Abstract
Mammalian sterile 20-like kinase-3b (Mst3b/Stk24) regulates axon outgrowth in embryonic cortical neurons in culture, but its role in vivo and in neural repair is unknown. Here we show that Mst3b mediates the axon-promoting effects of trophic factors in mature rat retinal ganglion cells (RGCs) and dorsal root ganglion (DRG) neurons, and is essential for axon regeneration in vivo. Reducing Mst3b levels using shRNA prevented RGCs and DRG neurons from regenerating axons in response to growth factors in culture, as did expression of a kinase-dead Mst3b mutant. Conversely, expression of constitutively active Mst3b enabled both types of neurons to extend axons without growth factors. In vivo, RGCs lacking Mst3b failed to regenerate injured axons when stimulated by intraocular inflammation. DRG neurons regenerating axons in vivo showed elevated Mst3b activity, and reducing Mst3b expression attenuated regeneration and p42/44 MAPK activation. Thus, Mst3b regulates axon regeneration in both CNS and PNS neurons.
Introduction
Axon regeneration occurs readily after injury in the mature peripheral nervous system (PNS) but not within the central nervous system (CNS). The optic nerve is a classic model of a CNS pathway that does not regenerate when injured. Mature retinal ganglion cells (RGCs) show an abortive sprouting reaction following nerve injury but no long-distance axon growth1. Surprisingly, however, significant regeneration can be achieved by inducing an inflammatory response in the eye. Implanting a peripheral nerve fragment into the vitreous2, injuring the lens3-5, or injecting zymosan6,7 or ciliary neurotrophic factor8,9 into the eye all lead to an influx of macrophages and enable RGCs to extend lengthy axons beyond the site of nerve injury. Macrophages release a growth factor, oncomodulin (Ocm), that stimulates optic nerve regeneration in vivo provided cAMP levels are elevated10. In the PNS, sensory neurons of the dorsal root ganglia (DRG) regenerate their peripherally directed axons readily after injury. The signals that initiate this process remain uncertain, but may include disruption of retrograde signaling, calcium influx, exposure of the proximal stump to an inflammatory environment11,12, or generation of novel retrogradely transported signals at the injury site13.
Several lines of evidence suggest that axon outgrowth involves a purine-sensitive protein kinase. The purine analog 6-thioguanine (6-TG) blocks the neurite-promoting effects of trophic agents14-16 and inhibits the activity of a 45-50 kDa protein kinase17. Inosine, a naturally occurring purine nucleoside, reverses the inhibitory effects of 6-TG and can promote axon outgrowth in several types of neurons on its own15,16,18. Based on these observations, we isolated Mst3b as a purine-sensitive protein kinase and showed that it mediates the axon-promoting effects of inosine and neurotrophic factors in cell culture15,16. Mst3b was found to be rapidly activated when embryonic cortical neurons or PC12 cells are exposed to neurotrophins, and inhibiting the expression or activity of Mst3b prevents these cells from extending axons in response to inosine or trophic factors16. In vivo, inosine stimulates the growth of axon collaterals from intact neurons if other brain pathways have been injured19-21. This observation suggests the possibility that Mst3b might play a role in axon growth in the mature nervous system.
In the present study, we have investigated whether Mst3b is essential for axon growth in mature neurons. Our results show that Mst3b plays an essential role in the response of both RGCs and DRG neurons to neurotrophic factors through changes in its kinase activity. In addition, suppressing Mst3b expression attenuated axon regeneration in the mature optic nerve and radial nerve. These findings place Mst3b as a key regulator of axon regeneration in the CNS and PNS, and as a potential therapeutic target for improving outcome after axonal injury.
Results
As a model for CNS regeneration, we examined the ability of retinal ganglion cells to extend axons past the site of injury in the mature rat optic nerve when stimulated by intraocular inflammation3,6. For the PNS, we utilized the radial nerve, in which axon regeneration occurs readily after injury in the absence of exogenous stimulation22. In both cases, we investigated whether reducing Mst3b expression suppressed the ability of the relevant projection neurons to extend axons in cell culture, whether Mst3b regulated axon growth via changes in its kinase activity, and whether altering Mst3b levels suppressed axon regeneration in vivo.
Mst3b is essential for axon outgrowth in RGCs
We transfected RGCs with plasmids expressing mst3b shRNA to investigate whether these cells require Mst3b to regenerate axons in response to appropriate growth factors in culture. We dissociated retinas from normal, intact animals, placed these cells into culture, and transfected them with plasmids expressing enhanced green fluorescent protein (EGFP) and either a control shRNA or an mst3b-specific shRNA that was previously shown to knock down Mst3b expression16. The control shRNA differs from mst3b shRNA in 2 nucleotide positions and does not affect axon outgrowth16. After 3 days of treatment with the combination of oncomodulin (Ocm), mannose, and forskolin, RGCs expressing the control shRNA showed a 3-fold increase in axon outgrowth relative to untreated controls (difference significant at P < 0.001: Fig. 1a,b,h). In contrast, RGCs expressing mst3b shRNA showed little response to these factors (Fig. 1,c,h).
Figure 1.
Mst3b mediates the response of retinal ganglion cells (RGCs) to growth factors. (a–g) Representative photos of RGCs transfected with plasmids expressing the shRNA constructs and/or Mst3b variants shown on the left. Cells were exposed to media alone or to a combination of oncomodulin, mannose, and forskolin (Ocm/m/f) as indicated. Cells were fixed 3 days later and immunostained for EGFP, a marker for transfected cells, and either βIII-tubulin, a marker for RGCs (antibody TuJ1), or the myc-his-tag present on recombinant human Mst3b. (h) Quantitation of axon outgrowth. RGCs transfected with control shRNA extended axons when exposed to Ocm/m/f (a,b,h), whereas cells expressing mst3b shRNA did not (c,h). Outgrowth was restored when RGCs expressing mst3b shRNA were co-transfected with a plasmid expressing his-tagged human Mst3b (d,h). Expression of human Mst3b was verified by immunostaining for the his tag (e). Expression of kinase-dead (k/d) Mst3b blocked the effects of growth factors (f,h), whereas expression of constitutively active (c/a) Mst3b resulted in outgrowth even with growth factors absent (g,h). Scale bar for a–g: 40 μm. Each experiment included 4 blinded, independent observations (10–100 cells per well), and each experiment was repeated 3 times. *, **, ***increase relative to untreated controls significant at P < 0.05, P < 0.01, or P < 0.001, respectively. †††decrease relative to control cells treated with growth factors significant at P < 0.001. Error bars represent s.e.m.
