Abstract
Host-guest interactions between α-, β-, and γ-cyclodextrins and vanadocene dichloride (Cp2VCl2) have been investigated by a combination of thermogravimetric analysis, differential scanning calorimeters, PXRD and solid state and solution EPR spectroscopy. The solid state results demonstrated that only β- and γ-cyclodextrins form 1:1 inclusion complexes, while α-cyclodextrin does not form an inclusion complex with Cp2VCl2. The β- and γ-CD-Cp2VCl2 inclusion complexes exhibited anisotropic electron-51V (I = 7/2) hyperfine coupling constants whereas the α-CD- Cp2VCl2 system showed only an asymmetric peak with no anisotropic hyperfine constant. On the other hand, solution EPR spectroscopy showed that α-CD may be involved in weak host-guest interactions in equilibrium with free vanadocene species.
Keywords: cyclodextrin, vanadocene dichloride, EPR, inclusion complex
1. Introduction
Cyclodextrins (CDs) are cyclic oligosaccharides that have α-1,4 linked D-glucose units. CDs are named according the number of the glucose units, α (six),β (seven) and γ (eight). They act as molecular hosts to a variety of guests: ions, metal complexes, polar and non-polar organic molecules [1]. These inclusion complexes have found pharmaceutical applications due to the increased aqueous solubility of the drugs, better oral absorption and their improved stability towards heat, light, oxidizing reagents and acidic conditions. CDs are known to form stable inclusion compounds with a variety of organometallic species including ferrocene and its derivatives [2–5], and sandwich complexes of molybdenum [6–8].
Metallocene dihalides and pseudo halides of general formula Cp2MX2 (M = Ti, V, Nb, Mo; X= Cl, Br, CN, SCN) have shown activity on a wide variety of murine and human tumors [9–25]. Although they belong to the same class of complexes, they have different chemical and biochemical behaviors. One of the major complications of these metallocene complexes is the low hydrolytic stability at physiological pH [26,27]. This has hindered the study of these complexes mechanistically and as a result limited their pharmacological use. One way to protect these species from extensive hydrolysis is to encapsulate them into macromolecules such as cyclodextrins.
There are few reports on the encapsulation of metallocene dichlorides in cyclodextrins. For instance, the encapsulation of Cp2TiCl2 in cyclodextrins was reported in 1999 by Turel and coworkers [28]. This group has shown that the inclusion complexes are formed by the interaction of the metallocenes in the CD cavity and their penetration depends of the length of the cyclic oligosaccharides. According to this report, titanocene dichloride can be encapsulated in the larger β- and γ-cyclodextrins, and not in the smaller α-cyclodextrin. In another report, the β-CD-molybdenocene dichloride inclusion complex was characterized by physical methods and ab initio calculations [29]. The predicted geometry of β-CD-molybdenocene dichloride inclusion complex is that only one of the Cp ligands inside the cavity of the cyclodextrin D-glucopyranose units.
More recently, the CD-Cp2VCl2 inclusion complexes have been studied by EPR spectroscopic methods [30]. The g-tensor and the anisotropic hyperfine coupling constants (Ax, Ay, Az) demonstrated that vanadocene dichloride and 1,1′-dimethylvanadocene dichloride were encapsulated in the β- and β-cyclodextrins and the rhombic symmetry was distorted as expected for encapsulated vanadium species, while the α-cyclodextrin can not encapsulate vanadocene complexes. In this regard, the anisotropic EPR spectral data demonstrated that only in the β- and γ-cyclodextrins, the hyperfine interactions with the vanadium nucleus can be observed as a result of the vanadocene inclusion. However, no thermal analysis, powder X-ray diffraction (PXRD) and solution EPR spectroscopies were presented. Herein we report a more detailed thermal and spectroscopic characterization of the CD-Cp2VCl2 inclusion complexes. While in the previous report the α-cyclodextrin-Cp2VCl2 was completely ruled out as inclusion complex, we obtained different results in solution, using EPR spectroscopy. Herein we report our findings on the CD-Cp2VCl2 host-guest interactions.
2. Experimental
2.1 MATERIAL AND METHODS
α-CD (Sigma-Aldrich), β-CD (Aldrich) and γ-CD (Fluka) were obtained commercially. The water contents of cyclodextrins were determined by thermal gravimetric analysis (TGA), α-CD (9.3 %), β-CD (13.95 %) and γ-CD (8.5 %). Cp2VCl2 was purchased from Aldrich and used as received. The inclusion complexes were prepared using CDs (α-, β-, and γ corrected for water content) and vanadocene dichloride.
Elemental analyses were performed by Atlantic Microlab. The thermal analysis experiments were performed using a TAQ100 (DSC) and TAQ500 (TGA) instrument. The heating rate was 3 °C/min for DSC analysis and 10°C/min for TGA analysis. A differential scanning calorimetry interfaced to a PC was used to measure the thermal properties of the inclusion compounds. The calorimetry operated with a nitrogen flow of 50 mL/min. The temperature of the calorimeter was calibrated from the observed melting points of indium. Powder x-ray diffraction (PXRD) data were collected on a Siemens D5000 diffractometer using Cu Kα radiation = 1.5418 Å. The diffractograms were acquired between 2θ angles of 2° and 60° with a step of 0.020°and step time of 2 s at 25°C.
FTIR data were collected on a Nexus 670 spectrometer using Thunderdome ATR. EPR spectra were recorded with an Elexsys E 500 spectrometer with an ER 4122SHQE resonator. Magnetic fields were measured with an E036 TM Teslameter. Solid sample spectra were acquired with 3 mm ID sample tubes. Solution samples were acquired with ER 106FC-Q flat cells. Simulations were optimized with XSophe version 1.114.
2.2 SYNTHESIS OF CD-Cp2VCl2 INCLUSION COMPLEXES
0.2 mmol of CD (corrected for water content) was dissolved in 30 mL of deionized water and 0.2 mmol of solid Cp2VCl2 was added. After stirring for 30 minutes, the green solution was filtered in a fritted funnel of fine porosity and the resulting solution was lyophilized to obtain an amorphous voluminous solid product.
Anal. Calc. for “α-CD-Cp2VCl2·13H2O” [(C36H60O30)-(C10H10VCl2)·13H2O]: C, 37.86; H, 6.63; Cl, 4.86. Found: C, 37.41; H, 5.98; Cl, 4.28. IR(KBr) cm−1: 3272(bm), 2928(w), 1685(vw), 1330(w), 1151(m), 1076(m), 1024(s), 950(w), 937(w), 825(w).
