Abstract
Background
The goal of this study was to test the hypothesis that intrarenal Ang II has a proinflammatory effect leading to renal damage and dysfunction in Dahl S rats on high Na intake.
Methods
Forty-six 7-to 8-week old Dahl S or R/Rapp strain rats were maintained for 5 weeks on high sodium (8%) with or without candesartan cilexetil in daily doses of 10–15 mg/kg/day. Arterial catheters were implanted at day 28.
Results
By day 35 in the high Na S + candesartan rats, renal tissue Ang II concentration, renal monocytes/macrophages, TNFα, and MCP-1 significantly decreased. Plasma Ang II remained at very low levels in all groups. Reduced renal damage in candesartan-treated Dahl S rats was demonstrated by marked decreases in urinary protein excretion and renal glomerular and interstitial damage. After 5 weeks of high Na, compared to high Na Dahl S rats, arterial pressure was unchanged in candesartan S rats, but creatinine clearance was increased.
Conclusions
Therefore, candesartan reduced renal tissue Ang II, renal damage, infiltration of immune cells, cytokines, chemokines, and improved renal hemodynamics. These data suggest that intrarenal Ang II plays an important role in causing renal inflammation which leads to renal cortical damage, proteinuria, and decreases in renal hemodynamics.
Keywords: Renal failure, cytokines, macrophages, renal hemodynamics, inflammation
INTRODUCTION
Progressive renal damage and dysfunction occurs in several types of salt-sensitive hypertension in humans and experimental models leading to end-stage renal disease. The Dahl salt-sensitive (S) rat is a good model of human salt-sensitive hypertension, and several studies indicate that the intrarenal renin-angiotensin system may play a role in this hypertension. Previous studies have shown low levels of plasma renin activity or plasma angiotensin II (Ang II) levels1, 2. However, renal tissue levels of Ang II have been reported to be elevated in salt-sensitive hypertension including post L-Name hypertension3, post Ang II hypertension4 and during Ang II infusion5. During high Na intake, intrarenal Ang II was suppressed in Dahl R rats but not in Dahl S rats6.
Even though the circulating levels of Ang II are especially low in Dahl S rats, treatment with angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) have proven to decrease renal damage and dysfunction7–10, thus implicating changes in intrarenal Ang II. In fact, the ARB, candesartan, markedly reduced intrarenal Ang II in Dahl S rats but not Dahl R rats2.
Dahl S rats on high Na intake have been shown to have renal infiltration of immune cells, elevated cytokines and chemokines11, 12, and treatment with antiimmune drugs decreases the immune response and the associated renal damage13, 14. Ang II has been shown to elicit a potent immune response4, 5, 12; however, it is not known if ARB will attenuate the proinflammatory effects of intrarenal Ang II in Dahl S hypertension.
The goal of the present study was to test the hypothesis in Dahl S rats on high Na intake that intrarenal Ang II has a proinflammatory effect leading to renal damage and dysfunction. These goals were met in studies in Dahl R and S rats on an 8% Na diet over a 5 week period with and without candesartan, and we determined changes in renal tissue Ang II, renal cytokines, chemokines, oxidative stress, creatinine clearance, blood pressure, renal monocytes/macrophages and renal damage.
METHODS
Animal Protocol and Experimental Measurements
Studies were conducted over five weeks in forty-six conscious 7- to 8-wk-old male Dahl salt-sensitive (S) rats and Dahl salt-resistant (R) rats, Rapp strain (Harlan Sprague Dawley, Indianapolis, IN) with the authorization of the Institutional Animal Committee. Rats arrived at our laboratory when they were 6–7 wk old, were afforded a 1-wk recovery, and then were fed an 8% NaCl diet. Dahl S and R rats were divided randomly into the following groups: S high Na + vehicle (n=10); S high Na + candesartan (n=8); R high Na + vehicle (n=14); R high Na + candesartan (n=14). Candesartan treatment groups received candesartan cilexetil (Astra Zeneca, Wilmington, DE) in daily doses of 10–15 mg/kg/day based on results from previous rat studies2. Candesartan was dissolved in drinking water containing ethanol (0.05% to 0.075% v/v), polyethylene glycol 300 (0.05% to 0.075% v/v), and sodium bicarbonate (0.75 to 1.13 mmol/L), and this vehicle has been shown to have no effect on blood pressure, renal collagen content, urine protein excretion and plasma and kidney Ang II levels in Dahl R or S rats2.
After 4 wk on the various diets, chronic catheters were implanted through the femoral artery with aseptic technique and isoflurane anesthesia (1%). Catheters exited the scapular region through a swivel apparatus. Rats recovered for 5–6 days in metabolic cages before the beginning of a conscious collection of arterial pressure, heart rate, 24 hour urine samples and creatinine clearance14–17. On each of these days, pulsatile arterial pressure signals were sent to a computer at 500 Hz for 4 s of each minute during a 3 hour measurement period to determine arterial pressure and heart rate.