To investigate whether the reduced outgrowth seen in this study is specifically related to a reduction in Mst3b levels, we co-transfected cells with 2 plasmids, one expressing mst3b shRNA and the other expressing his-tagged human Mst3b. The mRNA sequence of human mst3b differs significantly from that of the rat and its expression is not blocked by the shRNA construct used here16. We verified the presence of exogenous human Mst3b by staining cells with an anti-his antibody that recognizes his-tagged Mst3b (Fig. 1e). Co-transfecting RGCs with the two plasmids restored RGCs' ability to extend axons in response to growth factors (P < 0.01; Fig. 1d,e,h). Thus, the effect of mst3b shRNA in blocking outgrowth depends specifically on its ability to knock down Mst3b expression and not on off-target effects.
Mst3b regulates growth via changes in kinase activity
To determine whether Mst3b exerts its effects through its kinase activity, we transfected RGCs with a plasmid expressing a mutant form of the protein with an amino acid change in the ATP-binding site16. Expression of this kinase-dead (k/d) Mst3b mutant eliminated the response of RGCs to growth factors (Fig. 1f,h).
To investigate whether the kinase activity of Mst3b plays an active role in axon growth, we next transfected RGCs with a plasmid expressing a constitutively active (c/a) mutant of Mst3b. To create c/a Mst3b, we mutated threonine190 to aspartate, a change that has been shown to activate Mst3, an isotype of Mst3b23. Expression of c/a Mst3b enabled RGCs to grow axons even with growth factors absent (Fig 1g,h). Thus, the kinase activity of Mst3b is necessary and sufficient for axon outgrowth in RGCs. Low magnification photographs illustrating the transfection efficiency and outgrowth in these cultures are shown in Supplementary Fig.1 online.
Mst3b depletion blocks optic nerve regeneration in vivo
To investigate the role of Mst3b in vivo, we injected adult rats intravitreally with AAV2 expressing EGFP from a CMV promoter and, from a U6 promoter, either the shRNA that blocks Mst3b expression or the control shRNA. Animals survived for 4 weeks after viral infections prior to undergoing surgery to injure the optic nerve and induce intravitreal inflammation. The 4-week delay allowed for high levels of shRNA expression and degradation of preexisting Mst3b. In conformity with earlier reports on the high infection efficiency of AAV224-26, we found that AAV2 expressing EGFP and either of the two shRNAs enabled us to visualize EGFP in up to 68% of RGCs 4 weeks after infection (Fig 2a). Infecting RGCs with AAV2 expressing mst3b shRNA strongly suppressed Mst3b expression. Using an antibody that recognizes the N-terminal region of Mst3b16, we detected the protein in essentially every EGFP-positive RGC expressing control shRNA, but observed an ∼85% reduction in EGFP-positive RGCs expressing mst3b shRNA (Fig. 2b,c). As expected from the known tropism of AAV2 and the 4 week separation between viral injections and macrophage induction, intraocular injections of AAV2 expressing EGFP and mst3b shRNA did not infect macrophages nor block their activation (Fig. 2d).
Figure 2.
Infection of RGCs with AAV2 expressing shRNAs in vivo. (a) Animals received intraocular injections of AAV2 expressing EGFP and either a control shRNA or mst3b shRNA 4 weeks prior to dissection. Cross-sections through the retina show double-labeled cells (arrows) expressing TuJ1 (to identify RGCs: red) and EGFP (a marker for transfected cells: green). Transfection efficiencies averaged 58% for the virus expressing control shRNA and 68% for the virus expressing mst3b shRNA. (b) Suppression of Mst3b expression. Cross-sections through the retinas of animals injected 4 weeks earlier with AAV2 expressing EGFP and either control shRNA (top) or mst3b shRNA (bottom). Double-immunostaining with antibodies to Mst3b (red) and EGFP (green) shows co-expression of the two markers in cells expressing control shRNA (arrows, top), but a loss of Mst3b in RGCs expressing mst3b shRNA (arrowheads, bottom). Scale bar: 20 μm. (c) Quantitation of Mst3b expression. EGFP-positive cells expressing either control or mst3b shRNA were analyzed for intensity of Mst3b immunostaining in cross-sections through all cases using Image J. (d) Lack of macrophage infection: Four weeks after intravitreal injections of AAV2 expressing mst3b shRNA and EGFP, the lens was injured to induce an inflammatory response. Double immunostaining shows ED-1-positive macrophages to be uninfected, as evidenced by an absence of EGFP. Scale bar: 40 μm. Error bars represent s.e.m.
To verify that virally mediated Mst3b reduction still suppressed axon growth after the time delay used in our in vivo studies, we waited 4 weeks after injecting AAVs, euthanized animals, dissected and dissociated retinas, and cultured cells6 in the presence or absence of Ocm, mannose, and forskolin6,10,27. In RGCs expressing the control shRNA, the addition of Ocm, forskolin and mannose caused a two-fold increase in average axon length over baseline (difference significant at P < 0.001: Supplementary Fig. 2 on line). RGCs expressing mst3b shRNA extended much shorter axons with or without growth factors present (difference from control-infected RGCs significant at P < 0.001: Supplementary Fig. 2 online). Thus, the effect of Mst3b knockdown remains strong several weeks after viral infection and results in a reduction of both baseline- and growth factor-stimulated levels of axon outgrowth. The lower overall outgrowth seen in Fig. 1h compared to that in Supplementary Fig. 2e may reflect a general decrease in RGCs' ability to extend axons after being exposed to the reagents used for the transient transfections.