Anal. Calc. for β-CD-Cp2VCl2·14H2O [(C42H70O35)-( C10H10VCl2)·14H2O]: C, 38.10; H, 6.64; Cl, 4.33. Found: C, 37.90; H, 6.24; Cl, 3.90. IR(KBr) cm−1: 3278(bm), 2929(w), 1686(w), 1331(w), 1151(m), 1076(s), 1024(s), 950(w), 938(w), 826(w).
Anal. Calc. for γ-CD-Cp2VCl2·10H2O [(C48H80O40)-( C10H10VCl2)·10H2O]: C, 40.29; H, 6.41; Cl, 4.10. Found: C, 40.65; H, 5.94; Cl, 3.92. IR(KBr) cm−1: 3279(bm), 2930(w), 1331(w), 1151(m), 1076(m), 1024(s), 950(w), 938(w), 826(w).
Physical mixtures were prepared by mixing equimolar amounts of cylodextrin and Cp2VCl2 in a bench top tumbler blender.
3. Results and Discussion
Vanadocene dichloride slowly hydrolyzes in water, at low pH, to form [Cp2V(OH2)2]2+ [31]. However, the inclusion complexes in solution and solid states have shown that Cp2VCl2 is the predominant species, as corroborated by a series of spectroscopic and analytical techniques. First, we will discuss the solid state results. The inclusion complexes were characterized by elemental analysis, thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and, IR, powder X-ray diffraction (PXRD) and EPR spectroscopies.
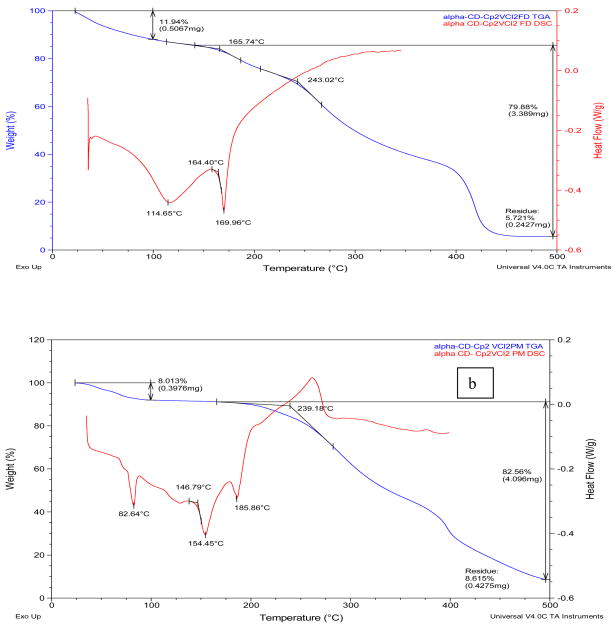
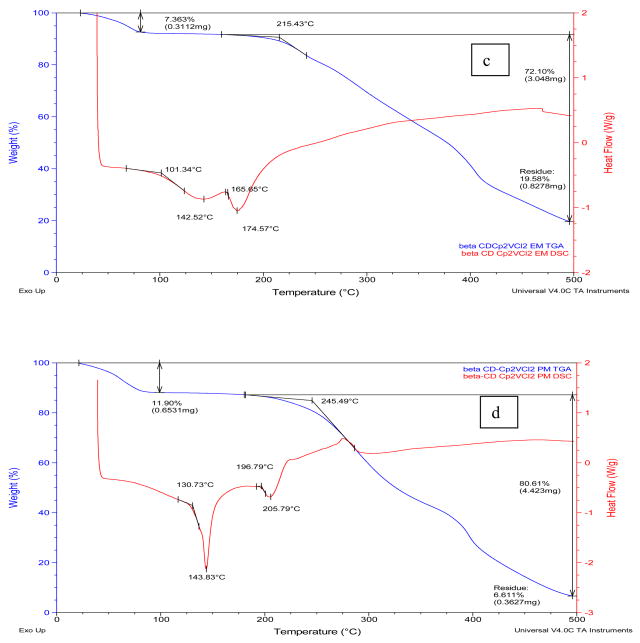
Table I summarizes the thermal properties (TGA and DSC) of the drug, cyclodextrins and the inclusion complexes and Figures 1 and 1S (Supplementary Material) show representative TGA and DSC thermograms for α-, β- and γ-cyclodextrin complexes. In general, pure α-, β- and γ-cyclodextrins loose 8–11 % of mass in the range of 25–108° C (see Supplementary Material), indicating loss of hydrated water in the cyclodextrins. The TGA and DSC thermograms of the inclusion complexes demonstrated mass loss of 7–12 % between 25–100° C, also indicating loss of water from the cyclodextrin inclusion complexes. Although we are aware that elemental analysis of lyophilized samples could be meaningless at some point, we compared the water calculated with elemental analysis and TGA for the new complexes. It can be observed that there is a discrepancy between the water content calculated by elemental analysis and the water content determined by thermal analysis. This suggests that in the thermal analysis, at low temperature (25–100° C) only the weakly bound water molecules are released. The more tightly bound water molecules are lost at higher temperatures and this process could be overlapped by other thermal events such as the melting/decomposition of the inclusion complexes. Similar results were obtained in the CD-Cp2TiX2 inclusion complexes reported by Turel and co- workers [28] and in CD-Cp2NbCl2 inclusion complexes by our group [32].
Table I.