After 5 weeks of the diets, blood samples for plasma Ang II measurement were collected quietly before the sacrifice surgery. Plasma Ang II levels were measured by radioimmunoassay (RIA) using the antibody-trapping technique. Urine and plasma creatinine were measured with an assay from Bioassay Systems. After rats were anesthetized with isoflurane (1%), the weights of the rats, the blood samples for plasma creatinine were taken, and the left kidneys were removed to provide tissue for pathology, intrarenal Ang II, and Lowry assays. Then, the right kidneys were perfused, removed and homogenized for assays on tumor necrosis factor-alpha (TNFα), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), and glutathione. Renal tissues were homogenized in appropriate buffers and protease inhibitors. The amounts of protein in renal tissues and urine were quantified with the Lowry and Bradford assays, respectively.
Measurement of renal glutathiones, interleukin-6 (IL-6), TNFα, and monocyte chemoattractant protein-1 (MCP-1), renal monocytes/macrophages and intrarenal Ang II
Reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured with the fluorescent detection of dansyl derivatives using HPLC according to the method of Jones et al.18 as we have done before11, 14, 17, 19. Renal cortical IL-6 and TNFα were measured using kits from R & D Systems according to the manufacturer’s instructions. Renal cortical MCP-1 was determined using a kit from Biosource. Monocytes/macrophages in the kidney sections collected at 5 wk of the various groups were measured by indirect immunoperoxidase methodology17, 18 using ED-1, a monoclonal antibody to monocytes/macrophages (Chemicon). Intrarenal ANGII concentration was determined by RIA on homogenized tissue extracted using Sep-Pak columns.
Analysis of glomerular necrosis and tubulointerstitial injury
A periodic acid Schiff renal section from each rat was examined at 100× magnification, and necrotic and sclerotic glomeruli were counted as a proportion of all consecutively examined glomeruli as we have done previously14, 16, 19. Tubulointerstitial injury was measured as the proportion of the number of points overlying damaged interstitial tissue, dilated cast-containing tubules, or tubules showing acute injury divided by the number of points on the grid overlying nonglomerular and nonvascular cortex, as we have done previously14, 16, 19.
Statistical analysis
Statistical comparisons of data from all groups were performed by analysis of variance followed by a Fisher least significant difference test for post hoc analysis or a T test and were considered to be statistically significant if P < 0.05. All data are expressed as means ±SE.
RESULTS
Renal tissue Ang II and plasma Ang II responses to high Na and candesartan
Figure 1 shows that renal tissue Ang II in vehicle-treated Dahl S rats on high Na intake had a value of 9.2 ± 1.4 pg/mg, and this value was 5.5 ± 0.7 pg/mg in candesartan S rats (P< 0.05 using Anovar and a Fisher LSD post hoc test). If renal tissue Ang II in high Na Dahl S rats is compared to high Na R rats using a T test, a significant difference is found. If renal tissue Ang II in high Na Dahl R rats is compared to high Na R rats with candesartan treatment using a T test, a significant difference is found. Compared to plasma Ang II, in the lower panel, the tissue Ang II in S rats on vehicle had about 1500 times the concentration. There were also no significant changes in plasma Ang II in any group when all four groups were included in the analysis using Anovar and a Fisher LSD post hoc test. However, if the plasma Ang II of S + candesartan rats is compared to the high Na S rats using a T test, a significant difference is found.
Figure 1.
Renal tissue Ang II concentration and plasma ANG II concentration in Dahl salt-sensitive (S) rats in 8% Na + vehicle (V), Dahl S 8% Na + Candesartan (C), Dahl salt-resistant (R) rats in 8% Na + V, and Dahl R 8% Na + C. S* P<0.05 when comparing two groups at the end of each bracket.
Renal monocytes/macrophages responses to high Na and candesartan
Figure 2 shows representative sections of renal monocytes/macrophages in the four groups of rats. In the high Na Dahl S rats in the upper left corner, monocyte/macrophage infiltration is evident. In the lower panel, average monocyte/macrophage counts are shown for each group. The average renal ED-1+ cells indicate that renal monocyte/macrophages had much higher values in vehicle S rats compared to vehicle R rats. S + V rats had a value of ED-1+ cells of 22 ± 1.6 cells/mm2, and candesartan treatment resulted in a lower value of 7.1 ± 0.6 cells/mm2 in S rats (P< 0.05).
Figure 2.
