Abstract
An electrically controlled drug release (ECDR) system based on sponge-like nanostructured conducting polymer (CP) polypyrrole (PPy) film was developed. The nanostructured PPy film was composed of template-synthesized nanoporous PPy covered with a thin protective PPy layer. The proposed controlled release system can load drug molecules in the polymer backbones and inside the nanoholes respectively. Electrical stimulation can release drugs from both the polymer backbones and the nanoholes, which significantly improves the drug load and release efficiency. Furthermore, with one drug incorporated in the polymer backbone during electrochemical polymerization, the nanoholes inside the polymer can act as containers to store a different drug, and simultaneous electrically triggered release of different drugs can be realized with this system.
Keywords: Electrically controlled drug release, Conducting polymers, Polypyrrole, Fluorescein, Dexamethasone
1. Introduction
For the creation of “smart” implantable devices that can monitor health status and provide therapeutic treatment, a key step is to build controllable drug release devices that can be miniaturized1–4. Drug release systems based on CPs are excellent potential candidates, as CPs can be flexibly synthesized on conductive surfaces even at the nanoscale5–7, and their inherent electroactivity provide the mechanism for electrically triggered molecule release8–11. In the past, different molecules have been incorporated into CP films and then released upon electrical stimulation12–15. In these cases, drug release is based on the electrical switching of the polymer redox states, accompanied by the movement of dopant ions in and out of the material16. Several limitations have prevented the broad use of CPs in controlled release systems. For instance, the drug molecules to be delivered were incorporated into the bulk of the CPs, which has limited capacity for drug loading. In addition, the range of drugs was restricted due to the charge and size requirements of dopant molecules.
In our previous work, we have significantly improved the drug release efficiency of a CP controlled release system by template-synthesizing high surface area nanoporous polypyrrole17. Here we report a further improved ECDR system based on sponge-like nanostructured PPy. With this system, drugs can be loaded during the PPy polymerization into the bulk, and extra drug or a different drug, not necessarily being a dopant, can be loaded into the nanopores inside the polymer film and sealed by a thin layer of polypyrrole on top. This design provides a CP based drug release system with higher loading capacity for a wider range of drugs.
2. Materials and methods
2.1. Chemicals
Pyrrole (98%), Fluorescein (Flu) sodium salt and Dexamethasone (Dex) 21-phosphate disodium salt were purchased from Sigma-Aldrich, and pyrrole was distilled under vacuum and stored frozen. Polystyrene (PS) nanobeads (mean diameter 46±2.0 nm) were obtained from Duke Scientific Corporation. Milli-Q water from a Millipore Q water purification system was used throughout.
2.2. Apparatus
Electrochemical experiments were performed on a Gamry Potentiostat, FAS2/Femtostat (Gamry Instruments) using a three-electrode system, with a glassy carbon (GC, diameter of 3.0 mm) electrode as the working electrode, a platinum wire as the counter electrode, and a Ag/AgCl electrode as reference electrode. SEM was performed with an XL30 SEM instrument (FEI Company). The concentration of Dex and Flu solutions were measured with a microplate reader SpectraMax M5 (Molecular Devices). The ultraviolet absorption of Dex was measured at 242 nm, and the fluorescence of Flu was measured at the excitation and emission wavelength of 405 and 538 nm, respectively.
2.3. Preparation of nanostructured PPy films loaded with drugs
The ECDR system was constructed by forming a thin PPy layer on top of the nanoporous PPy film, as shown in Scheme 1. Nanoporous PPy films loaded with Flu were prepared using a template method as previously described17. For comparison, conventional PPy films incorporated with Flu were synthesized similarly, but without the PS templates.