Thermoanalytical data of the inclusion complexes (FD = freeze dried), physical mixture (PM) and precursors. Note: D= Dehydration and M = Melting.
| Dehydration | Melting | DSC Endothermic max (°C) |
|||
|---|---|---|---|---|---|
| Temperature range (°C) |
Mass loss (%) |
Temperature range (°C) |
Mass loss (%) |
||
| α-CD | 25–100 | 9 | 245–496 | 88 | 76(D), 108(D), 136(D/glass transition), 289(M), 325(Decomposition/exothermic) |
| β-CD | 25–80 | 11 | 271–496 | 88 | 129(D/glass transition), 288(M), 325(Decomposition/exothermic) |
| γ-CD | 25–98 | 8 | 276–496 | 93 | 104(D/glass transition), 284 (M), 330(Decomposition/exothermic) |
| Cp2VCl2 | 25–100 | 1 | 220–496 | 63 | 291(M/Decomposition/exothermic) |
| αCD- Cp2VCl2 FD | 25–100 | 12 | 140–496 | 80 | 115 (D/glass transition), 170 (M) 243(Decomposition/exothermic) |
| αCD- Cp2VCl2 PM | 25–100 | 8 | 139–496 | 83 | 83(D), 154 (D/glass transition), 186 (M), 239 (Decomposition/exothermic) |
| β-CD- Cp2VCl2 FD | 25–80 | 7 | 162–496 | 72 | 110(D/glass transition), 175(M), 215(Decomposition/exothermic) |
| β-CD- Cp2VCl2 PM | 25–100 | 12 | 130–496 | 81 | 144(D/glass transition), 206(M), 245 (Decomposition/exothermic) |
| γ-CD- Cp2VCl2 FD | 25–100 | 11 | 140–496 | 82 | 167(D/glass transition), 174(M), 230(Decomposition/exothermic) |
| γ-CD- Cp2VCl2 PM | 25–100 | 8 | 117–496 | 84 | 141(D/glass transition), 193(M), 274(Decomposition/exothermic) |
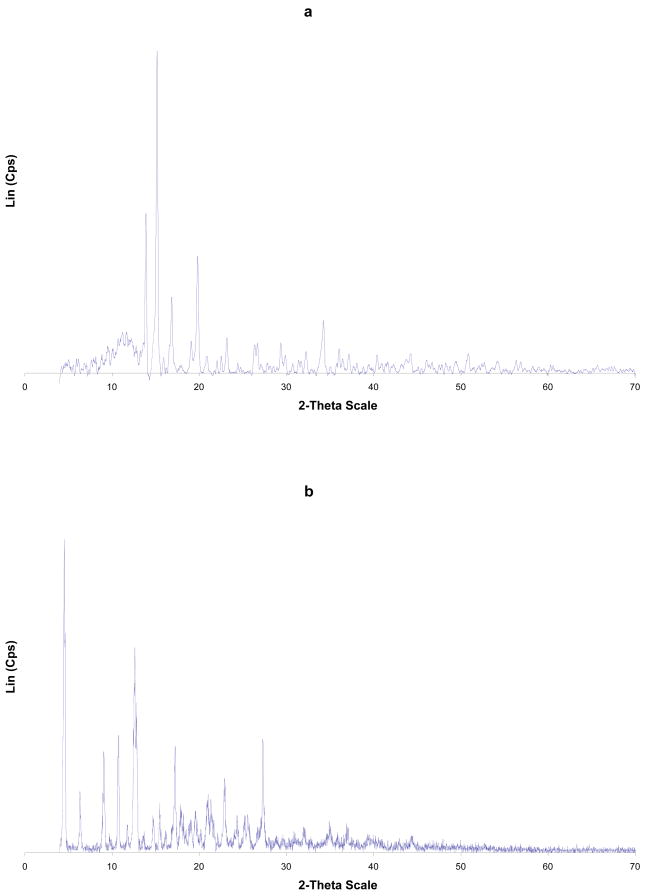
Figure 1.
TGA and MDSC curves of a) α-CD-Cp2VCl2 FD; (b) α-CD-Cp2VCl2 PM. TGA (red),; c) β-CD-Cp2VCl2 FD; (d) β-CD-Cp2VCl2 PM. TGA (red), MDSC (blue). FD=freeze dried (EM=enclosed mixture), PM=physical mixture.
According to the DSC analysis, the melting points of the cyclodextrins (284–289° C), Cp2VCl2 (291° C) and the physical mixtures (186–206° C) are substantially higher than those of the inclusion complexes (lyophilized samples, 170–175° C). Similarly, the decomposition temperatures of the free cyclodextrins, the physical mixtures and Cp2VCl2 are higher than those of the inclusion complexes. These thermal behaviors initially suggest that the lyophilized samples are inclusion complexes due to their distinct thermal behaviors.
The TGA and DSC curves of the α-CD-Cp2VCl2 freeze dried sample (α-CD-Cp2VCl2 FD, Figure 1a, top trace) showed some features different from the free α-cyclodextrin and vanadocene dichloride as well as from the α-CD-Cp2VCl2 physical mixture (α-CD-Cp2VCl2 PM). This suggests that α-CD might be able to encapsulate Cp2VCl2. However, this is not a conclusive analysis to determine if the inclusion complex exists, as this thermal behavior could be the result of a fine dispersion rather than an inclusion complex or a mixture of inclusion complex and free vanadocene dichloride, as will be shown below with EPR spectroscopy. Likewise, the β-CD- Cp2VCl2 FD (Figure 1, bottom traces) and the γ-CD-Cp2VCl2 FD (supplementary material) exhibited thermal behaviors different to the free CD, Cp2VCl2 or CD- Cp2VCl2 physical mixtures. In this regard, for β-CD-Cp2VCl2 (β-CD- Cp2VCl2·14H2O) to be a 1:1 inclusion compound, 69.3% of the mass must belong to β-CD, while 15.4% must be Cp2VCl2. Upon analysis of Figure 1d, there is a major mass loss of 72% above 215°C, which we believe belongs to the β-CD. There is a mass loss of 7.5% between 165–230°C which could involve more tightly bound water molecules and 19.6% residue that must belong to some sort of vanadium compound. For a 2:1 β-CD-Cp2VCl2 inclusion compound, about 90% of the total must belong to β-CD. Such evidence is not observed into the TGA and DSC curves. In any event, for the β- and γ-CDs cases, EPR spectroscopy demonstrated unambiguously that they can indeed form inclusion complexes with Cp2VCl2.
In the infrared spectral data of the inclusion complexes, the weak shoulder about 3100 cm−1 attributed to Cp ν(C–H) vibration is most likely overlapped by the broad peak of OH vibrations and cannot be observed. Only a new peak at 825 cm−1 is observed in the inclusion compounds which belong to the Cp2VCl2 ν(C–H) out of plane vibration [33]. The KBr IR spectrum of Cp2VCl2 shows a peak at 820 cm−1. In any event, all the α-, β-, γ-CD- Cp2VCl2 samples exhibited this ν(C–H) vibration. Therefore, we explored other analytical techniques to determine inclusion compounds.
