Abstract
Developing oligodendrocytes (pre-OLs) are highly vulnerable to hypoxic-ischemic injury and associated excitotoxicity and oxidative stress. 17β-Estradiol plays an important role in the development and function of the CNS and is neuroprotective. The sudden drop in circulating estrogen after birth may enhance the susceptibility of developing OLs to injury. Estrogen receptor (ER)– α and ER-β are both expressed in OLs. We examined the effect of 17β-estradiol on oxygen-glucose deprivation and oxidative stress–induced cell death in rat pre-OLs in vitro and on hypoxic-ischemic brain injury in vivo. Pre-OLs in culture were subjected to oxygen-glucose deprivation (OGD) or glutathione depletion in the presence or absence of 17β-estradiol. LDH release, the Alamar blue assay, and phase-contrast microscopy were used to assess cell viability. Hypoxic-ischemic injury was generated in 6-day-old rats (P6) by unilateral carotid ligation and hypoxia (6% O2 for 1 hr). Rat pups received one intraperitoneal injection of 300 or 600 µg/kg 17β-estradiol or vehicle 12 hr prior to the surgical procedure. Injury was assessed by myelin basic protein (MBP) immunocytochemistry at P10. 17β-Estradiol produced significant protection against OGD-induced cell death in primary OLs (EC50 = 1.3 ± 0.46 × 10−9 M) and against oxidative stress. Moreover, 17β-estradiol attenuated the loss of MBP labeling in P10 pups ipsilateral to the carotid ligation. These results suggest a potential role for estrogens in attenuation of hypoxic-is-chemic and oxidative injury to developing OLs and in the prevention of periventricular leukomalacia.
Keywords: oligodendrocyte, oxidative stress, development, periventricular leukomalacia, excitotoxicity
Periventricular leukomalacia (PVL), the main pathological substrate underlying the development of cerebral palsy in premature infants, is characterized by focal and diffuse injury to cerebral white matter. The classic neuropathology of PVL has given rise to several hypotheses about pathogenesis largely related to infection/inflammation and hypoxia-ischemia/reperfusion in the sick premature infant (Folkerth et al., 2004; Dammann and Leviton, 2006). These hypotheses include free-radical injury, cytokine toxicity, and excitotoxicity.
Excitotoxicity and oxidative stress play crucial roles in hypoxic-ischemic injury to developing white matter during the perinatal period (Yoshioka et al., 1996; McDonald et al., 1998; Follett et al., 2000; Matute et al., 2002; Deng et al., 2004). The injury can lead to devastating neurological consequences that are highly age dependent. In term infants, hypoxia-ischemia predominantly affects the cerebral cortex with principally neuronal injury. In contrast, hypoxic-ischemic brain injury in the premature infant is postulated to result in a chronic disturbance of myelination and white matter loss, described as periventricular leukomalacia (PVL; Volpe, 2001a). A key cellular target is the developing oligodendrocyte (pre-OL), which populates developing white matter during the period of greatest risk for PVL (Back et al., 2001; Jensen, 2005). We have shown that pre-OLs are more sensitive to excitotoxic and oxidative injury than are mature oligodendrocytes (OLs; Back et al., 1998; Deng et al., 2003; Rosenberg et al., 2003) and that excitotoxicity plays a role in a model of hypoxic/ischemic injury to developing white matter (Follett et al., 2000; Volpe, 2001b; Deng et al., 2004).
So far, no specific treatment exists for PVL (Volpe, 2001b, 2003). Estrogen has been shown to be neuroprotective in adult animal models of neurological disorders such as Parkinson’s and Alzheimer’s disease (Sawada et al., 2002; Shughrue, 2004; Baum, 2005; Zhao and Brinton, 2006), as well as in cerebral ischemia (Sampei et al., 2000; Merchenthaler et al., 2003; Choi et al., 2004).
During pregnancy, maternal 17β-estradiol (E2) plasma levels increase up to 100-fold (15,000 pg/mL, approximately 55 nM), largely because of placental aromatization of C-19 steroids produced by fetal adrenal glands (Ishida et al., 1985; Wood, 2005), compared with plasma estrogen concentrations in nonpregnant females (Tulchinsky et al., 1972). After birth, E2 decreases to low levels (5–35 pg/mL, or 20–130 pM) within 24 hr. Recently, we found that 17β-estradiol produced significant dose-dependent protection against oxygen-induced apoptotic cell death in primary OLs and attenuated loss of myelin basic protein (MBP) labeling in neonatal rat pups after oxygen exposure (Gerstner et al., 2007). Other groups have reported a neuroprotective effect of 17β-estradiol in an in vivo model of neonatal stroke (Nunez et al., 2007) and against kainic acid–induced hippocampal injury in the female newborn rat (Hilton et al., 2004; Nunez et al., 2007). To determine whether E2 will counteract injury to developing white matter, this study focused on the effect of E2 on developing OLs in established neonatal animal models of hypoxic/is-chemic injury in vitro and in vivo.
METHODS AND MATERIALS
Oligodendrocyte Primary Cultures
Primary rat OLs were prepared from the cerebral hemispheres of Sprague-Dawley rats on postnatal days 1–2 using a shaking method (Rosenberg et al., 2003) with modifications, as described previously (Li et al., 2003). Animals were sacrificed by decapitation. Purified OLs were cultured for 7–8 days in a serum-free basal-defined medium (BDM): DMEM (#11960-069; Invitrogen), 0.1% bovine serum albumin (#A9647; Sigma), 50 µg/mL human apo-transferrin (#T2252; Sigma), 50 µg/mL insulin (#I6634; Sigma), 30 nM sodium selenite (#S5261; Sigma), 10 nM D-biotin (#B4501; Sigma), 10 nM hydrocortisone (#H0888 Sigma), 200 µM l-cystine, 10 ng/mL human recombinant platelet-derived growth factor (#100-13A; Peprotech, Rocky Hill, NJ), and 10 ng/mL human recombinant basic fibroblast growth factor (Peprotech #100-18B)). At 7–8 days, the cultures were composed primarily of progenitors and pre-OLs (O4+, O1−, MBP−). The purity of OL cultures was consistently more than 95% OLs, with less than 5% astrocyte contamination. Primary OL cultures were subjected to oxygen-glucose deprivation (OGD) in the presence or absence of E2 (10−6−10−10 M; Sigma, St. Louis, MO).