In vivo, regenerating axons that arise from infected RGCs were distinguished by virtue of staining positively for both EGFP and GAP-43. The membrane phosphoprotein GAP-43 is normally undetectable in the mature optic nerve, but is strongly induced in axons undergoing regeneration2,3,28. Profiles that are GAP-43+/EGFP-, on the other hand, are expected to reflect regenerating axons that arise from uninfected neurons. Remnants of degenerating axons that already contained EGFP before injury, but which are not regenerating, would be GAP-43-/EGFP+. Animals infected with control shRNA (N = 7) showed many GAP-43+/EGFP+ axons regenerating ≥ 0.5 mm past the lesion site (Fig. 3a, top). In contrast, animals with Mst3b knocked down (N = 7) showed a 97% reduction in GAP-43+/GFP+ regenerating axons (Fig. 3b) (difference significant at P < 0.001: Fig. 3a, bottom and 3c). In all cases, the number of axons was normalized by the percentage of RGCs found to be EGFP-positive to account for individual variations in infection rates. As an internal control, we also quantified the number of regenerating axons arising from non-infected RGCs (GAP-43+/EGFP-) and found this number to be similar between animals infected with AAV2 expressing control vs. mst3b shRNA (Fig. 3c). Thus, Mst3b suppression essentially eliminated axon regeneration in the mature optic nerve, but did not alter the growth capacity of neighboring non-infected neurons.
Figure 3.
Mst3b is essential for optic nerve regeneration. RGCs were infected with AAV2 expressing EGFP plus either control or mst3b shRNA. Four weeks later, the optic nerve was crushed and the lens was injured to induce inflammation and transform RGCs into an active growth state. Regeneration was quantified 2 weeks later. (a) Longitudinal sections through the optic nerve stained for GAP-43 (red, a marker for regenerating axons) and EGFP (green, a marker for transfected neurons). Asterisk denotes the site of nerve injury; box shows the region magnified in b. Scale bar: 100 μm. (b) Area distal to the injury site from a case expressing mst3b shRNA. Merged image shows that GAP-43-positive axons that arise from uninfected RGCs (red), but not double-labeled axons (yellow) that arise from transfected neurons, extend distal to the injury site. Arrows indicate regenerating axons from uninfected RGCs. Scale bar: 100 μm. (c) Mean number of axons that regenerate ≥ 500 μm beyond the injury site. The set of bars on the left shows axons that arise from transfected RGCs. The number of regenerating axons was normalized by the percentage of transfected cells to account for differences in infection efficiency. The set of bars on the right shows axons of noninfected cells, normalized by the percentage of noninfected cells. Results are based on 7 animals per group, 4-6 sections per animal, and 0-24 axons per section. †††decrease relative to cases transfected with control shRNA significant at P < 0.001. Error bars represent s.e.m.
The effect of Mst3b knockdown on axon regeneration was not due to changes in cell survival. RGCs begin to undergo apoptotic cell death a few days after optic nerve injury, and by 2 weeks, ca. 80% will have died29,30. Lens injury enhances survival to allow 40-50% of RGCs to remain alive after 2 weeks. To investigate whether the loss of axon regeneration after Mst3b knockdown in vivo might reflect a selective loss of the infected RGCs, we examined the survival of these cells after infection with AAV2 carrying either control or mst3b shRNA. The number of surviving RGCs was found to be the same in both cases (8.8 ± 0.3 TuJ1+ cells per 200 microns per section with mst3b shRNA, and 7.3 ± 0.4 TuJ1+ cells per 200 microns per section with the control shRNA). We also calculated the percentage of RGCs that were EGFP positive with and without surgery. In animals expressing mst3b shRNA, the percentage of RGCs that was EGFP-positive was 61.1 ± 3.3% in animals that survived 6 weeks and did not have surgery (N = 4) and 68.3 ± 1.9% after optic nerve crush plus lens injury (ONC/LI: N = 7: difference not significant: t = 1.89, df = 10, P ∼ 0.09). Animals infected with the control shRNA showed similar results, although the infection rates were lower. In these cases, 16.2 ± 2.2% of RGCs were EGFP-positive in animals that did not have surgery (N = 4) compared to 12.1 ± 1.6% two weeks after ONC/LI (N = 7: difference not significant: t = 1.51, df = 10, P ∼ 0.16).
Mst3b knockdown blocks NGF-induced outgrowth in DRG neurons
In contrast to the optic nerve, axon regeneration occurs readily in peripheral nerves after injury. Axons that arise from neurons in cervical DRGs 7 and 8 begin to regenerate past a lesion site in the radial nerve within a day or two, with full recovery of function taking place over a few weeks22,31. As with RGCs, we first used transient transfection assays to evaluate whether Mst3b mediates the response of adult DRG neurons to trophic factors, in this case nerve growth factor (NGF). We dissociated DRG neurons from non-infected, non-lesioned animals and transfected these with plasmids expressing EGFP and either control or mst3b-specific shRNA. After being exposed to NGF for 3 days, DRG neurons expressing the control shRNA showed a 2-fold increase in average axon length relative to untreated controls (difference significant at P < 0.001: Fig. 4a,b,g), whereas DRG neurons expressing mst3b shRNA showed no response (Fig. 4c,g). To investigate whether this failure to respond to NGF was due specifically to reduced Mst3b expression, we co-transfected DRG neurons with plasmids expressing mst3b shRNA and human Mst3b, as above. Co-expression of the two plasmids restored DRG neurons' ability to respond to NGF (P < 0.01; Fig. 4d,g). Thus, the effect of mst3b shRNA in blocking outgrowth is due specifically to knockdown of Mst3b expression. These studies show that Mst3b is essential for DRG neurons' ability to extend axons in response to NGF.
Figure 4.