Top panels. Representative ED1 + cells (monocytes/macrophages) in the kidneys of high Na Dahl R or S rats with and without candesartan treatment. Kidneys were removed after 5 weeks of the Na diet. Renal infiltration of monocytes/macrophages decreased in S rats treated with candesartan. Bottom Panel bar graph. Average renal monocyte/macrophage infiltration in Dahl salt-sensitive (S) rats in 8% Na + vehicle (V), Dahl S 8% Na + Candesartan (C), Dahl salt-resistant (R) rats in 8% Na + V, and Dahl R 8% Na + C. * P<0.05 when comparing two groups at the end of each bracket.
IL-6, TNFα and MCP-1 responses to high Na and candesartan
Figure 3 shows that the IL-6 levels in the renal cortex of vehicle-treated high Na Dahl S rats were significantly higher than those of vehicle R rats. However, candesartan treatment in S rats did not lower the IL-6 levels. Renal cortical TNFα was significantly higher in vehicle S rats compared to vehicle R rats, and TNFα levels were significantly lower after candesartan treatment in S rats but not R rats. Renal cortical MCP-1 levels were higher in vehicle Dahl S rats compared to vehicle Dahl R rats, and candesartan treatment resulted in significantly lower values in S rats but not in R rats.
Figure 3.
Renal cortical IL-6, TNFα and MCP-1 in Dahl salt-sensitive (S) rats in 8% Na + vehicle (V), Dahl S 8% Na + Candesartan (C), Dahl salt-resistant (R) rats in 8% Na + V, and Dahl R 8% Na + C. * P<0.05 when comparing two groups at the end of each bracket.
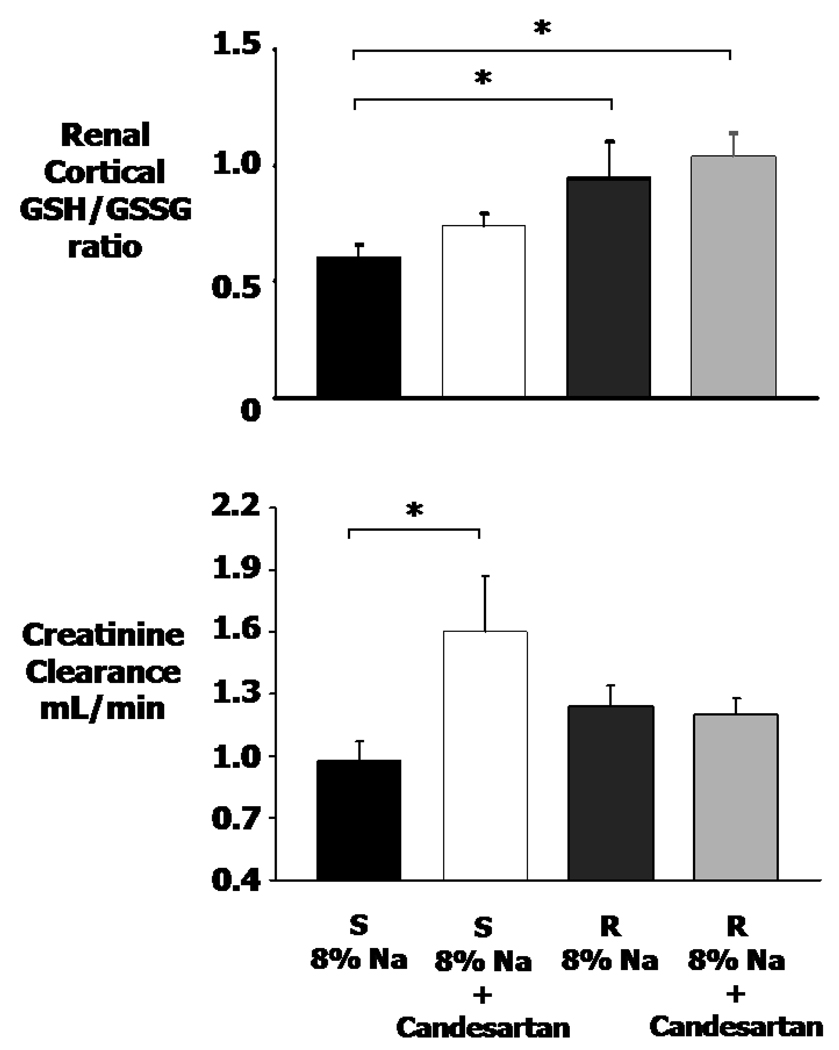
Renal cortical GSH/GSSG and renal creatinine clearance responses to high Na and candesartan
Figure 4 shows that vehicle Dahl S rats had a significantly lower GSH/GSSG ratio in the renal cortex compared to vehicle Dahl R rats. This ratio has been shown to be a highly reliable index of oxidative stress with higher values indicating less oxidative stress18. The GSH/GSSG ratio tended to increase in S rats treated with candesartan, but the differences did not reach significance. Therefore, candesartan showed no significant antioxidative effects in either R or S rats. Figure 4 also shows that creatinine clearance was significantly decreased in vehicle S rats compared to candesartan S rats, indicating that AT1 inhibition preserved the glomerular filtration.