Oxidative Injury in Cell Cultures
To induce oxidative injury, cells were incubated for 24 hr with BDM medium with low or absent cystine (normal concentration 200 µM, Cys+), unless specified otherwise. In experiments involving the addition of drugs dissolved in absolute ethanol, the final concentration of ethanol in the culture medium was 0.1% and had no effect on cell viability, proliferation, or morphology.
Oxygen-Glucose Deprivation
Cultures were switched to BDM medium that lacked glucose (Invitrogen) and were transferred to an anaerobic chamber filled with 95% N2 plus 5% CO2 at 37°C (Deng et al., 2004). Following OGD for 3.5 hr, D-glucose from a concentrated stock solution made in the glucose-free BDM medium was added back to the cultures to a final concentration of 25 mM, and the cultures were returned to an air/5% CO2 incubator at 37°C. Cell death was assessed at 24 hr by measuring lactate dehydrogenase release from the cells into the medium, as previously described (Deng et al., 2004).
Survival Assays
LDH Release
After exposure to OGD, cell culture medium samples were collected and centrifuged, and lactate dehydrogenase (LDH) activity was quantified in 50-µL samples of medium supernatant, using a colorimetric cytotoxicity assay kit according to the manufacturer’s directions (Roche). Absorbance data were obtained using a 96-well plate reader (Molecular Devices) with a 450-nm filter and 650 nm as a reference wavelength. Maximum LDH release was determined by cell lysis of untreated cells with cell culture media containing 1% Triton X-100.
Alamar Blue Assay
After 24 hr of cystine deprivation, cell survival was determined using Alamar Blue (Southern Biotechnology, Birmingham, AL), a tetrazolium dye reduced by living cells to a colored product (Back et al., 1998). All results of cell death assays were also confirmed by visual inspection using phase-contrast microscopy. The fluorescence of the assay solution, reflecting cell viability, was measured with a fluorescence plate reader (FluoroCount, Packard Instrument Co.) using an excitation wavelength of 560 nm and an emission wavelength of 590 nm.
Subjects
Litters of male Long-Evans rat pups (Charles River Laboratories, Wilmington, MA) were raised with dams in a temperature-controlled environment with 12-hr light/12-hr dark cycles. All procedures were approved and in accordance with guidelines set by the institutional animal care and use committee. Pups underwent unilateral carotid ligation (UCL) and hypoxia on postnatal day 6 (P6). They were allowed to recover on a thermal blanket at 33°C–34°C (baseline temperature) and returned to their dam for 96 hr before being killed.
Carotid Ligation with Hypoxia
Selective white matter injury was produced in P6 rats by unilateral carotid ligation followed by hypoxia (6% O2 for 1 hr), as described previously (Follett et al., 2000, 2004). To determine the protective effect of E2 in hypoxic/ischemic white matter injury, rats were randomized for treatment with 17β-estradiol (n = 15; Sigma, St. Louis, MO), 300 µg/kg (n = 7) and 600 µg/kg (n = 8), or vehicle (2-hydroxypropyl-β-cyclodextrin, n = 14; Sigma, St. Louis, MO). Twelve hours before UCL hypoxia, animals received a single injection of E2 intraperitoneally diluted in sterile water at a volume of 0.1 mL/10 g. Control animals received an injection with vehicle. Rats were anesthetized with ether, and the proximal internal carotid artery was isolated from the sympathetic chain, clamped, and cauterized. The neck wound was closed, and the animals were allowed to recover for 1 hr on a thermal blanket, maintaining body temperature at 33°C–34°C. The rats were then placed in a sealed chamber infused with nitrogen to a level of 6% O2 and also on a thermal blanket, maintaining body temperature at 33°C–34°C throughout the hypoxia period. Body temperature was monitored by rectal probe before and after surgery, and hypoxia did not differ between groups. After a 1- to 2-hr period of recovery, the rats were returned to their dam. Rats were killed 96 hr after injection, and brains were perfused with 4% paraformaldehyde, postfixed for 24 hr, and then cryoprotected in 30% sucrose in PBS.
Immunocytochemistry and Histological Analysis
OL maturation was evaluated by immunocytochemistry (ICC) with antibodies to the specific OL marker myelin basic protein (MBP) in rats killed at P10. Histological analysis using hematoxylin and eosin staining and ICC analysis was performed on alternating serial 16-µm coronal sections, as detailed previously (Follett et al., 2000, 2004). Sections were blocked and incubated overnight with anti-MBP (1:1,000, SMI-99; Sternberger Monoclonals, Baltimore, MD, rinsed, and then incubated with the appropriate secondary Ab (Molecular Probes, Eugene, OR) for 1 hr at room temperature. MBP was used as a marker for white matter injury. Lesion size was assessed in three similar sections per animal by a blinded observer, with reproducibility demonstrated by a second blinded observer using a semiquantitative scale of 1–5 (corresponding to 100%–0%): 0, no abnormality; 1, any loss of processes radiating from the capsule into the cortex; 2, some loss of processes without any capsule involvement; 3, loss of processes and fragmenting or thinning of capsule; 4, ragged-appearing capsule and thinning; 5, discontinuity in capsule because of massive thinning, full thickness loss in areas. Scores were analyzed for significance by one-way analysis of variance (P < 0.05). Histological assessment (H&E staining) was performed to assess for the presence of large infarcts, in which case animals would have been excluded from the analysis. In fact, no large infarcts occurred in any of the sample populations, and no animals were excluded from the study.
Statistical Analysis
Statistical differences were assessed by one- or two-way (Fig. 4) analysis of variance (ANOVA) with Tukey-Kramer post hoc analysis for multiple comparisons. The Student t test was used when only two independent groups were compared. Differences were considered statistically significant at P < 0.05.
