Abstract
Etravirine, a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI), was approved in the USA in January, 2008, with approval in Europe expected later this year. It is dosed at 200 mg (two 100 mg tablets) twice daily foll owing a meal. It is approved for treatment of HIV-1 infection in adults failing a stable antiretroviral regimen with resistance to other NNRTIs and other antiretroviral agents. Etravirine is active against HIV with single mutations in the reverse transcriptase (e.g., K103N) that confer class resistance to first-generation NNRTIs. Clinical efficacy in Phase III trials has been demonstrated for up to 48 weeks of follow-up. In these Phase III trials, rash was the only adverse event that was significantly more prevalent with etravirine than with placebo. Etravirine has a tolerability and safety profile comparable to placebo with the exception of rash. Rash was generally grade 1 or 2, was not associated with prior NNRTI-related rash, was more common in women than in men, appeared a median of 12 days after treatment initiation and resolved spontaneously with continued therapy. Etravirine is the first agent in the NNRTI class that can be used for HIV-1 virus with resistance to other NNRTIs owing to a higher genetic barrier to resistance.
Keywords: etravirine, HIV, Intelence™, non-nucleoside reverse transcriptase inhibitor, TMC125
The evolution of antiretroviral (ARV) therapy for HIV-1 over the last decade has produced combinations of drugs that can successfully suppress viral replication and preserve immune function, thus greatly prolonging survival and reducing HIV-related complications [1,2]. The updated goal of ARV therapy is to achieve and maintain HIV-1 RNA levels below 50 copies/ml for all patients [3,101]. To reach the virologic goals of therapy, the current recommendations of the International Aids Society–USA Panel for initial drug regimens include a combination of two nucleoside reverse transcriptase inhibitors (NRTIs) in addition to either a non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI) and low dose ritonavir (‘boosted PI’) [3]. Not all patients are able to maintain suppression on initial therapy either due to acquired or transmitted viral drug resistance and subsequent virologic failure, thereby necessitating a change in the treatment regimen.
The first-generation NNRTIs, efavirenz, nevirapine and delavirdine, have a low genetic barrier to resistance, with class-wide resistance developing from single mutations in the reverse transcriptase [4,5]. The K103N and Y181C mutations, responsible for much of the first-generation NNRTI resistance, often appear early after virologic breakthrough. Use of nevirapine monotherapy to prevent mother-to-child transmission of HIV during delivery demonstrated development of resistance to the NNRTI class after a single dose [6]. Overcoming this ‘Achillesheel’ of the NNRTI class would allow for sequential NNRTI-based regimens. To date, virologic failure of an original NNRTI-based regimen has limited the clinical utility of treatment with a subsequent NNRTI-containing regimen [7].
Overview of the market
Etravirine is the first new NNRTI to be successfully developed since efavirenz was released in 1998. The inflexible chemical structure of older NNRTIs prevents them from interacting effectively with the viral reverse transcriptase once certain single mutations in the drugs binding pocket develop (particularly the K103N mutation) [8]. This conformational change in the drugs' target often yields class-wide resistance to the older NNRTIs, which limits treatment options for treatment-experienced patients. Transmitted drug resistance in treatment-naive patients is also a growing concern [9]. In a multicenter study of 228 treatment-naive patients in US cities, 9.8% of patients had primary NNRTI resistance [10]. In a similar multicenter study of primary resistance in the UK, 14.2% of 2357 treatment-naive patients had resistance to at least one agent, with 8% of the study population demonstrating primary resistance to NNRTIs [11]. The authors of the UK study, however, suggest that these estimates may be artificially elevated as a result of sampling bias and the actual rates of transmitted drug resistance in the UK are likely to be similar to US rates.
The use of the older NNRTIs is also limited by drug toxicity, including neurological side effects and teratogenicity with efavirenz, and hepatotoxicity with nevirapine. All of the first-generation NNRTIs can cause a rash, although most cases are mild and resolve spontaneously on continued therapy. A small percentage of patients (1–2% with efavirenz, 4–5% with delavirdine and 7% with nevirapine) experience a severe rash necessitating drug discontinuation [102].
Newer agents are needed that circumvent these barriers to treatment. Many treatment-experienced patients also have multidrug resistant virus, which limits the options for active agents from which to build an ARV regimen [12]. The addition of a new NNRTI with an efficacy that is not abrogated by previously described NNRTI mutations would expand the options to create ARV treatment regimens that are most effective at suppressing viral replication in the face of transmitted or acquired viral drug resistance.
Etravirine
Etravirine (Intelence®, TMC125, Tibotec Pharmaceuticals, Mechelen, Belgium), a flexible diarylpyrimidine compound, is a new second-generation NNRTI (Figure 1). It is the first NNRTI to have activity against both wild-type virus and NNRTI-resistant HIV-1 [13]. Etravirine binds to the viral reverse transcriptase at a site remote from the active enzyme site, hindering the necessary movement of the enzyme's dual subdomains. Initial modeling studies of etravirine demonstrated that its specific molecular structure, including four key pivoting bonds, allows for a degree of flexibility novel to the NNRTI class [14]. As a result, the drug is able to mold itself to binding pockets altered by mutation at a low energetic cost, while maintaining important intermolecular interactions that inhibit the activity of the reverse transcriptase [8,15].
Figure 1.
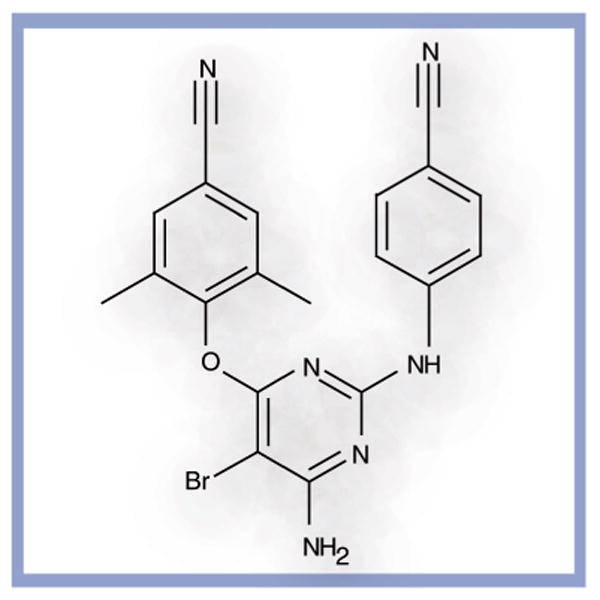
Etravirine.
Etravirine is available in 100 mg tablets. The approved dosing regimen is two 100 mg tablets twice daily following a meal. Etravirine is indicated for treatment of HIV-1 infection in treatment-experienced adults who have evidence of treatment failure and genotypic resistance to the first-generation NNRTIs and other ARV medications. A regimen consisting of etravirine plus NRTIs alone should not be used in patients who have failed an NNRTI-containing regimen. Suboptimal performance was seen in a clinical trial comparing etravirine plus two NRTIs alone with a boosted PI plus an NRTI in PI-naive patients with high levels of baseline resistance to NRTIs and NNRTIs [16].