Figure 4.
Reduced to oxidized glutathione ratio (GSH/GSSG ratio) in renal cortical tissue and creatinine clearance in Dahl salt-sensitive (S) rats in 8% Na + vehicle (V), Dahl S 8% Na + Candesartan (C), Dahl salt-resistant (R) rats in 8% Na + V, and Dahl R 8% Na + C. * P<0.05 when comparing two groups at the end of each bracket.
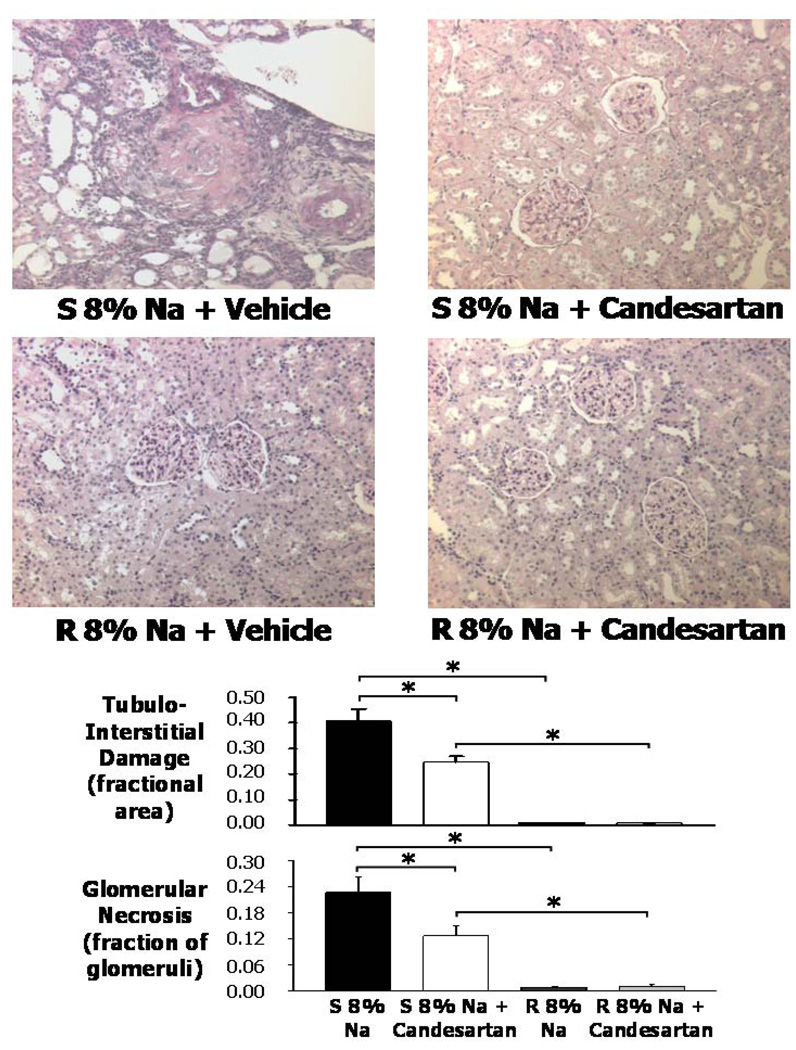
Histological analyses of kidneys with or without candesartan
Representative kidney sections for each group of rats are shown in Figure 5. In the 8% Na + vehicle Dahl S rats (Fig. 5, top left), the kidneys exhibited fibrinoid arterial necrosis and fibrinoid glomerular necrosis with dilated tubules and invasion of immune cells. Some glomeruli at all levels of the cortex showed global or segmental glomerular sclerosis. In the 8% Na + candesartan Dahl S rats, the kidneys had a significantly smaller number of glomeruli that showed fibrinoid glomerular and arteriolar necrosis, and tubulointerstitial injury, and did contain some areas with dilated tubules. In the Dahl R rat groups (Fig. 5, bottom), the kidneys had a normal appearance, with only occasional sclerotic or necrotic glomeruli.
Figure 5.