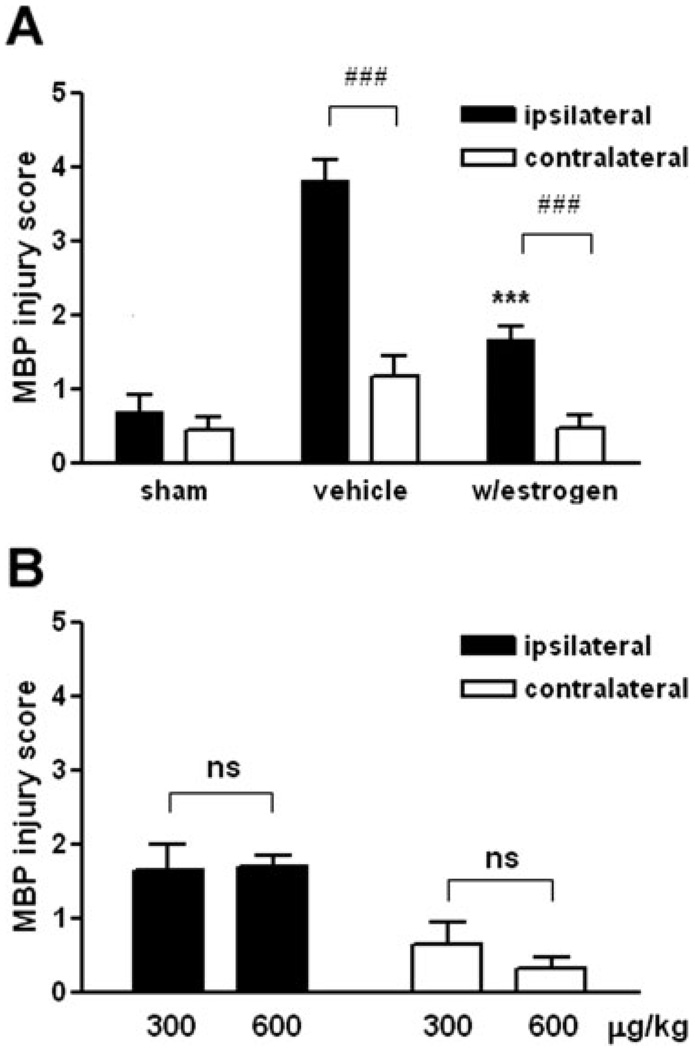
Fig. 4.
E2 was protective against hypoxia/ischemia-induced white matter injury. A: Of 14 cases treated with vehicle, injury was significantly higher on the ipsilateral than on the contralateral side (mean 3.8 vs. 1.2 ± SEM, # # #P < 0.001). Of the 15 cases treated with estrogen, injury was also significantly higher on the ipsilateral than on the contralateral side (mean 1.7 vs. 0.5 ± SEM, # # #P < 0.001). Thus, injury was always higher on the ipsilateral side than the contralateral side regardless of treatment. Injury on the ipsilateral side was significantly lower in the group treated with estrogen (***P < 0.001). B: In the cases treated with estrogen, there was no significant difference in injury by dose: 300 versus 600 µg/kg (ipsilateral: mean 1.6 vs. 1.7 ± SEM, P = 0.90; contralateral: mean 0.6 vs. 0.3 ± SEM, P = 0.34).
RESULTS
17β-Estradiol Protects Against Oxidative Stress Induced by Cystine Deprivation in Developing OLs
Developing OLs in vitro are absolutely dependent on extracellular cystine for survival (Yonezawa et al., 1996; Back et al., 1998; Baud et al., 2004). The EC50 value for this effect of cystine was 4.3 ± 1.9 µM (n = 3; Fig. 1A), similar to the value described previously (Baud et al., 2004). Cell death occurred slowly during the first 12 hr and rapidly accelerated between 12 and 16 hr following exposure to cystine-depleted medium. We previously observed that estrogen receptor (ER)–α and ER-β are preferentially expressed in immature oligodendrocytes and showed down-regulation in mature oligodendrocytes (Gerstner et al., 2007). To investigate whether E2 treatment can reduce oxidative stress–mediated cell death in oligodendrocytes, we administered 1 µM E2 or vehicle 12 hr prior to cystine deprivation (0, 2.5, 5, 7.5, 10, and 200 µM cystine) and for an additional 24 hr to primary oligodendrocyte (O4+) cultures. This concentration and schedule for treatment with E2 was chosen because 1 µM E2 and 12-hr pretreatment were previously found to produce maximal protection against hyperoxia-induced cell death (Gerstner et al., 2007). The cell survival assay demonstrated significant protection by 1 µM E2 in response to mild cystine deprivation (2.5–7.5 µM). At zero-added cystine concentration, E2 was no longer protective (Fig. 1B).
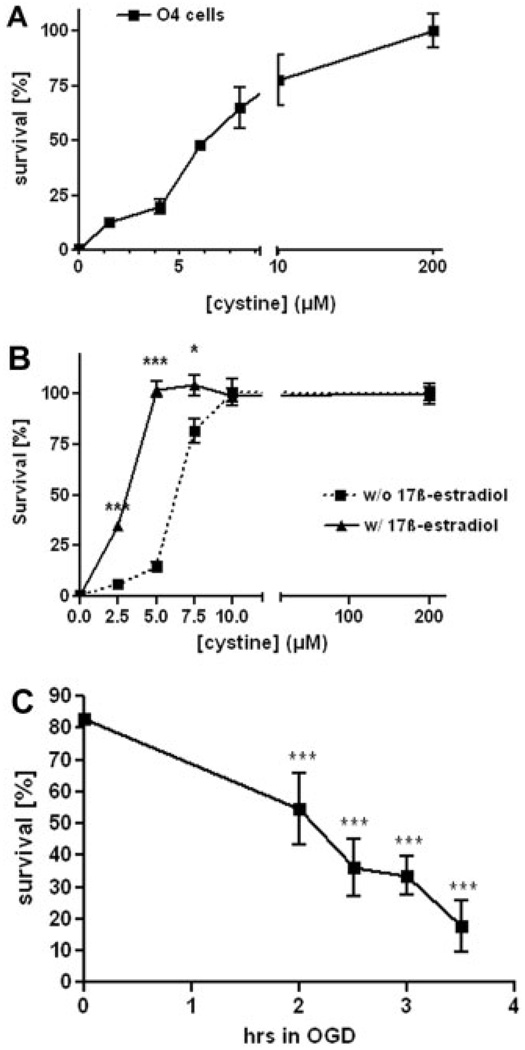
Fig. 1.
