and fear-relevant stimuli suggests, albeit tentatively, that the greater fear and phobic incidence in female children and adults may be partially based on differently functioning fear mechanisms. According to evolutionary theory, and in particular Error Management Theory (e.g., Haselton & Buss, 2000; see also Nesse, 2001), there would have been powerful selection pressure for women to err on the side of caution with regard to recurrent threats such as snakes and spiders. This may have been they would likely have encountered them often during foraging and gathering and because of the potential cost to themselves and their offspring. There would also have been less selection pressure for men to avoid these threats because of the need for risk-taking behavior such as hunting (Buss, 2008). Unfortunately, the current data do not help to determine which, if any, of these explanations are more veridical.
It could be argued that the current findings do not provide evidence of a fear mechanism for snakes and spider because women and girls have substantially more phobias and fears of all types than do men and boys. Thus, it is not that women or girls have a specific spider and snake fear mechanism but rather they have a general bias to develop fears and phobias more than men and boys. There are at least two reasons to reject this claim, however. First, although women and girls tend to have a greater number of phobias than men and boys, significant sex differences are found only for evolutionarily relevant stimuli (e.g., snakes, spiders, closed spaces, darkness) and not for more modern fear-related stimuli (e.g., injections, flying, dentists) (Fredrikson et al., 1996). This suggests that women – and men - may have a number of specialized evolved fear mechanisms rather than a general tendency to develop phobias for any and all stimuli. As discussed above, it is possible that women are more likely than men to develop phobias for these evolutionarily relevant stimuli because of the potential survival cost to their child.
Second, if women – and female infants –have a general tendency to develop fears and phobias for all stimuli that are paired with a negative emotion then in the current experiments they should have associated the fearful face with the mushroom or flower. However, infant girls tested here associated only the snakes and spiders with the fearful face and not the mushrooms and flowers with the fearful face. Why, then, does an evolved fear mechanism cause sex differences in learning at such an early age? One compelling possibility is that they are preparatory for the development of the later sex differences in fear learning found in human adults. An alternative, though not mutually exclusive, explanation is that our ancestors were highly likely to encounter recurrent threats in infancy and early childhood and it is therefore adaptive for a fear mechanism to be on-line early in life.
To conclude, the current experiments provide the first evidence that the greater incidence of snake and spider fears and phobias in women may have its origins in infancy. Clearly, however, caution is necessary at this point because the current experiments show only that female infants have an advantage over male infants in associating fearful schematic faces with fear-relevant stimuli. It remains to be seen whether this form of associative learning is the same as that involved in fear learning in infancy and beyond. Nonetheless, the approach taken here, in conjunction with recent work with infants and young children (DeLoache & LoBue, 2009; LoBue & DeLoache, 2008; Rakison & Derringer, 2008), demonstrates that a developmental perspective can provide considerable insight into the evolved psychological mechanisms of adults.
Acknowledgments
The author would like to thank Gabriel Smith, Jessica Jankowitsch, Katie Andreasson, and the rest of the staff of the Infant Cognition Laboratory at Carnegie Mellon University for their help with data collection and participant recruitment. He would also like to thank two anonymous reviewers and the Martie Haselton for excellent suggestions and critiques during the review process. This work was supported by a grant from the National Institute of Child Health and Human Development (R03HD049511-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buss DM. Evolutionary psychology: The new science of the mind. 3. Boston, MA: Allyn & Bacon; 2008. [Google Scholar]
- Cook M, Mineka S. Selective associations in the observational conditioning of fear in rhesus monkeys. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16:372–389. [PubMed] [Google Scholar]
- Fredrikson M, Annas P, Fischer H, Wik G. Gender and age differences in the prevalence of specific fears and phobias. Behaviour Research and Therapy. 1996;34:33–39. doi: 10.1016/0005-7967(95)00048-3. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Annas P, Wik G. Parental history, aversive exposure and the development of snake and spider phobia in women. Behaviour, Research and Therapy. 1997;35:23–28. doi: 10.1016/s0005-7967(96)00076-9. [DOI] [PubMed] [Google Scholar]
- Haselton MG, Buss DM. Error management theory: A new perspective on biases in cross-sex mind reading. Journal of Personality and Social Psychology. 2000;78:81–91. doi: 10.1037//0022-3514.78.1.81. [DOI] [PubMed] [Google Scholar]
- LoBue V, DeLoache JS. Detecting the snake in the grass: Attention to fear-relevant stimuli by adults and young children. Psychological Science. 2008;19:284–289. doi: 10.1111/j.1467-9280.2008.02081.x. [DOI] [PubMed] [Google Scholar]
- DeLoache JS, LoBue V. The narrow fellow in the grass: Human infants associate snakes and fear. Developmental Science. 2009;12:201–207. doi: 10.1111/j.1467-7687.2008.00753.x. [DOI] [PubMed] [Google Scholar]
- Marks IM. Fears and Phobias. London: Heinemann; 1969. [Google Scholar]
- Muris P, Mercklebach H. The etiology of childhood specific phobia: A multifactorial model. In: Vasey M, Dadds M, editors. The developmental psychopathology of anxiety. New York: Oxford University Press; 2001. pp. 355–385. [Google Scholar]
- Nesse RM. The smoke detector principle: Natural selection and the regulation of defenses. Annals of the New York Academy of Sciences. 2001;935:75–85. [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fear, phobias and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. The malicious serpent: Snakes as a prototypical stimulus for an evolved module of fear. Current Directions in Psychological Science. 2003;12:5–8. [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Rakison DH, Derringer J. Do infants possess an evolved spider-detection mechanism? Cognition. 2008;107:381–393. doi: 10.1016/j.cognition.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Miller JZ, Pogue-Geile MF, Cardwell GF. Twin-family studies of common fears and phobias. In: Gedda L, Parisi P, Nance WE, editors. Twin research, 3: Intelligence, personality, and development. New York: Alan R. Liss Inc; 1981. [Google Scholar]