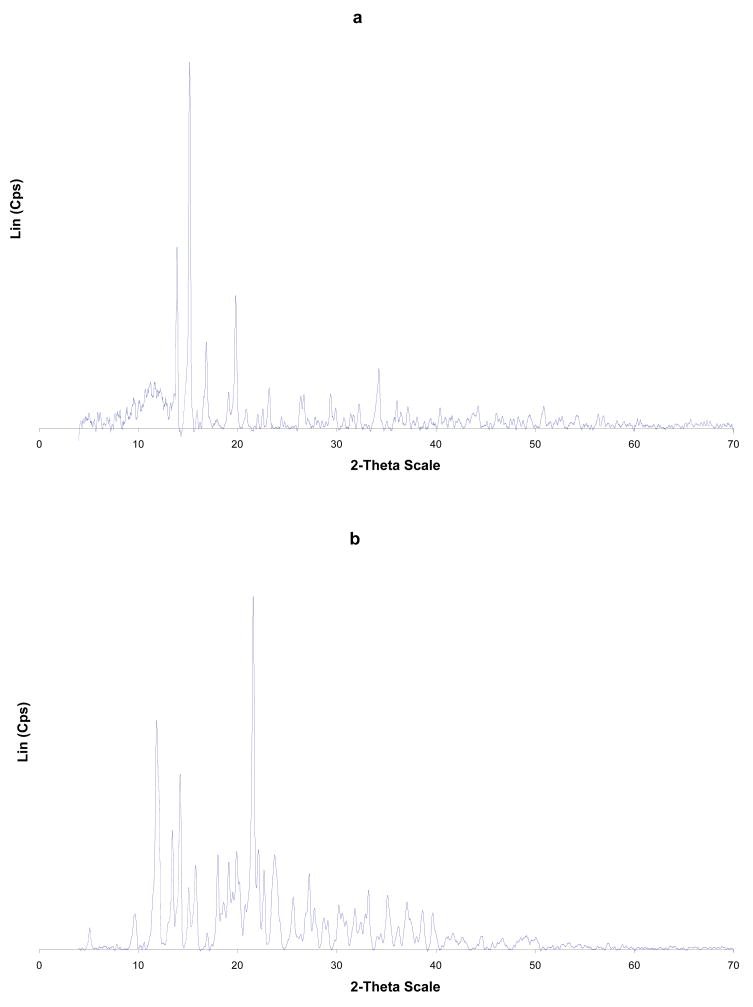
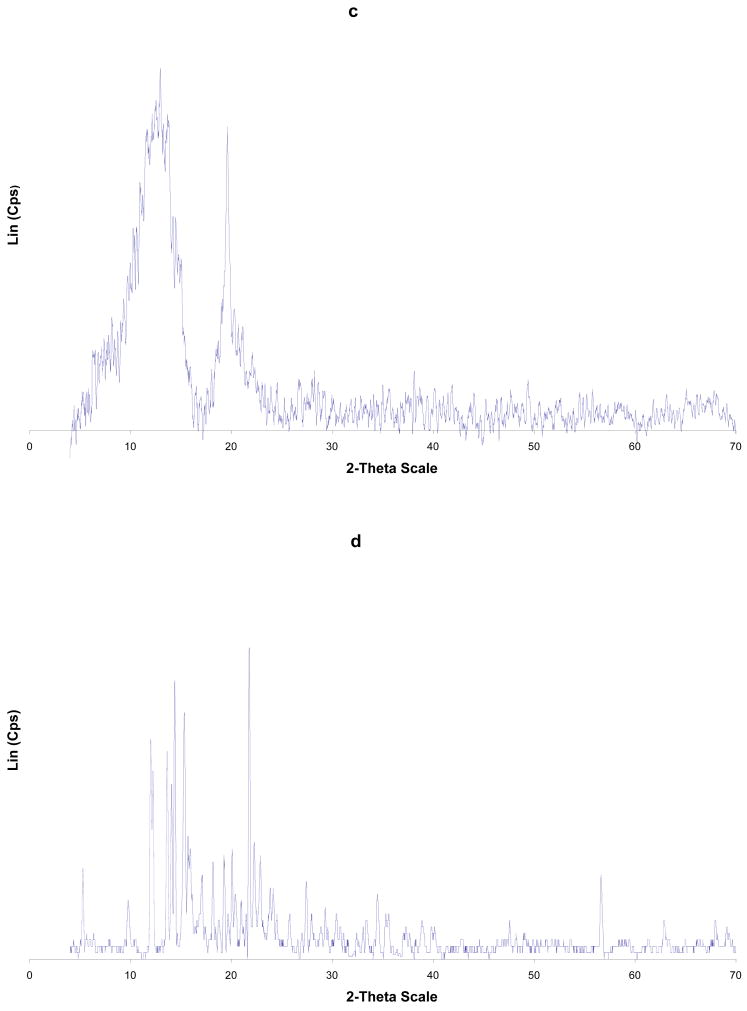
Powder x-ray powder diffraction (PXRD) analysis was undertaken on the investigated compounds. This is an importance technique to characterize inclusion complexes of cyclodextrins in the solid state. Table II shows the PXRD peaks of the hosts, guests, physical mixtures and inclusion complexes. Figures 2, 3 and Figure 2S (Supplementary Material) depict PXRD spectra of CD-vanadocene complexes.
Table II.
Powder x-ray diffraction spectral analysis of inclusion complexes (FD), physical mixtures (PM) and precursors. FD = freeze dried, PM = physical mixtures.