Mst3b mediates the response of DRG neurons to NGF. (a–f) Representative photos of DRG neurons transfected with plasmids expressing shRNA constructs and/or variant forms of Mst3b as shown on the left. Cells were exposed to NGF (50 ng/ml) or media alone. Three days later, cells were fixed and immunostained to detect EGFP, a marker for transfected cells, and βIII-tubulin to identify neurons (antibody TuJ1). (g) Quantitation of axon growth. (a,b,g) Cells expressing the control shRNA extend neurites when treated with NGF (b,g). (c,g) Cells expressing mst3b shRNA (arrowheads) fail to respond to NGF; an nontransfected cell in the same field shows outgrowth (arrow). (d,g) NGF-induced outgrowth is restored when cells expressing mst3b shRNA (green) are co-transfected with a plasmid expressing human Mst3b. (e,g) Cells expressing kinase-dead (k/d) Mst3b (arrowheads) fail to extend neurites when treated with NGF; a non-transfected cell (arrows) in the same field shows outgrowth. (f,g) expression of constitutively active (c/a) Mst3b (arrowhead) is sufficient to induce outgrowth in the absence of NGF; arrow points to an untransfected cell in the same field that is not growing. Scale bar in a–f: 100 μm. Each experiment included 4 blinded, independent observations of about 10 cells per well, and each experiment was performed three times. **,***increase relative to baseline (control shRNA-transfected cells without NGF) significant at P < 0.01 or P < 0.001, respectively. †, ††† decrease relative to control shRNA-transfected cells treated with NGF significant at P<0.05 or P<0.001, respectively. Error bars represent s.e.m.
As in RGCs, we next investigated whether Mst3b participates in axon outgrowth through changes in its kinase activity. To do this, we transfected DRG neurons with either the kinase-dead or constitutively active form of Mst3b described above. Expression of k/d Mst3b eliminated the ability of DRG neurons to extend axons in response to NGF (Fig. 4e,g). In contrast, transfection with c/a Mst3b caused these cells to extend long axons even in the absence of NGF (Fig. 4f,g). Thus, the axon-promoting effect of NGF on DRG neurons appears to be mediated via changes in the kinase activity of Mst3b.
Mst3b knockdown attenuates peripheral nerve regeneration in vivo
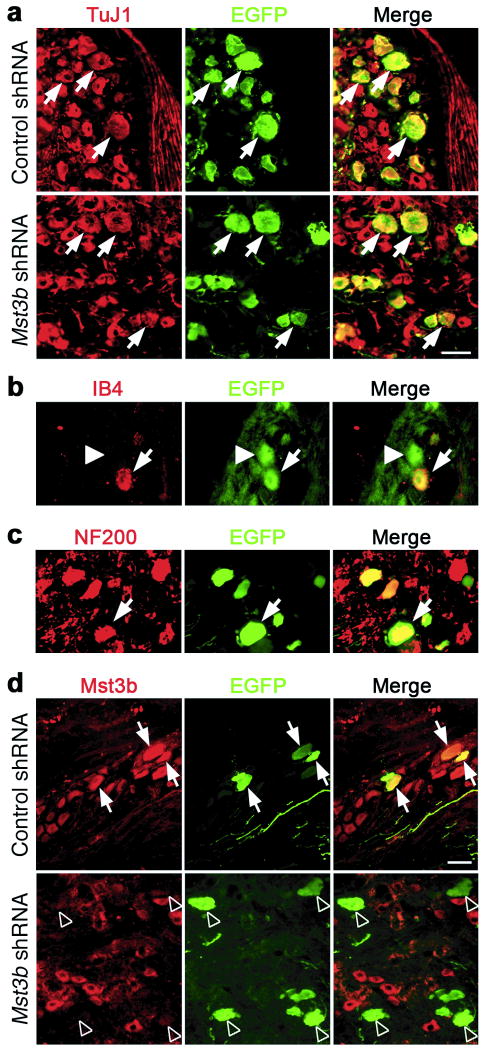
Four weeks after injecting AAV2 expressing EGFP and either control shRNA or mst3b shRNA into cervical ganglia, the percentage of TuJ1+ neurons found to be transfected was 21.4 ± 2.9% and 34.9 ± 2.1%, respectively. Transduction was neuron-specific (Fig. 5a) and included small peptidergic (IB4-) and non-peptidergic (IB4+) neurons, as well as large NF200+ neurons (Fig. 5b,c). Compared to cells expressing control shRNA, Mst3b levels were reduced by ∼ 85% in DRG neurons transfected with AAV2 expressing mst3b shRNA (Fig. 5d).
Figure 5.
Mst3b knockdown in DRG neurons. Sections through the rat DRG four weeks after injecting AAV2 expressing EGFP and either control or mst3b shRNA. (a) Infection of DRG neurons is demonstrated by co-immunostaining (arrows) with antibodies to the neuronal marker, βIII tubulin (TuJ1, red), and to virally expressed EGFP (green). Overall transfection rates were 21% for the control shRNA group (N = 10) and 35% for the mst3b shRNA group (N = 13). (b) AAV2 expressing EGFP and mst3b shRNA infects small IB4-positive (red) non-peptidergic neurons (arrows) as well as small IB-4-negative neurons (arrowheads). (c) AAV2 also infects large neurons (arrow) that stain positively with antibodies to the neurofilament protein NF200 (red). Scale bar: 40 μm. (d) Knockdown of Mst3b in DRG neurons. Ganglia were immunostained with antibodies to Mst3b (red) and EGFP (green) 4 weeks after infecting DRGs with AAV2 expressing EGFP and either control (top) or mst3b shRNA (bottom). Mst3b can be visualized in cells expressing control shRNA (arrows) (top), but not in cells expressing mst3b shRNA (arrowheads) (bottom). Scale bar: 40 μm.
As with RGCs, we examined whether virally mediated knockdown of Mst3b would affect DRG neurons' ability to regenerate axons when tested several weeks later. Four weeks after infecting cervical DRGs in vivo with AAV2 expressing either control or mst3b-specific shRNA, we dissociated and cultured DRG neurons on poly-d-lysine-coated glass coverslips. In the absence of NGF, EGFP-positive DRG neurons showed similar levels of baseline outgrowth irrespective of which shRNA was being expressed. However, whereas neurons expressing the control shRNA showed a 3-fold increase in average axon length when exposed to NGF, neurons expressing mst3b shRNA showed no detectable response (Supplementary Fig. 3 online: difference between groups significant at P < 0.02).