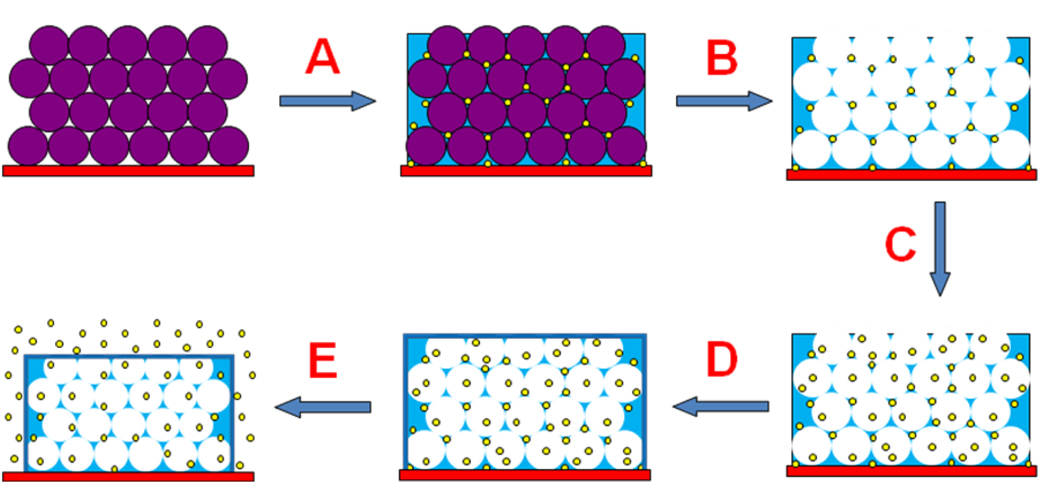
Scheme 1.
Schematic process of the synthesis and drug releasing of the nanosturctured PPy film. A) Immerse the template modified electrode into the pyrrole solution containing drugs and carry out electropolymerization; B) Dissolve the polystyrene nanobeads; C) Add extra drug or another kind of drug; D) Electrodeposit a thin PPy layer; E) Apply potentials to release the loaded drugs.
The prepared nanoporous PPy modified electrode was soaked in ethanol and water alternatively for several times to make the nanoporous film more hydrophilic, and 5.0 µL solution containing 0.01 M Flu was then dropped onto the electrode surface and dried in air. The added drugs can penetrate throughout the hydrophilized nanoporous PPy films, as the nanoholes resulted from closely packed PS nanobeads are interconnected18. To prevent the stored drugs from leaking, a protective thin PPy layer was electropolymerized on top of the nanoporous PPy. The electrode was immersed in the solution containing 0.2 M pyrrole and 0.01 M Flu, and electropolymerization was carried out immediately by cycling the potential from 0.5 V to 1.2 V at the scan rate of 50 mV s−1 for one cycle, which was optimized to form a uniform thin PPy layer.
A similar method was used for the preparation of the binary drug release system, in which Dex (0.02 M, 5.0 µL) was added instead of Flu. The resulted drug release system is denoted as NPPy-Flu/Dex/PPy-Flu.
2.4. Electrically controlled drug release
The ECDR was carried out in an electrochemical cell containing 1.6 mL 0.01 M PBS (pH 7.4). Before the drug release, the electrodes were immersed in 30 mL 0.01 M PBS under magnetic stirring for 15 min to remove any physically adsorbed drugs. The electrical stimulus for drug release was voltage application of −2.0 V or −0.5 V for 5s followed by 5 second of 0V period (for PPy recovery). Such stimulus was found to be most efficient in driving the drug out while minimizing the electrochemical damage to the polymer film.