| Compound | Major characteristic peaks at 2θ |
|---|---|
| α-CD | 14.4 (strongest), 12.3, 21.8 (secondly), 5.4, 13.7, 15.8, 19.4, 22.9, 56.6 |
| β-CD | 4.5 (strongest), 12.6, 12.8 (second), 9.1, 10.7, 12.8, 17.2, 22.9, 27.3 |
| γ-CD | 5.1 (strongest), 16.5 (second), 4.5, 6.2, 9.2, 11.3, 12.3, 15.4, 15.9, 17.1, 17.8, 18.7, 22.5 |
| Cp2VCl2 | 14.2 (strongest), 13.8, 15.5 (secondly), 20.2 |
| αCD- Cp2VCl2 FD | 14.2 (strongest), 13.8, 15.5 (secondly), 20.2 |
| αCD- Cp2VCl2 PM | 21.8 (strongest), 14.2, 15.5 (secondly), 12.3, 13.7 |
| β-CD- Cp2VCl2 FD | 12.3 (strongest), 6.0, 11.9, 17.8, 18.9, (secondly), 7.3, 15.8, 23.9 |
| β-CD- Cp2VCl2 PM | 12.7 (strongest), 15.5, 21.2, (secondly), 4.7, 9.2, 17.9, 19.0, 19.7, 25.9 |
| γ-CD- Cp2VCl2 FD | 12.1 (strongest), 16.6 (secondly), 7.4, 11.5, 14.2, 15.7, 20.4, 21.7, 22.4, 23.5 |
| γ-CD- Cp2VCl2 PM | 13.7 (strongest), 15.5 (secondly), 5.3, 16.5, 18.9 |
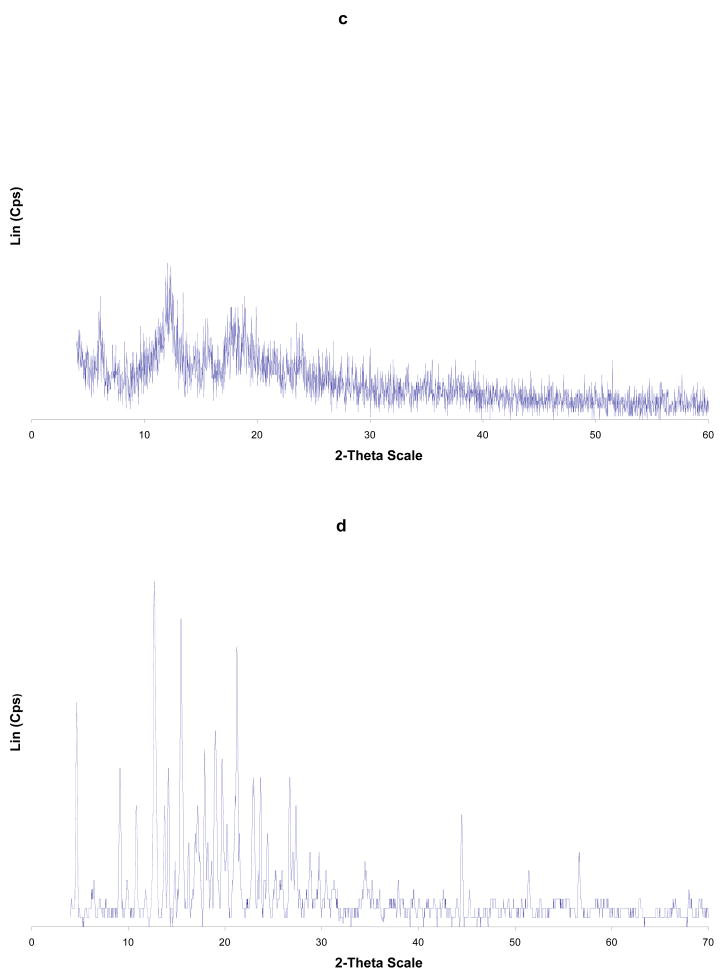
Figure 2.
PXRD of: (a) Cp2VCl2; (b) α-CD; (c) α-CD-Cp2VCl2 FD; (d) α-CD-Cp2VCl2 PM. FD = freeze dried and PM = physical mixture.
Figure 3.
PXRD of: (a) Cp2VCl2; (b) β-CD; (c) β-CD-Cp2VCl2 FD; (d) β-CD-Cp2VCl2 PM. FD = freeze dried and PM = physical mixture.
Upon analysis of Figure 2 and Table II, it is evident that the PXRD of the free α-CD, Cp2VCl2, physical mixture and freeze dried samples have some common features. In particular, the lyophilized sample has features corresponding mainly to free Cp2VCl2. This is supported by the fact that α-CD-Cp2VCl2 freeze dried sample has x- ray diffraction peaks at 2θ (13.8, 14.2, 15.5 and 20.2) corresponding to free Cp2VCl2. This evidence suggests that α-CD-Cp2VCl2, freeze dried sample is not an inclusion complex. For β- and γ-CD-Cp2VCl2 freeze dried samples, diffraction peaks corresponding to the free Cp2VCl2 and CDs are not observed. In this regard, the PXRD spectra of the β- and γ-CD- Cp2VCl2 freeze dried samples have diffraction patterns (see Figure 3 and Figure 2S Supplementary Material) completely different from their initial components and the physical mixtures. These new diffraction patterns observed in the PXRD spectra can only be explained as new, more amorphous solid materials containing the Cp2VCl2 encapsulated into their CD hydrophobic cavities.
To explore the possibility of other types of inclusion compounds with α-CD, we studied the diffraction patterns of 1:2 and 2:1 α-CD-Cp2VCl2 systems (Supplementary Material). None of these systems exhibited diffraction patterns that can be attributed to an inclusion compound.
Electron Paramagnetic Resonance (EPR) spectroscopy in the solid state has been used to elucidate the electronic and magnetic environments around the vanadium (d1) metal center and determine if Cp2VCl2 is encapsulated into the CD cavity [30]. We have pursued solution and solid state EPR experiments to determine the inclusion complexes in both states (see Tables III and IV).
Table III.
Anisotropic hyperfine coupling constants and g factors of the inclusion complexes.
| Complex | A⊥ | A|| | g⊥ | g|| |
|---|---|---|---|---|
| Cp2VCl2 | Single | asymmetric | Peak | |
| α–CD- Cp2VCl2 | Single | asymmetric | peak | |
| β-CD- Cp2VCl2 | 74.7 G | 197.1 G | 1.980 | 1.937 |
| γ-CD- Cp2VCl2 | 74.0 G | 198.8 G | 1.980 | 1.936 |
Table IV.