Protective effect of E2 against oxidative injury caused by cystine deprivation. A: Concentration dependence of cystine deprivation toxicity on rat OLs in culture. Developing OLs (O4+) were cultured for 24 hr in culture medium containing cystine at selected concentrations and then were assayed for cell survival using Alamar Blue. Normal cystine content in the culture medium was 200 µm. Values shown are mean ± SEM from a single representative experiment of three that was performed. The EC50 value for cystine as a survival agent in this experiment was 4.3 ± 1.9 µM. B: E2 (1 µM) significantly protected against oxidative injury induced by cystine deprivation in culture medium containing 2.5–7.5 µM cystine. At lower cystine concentrations the protective effect of E2 was no longer detectable. At higher concentrations the induced oxidative stress was negligible and did not lead to significant cell death in comparison with cells kept in normal culture medium. Values shown are mean ± SEM from a single representative experiment of three that were performed with one-way ANOVA with Tukey’s multiple-comparison test (*P < 0.05, ***P < 0.001). C: Time dependence of OGD-induced death in developing oligodendrocytes. Primary oligodendrocyte viability was measured by LDH release, after 2, 2.5, 3, and 3.5 hr of OGD. Values shown are mean ± SEM of three independent experiments performed, anlayzed by one-way ANOVA with Tukey’s multiple-comparison test (***P < 0.001).
Oxygen-Glucose Deprivation Reduced Oligodendrocyte Cell Viability in a Time-Dependent Fashion
To estimate the time course of oxygen-glucose deprivation (OGD)–induced toxicity, primary immature OLs were exposed to OGD for 2, 2.5, 3, and 3.5 hr. Cell viability was assessed by LDH release. Survival of pre-OLs decreased with time of exposure (Fig. 1C). After 2 hr of OGD, cell survival at 24 hr was reduced to 70%, and after 3.5 hr of OGD, only 25% of the cells remained viable at 24 hr.
17β-Estradiol Protects Against Oxygen-Glucose Deprivation–Induced Cell Death in Developing Oligodendrocytes
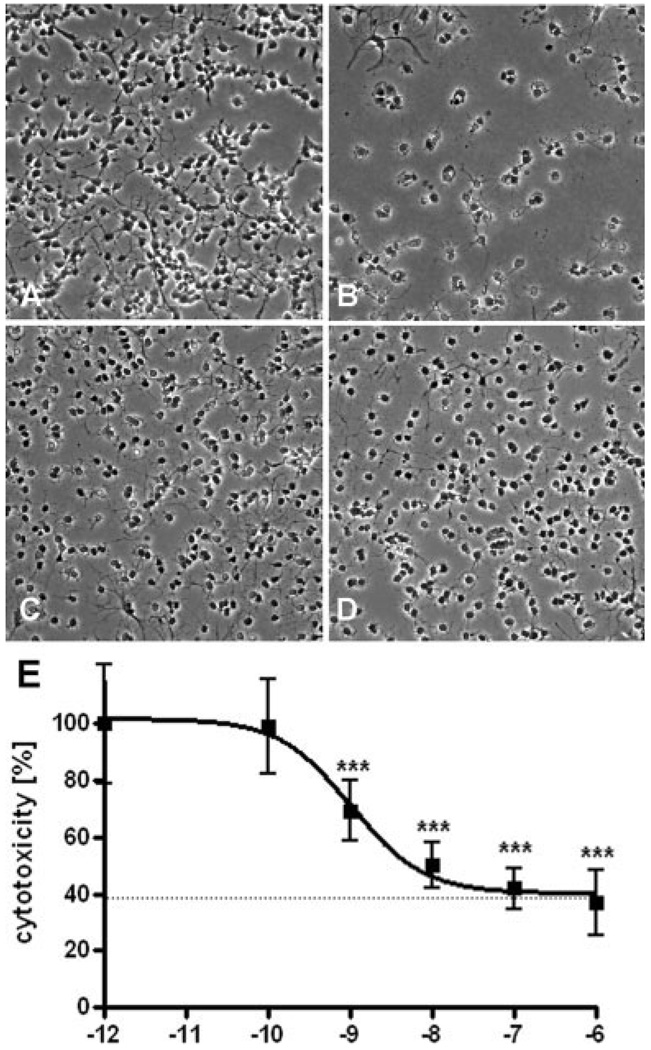
To investigate whether E2 treatment can reduce OGD-induced cell death in oligodendrocytes, we administered E2 or vehicle 12 hr prior to OGD to primary oligodendrocyte (O4+) cultures. Representative phase-contrast photomicrographs are shown (Fig. 2A– D). After 3.5 hr of OGD, considerable death of pre-OLs had occurred (compare Fig. 2A,B). Death occurred less frequently to cells treated with E2 (Fig. 2D). E2-containing culture medium had no obvious effect on its own (Fig. 2C). The LDH cell survival assay demonstrated significant dose-dependent protection of E2, with an EC50 of 1.3 ± 0.46 × 10−9 M (Fig. 2E). Interestingly, the protective effect of estrogen did not extend to all cells, saturating at approximately 60% protection. This result is similar to what was observed in studies of the protective effect of estrogen against hyperoxia (Gerstner et al., 2007) and suggests there may be two populations of cells with different sensitivities to estrogen, possibly because of differences in receptor expression, redox state, developmental stage, or other parameters.
Fig. 2.
E2 protected against OGD in developing oligodendrocytes. Representative pre-OL cell phase contrast images under normoxic condition without (A, B), or with (C, D) 1 µM E2 treatment are shown. Most pre-OLs were killed 24 hr following 3.5 hr of OGD (B). E2 treatment (1 µM) partially attenuated this cell death (D). The protective effect of E2 was dose dependent, with an EC50 value of 1.3 ± 0.46 × 10−9 M (E). Values shown are means ± SEMs from three independent experiments performed, analyzed by one-way ANOVA with Tukey’s multiple comparison test (***P < 0.001).
Preinsult Treatment of 17β-Estradiol Attenuates White Matter Injury in a Rodent Model of PVL
The effect of E2 treatment was evaluated in an immature rat model of PVL. We have previously adapted a well-described model of unilateral carotid ligation (UCL)/hypoxia (6% O2, 1 hr) in the P6 rat to produce selective subcortical white matter injury (Follett et al., 2000). Major features of the resulting lesion include initial loss of pre-OLs and subsequent attenuation of MBP expression, with white matter gliosis but relative axonal and cortical neuron sparing. Impairment of skilled limb use correlates with the contralateral white matter injury in the developing rodent (Tomimatsu et al., 2002), and white matter injury appears to persist into adulthood (Yager and Asselin, 1999). Twelve hours before UCL hypoxia, rat pups were treated intraperitoneally with E2 (n = 15), 600 (n = 8) or 300 (n = 7) µg/kg, or vehicle (2-hydroxypropyl-β-cyclodextrin; n = 14). These doses were found to completely block hyper-oxia-induced apoptosis in a previous study (Gerstner et al, 2007). Injury was assessed by two blinded observers for degree of MBP loss in ipsilateral cerebral white matter (Fig. 3A–F) and by hematoxylin and eosin histology on serial sections. There was significant attenuation of lesion severity in rats treated preinsult with E2 (Fig. 3G,H), compared with that in vehicle-treated controls (Fig. 3E,F versus C,D). Injury was significantly higher ipsilaterally than contralaterally (# # #P < 0.001) and significantly lower ipsilaterally in the group treated with estrogen (***P < 0.001; Fig. 4). In the cases treated with estrogen, there was no significant difference in injury by dose: 300 versus 600 µg/kg E2 (Fig. 4B). There was no difference in mortality or weight gain among any of the treatment groups.