Top panels. Representative renal histology (all 200X) in the renal cortex of Dahl salt-sensitive (S) rats in 8% Na + vehicle (V), Dahl S 8% Na + Candesartan (C), Dahl salt-resistant (R) rats in 8% Na + V, and Dahl R 8% Na + C. Bottom panel bar graphs. Average tubulointerstitial damage (fractional area) and glomerular necrosis (fraction of glomeruli) in the kidneys of the four groups of rats. * P<0.05 when comparing two groups at the end of each bracket.
The next to bottom panel of figure 5 shows that tubulointerstitial damage averaged 0.41 ± 0.05 (fractional area) in the high Na S group, and this area decreased significantly in high Na S rats on candesartan treatment to 0.25 ± 0.02. The bottom panel of figure 5 shows that the average glomerular necrosis was markedly increased in the high Na S rats when compared to either the S high Na + candesartan rats or to high Na R rats. The candesartan treatment decreased the fraction of glomerular necrosis in the Dahl S rats on a high Na intake by nearly 50%.
Table 1 shows that candesartan treatment caused no significant changes in arterial pressure in high Na Dahl S rats or Dahl R rats. Heart rate did not change significantly in any group. The bottom part of Table 1 shows that in Dahl S rats the high Na + V rats had elevated values of urinary protein excretion compared to vehicle-treated Dahl R rats. Candesartan treatment in S rats resulted in lower values of protein excretion compared to vehicle-treated S rats. Candesartan treatment thus prevented renal damage in high Na S rats.
Table 1.
Blood Pressure, heart rate and urinary protein excretion response to 5 weeks of high salt with or without candesartan.
| S-vehicle | S-candesartan | R-vehicle | R-candesartan | |
|---|---|---|---|---|
| MAP (mmHg) | 168 ± 8 | 154 ± 6 | 87 ± 3* | 82 ± 1 |
| HR (bpm) | 390 ± 6 | 397 ± 16 | 376 ± 15 | 368 ± 6 |
| Urine Prot Excret (mg/d) | 318 ± 43 | 168 ± 20* | 19 ± 2* | 15 ± 2 |
Responses of mean arterial pressure (MAP), heart rate (HR), and urinary protein excretion (Urine Prot Excret) to 5 weeks of high salt and vehicle or candesartan in Dahl S or R rats.
P<0.05 compared to S vehicle group.
DISCUSSION
The major new finding in this study was that candesartan treatment of high Na Dahl S rats caused a significant decrease in renal tissue Ang II which was associated with a decrease in renal inflammation. Renal cortical TNFα and MCP-1 decreased in S rats with candesartan treatment, and renal monocytes/macrophages decreased markedly. During this decrease in inflammation, renal pathological damage and urinary protein excretion decreased, and creatinine clearance increased. Also, renal oxidative stress was lower than in R rats. Overall, the data indicate that that intrarenal Ang II has a proinflammatory effect leading to renal damage and dysfunction in Dahl salt-sensitive hypertension.
Accumulating evidence indicates that Ang II is locally formed in the kidney and is an important mediator of renal inflammation and damage. Several studies have shown an elevated amount of intrarenal Ang II in different types of salt-sensitive hypertension. L-Name was given to rats to inhibit NO formation for 3 weeks, and this was followed by a 1 week period of without L-Name infusion and then high Na intake for 4 weeks, and salt-sensitive hypertension occurred3. During the high Na period, Ang II positive cells were found in the renal interstitium accompanied by activated lymphocytes. Treatment with the antiimmune substance, mycophenolate mofetil, lowered activated lymphocytes and Ang II positive cells3, suggesting that the lymphocytes could be a source of the interstitial Ang II. Infusion of Ang II into Sprague-Dawley rats has been shown to increase intrarenal Ang II which was accompanied by renal macrophage infiltration and increases in renal MCP-1, TGFβ and NFκB12. Cessation of the Ang II infusion resulted in a return of renal tissue Ang II levels within 3 days12. In another study, Ang II was infused into Sprague-Dawley rats for 2 weeks, followed by a 5 day washout period and a 5 week period of a high Na diet; this resulted in a salt-sensitive hypertension accompanied by increases in renal Ang II levels, macrophages, lymphocytes and Ang II positive cells during high Na4. There were positive correlations between renal macrophages and renal Ang II concentration and between renal Ang II concentration and Ang II positive cells in the renal interstitium4. A study in Dahl S rats has shown an increase in renal Ang II positive cells and macrophages during high Na intake20. Taken together, these studies suggest that renal tissue Ang II levels are associated with increased renal inflammation in salt-sensitive hypertension, which supports the results of the present study in high Na Dahl S rats which showed high renal Ang II levels, renal immune cell infiltration, and increased cytokines and chemokines.