Pharmacodynamics
Etravirine decreases viral replication by binding to the viral reverse transcriptase and impeding the virus' polymerase activity. No prolongation of the QT interval corrected for heart rate (QTc) was seen with daily oral etravirine doses at 200 mg twice daily or 400 mg once daily [17].
Pharmacokinetics & metabolism
Etravirine is administered orally following a meal, as fasting reduced the systemic exposure (AUC) by approximately 50%. Tmax was achieved 2.5–4 h after oral dosing (Table 1). Steady-state levels were reached by day 6, and mean elimination half-life was 41 h [17,18]. Despite its long half-life and pharmacokinetic potential for once-daily dosing, etravirine was dosed twice daily in Phase II trials in an effort to distribute the pill burden [18]. Etravirine absorption is unaffected by medications that increase gastric pH. A total of 99.9% of the active drug remains bound to plasma proteins, and distribution beyond the plasma compartment has not been studied in humans. Etravirine penetration into the CNS or the cerebrospinal fluid has not been studied. Plasma drug levels are unaffected by age, race or gender [19]. Etravirine has not been sufficiently studied in children and pregnant or nursing women.
Table 1. Pharmacokinetics and drug interactions of etravirine.
| Pharmacokinetic variable | Value or corresponding parameter(s) |
|---|---|
| Tmax | 2.5–4 h |
| Half-life (T1/2) | 41 h |
| Primary metabolism | Hepatic |
| Substrate of enzymes | CYP3A4, CYP2C9 and CYP2C19 |
| Enzymes induced | CYP3A4 |
| Enzymes inhibited | CYP2C9 and CYP2C19 |
| Plasma protein binding | 99.9%, with 99.6% bound to albumin |
| Dose adjustment in hepatic failure | Child–Pugh Class A and B: no dose adjustment Child–Pugh Class C: not evaluated |
| Hepatitis B and/or C coinfection | No dose adjustment |
| Renal failure | No dose adjustment <1.2% of the dose is excreted in the urine, with no secretion of active drug |
| Hemodialysis | Highly bound to plasma proteins and unlikely to be removed by dialysis |
| Pregnant women, nursing mothers and pediatric population | Not studied |
| Do not coadminister with | Tipranavir/ritonavir, fosamprenavir/ritonavir and atazanavir/ritonavir Ritonavir at full dose (600 mg twice daily) Protease inhibitors without low-dose ritonavir Other non-nucleoside reverse transcriptase inhibitors Carbamazepine, phenobarbital, phenytoin, rifampin and rifapentine Rifabutin (when combined with a boosted protease inhibitor) St John's Wort (Hypericum perforatum) |
| Coadminister with caution | Lopinavir/ritonavir Antiarrhythmics (measurement of antiarrhythmic drug levels is recommended) Systemic dexamethasone, cyclosporine, sirolimus and tacrolimus Other substrates, inhibitors, or inducers of CYP3A4, CYP2C9 or CYP2C19 |
Taken from [17].
Metabolism of the active compound occurs primarily in the liver, via the CYP3A4, CYP2C9 and CYP2C19 enzyme pathways. In cell culture, the major metabolites were over 90% less active than the active drug. Only a small percentage (<1.2% of the dose) of the drug metabolite, and no active drug, was recovered from the urine. Dose adjustment is unnecessary in renal impairment and etravirine levels are unlikely to be affected by hemodialysis due to the degree of plasma protein binding [17]. After a single oral dose, 93.7% of the administered dose was recovered from the stool, primarily in the form of the active compound. No dose adjustment is required for mild-to-moderate hepatic impairment (Child–Pugh classes A or B). Insufficient data exists for severe (Child–Pugh Class C) hepatic failure, and the drug should be used with caution in this population. The AUC of etravirine increased by 35% in hepatitis B and/or C patients compared with patients without viral hepatitis (p = 0.0028). Drug clearance was reduced by 24% in hepatitis C coinfected patients [19]. Despite these differences, no dose adjustment is recommended in hepatitis B and/or C coinfected patients without severe hepatic dysfunction.
Drug interactions
Etravirine is a substrate of CYP3A4, CYP2C9 and CYP2C19. Co-administration of other substrates, inhibitors or inducers of these enzymes may influence etravirine and other drug levels [17]. Etravirine can be co-administered with NRTIs without dose adjustments. A list of drugs with which etravirine should not be co-administered and the rationale for those recommendations, based on USA labeling information, are listed in Table 2. Etravirine should not be co-administered with other NNRTIs, unboosted PIs (‘unboosted’ – PI without low-dose ritonavir), high-dose ritonavir (600 mg twice daily), ritonavir-boosted atazanavir, ritonavir-boosted tipranavir and ritonavir-boosted fosamprenavir, carbamazepine, phenobarbitol, phenytoin, rifampin, rifapentine or St. John's Wort (Hypericum perforatum). In the event that etravirine is not co-administered with a boosted PI, then a dose of rifabutin 300 mg once daily is recommended. Rifabutin should not be co-administered in patients on etravirine and darunavir/ritonavir or saquinavir/ritonavir. No dose adjustment is required with enfuvirtide or raltegravir. Maraviroc should be dose adjusted to 600 mg twice daily when given with etravirine without a boosted PI, and 150 mg twice daily when given with a combination of etravirine and a boosted PI [20]. Co-administration of other medications may require dose adjustment or clinical monitoring.
Table 2. Etravirine drug interactions preventing safe co-administration of the indicated agent.
| Co-administered drug | Change in ETR concentration | Change in co-administered drug concentration | Other considerations |
|---|---|---|---|
| NNRTIs | |||
| Efavirenz | Decreases ETR | No clinical benefit to combining NNRTIs | |
| Nevirapine | Decreases ETR | No clinical benefit to combining NNRTIs | |
| Delavirdine | Increases ETR | No clinical benefit to combining NNRTIs | |
| Unboosted PI* | |||
| Atazanavir | Decreases atazanavir | ||
| Fosamprenavir | Increases amprenavir | ||
| Nelfinavir | Increases nelfinavir | ||
| Indinavir | Decreases indinavir | ||
| Boosted PI‡ | |||
| Atazanavir/ritonavir | Increases ETR | Decreases atazanavir | |
| Tipranavir/ritonavir | Decreases ETR | ||
| Fosamprenavir/ritonavir | Increases amprenavir | ||
| High dose ritonavir (600 mg twice daily) | Decreases ETR | ||
| Anticonvulsants | |||
| Carbamazepine | Decreases ETR | Potent CYP450 inducer | |
| Phenobarbitol | Decreases ETR | Potent CYP450 inducer | |
| Phenytoin | Decreases ETR | Potent CYP450 inducer | |
| Antimycobacterials | |||
| Rifampin | Decreases ETR | Potent CYP450 inducer | |
| Rifapentine | Decreases ETR | Potent CYP450 inducer | |
| St. John's Wort | Decreases ETR | ||
PI without addition of low dose ritonavir.
PI in combination with low dose ritonavir.
ETR: Etravirine; NNRTI: Non-nucleoside reverse transcriptase inhibitor; PI: Protease inhibitor.