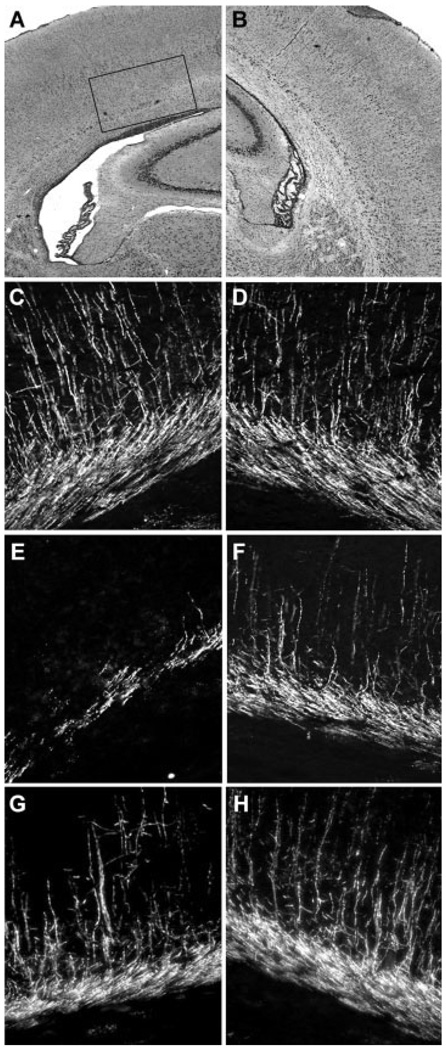
Fig. 3.
E2 protected against hypoxic–ischemic white matter injury in vivo. A, B: Hematoxylin and eosin staining of a coronal brain section from a pup killed at P10 after UCL hypoxia at P6, demonstrating ipsilateral (A) white matter injury with relative sparing of the overlying cortex and normal ventricle size on the contralateral side (B). Note the enlargement of the lateral ventricle on the ipsilateral side. Inset demonstrates regions shown in C–G. C, D: Normal MBP expression seen in the P10 pup (score = 0, no abnormality). E, F: Loss of MBP seen in the P10 pup ipsilateral to carotid ligation (E, score = 5, discontinuity in capsule because of massive thinning, full thickness loss in areas) after UCL/hypoxia at P6 and, less so, on the contralateral side (F, score = 3, loss of processes and fragmenting or thinning of capsule). Systemic treatment with E2 (600 µg/kg) 12 hr prior to the insult attenuated the injury in a littermate pup, ipsilateral (G, score = 3) and contralateral (H, score = 1, any loss of processes radiating from the capsule into the cortex).
DISCUSSION
The present study demonstrates that E2 treatment is protective against OGD-induced cell death in OLs in vitro and against hypoxic-ischemic white matter injury in a neonatal model of PVL. E2 is also effective in vitro in primary rat oligodendroglial cell cultures against glutathione depletion—induced oxidative stress.
E2 and ER play important roles in the development, plasticity, and function of the CNS (Arai and Matsumoto, 1978; Lustig, 1994; Levy et al., 1996; de Lacalle, 2006). Proliferation, differentiation, and migration of neuronal cells are controlled by E2 (Brannvall et al., 2002). Because white matter defects are the predominant type of injury in prematurely born infants, we focused in this study on the effect of estrogen on oxygen-glucose deprivation and oxidative stress–induced cell death in pre-OLs. Migration and differentiation of OLs take place during the last months of pregnancy (Levison and Goldman, 1993). Pre-OLs are the predominant OL stage in human cerebral white matter during the peak incidence of PVL (Back et al., 2001). ERs are expressed in the OL plasma membrane and within the myelin sheath (Arvanitis et al., 2004), and E2 stimulates ER-α and ER-β on both estrogen response element– and AP-1-driven promoters (Guzman et al., 2005), indicating a potential role for estrogen in myelin maintenance or functions. Using primary cultures of rat glial cells, Jung-Testas et al. (1992) found that cell growth was stimulated by E2, inducing dramatic morphologic changes in OLs and increasing the synthesis of myelin basic protein. Interestingly, Prayer et al. (1997) demonstrated that starting at P2, rat pups showed accelerated white matter maturation of the anterior optic pathways and hemisphere commissures after treatment with E2.
Back et al. found that the mechanism of perinatal white matter injury involved maturation-dependent vulnerability in the OL lineage by using a neonatal rat model of hypoxic-ischemic injury (Back et al., 2002; Follett et al., 2004). Late OL progenitors and immature OLs are the predominant OL stage in human cerebral white matter during the peak incidence of PVL (Back et al., 2001). In the neonatal rat brain, mainly O4+O1− OLs are found in the cerebral hemispheres (corpus callosum and cortex) between postnatal days 1 and 5 (Gard and Pfeiffer, 1989), whereas by postnatal day 7 most of these cells have developed into O4+O1+OLs. In late pregnancy OLs are exposed to high levels of estrogen (Wood, 2005) during the stage of development when OL differentiation is proceeding. In the preterm baby, withdrawal from estrogen is occurring just at the stage of development when OLs are most vulnerable to excitotoxic and oxidative injury.