To investigate the role of Mst3b in peripheral nerve regeneration in vivo, we prepared a separate group of animals in which cervical DRG neurons were injected with AAV2 expressing EGFP and either the control or mst3b-specific shRNA. We then crushed the radial nerve 4 weeks later and evaluated axon regeneration after three days, a time point at which injured axons have normally grown several millimeters beyond the injury site (Fig. 6). As in the optic nerve, we assessed regeneration arising from infected neurons as the number of axons beyond the injury site that were positive for both GAP-43 and EGFP, and then normalized this number to the percentage of cells that were transfected to account for individual variations in transfection efficiency. In cases injected with AAV2 expressing the control shRNA, many EGFP+/GAP-43+ axons extended 1-2 mm distal to the injury site, and some extended as far as 5 mm (Fig. 6a,b,e,f: N = 8). Animals expressing mst3b shRNA showed an 80 – 85% reduction in axon growth (N = 9; difference from controls significant at P < 0.001 at 0.5 mm, P < 0.01 at 1, and 2 mm; N.S. at 3, 4, and 5 mm: Fig. 6c,d,f). We also evaluated regeneration in non-infected DRG neurons in these same cases, that is, the number of axons that were GAP-43-positive and EGFP-negative, normalized by the percentage of cells that were uninfected. Axons arising from non-infected DRG neurons regenerated to a similar extent regardless of whether their neighbors expressed the control or mst3b shRNA (Fig. 6g). In sum, knocking down Mst3b expression in DRG neurons strongly attenuated, but did not eliminate, the regeneration of injured axons in vivo.
Figure 6.
Mst3b knockdown attenuates peripheral nerve regeneration. (a–d) The radial nerve was crushed 4 weeks after infecting cervical DRG neurons with AAV2 expressing EGFP and either mst3b- or control shRNA. Axons extending beyond the injury site (arrowheads) were visualized 3 days later in sections stained with antibodies to detect GAP-43 (red) and EGFP (green). The box in b is magnified in panel e. Only profiles that are labeled with both markers are considered as regenerating axons that arise from infected neurons (arrows). Scale bar for a–d: 200 μm. (e) High-power images show regenerating axons that arise from neurons expressing control shRNA: many axons are positive for both GAP-43 (red) and EGFP (green) (arrows). Scale bar: 100μm. (f) Quantitation of GAP-43+/EGFP+ axons. The number of regenerating axons was normalized by the percent of infected cells to account for differences in infection efficiency. Note the marked attenuation of axon growth in cells expressing mst3b shRNA. (g) Quantitation of GAP-43+/EGFP- axons from non-infected neurons shows similar levels of regeneration irrespective of the virus infecting neighboring neurons. Data show mean number of axons at the indicated distances from the injury site (8-9 animals per group, 4-6 sections per animal, 0-10 axons per section). **,***Differences between number of axons arising from cells expressing control vs. mst3b shRNA are significant at P < 0.01, P < 0.001, respectively. Error bars represent s.e.m.
The number of DRG neurons (TuJ1+) that survived infection with AAV carrying mst3b shRNA (142.8+/- 6.0 per mm2) was similar to that seen after infection with AAV carrying control shRNA (139.5+/-8.8 per mm2). Thus, knocking down Mst3b expression did not differentially impair DRG neuron survival.
Mst3b kinase activity changes during regeneration
To determine whether the activity of Mst3b in DRG neurons increases during regeneration, we isolated ganglia 3 days after peripheral nerve injury and compared their Mst3b kinase activity with that of control DRG neurons using immunoprecipitation-kinase (IP-kinase) assays as described16. We precipitated Mst3b from DRG lysates using the antibody to the N-terminal region of the protein, and evaluated kinase activity using histone protein HF1 as an experimental substrate. DRG neurons undergoing axon regeneration showed a 2.5-fold increase in Mst3b kinase activity compared with control neurons (P < 0.01). 6-thioguanine, an inhibitor of Mst3b, attenuated this increase (P < 0.05: Fig. 7a,b).
Figure 7.
Mst3b activation and regulation of p42/44 MAPK phosphorylation in vivo. (a) Mst3b was immunoprecipitated from lysates of DRG neurons 3 days after peripheral nerve crush or sham surgery. Kinase assays were performed using HF1 as a pseudo-substrate and γ-[32P]-ATP, with or without 6-thioguanine present. Reaction mixes were separated by SDS-PAGE and kinase activity was visualized by autoradiography (top). Protein loading was visualized by Coomassie Blue staining (bottom). Position of molecular weight markers are shown on the right. (b) Quantitation of Mst3b kinase activity based on densitometry of autoradiograms using Image J (NIH) averaged from 3 separate experiments. (c,d) Cross-sections through the DRG of animals injected 4 weeks earlier with AAV2 expressing EGFP and either control shRNA (top) or mst3b shRNA (bottom). Arrows point to infected, EGFP-positive neurons. (c) Double-immunostaining with antibodies to phospho-p42/44 MAPK (red) and EGFP (green) reveal a selective reduction of phospho-MAPK in DRG neurons expressing the mst3b shRNA. (d) Double-immunostaining with antibodies to p42/p44 MAPK (red) and EGFP (green) reveal that Mst3b knock-down does not alter overall p42/44 MAPK levels. (e) Quantitation of changes in the overall expression and phosphorylation of p42/p44 MAPK based on 4-6 sections from 3 animals per condition. Scale bar: 40 μm. **Increase relative to controls significant at P < 0.01; †, ††Decrease relative to induced state significant at P < 0.05 or P < 0.01, respectively. Error bars represent s.e.m.
The MAP kinase pathway contributes to the development and regeneration of peripheral axons32,33 and to the effect of NGF in inducing outgrowth in PC12 cells34,35. The observation that knocking down Mst3b inhibits outgrowth in both sensory neurons and PC12 cells16 is consistent with the possibility that it acts via a MAPK pathway. To examine how Mst3b fits into the signal transduction cascade that leads to axon outgrowth, we compared p42/44 MAPK phosphorylation in neurons expressing control vs. mst3b shRNA. In sections through DRGs expressing mst3b shRNA, immunostaining for phospho-p42/44 MAPK was detected in 11% of neurons, compared to 87% of neurons expressing the control shRNA (Fig. 7c–e). The level of total p42/44 MAPK did not differ between the two groups.
Discussion
Mst3b was recently shown to be a neuron-specific protein kinase that is essential for axon outgrowth in embryonic cortical neurons and PC12 cells in culture16. The present study demonstrates that Mst3b also mediates the effects of trophic factors in stimulating axon outgrowth in adult RGCs and DRG neurons, that it exerts these effects through changes in its kinase activity, and that this kinase plays an essential role in enabling mature RGCs and DRG neurons to regenerate injured axons in vivo.