Taken from [17].
Clinical efficacy
In vitro studies
Initial drug development employed a parallel screening strategy of candidate compounds from the diarylpyrimidines, testing their activity against both wild-type HIV-1 as well as single and double mutants (including K101E + K103N and K103N + Y181C) [13]. Etravirine emerged as a highly active compound for wild-type HIV-1 (EC50:1.4–4.8 nM) and its efficacy remained despite first-generation NNRTI resistance (EC50: <100 nM) in 97% of over 1000 recombinant viruses from clinical samples with resistance to first-generation NNRTIs [21].
Phase I/II studies
During Phase II studies, the original etravirine formulation (TF035) was improved to increase its bioavailability. The currently approved formulation (F060) at a dose of 200 mg twice daily correlates to a dose of 800 mg twice daily of the TF035 formulation used in Phase I/II trials [22].
An open-label, Phase IIa, proof-of-concept trial in HIV-infected patients failing an ARV regimen containing efavirenz or nevirapine (HIV-1 viral load >2000 copies/ml) substituted etravirine for the NNRTI of their failing regimen, and measured viral decay dynamics, side effects and pharmacokinetics over 7 days [18]. Seven of 15 patients (44%) had a greater than 1 log10 drop in HIV-1 RNA, with a median decline of 0.89 log10 over 7 days, despite phenotypic first-generation NNRTI resistance. The HIV-1 RNA decay rate was 0.13 log10 RNA copies/ml/day.
The second Phase IIa study, TMC125-C208, was a double-blind, placebo-controlled trial of etravirine monotherapy in 19 ARV-naive adult men with HIV-1 RNA levels of 5000–125,000 copies/ml [23]. Results from the intent-to-treat analysis (ITT) demonstrated a mean decrease of 1.99 log10 copies/ml in HIV-1 RNA in the etravirine group at the end of 7 days compared with 0.06 log10 copies/ml with placebo (p <0.001). The viral decay rate for the etravirine group was 0.33 log10 copies/ml/day compared with 0.02 copies/ml/day for placebo (p <0.001). Steady state concentrations were reached within 4 days.
The viral load decay rate of etravirine monotherapy in the TMC125-C208 trial (n = 12) was then compared retrospectively to that of a triple-class, five-drug regimen of zidovudine, lamivudine, abacavir, indinavir and nevirapine from the ERA study (n = 11) [24,25]. With the goal of a viral load decrease of 1.59 copies/ml or more in the first 7 days for a completely efficacious regimen [25], etravirine monotherapy matched the efficacy of the five-drug regimen (1.92 vs 1.76 log10 copies/ml, p = 0.77). The success of etravirine monotherapy in the first 7 days may partly be ascribed to a fourfold higher distribution in lymph nodes than in plasma, possibly allowing targeting of compartmentalized viral populations [26].
In the open-label, active control, randomized, 48-week Phase II trial TMC125-C227, regimens of two NRTIs plus one investigator-selected PI (61% lopinavir/ritonavir, 32% ATV/ritonavir, 7% unboosted PI; n = 57) were compared with two NRTIs plus etravirine (n = 59) [16]. Study patients were PI naive, had at least one NNRTI resistance mutation, and were failing first-line therapy including a first-generation NNRTI. The etravirine arm was discontinued prematurely following a review by the Drug Safety Monitoring Board because the 1.3 log10 drop in viral load seen with etravirine in the first 8 weeks was not sustained. Viral rebound correlated with baseline thymidine-associated mutations and/or M184V, as well as the number of baseline NNRTI mutations. In a multivariate analysis, the total number of baseline NRTI and/or NNRTI mutations, and not K103N or Y181C in particular, predicted virologic response. These results are the basis for the recommendation to avoid constructing a regimen solely from NRTIs plus etravirine in NNRTI-experienced patients; underlying NRTI resistance may limit the number of active agents in such a treatment regimen. The PI arm demonstrated an approximately 2 log10 drop in HIV-1 RNA at week 8, which continued to fall to near 2.5 log10 by week 16.
The open-label, triple-arm, randomized trial TMC125-C223 compared etravirine (dosed at 400 or 800 mg twice daily of the TF035 formulation) with an optimized background of two NRTIs and/or lopinavir/ritonavir and/or enfuvirtide to an active comparator arm of at least three drugs, including NRTIs or PIs and/or enfuvirtide [27]. Investigators and participants were blinded to the etravirine dose, but not to the etravirine versus control arm. The 199 patients studied had genotypic resistance to first-generation NNRTIs and at least three primary PI mutations, were randomized 2:2:1 to low dose etravirine, high dose etravirine and control groups, and analyzed by ITT. The mean decreases in HIV-1 RNA at 24 weeks for the etravirine 400 mg, etravirine 800 mg and control groups were 1.04 (p = 0.005 vs control), 1.18 (p <0.001 vs control) and 0.19 log10 copies/ml in ITT, respectively. Viral suppression (HIV-1 RNA <400 and <50 copies/ml) was also greater in the etravirine arms versus placebo (Table 3).
Table 3. Summary of Phase I/II trials.
| Phase I/II study | Sample size | Study design | Treatment arms (n) | Primary outcome | HIV RNA reduction from baseline or viral decay rate | Categorical HIV RNA change | Change in CD4 cell count (cells/μl) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Gazzard Phase IIa | n = 16 | OL SA |
|
HIV-1 RNA decay rate per day |
|
7 (44%) had a greater than 1 log10 decline in HIV-1 RNA | No significant change in 7-day study period | [18] |
| Gruzdev TMC125-C208 Phase IIa | n = 19 | DB PC DA 2:1 R |
|
|
Mean HIV-1 RNA decline:
|
|
ETR: +104 PLB: -150 (p = 0.016) |
[23] |
Viral decay rate:
|
|
|||||||
| Sankatsing | n = 23 | Retrospective DA |
|
Median plasma HIV RNA elimination rate | Median HIV-1 RNA decline:
|
Not reported | ETR: +119 ERA: +60 (p = 0.29) |
[24] |
Viral decay rate:
|
||||||||
| Nadler TMC125-C223 Phase IIb | n = 199 | OL Partially-blinded 2:2:1 R |
|
Change in HIV-1 RNA from baseline values at week 24 ITT |
Mean decline in HIV-1 RNA at week 24:
|
Proportion with HIV-1 RNA <400 c/ml: ETR 400: 30% (p = 0.018 vs control) ETR 800: 39% (p = 0.002 vs control) Control: 7.5% |
ETR 400: +47 (p = NS vs control) ETR 800: +48 |
[27] |
Proportion with decrease in HIV-1 RNA by ≥1 log10 c/ml:
|
Proportion with HIV-1 RNA <50 c/ml: ETR 400: 21.3% (p = 0.133 vs control) ETR 800: 17.7% (p = 0.218 vs control) Control: 7.5% |
(p = NS vs control) Control: +10 |
||||||
ARV: Antiretroviral; B: Blinded; BR: Background regimen; c/ml/day = HIV-1 RNA copies/milliliter of blood/day; c/ml: HIV-1 RNA copies/milliliter of blood; DA: Double arm; DB: Double blinded; DRV/r: Darunavir/ritonavir; EFV: Efavirenz; ERA: ERA study historical control arm; ETR: Etravirine; ITT: Intent-to-treat analysis; LPV/r: Lopinavir/ritonavir; NRTI: Nucleoside reverse transcriptase inhibitor; NS: Not significant; NVP: Nevirapine; OL: Open label; PC: Placebo controlled; PI: Protease inhibitor; PLB: Placebo; R: Randomized; SA: Single Arm.