Previous studies have suggested that estrogens are neuroprotective (Lustig, 1994; Weaver et al., 1997; Sawada et al., 2002; Baum, 2005; Perrella and Bhavnani, 2005; Zhao and Brinton, 2006). The protective effect of E2 against hypoxic-ischemic injury to developing OLs and white matter is a novel finding. When the umbilical cord is clamped, high placental estrogen, which increases to 15 ng/mL (55 nM) during the last trimester, is no longer available for the premature infant (Tulchinsky et al., 1972), whose circulating E2 level is reduced approximately 1,000-fold (5–35 pg/mL, 20–130 pM; Thomas, 2005). Our data suggest that this decline may lead to a higher risk for developing PVL and the associated motor and cognitive deficits. The maternal level of E2 is approximately 40-fold higher than the EC50 value for the protective effects of E2 against OGD shown in this study. However, because the relation of serum hormone concentrations in maternal versus umbilical cord samples is about 0.3 (Troisi et al., 2003), the EC50 value in this study correlates well with the E2 levels the fetus is exposed to in the last trimester.
Because of differences in species, our experimental paradigm cannot exactly mimic the human situation. Although the brain develops mainly perinatally in human beings, the major growth spurt of the rodent brain occurs postnatally (Dobbing and Sands, 1979). To have a model relevant for understanding injury to white matter in a premature baby less than 34 weeks of gestation, we had to inject the rat pups on postnatal day 6. In addition, the rodent model used in the present study is not an exact replica of the human preterm infant regarding exposure to high estrogen levels, in that in the rodent model, estrogen is administered 6–7 days after birth, whereas in humans estrogen exposure is continuous during in utero development. Another caveat is that male mice were used exclusively in the present study. Gender differences have been described in human studies of mortality and morbidity in premature babies (Naeye et al., 1971; Ingemarsson, 2003; Nunez and McCarthy, 2003; Zhu et al., 2006b). We did not want to have sex-related differences affect the outcome of the experiments in the present study. Nonetheless, it would be of considerable interest for the future to investigate sex differences in the response to estrogen.
So far, two ERs have been identified. ERs are found in various regions of the human and rodent brains (Shughrue et al., 1997; Laflamme et al., 1998), but these studies did not identify specific cell types. Our previous data demonstrated that ER-α and ER-β proteins are expressed in the oligodendrocyte lineage and decrease with maturation, suggesting an important role of ER-α and ER-β during oligodendroglial development. E2 has equal binding affinity for the human ERs ER-α and ER-β (Zhu et al., 2006a). The Kd values of E2 for recombinant human ER-α and ER-β are 0.7 and 0.75 nM, comparable to the EC50 value of 1.3 nM obtained in this study.
A previous study suggested a crucial role for ER-α in providing neuroprotection in a stroke model (Dubal et al., 2001). Activation of protective signaling pathways through estrogen could depend on localization of the ER, which has been found in the nucleus (Deroo and Korach, 2006), in the cytoplasm (Levin, 2005), and on the plasma membrane (Xu et al., 2004). Which of these sites of expression account for the neuroprotective effect of E2 in developing white matter requires investigation.
Developing oligodendrocytes are highly vulnerable to excitotoxicity and oxidative stress, both of which are important in the pathogenesis of hypoxic-ischemic brain injury (Deng et al., 2006). OL excitotoxicity is mediated by ionotropic glutamate receptors of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate type in these cells. Deng et al. (2004) reported that activation of group 1 mGluRs attenuates OL excitotoxicity by controlling downstream oxidative stress after iGluR overactivation. The modulating effect of group 1 mGluRs on hypoxic-ischemic OL injury requires activation of PKC alpha after G-protein coupling to phospholipase C. Metabotropic GluRs have long been linked to numerous signaling events, such as phosphorylation and dephosphorylation of proteins, protein–protein interaction, and transactivation of genes (Conn and Pin, 1997; Michaelis, 1998), all of which may contribute to the regulation of apoptotic/antiapoptotic molecules, cellular redox status, and glutathione metabolism. One possible clue to the protective effect of estrogen in models of excitotoxic injury comes from evidence that activation of ER-α leads to mGluR1a signaling in hippocampal neurons, which triggers cAMP response element–binding (CREB) protein phosphorylation through phospholipase C regulation of MAPK (Boulware et al., 2005). The mechanism by which ER-α activation leads to mGluR1 activation is unclear. A previous study has determined that E2 induces CREB phosphorylation via activation of MAPK in an adult rat model of focal cerebral ischemia (Choi et al., 2004). Future research will need to determine whether E2 binding to membrane-associated estrogen receptors and activation of group I mGluR signaling also account for the protective effect of estrogen in the oligodendroglial lineage.
There may be indirect effects of estrogen that promote the survival of oligodendrocytes in vivo. The conditions used for the in vivo experiments in this study were carefully determined to produce a relatively selective white matter lesion with minimal neuronal injury (Follett et al., 2000). Therefore, although it has been shown that estrogen is neuroprotective in a neonatal stroke model (Nunez et al., 2007), under the conditions used for our study, there is little neuronal death, and so it seems unlikely that neuroprotection plays a major role in the protection against white matter injury seen with estrogen. This does not exclude the possibility of a contributing role of injury to neurons, astrocytes, or microglia to the white matter injury investigated in the present study or a contribution of protection against those forms of injury to the protection afforded by estrogen. However, that oligodendrocytes have estrogen receptors and that the protective effects of estrogen can be shown in relatively pure cultures of oligodendrocytes strongly suggest direct effects on oligodendrocytes as well.
Several studies, including the present one, have demonstrated the protective impact of E2 on the developing brain and raise the interesting hypothesis that specific hormonal replacement therapy for premature infants could improve neurological outcome. E2 might be suitable as a preventive treatment in premature infants and asphyxiated term infants, who are at risk for diffuse injury of developing cerebral white matter. E2 replacement therapy in extremely low-birth-weight infants has been introduced in some centers, with the goal of improving bone mineralization, and no adverse side effects have been observed thus far (Trotter et al., 2001). Therefore, we suggest that E2 supplementation could be an effective treatment to protect neonates from brain injury. More data are urgently needed concerning the safety and efficacy of estrogen supplementation in the neonatal period, as well as the long-term protective effect of E2 on white matter injury in rats.
ACKNOWLEDGMENTS
We thank Dr. Felicia M. Trachtenberg of the New England Research Institute, Watertown, Massachusetts, and Dr. Matt Gregas from Children’s Hospital Boston for advice regarding statistical analysis. We are very grateful to Drs. Delia Talos, Nikolaus Sucher, and Wenbin Deng for helpful discussion and support.