Previous studies in our laboratory have shown that renal damage in Dahl salt-sensitive hypertension was caused by both oxidative stress and inflammation in the kidney. Treatment of Dahl S rats during high Na intake with antioxidants decreased renal macrophages, chemokines, cytokines, glomerular and tubulointerstitial damage and improved renal hemodynamics11, 17, 19. Treatment with the antiinflammatory agent, mycophenolate mofetil, also prevented renal damage and dysfunction14. However, the mechanisms of the increases in oxidative stress and inflammation in this rat model are not clear. The present study presents evidence that the intrarenal angiotensin system is activated in high Na Dahl S rats, and this is associated with renal inflammation and oxidative stress. The ARB, candesartan, effectively reduced intrarenal Ang II and decreased renal inflammatory cells and cytokines and increased glomerular filtration. To the best of our knowledge, this is the first report of an ARB causing a reduction in renal inflammatory cells and cytokines in Dahl S hypertension.
ACE inhibitors (CEI) and ARBs have been administered to high Na Dahl S rats in several studies. In two studies with Dahl S rats on a 4% NaCl diet and an ARB, neither systolic pressure (measured by a tail cuff) nor urinary protein excretion significantly changed21, 22. However, another study in Dahl S rats treated with 4% NaCl and a CEI, found no change in systolic blood pressure, but a significant decrease in proteinuria and an improvement in glomerular histology occurred7. In a study in Dahl S rats on 6% NaCl and a CEI, a marked increase in survival rate occurred compared with untreated high Na S rats; systolic blood pressure was unchanged; and glomerular and tubular renal damage improved8. Candesartan did not reduce systolic pressure of Dahl S rats on 8% NaCl, but renal damage significantly decreased compared to untreated S rats2. In all the above studies, the tail cuff method was used to measure systolic pressure. In the present study, the rats were equipped with indwelling catheters, and no significant change in mean arterial pressure occurred in candesartan-treated Dahl S rats after 5 weeks of high Na.
There could be several reasons why arterial pressure did not decrease in high Na S rats treated with an ARB in the present study. First, treatment of Dahl S rats with a high Na diet decreased plasma Ang II to very low levels; therefore, the candesartan would not be expected to have a significant systemic effect, but only an effect on intrarenal Ang II. The lack of a systemic effect would minimize the blood pressure-lowering effect of candesartan. As mentioned above, other studies in high Na S rats using either a CEI or an ARB reported no significant change in blood pressure2, 7, 8, 21, 22. Second, there were no significant decreases in renal oxidative stress in the candesartan-treated S rats in the present study. Previous studies in our laboratory demonstrated that treatment of Dahl S rats with the antioxidants, vitamin C and E, decreased renal oxidative stress16 and interestingly decreased renal cytokines and chemokines and moderately decreased arterial pressure19. Antiimmune treatment of high Na S rats decreased renal cytokines and immune cells but also decreased oxidative stress in the kidney, resulting in decreases in arterial pressure and renal damage14. In the present study, there was a milder antiimmune effect on TNFα compared to our studies using vitamins C and E as an antioxidant substance19. Therefore, vitamin or antiimmune treatment of high Na S rats decreased both oxidative stress and renal cytokines or macrophages, but candesartan only decreased renal inflammation moderately. The result was a reduction in renal damage but no significant change in arterial pressure.
Renal tissue Ang II levels in the present study were about 1500 times the plasma Ang II levels, which is comparable to the tissue to plasma Ang II ratio of 553 found in post-Ang II salt–sensitive hypertension4. The renal Ang II levels in candesartan-treated S rats in the present study were significantly lower as also occurred in another study2. Previous studies have shown that AT1 receptor blockade during Ang II hypertension resulted in increases in plasma Ang II but a marked attenuation in intrarenal Ang II23. This dissociation between plasma and intrarenal Ang II demonstrated a differential regulation of kidney and plasma Ang II. One method by which AT1 receptor blockade can decrease intrarenal Ang II is by decreased cellular uptake of Ang II. Indeed, this occurred in Ang II infused rats treated with candesartan. In these rats candesartan prevented the increases in Ang II levels in renal endosomes and intermicrovillar clefts24. Another way that AT1 receptor blockade can decrease intrarenal Ang II is by decreases in angiotensinogen23 and collecting duct renin25. In addition, two recent studies have showed that the AT1 receptor levels in the renal cortex of high Na Dahl R rats is significantly lower that of Dahl S rats on high Na2, 20. Therefore, AT1 receptor inhibition should have a greater effect in Dahl S rats which would contribute to renal protection. In the present study, the 1500 fold increase in renal tissue Ang II compared to plasma Ang II in high Na S rats and the decrease in intrarenal Ang II in candesartan-treated Dahl S rats provides evidence that the intrarenal Ang II system is actively involved in Dahl S hypertension.