Phase III studies
In Phase III trials, DUET-1 (TMC125-C206) and DUET-2 (TMC125-C216) evaluated the efficacy and safety of etravirine (200 mg twice daily of the F060 formulation) combined with darunavir/ritonavir, NRTIs and optional enfuvirtide. These double-blind, randomized, placebo-controlled trials were conducted in different geographical regions with an identical design. Randomization was 1:1, and stratified for intended enfuvirtide use in the study, previous darunavir exposure, and screening plasma HIV-1 RNA level (<30,000 or ≥30,000 copies/ml). The trials compared etravirine to placebo in treatment-experienced adult patients failing a stable treatment regimen after 8 weeks or more of therapy (HIV-1 RNA >5000 copies/ml), and with genotypic evidence of NNRTI resistance and three or more primary PI mutations. The primary ITT end point was an HIV-1 RNA level less than 50 copies/ml at 24 weeks using the US FDA time-to-loss of virologic response TLOVR algorithm. Pooled DUET demographics for the etravirine and placebo groups included median baseline HIV-1 RNA (4.8 vs 4.8 log10 copies/ml, respectively), and median CD4 cell counts (99 vs 109 cells/μl, respectively) [28]. Both study groups had a median age of 45 years, and were mostly male (etravirine 90%, placebo 89%) and Caucasian (etravirine 70%, placebo 70%).
In DUET-1, 170 out of 304 (56%) patients receiving etravirine reached a viral load of less than 50 copies/ml at 24 weeks compared with 119 out of 308 (39%) in the placebo group (p = 0.005) [29]. The etravirine group had a larger proportion of patients achieve a viral load of less than 400 copies/ml (224 out of 304 [74%] vs 158 out of 308 [51%]; p = 0.0001), a greater mean viral load decrease (2.41 vs 1.70 log10 copies/ml, p <0.0001), and a larger mean increase in CD4 cell count (89 vs 64 cells/μl, p = 0.0002). In DUET-2, 183 out of 295 (62%) patients receiving etravirine reached a viral load of less than 50 copies/ml compared with 129 out of 296 (44%) in the placebo group (p = 0.0003) [30]. Other end points were similar to DUET-1 (Table 4).
Table 4. Summary of Phase III trials.
| Phase III Study (sample size) | Proportion of patients with HIV-1 RNA <50 (copies/ml) | Proportion of patients with HIV-1 RNA <400 (copies/ml) | Mean decline in HIV-1 RNA (log10 copies/ml) | CD4 cell count increase from baseline (cells/μl) | Ref. |
|---|---|---|---|---|---|
| DUET-1 – 24 weeks | ETR: 170/304 (56%) | ETR: 224/304 (74%) | ETR: 2.41 | ETR: 89 | [29] |
| (n = 612) | PLB: 119/308 (39%) | PLB: 158/308 (51%) | PLB: 1.70 | PLB: 64 | |
| p = 0.005 | p = 0.0001 | p <0.0001 | p = 0.0002 | ||
| DUET-2 – 24 weeks | ETR: 183/295 (62%) | ETR: 221/295 (75%) | ETR: 2.34 | ETR: 78 | [30] |
| (n = 591) | PLB: 129/296 (44%) | PLB: 159/296 (54%) | PLB: 1.68 | PLB: 66 | |
| p = 0.0003 | p = 0.0001 | p <0.0001 | p = 0.3692 | ||
| Pooled DUET-1 & DUET-2 – 24 weeks | ETR: 353/599 (59%) | ETR: 443/599 (74%) | ETR: 2.37 | ETR: 81 | [31] |
| (n = 1203) | PLB: 248/604 (41%) | PLB: 317/604 (53%) | PLB: 1.69 | PLB: 64 | |
| p <0.0001 | p <0.0001 | p <0.0001 | p = 0.0022 | ||
| Pooled DUET-1 & DUET-2 – 48 weeks | ETR: 363/599 (61%) | ETR: 424/599 (72%) | ETR: 2.25 | ETR: 98 | [28] |
| (n = 1203) | PLB: 239/604 (40%) | PLB: 283/604 (47%) | PLB: 1.49 | PLB: 73 | |
| p <0.0001 | p < 0.0001 | p <0.0001 | p = 0.0006 | ||
Study design is identical for all the above Phase III studies: double blind, placebo controlled, 1:1 randomization of treatment experienced patients with first-generation non-nucleoside reverse transcriptase inhibitor resistance and at least three primary protease inhibitor mutations; randomized to ETR or PLB arms with a background regimen of darunavir/ritonavir, optimized nucleoside reverse transcriptase inhibitors, and optional enfuvirtide. Primary outcome: HIV RNA <50 copies/ml at week 24, intent-to-treat analysis. Note that the overall DUET-1 and DUET-2 primary outcomes were the week 24 analyses. Pooled and 48-week results were from subsequent analyses.
ETR: Etravirine; PLB: Placebo.
Planned, pooled analyses were performed for the DUET studies at 24 and 48 weeks [28,31] Table 4. In the 48-week analysis, 363 out of 599 etravirine-arm patients (61%) maintained an HIV-1 RNA level of less than 50 copies/ml at 48 weeks compared with 239 out of 604 placebo-arm patients (40%; p <0.0001). Mean CD4 cell count increase at 48 weeks was significantly greater for the etravirine arm compared with placebo (98 vs 73 cells/μl, p = 0.0006). The addition of enfuvirtide in enfuvirtide-naive patients and greater numbers of active ARV drugs in the background regimen contributed to achievement of this primary end point. Etravirine patients achieved the primary end point of HIV-1 RNA less than 50 copies/ml more readily than placebo patients, including 46 versus 6% of patients with no other active drugs in the regimen, 63 versus 32% with one other active drug, and 78 versus 67% with two or more active other drugs. Enfuvirtide was considered active if used de novo; darunavir was considered active if the darunavir fold change (FC) was less than 10.
Due to the recent accelerated approval of etravirine by the FDA, prolonged follow-up of patients' end points is not yet available beyond the 48-week Phase III trial observational period. Postmarketing experience with etravirine and continued monitoring of patients from Phase III trials will be critical in assessing the long-term efficacy and safety of etravirine.