To study the role of Mst3b in the CNS, we used the adult rat optic nerve as an accessible and widely studied example of a CNS pathway in which some regeneration can be achieved by inducing an inflammatory reaction in the eye3,6. A macrophage-derived growth factor, Ocm, can mimic the effect of intravitreal inflammation when introduced into the eye together with an agent to elevate intracellular cAMP; mannose, which is also required for the effects of Ocm, is normally abundant in the eye6,10,27. As shown here, depletion of Mst3b greatly reduced the ability of adult rat RGCs to extend axons in response to Ocm, mannose and forskolin in culture, and eliminated axon regeneration in vivo. The effect of mst3b shRNA on axon outgrowth can be attributed specifically to the loss of Mst3b protein, in that responsiveness to growth factors was rescued when RGCs expressing mst3b shRNA were co-transfected with plasmids expressing human Mst3b16. The role of Mst3b in axon growth was further demonstrated by the inability of RGCs expressing a kinase-dead Mst3b mutant to extend axons in the presence of growth factors. Conversely, expression of a constitutively active form of Mst3b caused RGCs to extend axons even in the absence of growth factors. Thus, changes in Mst3b kinase activity play a key role in controlling axon outgrowth in RGCs.
Mst3b is also important for axon regeneration in the peripheral nervous system. Depletion of Mst3b eliminated the ability of DRG neurons to respond to NGF in culture and attenuated axon regeneration following radial nerve crush in vivo. As with RGCs, the role of Mst3b in DRG neurons was shown to require its kinase activity. Expression of a kinase-dead mutant form of Mst3b blocked NGF-induced outgrowth in culture, and strikingly, expression of a constitutively active Mst3b mutant enabled DRG neurons to extend axons even in the absence of growth factors.
In vivo, DRG neurons expressing mst3b shRNA showed 80% less axon regeneration 3 days after nerve injury than neurons expressing the control shRNA or than nontransfected neurons in the same ganglia. Nonetheless, the fact that some regeneration continued to occur even when Mst3b levels were reduced suggests that there is enough Mst3b remaining to allow some regeneration or that axon regeneration in the PNS may utilize both Mst3b-dependent and Mst3b-independent mechanisms. DRG neurons extend processes in response to trophic factors and, via integrin signaling, in response to substrate molecules36. In culture, DRG neurons expressing mst3b shRNA continued to show basal outgrowth when cultured on poly-D-lysine, but were unable to respond to NGF. Peripheral nerves, but not optic nerves, are rich in laminin, and this may contribute to the ability of DRG neurons expressing mst3b shRNA, but not similarly infected RGCs, to exhibit some regeneration in vivo. The different amounts of regeneration in the two systems may also be due in part to the marked differences in inhibitory signals between the optic nerve and peripheral nerve37,38.
Ste20, the prototypic member of the kinase family that includes Mst3b, functions as a MAP4K to regulate budding in yeast. Ste20 relays the signal from a pheromone-sensitive G-protein coupled receptor to Ste11, a MAP3K; Ste11 in turn phosphorylates the MAP2K Ste7, which then phosphorylates Fus3, a MAPK39,40. MAP kinase pathways are known to contribute to the development and regeneration of peripheral axons32,33 and to the effect of NGF in inducing outgrowth in PC12 cells34,35. Thus, the observation that Mst3b depletion inhibits outgrowth in sensory neurons and PC12 cells16 is consistent with the possibility that Mst3b activates a MAPK pathway in neurons. In conformity with this idea, we found that the kinase activity of Mst3b was elevated in DRG neurons undergoing axon regeneration in vivo, and that reduction of Mst3b expression diminished p42/44 MAPK phosphorylation in these neurons. Together, these data support the idea that Mst3b controls axon outgrowth through changes in its kinase activity and subsequent activation of a MAP kinase-signaling cascade.
When stimulated to go into an active growth state in vivo, RGCs show marked changes in the expression of genes associated with axon growth and cell survival41, and these changes resemble those seen in DRG neurons undergoing axon regeneration in the PNS42-44. The results of the present study suggest that Mst3b could be part of a common signaling pathway that mediates the effects of trophic factors in controlling the expression of axon-outgrowth-related genes in CNS and PNS neurons.
In summary, we have shown that Mst3b, through its kinase activity, is a key regulator of axon regeneration in the adult optic nerve and radial nerve. It will be important to investigate whether Mst3b regulates axon regeneration in other parts of the CNS and PNS, and whether expression of a constitutively active form of Mst3b can augment the limited amount of growth that is currently achievable following CNS injury. These and further studies into the molecular mechanisms by which Mst3b functions may open up new avenues for the treatment of CNS injuries.
Supplementary Material
Supplementary Figure 1. Axon outgrowth in RGC cultures: Low-magnification images. We transfected RGCs with plasmids expressing control or mst3b shRNA, c/a Mst3b, or k/d Mst3b, and cultured the neurons in the presence or absence of Ocm, mannose, and forskolin as indicated. Cells were double-stained to visualize EGFP (green) and βIII tubulin (red). Arrowheads in a,c, and d point to transfected neurons; arrows in b and e point to transfected cells extending axons. Asterisks indicate axons in non-transfected neurons.
Supplementary Figure 2. RGCs fail to respond to growth factors after being infected 4 weeks earlier with AAV2 expressing mst3b shRNA in vivo. (a–d) RGCs expressing control shRNA (a,b) or mst3b shRNA (c,d) from animals infected with AAV2 shRNA were dissociated and placed in culture for 3 days in the absence (a,c) or presence (b,d) of Ocm, mannose, and forskolin. Scale bar: 50 μm. (e) Axon growth was averaged over 4 culture wells per condition (5–30 cells per well) in each experiment and then averaged from 3 independent experiments. ***increase relative to baseline significant at P < 0.001; †††decrease relative to corresponding control-transfected cells significant at P < 0.001. Error bars represent s.e.m.