3. Results and discussion
3.1. Morphology characterization of the nanostructured film
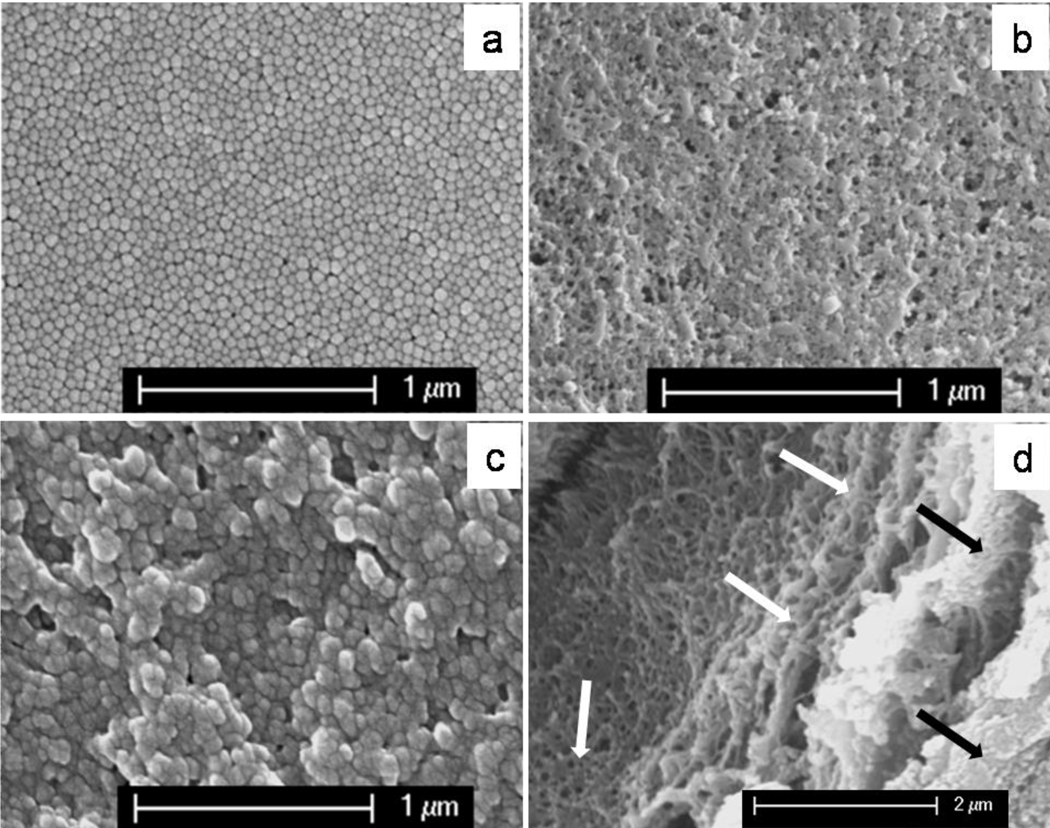
SEM images of the PS nanobead template and the nanoporous PPy film are shown in Figure 1a and 1b. It is clear that the template is composed of tightly packed PS nanobeads, and the sponge-like PPy film is composed of numerous nanoholes resulting from the removal of the PS nanobeads.
Fig. 1.
SEM images of the polystyrene nanobead template (a), the nanoporous PPy film (b), the nanoporous PPy film covered with an additional PPy layer (c), and the cross-sectional image of the nanoporous PPy film (white arrows) covered with an additional PPy layer(black arrows).
Figure 1c shows the subsequently polymerized PPy protective layer in which the top layer nanoholes of the porous PPy film were clearly covered. Similar results have also been reported by Bajpai et al.19, and they found that the opened mouths of PPy micro-containers could be sealed through further polymerization of pyrrole. As can be seen from the cross-section image (Figure 1d), a thin layer of dense PPy lays on top of the nanoporous structure. This image verified that the second PPy thin film layer was formed on top of the nanoporous PPy film, and the nanoporous morphology of the bottom layer remained unchanged.
3.2. Electrically Controlled Drug Release
3.2.1. Effect of Potential Amplitude
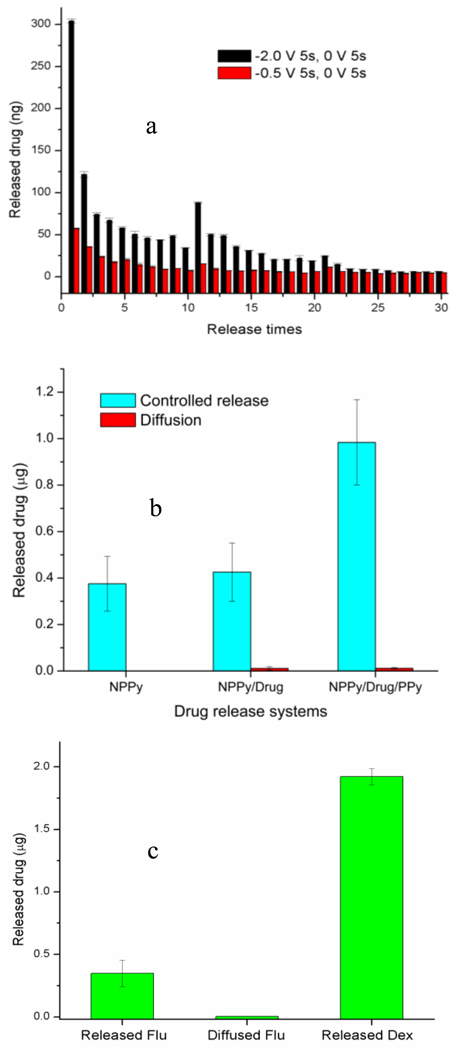
The effect of the potential amplitude was studied. As shown in Figure 2a, with each stimulus, the drug release at −2.0 V was significantly more than at −0.5 V. The drug release is indeed electrically controlled and the amount released is dependent on the strength of the stimulation. Interestingly, within the 30 stimuli tested, the amount of released drug after each stimulus decreases rapidly when the stimulation potential is high, while it was considerably steady at the lower potential. Therefore, higher potential can be chosen to release drugs more rapidly and lower potential allows more linear and steady drug release over a longer period of time.