Resistance
Genotype assays
In vitro studies have identified a number of mutations that emerge in the presence of etravirine, the clinical significance of which will be discussed here [32]. Specific analyses were performed to associate baseline mutations in HIV-1 reverse transcriptase to etravirine activity. All known NNRTI mutations were considered as possible etravirine-associated mutations; of the 44 possible mutations studied, 26 were included in the final analysis based on their presence as baseline mutations in five patients or more. Of those 26, a mutation was included in the final list of etravirine-associated mutations if the presence of the mutation resulted in decreased virologic response (≤75% of the response of patients free of baseline NNRTI mutations) compared with the absence of the mutation. These mutations were named etravirine resistance-associated mutations (etravirine RAMs). A total of 13 mutations were initially identified as etravirine RAMs, including V90I, A98G, L100I, K101E/P, V106I, V179D/F, Y181C/I/V and G190A/S (Table 5) [33]. No single mutation, including K103N, significantly decreased etravirine efficacy.
Table 5. Etravirine resistance-associated mutations: prevalence and impact on response.
The 17 currently recognized ETR RAMs (the four mutations identified most recently are in bold) [34]:
V90I, A98G, L100I, K101E, K101H, K101P, V106I, E138A, V179D, V179F, V179T, Y181C, Y181I, Y181V, G190A, G190S, M230L
Weighted Score Categories:
Score 3.0: Y181I, Y181V
Score 2.5: K101P, L100I, Y181C, M230L
Score 1.5: E138A, V106I, G190S, V179F
Score 1.0: V90I, V179D, K101E, K101H, A98G, V179T, G190A
| Study characteristic | Number of baseline ETR RAMs: | Ref. | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Patients with <50 copies/ml of HIV-1 RNA at 24 weeks | ETR: 121/161 | ETR: 73/121 | ETR: 37/64 | ETR: 13/32 | ETR: 7/28 | [33] |
| (75%) | (60%) | (58%) | (41%) | (25%) | ||
| PLB: 64/147 | PLB: 59/157 | PLB: 17/68 | PLB: 6/24 | PLB: 3/18 | ||
| (44%) | (38%) | (25%) | (25%) | (17%) | ||
| Prevalence of baseline ETR RAMs in a clinical cohort of 1470 patients from January 1999 to May 2007* | 63.1 | 22.2 | 10.1 | 4.0 | 0.6 | [35] |
Of the patients without baseline ETR RAMs, 38.1% also had no non-nucleoside reverse transcriptase inhibitor (NNRTI) RAMs per the International AIDS Society–USA mutation list. The proportion of patients without baseline ETR RAMs may be falsely elevated if an absence of NNRTI selection pressure at the time of sampling led to a failure to identify archived resistance mutations.
ETR: Etravirine; PLB: Placebo; RAM: Resistance-associated mutation.
More recently, further statistical analysis of the pooled 24-week DUET data expanded the original list of 13 etravirine RAMs to 17 with the addition of K101H, E138A, V179T and M23L (Table 5). Additional NNRTI RAMs were also identified, increasing their number from 44 to 57 mutations (box 1). A weighted scoring system for etravirine RAMs was subsequently developed, based on phenotype assays (etravirine FC) and decreased virologic response in DUET patients at week 24 who had experienced virologic failure [34]. This improved scoring system for etravirine RAMs allows for more reliable prediction of sustained virologic responses up to 24 weeks in patients with baseline etravirine RAMs who are being considered for treatment with a regimen containing etravirine. Weighted score ranges of 0–2, 2.5–3.5 and 4 or more correlated to response rates of 74, 52 and 38%, respectively. The mutations with the highest weights were Y181I and Y181V, followed by L100I, K101P, Y181C and M230L (Table 5). In general, the mutations with the highest weights tended to be the least prevalent in the study population. The total number of baseline etravirine RAMs and increasing mutation weights were inversely proportional to the subsequent virologic response.
Box 1. NNRTI resistance-associated mutations list
The 57 currently identified NNRTI RAMs [34]:
V90I, A98G/S, L100I, K101E/H/N/P/Q/R, K103H/N/R/S/T, V106A/I/M, V108I, E138A/G/K/Q, V179A/D/E/F/G/I/T, Y181C/F/I/V, Y188C/F/H/L, V189I, G190A/C/E/Q/R/S, H221Y, P225H, F227C/L, M230I/L, P236L, K238N/T, Y318F, N348I/T
NNRTI: Non-nucleoside reverse transcriptase inhibitor; RAM: Resistance-associated mutation.
In a retrospective analysis from a different population than the DUET trials, 1470 genotypes from clinical HIV-1 isolates from NNRTI-experienced patients demonstrated a low overall prevalence of etravirine RAMs [35]. Of the mutations that had most significantly affected response to etravirine in the DUET trials, Y181C was present in 17.5% of the isolates, G190S in less than 2.5%, and V179F was not found in any isolates. The most frequent mutations were Y181C (17.5%) and G190A (15.3%). Only 68 out of 1470 patients (4.6%) had three or more etravirine RAMs, whereas 63.1% of patients had none, although these data included patients who may have archived resistance which was not identified by genotyping at the time (38.3% had no NNRTI RAMs despite prior NNRTI exposure and HIV-1 RNA >1000 copies/ml) (Table 5). Patients with prior nevirapine exposure had significantly more etravirine RAMs (0.66 ± 0.92 vs 0.43 ± 0.78; p < 0.001) and a higher prevalence of the Y181C mutation (23.7 vs 8.7%; p < 0.001) than those who had used efavirenz. It should be noted, however, that in clinical settings where the access to or frequency of laboratory monitoring is limited, delayed diagnosis of virologic failure and the resultant prolongation of exposure to a failing regimen may increase the proportion of patients with larger numbers of etravirine RAMs in those populations.
Phenotype assays
Three sets of analyses have been performed to determine phenotypic etravirine cutoffs using two phenotype assays and one phenotypic interpretation of a genotype assay. In general, the goal is to determine a lower clinical cutoff below which a full clinical response would be expected, and an upper cutoff above which little ARV activity of a drug would be expected against the isolate.
Data from four Phase IIb studies (TMC125-C203, -C209, -C223 and -C227) and the Phase III DUET trials were used to define clinical cutoffs for etravirine based on predicted phenotypes derived from genotypic analysis (‘virtual phenotyping’, virco®TYPE HIV-1 assay, Virco, Mechelen, Belgium) [36]. A lower clinical cutoff of 1.6 FC and a higher clinical cutoff of 27.6 FC were defined. Using these cutoffs, the virologic response to etravirine at week 24 was 55% in the subjects with etravirine FC of less than 1.6 and 26% for those with an FC greater than 27.6. To evaluate the clinical cutoffs from the Virco phenotype assay, pooled DUET data from the 599 patients who received etravirine was analyzed by analysis of covariance (ANCOVA) and data mining (Antivirogram® assay, Virco, Mechelen, Belgium) [37]. The subset of patients studied included those not receiving enfuvirtide de novo and excluded patients who discontinued the study for reasons other than virologic failure. The highest rate of virologic response (71%) was seen for patients with a baseline etravirine FC of less than three, which was determined to be the lower clinical cutoff threshold. Of all DUET patients, 779 out of 1190 (66%) had a baseline FC for etravirine of three or less. FC values between three and 13 were associated with a 50% virologic response at week 24. There were few patients with an FC greater than 13 (n = 60), and despite their baseline FC, 22 out of 60 (37%) had an HIV-1 RNA level of less than 50 copies/ml at week 24. No upper clinical cutoff could be defined due to the small numbers of patients with baseline FC values greater than 13, and the lack of a threshold above which etravirine conferred no benefit in the data set studied.