Isotropic hyperfine coupling constants and g factors inclusion complexes.
| Complex | Aiso | giso |
|---|---|---|
| Cp2VCl2 | 78.4 G | 1.978 |
| α–CD- Cp2VCl2 | 79.6 G (62%), 115.7 G (35%), 68 G (3%) | 1.967, 1.979, 1.980 |
| β-CD- Cp2VCl2 | 114.4 G | 1.967 |
| γ-CD- Cp2VCl2 | 115.1 G | 1.966 |
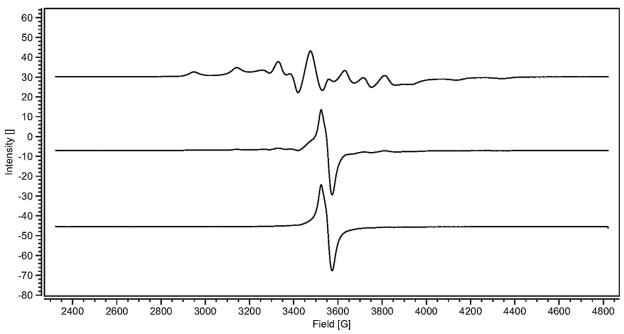
The solid state EPR spectra of the α- and β-CD-Cp2VCl2 host-guest interactions are depicted in Figures 4 and 5. Figure 4 presents the EPR spectra of the α-CD- Cp2VCl2 physical mixture, α-CD-Cp2VCl2 freeze dried sample and the subtraction of both spectra. It is evident that in the α-CD-Cp2VCl2 freeze dried sample, the major species present is free Cp2VCl2 since only a single asymmetric peak with no electron- 51 V (I = 7/2) anisotropic hyperfine coupling is observed. This spectrum has identical features to the solid state EPR spectrum of Cp2VCl2. There is a second species but its concentration is too low to determine the anisotropic hyperfine coupling accurately. Thus, this is evidence that the major component in the α-CD-Cp2VCl2 is mainly free vanadocene and the EPR spectrum (Figure 4) is mainly a dispersion mixture rather than an inclusion complex. On the hand, the β- and γ-CD- Cp2VCl2 freeze dried samples exhibited anisotropic hyperfine coupling on their EPR spectra (Table III). Similar results were obtained by Vinklarek and co-workers [30]. These results can be rationalized in terms of dilution of the paramagnetic d1 center. In this regard, magnetically diluted samples arise from the inclusion of Cp2VCl2 into the β- and γ-CD cavities. In other words, the d1 (Cp2VCl2) center has been diluted into the diamagnetic matrix, β-CD or γ-CD.
Figure 4.
Solid state EPR spectra of α-CD-Cp2VCl2. Physical mixture (top trace, green), freeze dried sample (middle trace, red) and subtraction of the top spectrum to the middle spectrum (bottom, blue).
Figure 5.
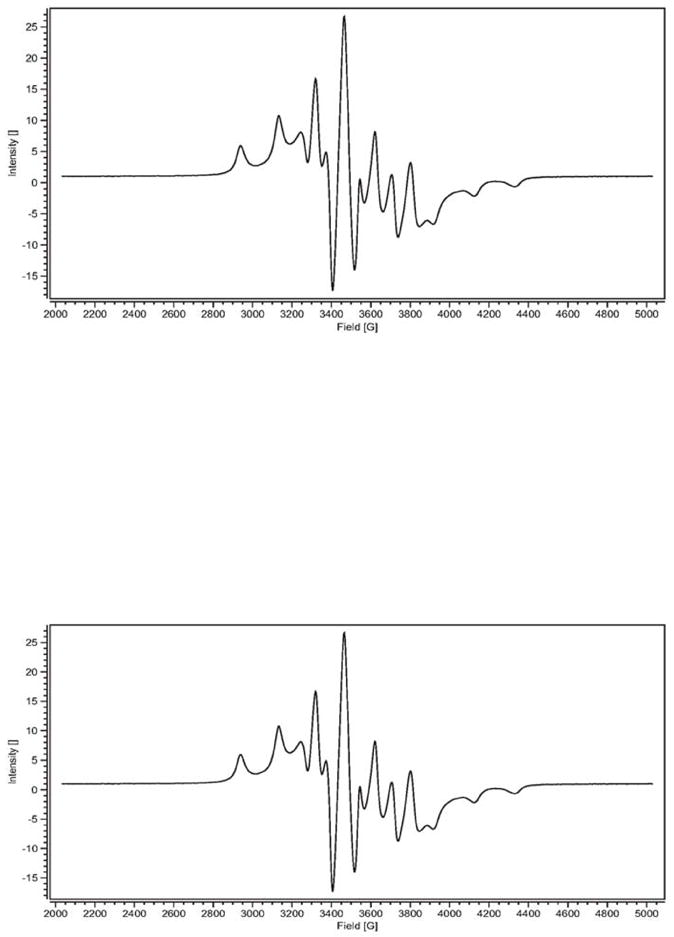
Solid state EPR spectra of β-CD-Cp2VCl2 FD (top) and γ-CD-Cp2VCl2 FD (bottom). FD = freeze dried sample.
Solution EPR spectra were recorded on the α-, β- and γ-CD-Cp2VCl2 host-guest interactions to further investigate the possible species that may exist, in solution and not in the solid state, between vanadocene species and cyclodextrins (Table IV). In aqueous solution, the EPR spectrum of the α-CD-Cp2VCl2 host-guest interaction (Figure 6) is a superposition of three paramagnetic species. The major component (62%) has an isotropic hyperfine coupling constant of 79.6 G. Based on previous report, the isotropic hyperfine coupling constant of Cp2VCl2 in aqueous solution is 75 G (there is a slight pH dependence) [31]. Our EPR spectrum of Cp2VCl2 in aqueous solution showed an isotropic hyperfine coupling constant (Aiso) of 78.4 G. Since it is known that Cp2VCl2 dissolved in water, at low pH, forms [Cp2V(H2O)2]2+ [27,31], we believe that the major species present in the α-CD-Cp2VCl2 system is free [Cp2V(H2O)2]2+. The second component (35%), has an Aiso of 115.7 G. Upon comparison with the solution EPR spectra of the β- and γ-CD-Cp2VCl2 host-guest interactions we found that these inclusion complexes have Aiso values of 114.4 G and 115.1 G respectively. Since these CDs form host-guest inclusion complexes with Cp2VCl2, we believe that some sort of host-guest interactions exist between the α-CD and Cp2VCl2, albeit weak. However, to pinpoint what type of interaction is present, we analyze the EPR spectrum of [Cp2V(MeOH)]2Cl2 in water. Surprisingly the Aiso of this complex which involves V-O (V-MeOH) coordination is 114 G. Therefore, it seems that in solution, vanadocene is being coordinated weakly by the secondary alcohols ((C-2 and C-3 OHs) on the wider edge of the cavity and that this interaction also exists in the β- and γ-CD-Cp2VCl2 host-guest interactions when the inclusion complexes are dissolved in water and the cyclodextrin releases the vanadocene species into the aqueous media. On the other hand once the water is removed from the medium (lyophilized sample), the vanadocene is enclosed into the β-CD and γ-CD but not into the smaller α-CD cavity. There is a third species (in the Cp2VCl2- α-CD solution) in about 3% with isotropic hyperfine coupling constant of 68 G. It is not clear what species is present but based on the Aiso it is possible that sort of vanadocene complex (perhaps [Cp2VCl(H2O)]+]) is present in the solution as a free species.