Fig. 2.
(a) Controlled release of fluorescein at different applied potentials. Each drug-releasing stimulus was 5 second of −0.5 or −2 V. The polymers were stimulated 10 times a day for 3 consecutive days. (b) Electrically controlled release and diffusion of fluorescein from different release systems. (c) Simultaneously controlled release of fluorescein and dexamethasone. In (b) and (c), the released amount was sampled after 10 stimuli. Each stimulus was the application of −2V for 5 second followed by 5 second of 0V application. Drug diffusion was tested similarly but without electrical stimulation.
3.2.2. Drug Release Comparison of Different Systems
The drug release of different systems was tested (Figure 2b) to investigate the mechanisms of drug loading and release. Comparing the amount of drug released from the nanoporous PPy film (NPPy) and the NPPy with added drug (NPPy/Drug), there was no significant increase in drug release. When a protective PPy layer was formed (NPPy/Drug/PPy), the amount of released drug was more than doubled. Two possibilities could lead to this effect. First, the additional layer of PPy may have contained additional drug thereby increasing the drug release. Second, the top layer of PPy helps to prevent the added drug from leaking out. The first possibility has been proven to be unlikely because adding a layer of PPy without the intermediate step of loading extra drug did not result in obvious increased drug release. Therefore, the main reason for the enhanced drug release is that the nanopores in the polymer film acted as containers to hold more drugs while the protective layer on top prevented the leakage. The nanoporous morphology is critical for extra drug load as adding extra drug and a protective layer to the conventional PPy film did not result in any increase in drug release. Without the nanopores, drug dropped on surface was easily washed out during the next step of polymerization.
In past studies, drug release from CPs is primarily based on the electrochemical reduction and dedoping process16. Recent efforts have been taken to design release systems utilizing the actuation effect of CPs, which relies on volume changes of the polymer matrix associated with the movement of ions and water under electrochemical switching20–24. For example, Abidian et al.25 have reported the release of Dex from poly(3,4-ethylenedioxythiophene) nanotubes based on actuation. In our system, we hypothesize that both mechanisms of release may be at play. Upon electrical stimulation, drug molecules incorporated in the backbone as dopants are released via the dedoping process, while those loaded in the nanopores may be squeezed out due to the actuation of the sponge-like PPy film. Although the protective layer was able to prevent the passive drug diffusion, the squeezing pressure and the shrink of the protective layer itself might allow drug solution to leach out.
3.2.3. Simultaneous Release of Different Drugs
As the nanoholes inside the nanoporous PPy film were used as nanocontainers, the porous film could theoretically be loaded with any drugs. Simply adding Dex solution to the nanoholes instead of Flu solution, a binary drug release system (NPPy-Flu/Dex/PPy-Flu) was constructed. As shown in Figure 2c, both Flu and Dex were released after electrical stimulation, and there was negligible diffusion of Flu, while the diffusion of Dex was not detectable. This experiment result is interesting in three ways. First, we verified that the drug added after nanoporous film synthesis was indeed loaded and released via electrical stimulation. Secondly, we can differentiate the amount of drug released from the PPy film as dopants vs. those stored in the pores. Thirdly, we have demonstrated the proof of concept in delivering two different drugs simultaneously.