Contract grant sponsor: Ernst Schering Research Foundation, Berlin, Germany; Contract grant sponsor: Metzler-Foundation, Frankfurt/Main, Germany; Contract grant sponsor: National Institutes of Health; Contract grant numbers: NS38475 and HD18655.
Abbreviations
- E2
17β-estradiol
- ER
estrogen receptor
- MBP
myelin basic protein
- OL
oligodendrocyte
- OGD
oxygen-glucose deprivation
- pre-OL
preoligodendrocyte (O4+, O1−, MBP−)
- PVL
periventricular leukomalacia
- UCL
unilateral carotid ligation
REFERENCES
- Arai Y, Matsumoto A. Synapse formation of the hypothalamic arcuate nucleus during post-natal development in the female rat and its modification by neonatal estrogen treatment. Psychoneuroendocrinology. 1978;3:31–45. doi: 10.1016/0306-4530(78)90039-2. [DOI] [PubMed] [Google Scholar]
- Arvanitis DN, Wang H, Bagshaw RD, Callahan JW, Boggs JM. Membrane-associated estrogen receptor and caveolin-1 are present in central nervous system myelin and oligodendrocyte plasma membranes. J Neurosci Res. 2004;75:603–613. doi: 10.1002/jnr.20017. [DOI] [PubMed] [Google Scholar]
- Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud O, Haynes RF, Wang H, Folkerth RD, Li J, Volpe JJ, Rosenberg PA. Developmental up-regulation of MnSOD in rat oligodendrocytes confers protection against oxidative injury. Eur J Neurosci. 2004;20:29–40. doi: 10.1111/j.0953-816X.2004.03451.x. [DOI] [PubMed] [Google Scholar]
- Baum LW. Sex, hormones, and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2005;60:736–743. doi: 10.1093/gerona/60.6.736. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- Choi YC, Lee JH, Hong KW, Lee KS. 17 Beta-estradiol prevents focal cerebral ischemic damages via activation of Akt and CREB in association with reduced PTEN phosphorylation in rats. Fundam Clin Pharmacol. 2004;18:547–557. doi: 10.1111/j.1472-8206.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Inflammation, brain damage and visual dysfunction in preterm infants. Semin Fetal Neonatal Med. 2006;11:363–368. doi: 10.1016/j.siny.2006.02.003. [DOI] [PubMed] [Google Scholar]
- de Lacalle S. Estrogen effects on neuronal morphology. Endocrine. 2006;29:185–190. doi: 10.1385/ENDO:29:2:185. [DOI] [PubMed] [Google Scholar]
- Deng W, Rosenberg PA, Volpe JJ, Jensen FE. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci U S A. 2003;100:6801–6806. doi: 10.1073/pnas.1136624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Wang H, Rosenberg PA, Volpe JJ, Jensen FE. Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc Natl Acad Sci U S A. 2004;101:7751–7756. doi: 10.1073/pnas.0307850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Yue Q, Rosenberg PA, Volpe JJ, Jensen FE. Oligodendrocyte excitotoxicity determined by local glutamate accumulation and mitochondrial function. J Neurochem. 2006;98:213–222. doi: 10.1111/j.1471-4159.2006.03861.x. [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerth RD, Haynes RL, Borenstein NS, Belliveau RA, Trachtenberg F, Rosenberg PA, Volpe JJ, Kinney HC. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol. 2004;63:990–999. doi: 10.1093/jnen/63.9.990. [DOI] [PubMed] [Google Scholar]
- Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE. Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci. 2004;24:4412–4420. doi: 10.1523/JNEUROSCI.0477-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989;106:119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Gerstner B, Sifringer M, Dzietko M, Schuller A, Lee J, Simons S, Obladen M, Volpe JJ, Rosenberg PA, Felderhoff-Mueser U. Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann Neurol. 2007;61:562–573. doi: 10.1002/ana.21118. [DOI] [PubMed] [Google Scholar]
- Guzman CB, Deighton-Collins S, Martinez A, Kleerekoper M, Zhao C, Benjamins JA, Skafar DF. Activity of estradiol and selective estrogen receptor modulators in the mouse N20.1 oligodendrocyte/astrocytes cell line. Neuro Endocrinol Lett. 2005;26:526–532. [PubMed] [Google Scholar]
- Hilton GD, Ndubuizu AN, McCarthy MM. Neuroprotective effects of estradiol in newborn female rat hippocampus. Brain Res Dev Brain Res. 2004;150:191–198. doi: 10.1016/j.devbrainres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ingemarsson I. Gender aspects of preterm birth. Bjog. 2003;110(20) Suppl:34–38. doi: 10.1016/s1470-0328(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Ishida T, Seo F, Hirato K, Fukuda T, Yanaihara T, Araki H, Nakayama T. [Changes in placental enzymatic activities in relation to estrogen production during pregnancy] Nippon Sanka Fujinka Gakkai Zasshi. 1985;37:547–554. [PubMed] [Google Scholar]
- Jensen FE. Role of glutamate receptors in periventricular leukomalacia. J Child Neurol. 2005;20:950–959. doi: 10.1177/08830738050200120401. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Renoir M, Bugnard H, Greene GL, Baulieu EE. Demonstration of steroid hormone receptors and steroid action in primary cultures of rat glial cells. J Steroid Biochem Mol Biol. 1992;41:621–631. doi: 10.1016/0960-0760(92)90394-x. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Levy A, Garcia Segura M, Nevo Z, David Y, Shahar A, Naftolin F. Action of steroid hormones on growth and differentiation of CNS and spinal cord organotypic cultures. Cell Mol Neurobiol. 1996;16:445–450. doi: 10.1007/BF02088111. [DOI] [PubMed] [Google Scholar]
- Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ, Rosenberg PA. Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci. 2003;23:5816–5826. doi: 10.1523/JNEUROSCI.23-13-05816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig RH. Sex hormone modulation of neural development in vitro. Horm Behav. 1994;28:383–395. doi: 10.1006/hbeh.1994.1035. [DOI] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Ibarretxe G, Sanchez-Gomez MV. Excitotoxicity in glial cells. Eur J Pharmacol. 2002;447:239–246. doi: 10.1016/s0014-2999(02)01847-2. [DOI] [PubMed] [Google Scholar]