A third analysis of clinical cutoffs using the Monogram phenotype assay was performed on 199 samples from DUET-1 and DUET-2 patients who were not receiving enfuvirtide (PhenoSense® assay, Monogam, San Francisco, CA, USA) [38]. Baseline etravirine FC was compared with clinical data at weeks 2, 4, 8 and 24. The biologic cutoff was defined as the FC value that determined the 99th percentile for a viral population lacking NNRTI-, NRTI- or PI-selected resistance mutations. The biologic cutoff was found to be a FC of 2.9, and the lower clinical cutoff was also a FC of 2.9. Again, owing to limited numbers of high etravirine FC isolates, an upper clinical cutoff could not be determined for this study population. With increasing clinical experience with etravirine, more data can be collected to continue to refine the upper and lower clinical cutoff values for etravirine.
Selection of NNRTI RAMs
In vitro studies suggest that etravirine selects for L100I, V179F/I, Y181C, G190E, M230L and Y318F, which may contribute to etravirine resistance (FC >10). Other in vitro mutations of unknown clinical significance include V189I, E194G, L234I and T386A [21].
Postmarketing surveillance
There is currently no postmarketing data on etravirine due to its recent approval in the USA and its pending approval in Europe.
Safety & tolerability
Primary (24 week) ITT analysis of the pooled data from the DUET Phase III trials comparing the safety and tolerability of etravirine against placebo found that the most common adverse effects were rash, gastrointestinal symptoms, infections, diarrhea and nausea [39]. Only rash was significantly more prevalent in the treatment group (etravirine arm n = 599, 17% rash; placebo group n = 604, 9% rash; p = 0.0002). Rash in patients on etravirine was usually grade 1 or 2, appeared a median of 12 days after starting treatment, and resolved with continued therapy in most cases. Rash was more common in women (28%) than in men (16%). Prior rash from first-generation NNRTIs was not associated with etravirine-related rash [40]. There were no clinically significant changes in laboratory values or electrocardiogram patterns associated with etravirine. With the exception of rash, the safety and tolerability of etravirine was generally comparable to placebo.
Regulatory affairs
Etravirine was granted accelerated approval by the FDA on January 18, 2008 [103]. Approval by the European Medicines Agency is expected in the third quarter of 2008, but etravirine is currently available through expanded access programs.
Cost
The average wholesale cost of etravirine is currently US$6.54 per tablet. At a dose of two tablets twice daily, the annual wholesale cost is US$9417.60. These are USA costs, and costs in other regions may vary [41]. In comparison, the average annual wholesale cost in the USA for efavirenz and nevirapine are US$6232.80 and US$5796.00, respectively [42].
Conclusion
The NNRTI class of ARV drugs is a valuable tool in the effort to meet the goals of virological suppression. Toxicity and early development of class-wide resistance to older NNRTIs limits the use of these agents in treatment-experienced patients. Etravirine is a newer generation NNRTI with proven efficacy against HIV-1, which has developed resistance to earlier NNRTIs, including mutants with single K103N and Y181C mutations. The conformational versatility of etravirine results in a high genetic barrier to resistance. However, significant decreases in etravirine activity are seen with three or more of the 17 described etravirine RAMs when combined with NRTIs, boosted darunavir and optional enfuvirtide. Etravirine has demonstrated safety and tolerability comparable to placebo, with the exception of rash, with up to 48 weeks of follow-up. As in the first-generation NNRTIs, rash was present in a significantly larger proportion of patients than in the placebo groups, although rash was usually grade 1 or 2 and prior NNRTI rash was not a predisposing factor. Etravirine had a favorable neuropsychiatric and hepatic side-effect profile compared with efavirenz and nevirapine, respectively. Etravirine is Pregnancy Class B, and the efficacy and safety of etravirine in pregnant women and children has not been adequately studied to recommend its use in these populations. The availability of etravirine expands the options for treatment-experienced patients with HIV-1 and resistance to first-generation NNRTIs.
Executive summary.
Mechanism of action
Etravirine is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI).
Etravirine is a noncompetitive inhibitor, binding proximal to the active site of the viral reverse transcriptase (RT) and causing a conformational change in the viral RT.
Four mobile intramolecular bonds allow maintenance of binding through conformational changes despite single mutations in the RT that yield resistance to first-generation NNRTIs.
Pharmacokinetics
Food enhances the bioavailability of etravirine; dosing following a meal is recommended.
The half-life of etravirine is 41 h.
Etravirine is primarily metabolized by the liver, via CYP3A4, CYP2C9 and CYP2C19.
Less than 1.2% of the dose is excreted as inactive metabolite by the kidney.
99.9% is bound to plasma proteins.
Clinical efficacy
Effective against wild-type HIV-1 and HIV-1 resistant to first-generation NNRTIs due to single point mutations.
Rapid viral decay rate.
Etravirine maintained virologic suppression for 48 weeks when combined with darunavir/ritonavir, optimized NRTIs and optional enfuvirtide.
Etravirine significantly increased CD4 cell counts compared with placebo at week 48 when combined with darunavir/ritonavir, optimized NRTIs and optional enfuvirtide.
Effective in many treatment-experienced patients with multidrug resistance.
Safety & tolerability
Comparable to placebo for most adverse events, including neurologic and hepatic dysfunction.
The most common adverse event was rash, which was significantly more prevalent with etravirine than with placebo.
Rashes were mild-to-moderate, generally appeared within 2 weeks of treatment, and most often resolved with continued therapy. No cases of Stevens-Johnson syndrome were seen in DUET trials. Rash was more common in women than in men, although both genders had similar rash severity and rates of rash-related discontinuation of etravirine.
Prior NNRTI rash was not associated with an increased risk of rash from etravirine.
Resistance
Etravirine has a high genetic barrier to resistance, with no single mutation leading to etravirine resistance.
Efficacy is inversely proportional to the number of etravirine resistance-associated mutations (RAMs) present on the RT, as well as the weighted score of these etravirine RAMs.
Mutations most commonly associated with significant etravirine resistance include Y181I, Y181V, K101P and M230L, which were rarely present in clinical isolates.
Etravirine RAMs include V90I, A98G, L100I, K101E/H/P, V106I, E138A, V179D/F/T, Y181C/I/V, G190A/S and M230L.
Drug interactions
Co-administration with substrates, inhibitors, or inducers of CYP3A4, CYP2C9 and CYP2C19 may influence etravirine and other drug levels, affecting virologic response or the risk of adverse events.
Dosage & administration
Etravirine is administered as two 100 mg tablets twice daily with food.
Etravirine should be combined with other classes of antiretroviral drugs.
• Regimens containing etravirine and NRTIs alone should be avoided.