4. Conclusion
A controlled release system based on sponge-like nanostructured PPy film is developed, which can load drugs not only within the PPy bulk, but also into the nanoholes. Different from the conventional CPs based drug release, choices of drug added to the nanoholes in this sponge like system are not limited by the charge and size of the molecules. Furthermore, this system can be used for simultaneous release of multiple drugs. With one kind of drug incorporated in the polymer bulk as dopants, another kind of drug can be stored in the nanoholes inside the polymer film. This simultaneous drug release system may find applications in cases where combined drug delivery is necessary, for example the delivery of an enzyme and its cofactor, or the delivery of a drug and its adjuvant.
Acknowledgements
The project was supported by the National Science Foundation Grant 0748001 and 0729869, National Institute of Health R01NS062019 and the Department of Defense TATRC grant WB1XWH-07-1-0716.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LaVan DA, McGuire T, Langer R. Nature Biotechnolo. 2003;21:1184. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 2.Santini JT, Richards AC, Scheidt R, Cima MJ, Langer R. Angew. Chem. Int. Ed. 2000;39:2397. [PubMed] [Google Scholar]
- 3.LaVan DA, Lynn DM, Langer R. Nat. Rev. Drug Disc. 2002;1:77. doi: 10.1038/nrd707. [DOI] [PubMed] [Google Scholar]
- 4.Paunov VN, Mackenzie G, Stoyanov SD, Mater J. Chem. 2007;17:609. [Google Scholar]
- 5.Cao Y, Kovalev AE, Xiao R, Kim J, Mayer TS, Mallouk TE. Nano Lett. 2008;8:4653. doi: 10.1021/nl800940e. [DOI] [PubMed] [Google Scholar]
- 6.Meenach SA, Burdick J, Kunwar A, Wang J. Small. 2007;3:239. doi: 10.1002/smll.200600362. [DOI] [PubMed] [Google Scholar]
- 7.Woodson M, Liu J. J. Am. Chem. Soc. 2006;128:3760. doi: 10.1021/ja057500i. [DOI] [PubMed] [Google Scholar]
- 8.Moulton SE, Imisides MD, Shepherd RL, Wallace GG. J. Mater. Chem. 2008;18:3608. [Google Scholar]
- 9.Pernaut JM, Reynolds JR. J. Phys. Chem. B. 2000;104:4080. [Google Scholar]
- 10.Li LD, Huang CB. J. Am. Soc. Mass Spectrom. 2007;18:919. doi: 10.1016/j.jasms.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Lira LM, de Torresi SIC. Electrochem. Commun. 2005;7:717. [Google Scholar]
- 12.Wadhwa R, Lagenaur CF, Cui XT. J. Controlled Release. 2006;110:531. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Massoumi B, Entezami A. Eur. Polym. J. 2001;37:1015. [Google Scholar]
- 14.Kontturi K, Pentti P, Sundholm G. J. Electroanal. Chem. 1998;453:231. [Google Scholar]
- 15.Li YL, Neoh KG, Kang ET. J. Biomed. Mater. Res. Part A. 2005;73A:171. doi: 10.1002/jbm.a.30286. [DOI] [PubMed] [Google Scholar]
- 16.Wallace GG. Conductive electroactive polymers: intelligent materials systems. Boca Raton, Fla.: CRC Press; 2003. [Google Scholar]
- 17.Luo XL, Cui XT. Electrochem. Commun. 2009;11:402. doi: 10.1016/j.elecom.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartlett PN, Birkin PR, Ghanem MA, Toh CS. J. Mater. Chem. 2001;11:849. [Google Scholar]
- 19.Bajpai V, He PG, Dai LM. Adv. Funct. Mater. 2004;14:145. [Google Scholar]
- 20.Sansinena JM, Gao JB, Wang HL. Adv. Funct. Mater. 2003;13:703. [Google Scholar]
- 21.Berdichevsky Y, Lo YH. Adv. Mater. 2006;18:122. [Google Scholar]
- 22.Smela E. Mrs Bull. 2008;33:197. [Google Scholar]
- 23.Jager EWH, Smela E, Inganas O. Science. 2000;290:1540. doi: 10.1126/science.290.5496.1540. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Wang C, Wang CL, Zoval J, Madou M. Biosens. Bioelectron. 2006;21:2094. doi: 10.1016/j.bios.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Abidian MR, Kim DH, Martin DC. Adv. Mater. 2006;18:405. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]