Figure 4 shows that candesartan-treated S rats experienced a significantly higher creatinine clearance compared to vehicle S rats, but no significant difference from R rats occurred. Since plasma Ang II was suppressed to very low values by the high salt diet and was not affected by candesartan, the candesartan effect on the kidney had to come from its reduction in intrarenal Ang II and the associated reduction in renal immune cell infiltration, cytokines and chemokines. The increases in GFR were likely due to a decrease in renal damage as documented by the decreases in glomerular necrosis and tubulointerstitial damage as seen in Figure 5.
A previous study in our laboratory showed that when high Na Dahl S rats were treated with the antiimmune substance, mycophenolate mofetil (MMF), a reduction in renal macrophage infiltration occurred accompanied by lower renal cytokines and chemokines, lower urinary protein excretion, improved renal hemodynamics, lower arterial pressure and much lower renal pathological changes14. Other salt-sensitive hypertensive models have shown significant renal injury and renal immune cell infiltrate including aging Zucker rats26, high–protein–induced proteinuria27, DOCA-salt rats28, post angiotensin II hypertension 29, and L-Name hypertensive rats 30. Inhibition of the inflammatory response in each of the above models with MMF reduced the high blood pressure and lowered renal damage26. A recent study has provided more insight into the roles of renal Ang II/inflammation and blood pressure in the renal damage found in experimental hypertension. Two groups of SHR received either ARB with olmesartan or triple therapy with hydralazine, reserpine and hydrochlorothiazide31. Both ARB and triple therapy reduced blood pressure to normal in the SHR, but kidney Ang II only decreased in the ARB group. In addition, only the ARB group had a normalization of urinary protein excretion, glomerular sclerosis and renal inflammation31. Another study has shown that Dahl S rats on a 4% NaCl diet had a systolic pressure of 218 ± 9 mmHg, glomerular damage and proteinuria7. Treatment with the CEI, perindopril, did not affect systolic blood pressure but improved the proteinuria and glomerular damage. However, treatment with the diuretic, indapamide, reduced systolic pressure to 175 ± 3 mmHg, but this group showed no further improvement in glomerular histology or proteinuria compared to the CEI group7. Although many studies have demonstrated the importance of blood pressure reduction in limiting renal damage, several studies including the present study have also showed the importance of inhibiting the renin-angiotensin system without changing blood pressure2, 7, 8. Our results suggest that one of the main ways that candesartan lowered renal damage in high Na Dahl S rats is by lowering renal Ang II, renal macrophages, and proinflammatory renal cytokines and chemokines. Both renal tubulointerstitial damage and glomerular necrosis were reduced in candesartan-treated Dahl S rats, while arterial pressure was not significantly changed. In addition, candesartan treatment also resulted in a lower urinary protein excretion. These data indicate a reduction in the proinflammatory actions of intrarenal Ang II in high Na Dahl S rats significantly reduced renal damage.
In patients with chronic renal disease, a progressive fall in GFR occurs32. Also another effective predictor of progression of renal disease is the urinary excretion of protein33. One of the most effective treatments to limit the progression of human end stage renal disease is by ACE inhibitors or ARBs which have proven to be more effective than other antihypertensive agents. A recent meta-analysis showed that the antiproteinuric effect of ACE inhibitors was much greater than equal blood pressure reduction with other antihypertensive agents33. Yet, how the Ang II inhibitors work is not clear. The present study indicates that candesartan effectively decreased renal Ang II, kidney inflammation and renal damage while only moderately decreasing blood pressure. Perhaps in human hypertension, ACE inhibitors or ARBs attenuate the progression of renal damage in part by reducing renal tissue Ang II and the accompanying inflammation.