Figure 6.
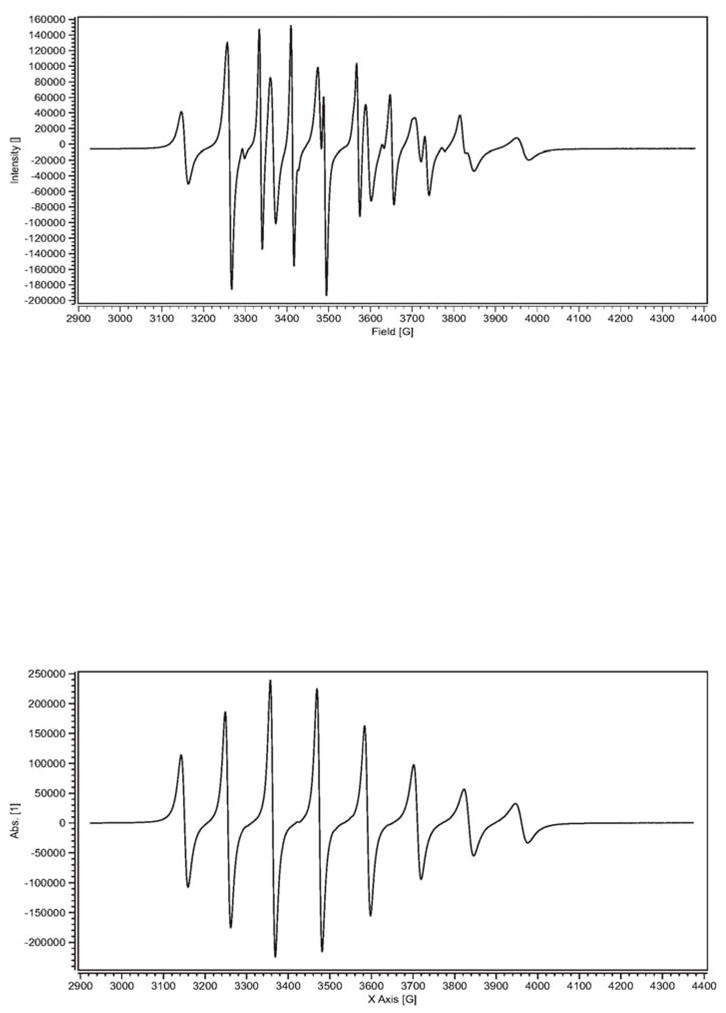
EPR spectra of α-CD-Cp2VCl2 mixture (top) and Cp2VCl2 in water (bottom).
4. Conclusion
The host-guest interactions between α-, β-, and γ-CDs and Cp2VCl2 have been characterized by a combination of solid-state physical methods and solid and solution EPR spectroscopy. The formation of a true inclusion complex in a 1:1 ratio has been detected unequivocally for β– and γ– cyclodextrins. In the solid state, α-CD does not form stable 1:1 inclusion complex. For the α-CD, solution EPR spectral data showed to have three species. Two of them should be free vanadocene species (Aiso = 68–79 G), while the third one has an Aiso = 115.7 G which suggest that some type of weak coordination between secondary alcohols of cyclodextrin and vanadocene. On the other hand, solution EPR spectroscopy showed that the vanadocene species involved in the β- and γ-CDs have only one species with high isotropic hyperfine coupling constants (Aiso 114–115 G). This should involve, must likely, some sort of intermediate species between free and encapsulated vanadocene, coordinated by the secondary alcohols analogous to α-CD. But upon removal of water, only β- and γ- CDs are able to enclose vanadocene.
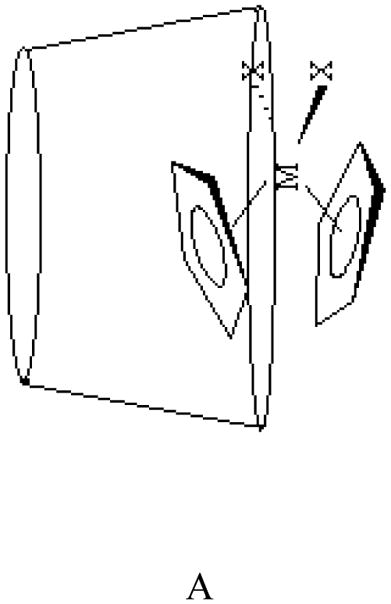
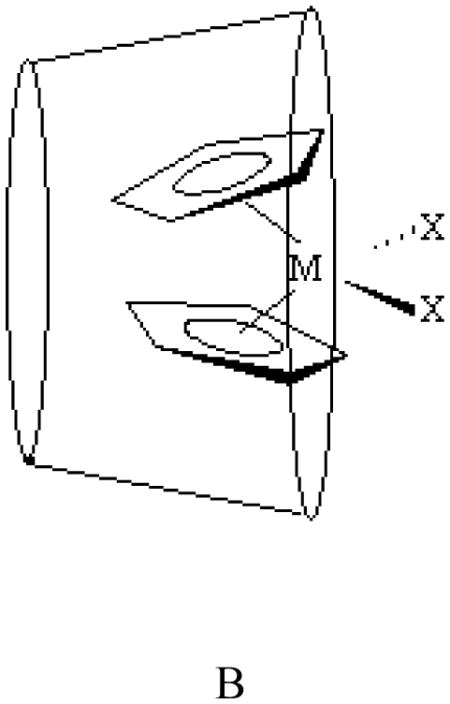
Two possible geometries for these inclusion complexes can be envisioned, A and B. According to the 13C CP MAS NMR spectral data on CD-niobocene [32] and CD-molybdenocene host-guest interactions [29], geometry A is the most likely conformation. On a recent study, Goncalves and co-workers found, using ab initio calculations, that geometry A and B as well as a third geometry where the chloride is inserted into the cavity with a shallow penetration of the Cp rings could exist at room temperature [34]. The most likely geometry of the CD-Cp2VCl2 host-guest complex will be investigated by molecular mechanics calculation.