- McClean J, Nunez JL. 17alpha-Estradiol is neuroprotective in male and female rats in a model of early brain injury. Exp Neurol. 2008;210:41–50. doi: 10.1016/j.expneurol.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Dellovade TL, Shughrue PJ. Neuroprotection by estrogen in animal models of global and focal ischemia. Ann N Y Acad Sci. 2003;1007:89–100. doi: 10.1196/annals.1286.009. [DOI] [PubMed] [Google Scholar]
- Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- Naeye RL, Burt LS, Wright DL, Blanc WA, Tatter D. Neonatal mortality, the male disadvantage. Pediatrics. 1971;48:902–906. [PubMed] [Google Scholar]
- Nunez J, Yang Z, Jiang Y, Grandys T, Mark I, Levison SW. 17beta-Estradiol protects the neonatal brain from hypoxia-ischemia. Experimental neurology. 2007;208:269–276. doi: 10.1016/j.expneurol.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Sex differences and hormonal effects in a model of preterm infant brain injury. Ann N Y Acad Sci. 2003;1008:281–284. doi: 10.1196/annals.1301.032. [DOI] [PubMed] [Google Scholar]
- Perrella J, Bhavnani BR. Protection of cortical cells by equine estrogens against glutamate-induced excitotoxicity is mediated through a calcium independent mechanism. BMC Neurosci. 2005;6:34. doi: 10.1186/1471-2202-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prayer D, Roberts T, Barkovich AJ, Prayer L, Kucharczyk J, Moseley M, Arieff A. Diffusion-weighted MRI of myelination in the rat brain following treatment with gonadal hormones. Neuroradiology. 1997;39:320–325. doi: 10.1007/s002340050416. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Dai W, Gan XD, Ali S, Fu J, Back SA, Sanchez RM, Segal MM, Follett PL, Jensen FE, Volpe JJ. Mature myelin basic protein-expressing oligodendrocytes are insensitive to kainate toxicity. J Neurosci Res. 2003;71:237–245. doi: 10.1002/jnr.10472. [DOI] [PubMed] [Google Scholar]
- Sampei K, Goto S, Alkayed NJ, Crain BJ, Korach KS, Traystman RJ, Demas GE, Nelson RJ, Hurn PD. Stroke in estrogen receptor-alpha-deficient mice. Stroke. 2000;31:738–743. doi: 10.1161/01.str.31.3.738. discussion 744. [DOI] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Honda K, Nakamizo T, Kanki R, Nakanishi M, Sakka N, Akaike A, Shimohama S. Estradiol protects dopaminergic neurons in a MPP+Parkinson’s disease model. Neuropharmacology. 2002;42:1056–1064. doi: 10.1016/s0028-3908(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Sawada H, Ibi M, Kihara T, Urushitani M, Akaike A, Shimohama S. Estradiol protects mesencephalic dopaminergic neurons from oxidative stress-induced neuronal death. J Neurosci Res. 1998;54:707–719. doi: 10.1002/(SICI)1097-4547(19981201)54:5<707::AID-JNR16>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ. Estrogen attenuates the MPTP-induced loss of dopamine neurons from the mouse SNc despite a lack of estrogen receptors (ERalpha and ERbeta) Exp Neurol. 2004;190:468–477. doi: 10.1016/j.expneurol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Thomas L. Frankfurt/Main. Germany: TH-Books; 2005. Labor und Diagnose. [Google Scholar]
- Tomimatsu T, Fukuda H, Endoh M, Mu J, Watanabe N, Kohzuki M, Fujii E, Kanzaki T, Oshima K, Doi K, Kubo T, Murata Y. Effects of neonatal hypoxic-ischemic brain injury on skilled motor tasks and brainstem function in adult rats. Brain Res. 2002;926:108–117. doi: 10.1016/s0006-8993(01)03311-x. [DOI] [PubMed] [Google Scholar]
- Troisi R, Potischman N, Roberts JM, Harger G, Markovic N, Cole B, Lykins D, Siiteri P, Hoover RN. Correlation of serum hormone concentrations in maternal and umbilical cord samples. Cancer Epidemiol Biomarkers Prev. 2003;12:452–456. [PubMed] [Google Scholar]
- Trotter A, Bokelmann B, Sorgo W, Bechinger-Kornhuber D, Heinemann H, Schmucker G, Oesterle M, Kohntop B, Brisch KH, Pohlandt F. Follow-up examination at the age of 15 months of extremely preterm infants after postnatal estradiol and progesterone replacement. J Clin Endocrinol Metab. 2001;86:601–603. doi: 10.1210/jcem.86.2.7176. [DOI] [PubMed] [Google Scholar]
- Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095–1100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001a;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the newborn. Philadelphia: W.B. Saunders; 2001b. p. 928. [Google Scholar]
- Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- Weaver CE, Jr, Park-Chung M, Gibbs TT, Farb DH. 17beta-Estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res. 1997;761:338–341. doi: 10.1016/s0006-8993(97)00449-6. [DOI] [PubMed] [Google Scholar]
- Wood CE. Estrogen/hypothalamus-pituitary-adrenal axis interactions in the fetus: The interplay between placenta and fetal brain. J Soc Gynecol Investig. 2005;12:67–76. doi: 10.1016/j.jsgi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Xu Y, Traystman RJ, Hurn PD, Wang MM. Membrane restraint of estrogen receptor alpha enhances estrogen-dependent nuclear localization and genomic function. Mol Endocrinol. 2004;18:86–96. doi: 10.1210/me.2003-0262. [DOI] [PubMed] [Google Scholar]
- Yager JY, Asselin J. The effect of pre hypoxic-ischemic (HI) hypo and hyperthermia on brain damage in the immature rat. Brain Res Dev Brain Res. 1999;117:139–143. doi: 10.1016/s0301-7516(99)00040-x. [DOI] [PubMed] [Google Scholar]
- Yonezawa M, Back SA, Gan X, Rosenberg PA, Volpe JJ. Cystine deprivation induces oligodendroglial death: rescue by free radical scavengers and by a diffusible glial factor. J Neurochem. 1996;67:566–573. doi: 10.1046/j.1471-4159.1996.67020566.x. [DOI] [PubMed] [Google Scholar]
- Yoshioka A, Bacskai B, Pleasure D. Pathophysiology of oligoden-droglial excitotoxicity. J Neurosci Res. 1996;46:427–437. doi: 10.1002/(SICI)1097-4547(19961115)46:4<427::AID-JNR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer’s disease. BMC Neurosci. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006a;147:4132–4150. doi: 10.1210/en.2006-0113. [DOI] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006b;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]