Acknowledgments
This research was supported by Grant HL-51971 from the National Heart, Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Iwai J, Dahl LK, Knudsen KD. Genetic influence on the renin-angiotensin system: low renin activities in hypertension-prone rats. Circ Res. 1973 June;32(6):678–684. doi: 10.1161/01.res.32.6.678. [DOI] [PubMed] [Google Scholar]
- 2.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int. 2004 March;65(3):972–981. doi: 10.1111/j.1523-1755.2004.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otsuka F, Yamauchi T, Kataoka H, Mimura Y, Ogura T, Makino H. Effects of chronic inhibition of ACE and AT1 receptors on glomerular injury in dahl salt-sensitive rats. Am J Physiol. 1998 June;274(6 Pt 2):R1797–R1806. doi: 10.1152/ajpregu.1998.274.6.R1797. [DOI] [PubMed] [Google Scholar]
- 4.Franco M, Martinez F, Rodriguez-Iturbe B, Johnson RJ, Santamaria J, Montoya A, Nepomuceno T, Bautista R, Tapia E, Herrera-Acosta J. Angiotensin II, interstitial inflammation, and the pathogenesis of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2006 December;291(6):F1281–F1287. doi: 10.1152/ajprenal.00221.2006. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008 September;295(3):F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003 March;41(3):592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation. 1997 October 7;96(7):2407–2413. doi: 10.1161/01.cir.96.7.2407. [DOI] [PubMed] [Google Scholar]
- 8.Kodama K, Adachi H, Sonoda J. Beneficial effects of long-term enalapril treatment and low-salt intake on survival rate of Dahl salt-sensitive rats with established hypertension. J Pharmacol Exp Ther. 1997 November;283(2):625–629. [PubMed] [Google Scholar]
- 9.Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol. 2008;28(1):158–167. doi: 10.1159/000110021. [DOI] [PubMed] [Google Scholar]
- 10.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007 September;59(3):251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 11.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008 December;295(6):R1858–R1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007 January;292(1):F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006 July;48(1):149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 14.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD., Jr Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007 February;292(2):H1018–H1025. doi: 10.1152/ajpheart.00487.2006. [DOI] [PubMed] [Google Scholar]
- 15.Meng S, Roberts LJ, Cason GW, Curry TS, Manning RD., Jr Superoxide dismutase and oxidative stress in Dahl salt-sensitive and - resistant rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R732–R738. doi: 10.1152/ajpregu.00346.2001. [DOI] [PubMed] [Google Scholar]
- 16.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005 May;45(5):934–939. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 17.Tian N, Rose RA, Jordan S, Dwyer TM, Hughson MD, Manning RD., Jr N-Acetylcysteine improves renal dysfunction, ameliorates kidney damage and decreases blood pressure in salt-sensitive hypertension. J Hypertens. 2006 November;24(11):2263–2270. doi: 10.1097/01.hjh.0000249705.42230.73. [DOI] [PubMed] [Google Scholar]
- 18.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 19.Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2007 December;293(6):H3388–H3395. doi: 10.1152/ajpheart.00981.2007. [DOI] [PubMed] [Google Scholar]
- 20.Chandramohan G, Bai Y, Norris K, Rodriguez-Iturbe B, Vaziri ND. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol. 2008;28(1):158–167. doi: 10.1159/000110021. [DOI] [PubMed] [Google Scholar]
- 21.Zhou MS, Adam AG, Jaimes EA, Raij L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension. 2003 November;42(5):945–951. doi: 10.1161/01.HYP.0000094220.06020.C8. [DOI] [PubMed] [Google Scholar]
- 22.Jaimes EA, Zhou MS, Pearse DD, Puzis L, Raij L. Upregulation of cortical COX-2 in salt-sensitive hypertension: role of angiotensin II and reactive oxygen species. Am J Physiol Renal Physiol. 2008 February;294(2):F385–F392. doi: 10.1152/ajprenal.00302.2007. [DOI] [PubMed] [Google Scholar]
- 23.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004 May;43(5):1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension. 2002 January;39(1):116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 25.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005 September;289(3):F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol. 2004 April;286(4):F606–F616. doi: 10.1152/ajprenal.00269.2003. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez V, Quiroz Y, Nava M, Pons H, Rodriguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol. 2002 November;283(5):F1132–F1141. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]
- 28.Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension. 2001;37:781–786. doi: 10.1161/01.hyp.37.2.781. [DOI] [PubMed] [Google Scholar]
- 29.Franco M, Tapia E, Santamaria J, Zafra I, Garcia-Torres R, Gordon KL, Pons H, Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Renal cortical vasoconstriction contributes to development of salt-sensitive hypertension after angiotensin II exposure. J Am Soc Nephrol. 2001 November;12(11):2263–2271. doi: 10.1681/ASN.V12112263. [DOI] [PubMed] [Google Scholar]
- 30.Quiroz Y, Pons H, Gordon KL, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ, Rodriguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am J Physiol Renal Physiol. 2001 July;281(1):F38–F47. doi: 10.1152/ajprenal.2001.281.1.F38. [DOI] [PubMed] [Google Scholar]
- 31.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005 July;16(7):2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitch WE, Walser M, Buffington GA, Lemann J., Jr A simple method of estimating progression of chronic renal failure. Lancet. 1976 December 18;2(7999):1326–1328. doi: 10.1016/s0140-6736(76)91974-7. [DOI] [PubMed] [Google Scholar]
- 33.Gansevoort RT, Sluiter WJ, Hemmelder MH, de ZD, De Jong PE. Antiproteinuric effect of blood-pressure-lowering agents: a meta-analysis of comparative trials. Nephrol Dial Transplant. 1995 November;10(11):1963–1974. [PubMed] [Google Scholar]