Supplementary Material
Acknowledgments
The authors are thankful to the National Institute of Health for financing this project and NSF-MRI for providing funds for the 500 MHz NMR instrument. AM is grateful to the Sloan Foundation – National Action Council for Minorities in Engineering for the pre-doctoral fellowship.
References
- 1.Martin Del Valle EM. Process Biochemistry. 2004;39:1033. [Google Scholar]
- 2.Harada A, Takahashi J Inclusion Phenom. 1984;2:791. [Google Scholar]
- 3.Odagaki Y, Hirotu K, Higuchi T, Harada A, Takahashi S. J Chem Soc Perlin Trans I. 1990:1230. [Google Scholar]
- 4.Ferreira P, Goncalves IS, Pillinger M, Rocha J, Santos P, Teixeira-Dias JJC. Organometallics. 2000;19:1455. [Google Scholar]
- 5.Hapiot F, Tilloy S, Monflier E. Chem Rev. 2006;106:767. doi: 10.1021/cr050576c. [DOI] [PubMed] [Google Scholar]
- 6.Braga SS, Goncalves IS, Lopes AD, Pillinger M, Rocha J, Romao CC, Teixeira-Dias JJC. J Chem Soc Dalton Trans. 2000:2964. [Google Scholar]
- 7.Lima S, Goncalves IS, Ribeiro-Claro P, Pillinger M, Lopes AD, Ferreira P, Teixeira-Dias JJC. Organometallics. 2001;20:2191. [Google Scholar]
- 8.Braga S, Marques MPM, Sousa JB, Pillinger M, Teixeira-Dias JJC, Goncalves IS. J Organometal Chem. 2005;690:2905. [Google Scholar]
- 9.Köpf-Maier P. Eur J Clin Pharmacol. 1994;47:1. doi: 10.1007/BF00193472. [DOI] [PubMed] [Google Scholar]
- 10.Köpf-Maier P, Köpf H. Structure and Bonding. 1988;70:103. [Google Scholar]
- 11.Köpf-Maier P, Köpf H. Chem Rev. 1987;87:1137. [Google Scholar]
- 12.Köpf-Maier P, Köpf H. In: Metal Compounds in Cancer Therapy, Organometallic Titanium, Vanadium, Niobium, Molybdenum and Rhenium Complexes - Early Transition Metal Antitumor Drugs. Fricker SP, editor. Chapman and Hall; London: 1994. pp. 109–146. [Google Scholar]
- 13.Harding MM, Mokdsi G. Current Medicinal Chemistry. 2000;7:1289. doi: 10.2174/0929867003374066. [DOI] [PubMed] [Google Scholar]
- 14.Meléndez E. Critical Review in Oncology and Hematology. 2002;42:309. doi: 10.1016/s1040-8428(01)00224-4. [DOI] [PubMed] [Google Scholar]
- 15.Köpf H, Köpf-Maier P. Angew Chem, Int Ed Engl. 1979;18:477. doi: 10.1002/anie.197904771. [DOI] [PubMed] [Google Scholar]
- 16.Köpf-Maier P, Köpf H. Z Naturforscher. 1979;34b:805. [Google Scholar]
- 17.Köpf-Maier P, Leitener M, Voitlander R, Köpf H. Z Naturforscher. 1979;34C:1174. [Google Scholar]
- 18.Köpf-Maier P, Köpf H. Naturwissenschaften. 1980;67:415. doi: 10.1007/BF00405494. [DOI] [PubMed] [Google Scholar]
- 19.Köpf-Maier P, Hesse B, Köpf H. J Cancer Res Clin Oncol. 1980;96:43. doi: 10.1007/BF00412896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Köpf-Maier P, Leitener PM, Köpf H. J Inorg Nucl Chem. 1980;42:1789. [Google Scholar]
- 21.Kurbacher CM, Bruckner HW, Andreotti G, Kurbacher PE, Saβ JA, Krebs D. Anti-Cancer Drugs. 1995;6:697–704. doi: 10.1097/00001813-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Kurbacher CM, Mallmann P, Kurbacher JA, Saβ G, Andreotti PE, Rahmun A, Hübner H, Krebs D. Anti-Cancer Research. 1994;14:1961–1966. [PubMed] [Google Scholar]
- 23.Villena-Heisen C, Friedich M, Ertan AK, Farnhammer C, Schmidt W. Anti-Cancer Drugs. 1998;9:557–563. doi: 10.1097/00001813-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Christodoulou CV, Ferry DR, Fyfe DW, Young A, Doran J, Sheehan TMT, Eliopoulos A, Hale K, Baumgart J, Sass G, Kerr DJ. J Clin Oncol 16. 1998;8:2761. doi: 10.1200/JCO.1998.16.8.2761. [DOI] [PubMed] [Google Scholar]
- 25.Köpf-Maier P, Köpf H. In: Cancer Therapy. Fricker SP, editor. Chapman & Hall; London: 1994. pp. 109–146. [Google Scholar]
- 26.Kuo LY, Kanatzidis MG, Sabat M, Tipton AL, Marks TJ. J Am Chem Soc. 1991;113:9027. [Google Scholar]
- 27.Toney JH, Marks TJ. J Am Chem Soc. 1985;107:947. [Google Scholar]
- 28.Turel I, Demsar A, Kosmrlj T. J Inclusion Phenom Macrocyclic Chem. 1999;35:595. [Google Scholar]
- 29.Braga SS, Goncalves IS, Pillinger M, Ribeiro-Claro P, Teixeira-Dias JJC. J Organomet Chem. 2001;632:11. [Google Scholar]
- 30.Vinklarek J, Honzicek J, Holubova J. Central Eur J Chem. 2005;1:72. [Google Scholar]
- 31.Toney JH, Brock CP, Marks TJ. J Am Chem Soc. 1986;108:7263. [Google Scholar]
- 32.Morales A, Struppe J, Melendez E. J Inc Phenom Macrocycl Chem. 2007 doi: 10.1007/s00775-008-0353-z. [DOI] [Google Scholar]
- 33.a Wilkinson G, Birmingham JM. J Am Chem Soc. 1954:4281. [Google Scholar]; b Diana E, Rossetti R, Stanghellini PL, Kettle SFA. Inorg Chem. 1997;36:382. doi: 10.1021/ic9807339. [DOI] [PubMed] [Google Scholar]
- 34.Pereira CCI, Nolasco M, Braga SS, Almeida Paz FA, Ribeiro-Claro P, Pillinger M, Goncalves IS. Organometallics. 2007;26:4220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.