Supplementary Figure 3. DRG neurons fail to respond to NGF 4 weeks after being infected with AAV2 expressing mst3b shRNA in vivo. (a–d) DRG neurons expressing EGFP and either control shRNA (a,b) or mst3b-specific shRNA (c,d) were dissociated and placed in culture 4 weeks after AAV2 infection in vivo. Cells were treated with NGF (b,d) or were left untreated (a,c). Scale bar: 100 μm. (f) Quantitation of axon growth. Each experiment included 4 blinded, independent observations of 5-30 cells per well, and each experiment was performed three times. *increase relative to baseline outgrowth (without growth factors) significant at P < 0.05; ††decrease relative to cells transfected with control shRNA and treated with NGF significant at P < 0.02. Error bars represent s.e.m.
Acknowledgments
We are grateful for the support of the NIH (EY05690 to LB), the European Union (Marie Curie Outgoing International Fellowship MOIF-CT-2004-008424 to BL), Alseres, Inc., and the Dr Miriam and Sheldon G. Adelson Medical Research Foundation. We wish to thank Drs. Ann Logan and Martin Berry for helpful advice on DRG injections and use of facilities, Dr. Yuqin Yin for surgical assistance in the preliminary optic nerve studies, Dan Kim and Laila Zai for surgical assistance in the Mst3b activation studies, and the Developmental Disabilities Research Center of Children's Hospital (NIH P30 HD018655) for use of core facilities and expertise.
Abbreviations
- AAV
adeno-associated virus
- CNS
central nervous system
- DRG
dorsal root ganglion
- EGFP
enhanced green fluorescent protein
- forsk
forskolin
- Mst3b
mammalian serine-threonine kinase-3b
- NGF
nerve growth factor
- Ocm
oncomodulin
- ON/LI
optic nerve crush plus lens injury
- PNS
peripheral nervous system
- RGCs
retinal ganglion cells
- shRNA
short hairpin RNA
- 6-TG
6-thioguanine
Footnotes
Author Contributions: BL helped design some of the experiments, carried out most in vivo and in vitro studies, drafted much of paper, and did most of the data analysis.
MH assisted with many cell culture studies and in vivo work.
LB helped conceptualize the overall structure of the study, supervised parts of it, carried out some of the data analysis, and wrote parts of the manuscript.
NI generated the core idea of the study, helped conceptualize the overall structure of the study, performed preliminary optic nerve studies, constructed the viral vectors, made the mutations in Mstb3, supervised parts of the study, did some of the data analysis, and wrote parts of the manuscript.
References
- 1.Ramon y Cajal S. Degeneration and Regeneration of the Nervous System. Oxford University Press; New York: 1991. [Google Scholar]
- 2.Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996;25:147–170. doi: 10.1007/BF02284793. [DOI] [PubMed] [Google Scholar]
- 3.Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer D, Heiduschka P, Thanos S. Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol. 2001;172:257–272. doi: 10.1006/exnr.2001.7822. [DOI] [PubMed] [Google Scholar]
- 5.Pernet V, Di Polo A. Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain. 2006;129:1014–1026. doi: 10.1093/brain/awl015. [DOI] [PubMed] [Google Scholar]
- 6.Yin Y, et al. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorber B, Berry M, Logan A. Lens injury stimulates adult mouse retinal ganglion cell axon regeneration via both macrophage- and lens-derived factors. Eur J Neurosci. 2005;21:2029–2034. doi: 10.1111/j.1460-9568.2005.04034.x. [DOI] [PubMed] [Google Scholar]
- 8.Leaver SG, et al. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006;13:1328–1341. doi: 10.1038/sj.gt.3302791. [DOI] [PubMed] [Google Scholar]
- 9.Cen LP, et al. Chemotactic effect of ciliary neurotrophic factor on macrophages in retinal ganglion cell survival and axonal regeneration. Invest Ophthalmol Vis Sci. 2007;48:4257–4266. doi: 10.1167/iovs.06-0791. [DOI] [PubMed] [Google Scholar]
- 10.Yin Y, et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- 11.Makwana M, Raivich G. Molecular mechanisms in successful peripheral regeneration. Febs J. 2005;272:2628–2638. doi: 10.1111/j.1742-4658.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- 12.Barrette B, et al. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yudin D, et al. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron. 2008;59:241–252. doi: 10.1016/j.neuron.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene LA, Volonte C, Chalazonitis A. Purine analogs inhibit nerve growth factor-promoted neurite outgrowth by sympathetic and sensory neurons. J Neurosci. 1990;10:1479–1485. doi: 10.1523/JNEUROSCI.10-05-01479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benowitz LI, et al. Axon outgrowth is regulated by an intracellular purine-sensitive mechanism in retinal ganglion cells. J Biol Chem. 1998;273:29626–29634. doi: 10.1074/jbc.273.45.29626. [DOI] [PubMed] [Google Scholar]
- 16.Irwin N, Li YM, O'Toole JE, Benowitz LI. Mst3b, a purine-sensitive Ste20-like protein kinase, regulates axon outgrowth. Proc Natl Acad Sci U S A. 2006;103:18320–18325. doi: 10.1073/pnas.0605135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volonte C, Greene LA. Nerve growth factor-activated protein kinase N. Characterization and rapid near homogeneity purification by nucleotide affinity-exchange chromatography. J Biol Chem. 1992;267:21663–21670. [PubMed] [Google Scholar]
- 18.Zurn A, Do K. Purine metabolite inosine is an adrenergic neurotrophic substance for cultured chicken sympathetic neurons. Proc Natl Acad Sci USA. 1988;85:8301–8305. doi: 10.1073/pnas.85.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci U S A. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JM, et al. Inosine promotes recovery of skilled motor function in a model of focal brain injury. Brain. 2007;130:915–925. doi: 10.1093/brain/awl393. [DOI] [PubMed] [Google Scholar]
- 21.Zai L, et al. Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J Neurosci. 2009;29:8187–8197. doi: 10.1523/JNEUROSCI.0414-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bontioti EN, Kanje M, Dahlin LB. Regeneration and functional recovery in the upper extremity of rats after various types of nerve injuries. J Peripher Nerv Syst. 2003;8:159–168. doi: 10.1046/j.1529-8027.2003.03023.x. [DOI] [PubMed] [Google Scholar]
- 23.Lu TJ, et al. Inhibition of cell migration by autophosphorylated mammalian sterile 20-like kinase 3 (MST3) involves paxillin and protein-tyrosine phosphatase-PEST. J Biol Chem. 2006;281:38405–38417. doi: 10.1074/jbc.M605035200. [DOI] [PubMed] [Google Scholar]