Future perspective
The approval of etravirine revives the option of employing the NNRTI class to attain virological suppression in treatment-experienced patients with first-generation NNRTI resistance. Future studies of combination regimens with other new agents and new drug classes (e.g., maraviroc, raltegravir, and so on) may identify powerful ARV combinations that exceed the activity of the more well-studied ARV regimens used today. More experience with etravirine and other newer agents may also identify toxicities or other limitations to their use that are unknown as yet. As the likelihood of transmitted drug resistance in newly HIV-infected patients rises, further experience with these newer agents may support their use in initial drug regimens in the future. Continued evaluation of the efficacy of etravirine in treatment-naive patients, pregnant women and children may support its use in these populations in the future.
Acknowledgments
Minuto and Haubrich are investigators in ongoing Tibotec studies. On behalf of Haubrich, the University of California receives research grants from Tibotec. The authors would like to acknowledge the financial support from the following grants and institutions: NIH: K24 AI064086; UCSD Center for AIDS Research (CFAR): AI36214; UCSD AIDS Clinical Trials Group unit: AI69432; and The California HIV/AIDS Research Program: CH05-SD-607.
Footnotes
Financial & competing interests disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Joshua J Minuto, University of California, San Diego, Division of Infectious Diseases, Antiviral Research Center, 150 Washington Street, Suite 100, San Diego, CA 92103, USA, Tel.: +1 619 543 8080; Fax: +1 619 298 0177; jminuto@ucsd.edu.
Richard Haubrich, University of California, San Diego, Division of Infectious Diseases, Antiviral Research Center, 150 Washington Street, Suite 100, San Diego, CA 92103, USA.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Tassie JM, Grabar S, Lancar R, Deloumeaux J, Bentata M, Costagliola D. Time to AIDS from 1992 to 1999 in HIV-1 infected subjects with known dates of infection. J Acquir Immune Defic Syndr. 2002;30(1):81–87. doi: 10.1097/00042560-200205010-00011. [DOI] [PubMed] [Google Scholar]; • Description of the changing face of HIV/AIDS care in the era of combination antiretroviral therapy with the goal of virologic suppression.
- 2.Schneider MF, Gange SJ, Williams CM, et al. Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984–2004. AIDS. 2005;19:2009–2018. doi: 10.1097/01.aids.0000189864.90053.22. [DOI] [PubMed] [Google Scholar]
- 3.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 Recommendations of the International AIDS Society–USA Panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 4.Wainberg MA. HIV resistance to nevirapine and other non-nucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr. 2003;34:S2–S7. doi: 10.1097/00126334-200309011-00002. [DOI] [PubMed] [Google Scholar]
- 5.Deeks SJ. Nonnucleoside reverse transcriptase inhibitor resistance. J Acquir Immune Defic Syndr. 2001;26:S25–S33. doi: 10.1097/00042560-200103011-00004. [DOI] [PubMed] [Google Scholar]; • Highlights the mechanism of HIV-1 resistance to the first-generation nonnucleoside reverse transcriptase inhibitors (NNRTIs) and the benefits and limitations of treatment regimens incorporating the older NNRTIs.
- 6.Cunningham CK, Chaix ML, Rekacewicz C, et al. Development of resistance mutations in women receiving standard antiretroviral therapy who received intrapartum nevirapine to prevent perinatal human immunodeficiency virus type 1 transmission: a substudy of pediatric AIDS clinical trials group protocol 316. J Infect Dis. 2002;186(2):181–188. doi: 10.1086/341300. [DOI] [PubMed] [Google Scholar]
- 7.Basavapathruni A, Vingerhoets J, De Bethune M, et al. Modulation of human immunodeficiency virus type 1 synergistic inhibition by reverse transcriptase mutations. Biochemistry. 2006;45:7334–7340. doi: 10.1021/bi052362v. [DOI] [PubMed] [Google Scholar]
- 8.Pauwels R. New non-nucleoside reverse transcriptase inhibitors (NNRTIs) in development for the treatment of HIV infections. Curr Opin Pharmacol. 2004;4(5):437–446. doi: 10.1016/j.coph.2004.07.005. [DOI] [PubMed] [Google Scholar]; • Thorough review of the drug development history of the new generation NNRTIs.
- 9.Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347(6):385–394. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- 10.Huang H, Daar ES, Sax PE, et al. The prevalence of transmitted antiretroviral drug resistance in treatment-naive patients and factors influencing first-line treatment regimen selection. HIV Medicine. 2008;9:285–293. doi: 10.1111/j.1468-1293.2008.00561.x. [DOI] [PubMed] [Google Scholar]
- 11.Cane P, Chrystie I, Dunn D, et al. Time trends in primary resistance to HIV drugs in the United Kingdom: multicentre observational study. BMJ. 2005;331:1368. doi: 10.1136/bmj.38665.534595.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richman DD, Morton SC, Wrin T, et al. The prevalence of antiretroviral resistance in the United States. AIDS. 2004;18:1393–1401. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]; • In a study of HIV infection in 1797 patients on antiretroviral therapy from 1996–1998 viremia was present in 63%, and drug resistance to at least one agent was found in 76%. This large study of the prevalence of drug resistance in large urban centers in the USA highlights the need for continuing development of novel antiretrovirals.
- 13.Andries K, Azijn H, Thielemans T, et al. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2004;48(12):4680–4686. doi: 10.1128/AAC.48.12.4680-4686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udier-Blagovic M, Tirado-Rives J, Jorgensen WL. Validation of a model for the complex of HIV-1 reverse transcriptase with nonnucleoside inhibitor TMC125. J Am Chem Soc. 2003;125:6016–6017. doi: 10.1021/ja034308c. [DOI] [PubMed] [Google Scholar]
- 15.Das K, Clark AD, Jr, Lewi PJ, et al. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J Med Chem. 2004;47(10):2550–2560. doi: 10.1021/jm030558s. [DOI] [PubMed] [Google Scholar]
- 16.Woodfall B, Vingerhoets J, Peeters M, et al. Impact of NNRTI and NRTI resistance on the response to the regimen of TMC125 plus two NRTIs in Study TMC125-C227. Proceedings of the 8th International Congress on Drug Therapy in HIV Infection; Glasgow, Scotland. 12–16 November 2006; Abstract PL5.6. [Google Scholar]
- 17.Intelence™ (etravirine) tablets, package insert. Tibotec; Raritan, NJ, USA: 2008. [Google Scholar]
- 18.Gazzard BG, Pozniak AL, Rosenbaum W, et al. An open-label assessment of TMC125 – a new, next-generation NNRTI, for 7 days in HIV-1 infected individuals with NNRTI resistance. AIDS. 2003;17:49–54. doi: 10.1097/00002030-200312050-00001. [DOI] [PubMed] [Google Scholar]