- 24.Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002;28:267–275. doi: 10.1016/s1046-2023(02)00232-3. [DOI] [PubMed] [Google Scholar]
- 25.Sapieha PS, Peltier M, Rendahl KG, Manning WC, Di Polo A. Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol Cell Neurosci. 2003;24:656–672. doi: 10.1016/s1044-7431(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 26.Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Irwin N, Yin Y, Lanser M, Benowitz LI. Axon regeneration in goldfish and rat retinal ganglion cells: differential responsiveness to carbohydrates and cAMP. J Neurosci. 2003;23:7830–7838. doi: 10.1523/JNEUROSCI.23-21-07830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaden H, Stuermer CA, Bahr M. GAP-43 immunoreactivity and axon regeneration in retinal ganglion cells of the rat. J Neurobiol. 1994;25:1570–1578. doi: 10.1002/neu.480251209. [DOI] [PubMed] [Google Scholar]
- 29.Kermer P, et al. Caspase-9: involvement in secondary death of axotomized rat retinal ganglion cells in vivo. Brain Res Mol Brain Res. 2000;85:144–150. doi: 10.1016/s0169-328x(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 30.Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ygge J. Central projections of the rat radial nerve investigated with transganglionic degeneration and transganglionic transport of horseradish peroxidase. J Comp Neurol. 1989;279:199–211. doi: 10.1002/cne.902790204. [DOI] [PubMed] [Google Scholar]
- 32.Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35:65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhong J, et al. Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci. 2007;10:598–607. doi: 10.1038/nn1898. [DOI] [PubMed] [Google Scholar]
- 34.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 36.Tucker BA, Rahimtula M, Mearow KM. A procedure for selecting and culturing subpopulations of neurons from rat dorsal root ganglia using magnetic beads. Brain Res Brain Res Protoc. 2005;16:50–57. doi: 10.1016/j.brainresprot.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- 38.Aguayo AJ, et al. Degenerative and regenerative responses of injured neurons in the central nervous system of adult mammals. Philos Trans R Soc Lond B Biol Sci. 1991;331:337–343. doi: 10.1098/rstb.1991.0025. [DOI] [PubMed] [Google Scholar]
- 39.Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 40.Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 41.Fischer D, Petkova V, Thanos S, Benowitz LI. Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J Neurosci. 2004;24:8726–8740. doi: 10.1523/JNEUROSCI.2774-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costigan M, et al. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan M, Mi R, Yew DT, Chan WY. Analysis of gene expression following sciatic nerve crush and spinal cord hemisection in the mouse by microarray expression profiling. Cell Mol Neurobiol. 2001;21:497–508. doi: 10.1023/A:1013867306555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benowitz LI, Apostolides PJ, Perrone-Bizzozero N, Finklestein SP, Zwiers H. Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci. 1988;8:339–352. doi: 10.1523/JNEUROSCI.08-01-00339.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui Q, Yip HK, Zhao RC, So KF, Harvey AR. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci. 2003;22:49–61. doi: 10.1016/s1044-7431(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 47.Moskowitz PF, Oblinger MM. Sensory neurons selectively upregulate synthesis and transport of the beta III-tubulin protein during axonal regeneration. J Neurosci. 1995;15:1545–1555. doi: 10.1523/JNEUROSCI.15-02-01545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwalb JM, et al. Two factors secreted by the goldfish optic nerve induce retinal ganglion cells to regenerate axons in culture. J Neurosci. 1995;15:5514–5525. doi: 10.1523/JNEUROSCI.15-08-05514.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leclere PG, et al. Effective gene delivery to adult neurons by a modified form of electroporation. J Neurosci Methods. 2005;142:137–143. doi: 10.1016/j.jneumeth.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Gavazzi I, Kumar RD, McMahon SB, Cohen J. Growth responses of different subpopulations of adult sensory neurons to neurotrophic factors in vitro. Eur J Neurosci. 1999;11:3405–3414. doi: 10.1046/j.1460-9568.1999.00756.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Axon outgrowth in RGC cultures: Low-magnification images. We transfected RGCs with plasmids expressing control or mst3b shRNA, c/a Mst3b, or k/d Mst3b, and cultured the neurons in the presence or absence of Ocm, mannose, and forskolin as indicated. Cells were double-stained to visualize EGFP (green) and βIII tubulin (red). Arrowheads in a,c, and d point to transfected neurons; arrows in b and e point to transfected cells extending axons. Asterisks indicate axons in non-transfected neurons.
Supplementary Figure 2. RGCs fail to respond to growth factors after being infected 4 weeks earlier with AAV2 expressing mst3b shRNA in vivo. (a–d) RGCs expressing control shRNA (a,b) or mst3b shRNA (c,d) from animals infected with AAV2 shRNA were dissociated and placed in culture for 3 days in the absence (a,c) or presence (b,d) of Ocm, mannose, and forskolin. Scale bar: 50 μm. (e) Axon growth was averaged over 4 culture wells per condition (5–30 cells per well) in each experiment and then averaged from 3 independent experiments. ***increase relative to baseline significant at P < 0.001; †††decrease relative to corresponding control-transfected cells significant at P < 0.001. Error bars represent s.e.m.
Supplementary Figure 3. DRG neurons fail to respond to NGF 4 weeks after being infected with AAV2 expressing mst3b shRNA in vivo. (a–d) DRG neurons expressing EGFP and either control shRNA (a,b) or mst3b-specific shRNA (c,d) were dissociated and placed in culture 4 weeks after AAV2 infection in vivo. Cells were treated with NGF (b,d) or were left untreated (a,c). Scale bar: 100 μm. (f) Quantitation of axon growth. Each experiment included 4 blinded, independent observations of 5-30 cells per well, and each experiment was performed three times. *increase relative to baseline outgrowth (without growth factors) significant at P < 0.05; ††decrease relative to cells transfected with control shRNA and treated with NGF significant at P < 0.02. Error bars represent s.e.m.