- 19.Kakuda TN, Scholler-Gyure M, Peeters M, et al. Pharmacokinetics of etravirine are not affected by sex, age, race, use of enfuvirtide or treatment duration in HIV-1 infected patients. Presented at: 9th International Workshop on Clinical Pharmacology of HIV Therapy; New Orleans, Louisiana, USA. 7–9 April 2008; Abstract O16. [Google Scholar]
- 20.Davis J, Scholler-Gyure M, Kakuda TN, et al. An open, randomized, two-period, crossover study in two cohorts to investigate the effect of steady-state TMC125 (etravirine) and the combination of TMC125/darunavir/ritonavir on the steady-state pharmacokinetics of oral maraviroc in healthy subjects. Presented at: 11th European AIDS Conference; Madrid, Spain. 24–27 October 2007; Abstract P4.3/02. [Google Scholar]
- 21.Vingerhoets J, Azijn H, Fransen E, et al. TMC125 displays a high genetic barrier to the development of resistance: evidence from in vitro selection experiments. J Virol. 2005;79(20):12773–12782. doi: 10.1128/JVI.79.20.12773-12782.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholler M, Hoetelmans R, Beets G, et al. Substantial improvement of oral bioavailability of TMC125 using new tablet formulations in healthy volunteers. Presented at: 6th International Workshop on Clinical Pharmacology of HIV Therapy; Quebec, Canada. 28–30 April 2005; Abstract 82. [Google Scholar]
- 23.Gruzdev B, Rakhmanova A, Doubovskaya E, et al. A randomized, double-blind, placebo-controlled trial of TMC125 as 7-day monotherapy in antiretroviral naive, HIV-1 infected subjects. AIDS. 2003;17:2487–2494. doi: 10.1097/00002030-200311210-00011. [DOI] [PubMed] [Google Scholar]
- 24.Sankatsing SUC, Weverling GJ, Peeters M, et al. TMC125 exerts similar initial antiviral potency as a five-drug, triple class antiretroviral regimen. AIDS. 2003;17:2623–2627. doi: 10.1097/00002030-200312050-00009. [DOI] [PubMed] [Google Scholar]
- 25.Weverling GJ, Lange JM, Jurriaans S, et al. Alternative multidrug regimen provides improved suppression of HIV-1 replication over triple therapy. AIDS. 1998;12:F177–F122. doi: 10.1097/00002030-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Piscitelli S, Baede P, DeGier K, Van T, Klooster G, Graham N. Pharmacokinetics of TMC 125 in HIV infected patients and healthy volunteers. Presented at: 3rd International Workshop on Clinical Pharmacology of HIV Therapy; Washington, DC, USA. 11–13 April 2002; Abstract 6.1. [Google Scholar]
- 27.Nadler JP, Berger DS, Blick G, et al. (TMC125-C223 Writing Group): efficacy and safety of etravirine (TMC125) in patients with highly resistant HIV-1: primary 24-week analysis. AIDS. 2007;21(6):F1–F10. doi: 10.1097/QAD.0b013e32805e8776. [DOI] [PubMed] [Google Scholar]
- 28.Haubrich R, Cahn P, Grinsztejn B, et al. DUET-1: week 48 results of a Phase III randomized double-blind trial to evaluate the efficacy and safety of etravirine (ETR; TMC125) versus placebo in 612 treatment-experienced HIV-1 patients. Presented at: 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 3–6 February 2008; Abstract 790. [Google Scholar]
- 29.Madruga JV, Cahn P, Grinsztejm B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1 infected patients in DUET-1: 24-week results from a randomized, double-blind, placebo-controlled trial. Lancet. 2007;370(9581):29–38. doi: 10.1016/S0140-6736(07)61047-2. [DOI] [PubMed] [Google Scholar]; •• Large international, multicenter, Phase III trial demonstrating the efficacy and safety of etravirine at 24 weeks.
- 30.Lazzarin A, Campbell T, Clotet B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1 infected patients in DUET-2: 24-week results from a randomized, double-blind, placebo-controlled trial. Lancet. 2007;370(9581):39–48. doi: 10.1016/S0140-6736(07)61048-4. [DOI] [PubMed] [Google Scholar]; •• Large international, multicenter, Phase III trial demonstrating the efficacy and safety of etravirine at 24 weeks.
- 31.Hicks C, Cahn P, Leider J, et al. Pooled 24-week results of DUET-1 and -2: efficacy of TMC125 in treatment-experienced patients. Presented at: 45th Meeting of the Infectious Diseases Society of America; San Diego, CA, USA. 4–7 October 2007; Abstract 1207. [Google Scholar]
- 32.Geretti AM. Shifting paradigms: the resistance profile of etravirine. J Antimicrob Chemother. 2008;62:643–647. doi: 10.1093/jac/dkn248. [DOI] [PubMed] [Google Scholar]
- 33.Vingerhoets J, Buelens A, Peeters M, et al. Impact of baseline NNRTI mutations on the virologic response to TMC125 in the phase III clinical trials DUET-1 and DUET-2. Presented at: 16th International HIV Drug Resistance Workshop; Barbados. 2007. abstract 32. [Google Scholar]
- 34.Vingerhoets J, Peeters M, Azijn H, et al. An update of the list of NNRTI mutations associated with decreased virologic response to etravirine (ETR): multivariate analyses on the pooled DUET-1 and DUET-2 clinical trial data. Presented at: 17th International HIV Drug Resistance Workshop; Sitges, Spain. 10–14 June 2008; Abstract 24. [Google Scholar]
- 35.Poveda E, Garrido C, De Mendoza C, et al. Prevalence of etravirine (TMC125) resistance mutations in HIV-infected patients with prior experience of non-nucleoside reverse transcriptase inhibitors. J Antimicrob Chemother. 2007;60(6):1409–1410. doi: 10.1093/jac/dkm372. [DOI] [PubMed] [Google Scholar]
- 36.Winters B, Villacian J, Van Craenenbroeck E, Bacheler L, Vingerhoets J, Peeters M. Development of Virco® type HIV-1 resistance analysis, including clinical cutoffs for TMC125 (Etravirine, ETR), a new NNRTI. Presented at: 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 3–6 February 2008; Abstract 873. [Google Scholar]
- 37.Peeters M, Nijs S, Vingerhoets J, et al. Determination of phenotypic clinical cut-offs for etravirine (ETR): pooled week 24 results of the DUET-1 and DUET-2 trials. Presented at: 17th International HIV Drug Resistance Workshop; Sitges, Spain. 10–14 June 2008; Abstract 121. [Google Scholar]
- 38.Coakley E, Chappey C, Benhamida J, et al. Biological and clinical cutoff analyses for ETR in the PhenoSense HIV Assay. Presented at: 17th International HIV Drug Resistance Workshop; Sitges, Spain. 10–14 June 2008; Abstract 122. [Google Scholar]
- 39.Haubrich R, Schechter M, Walmsley S, Peeters M, Janssens M, De Smedt G. TMC125 safety and tolerability in treatment-experienced, HIV infected patients – pooled DUET trial data. Presented at: 45th Meeting of the Infectious Diseases Society of America; San Diego, California, USA. 4–7 October 2007; Abstract 1210. [Google Scholar]
- 40.Di Perri G, Girard PM, Clumeck N, Peeters M, Janssens M, De Smedt G. Pooled 24-week results of DUET-1 and DUET-2: TMC125 (etravirine;ETR) safety and tolerability in treatment-experienced, HIV-1 infected patients. Presented at: 11th European AIDS Conference; Madrid, Spain. 24–27 October 2007; Abstract P7.3/I2. [Google Scholar]
- 41.Red Book. Pharmacy's Fundamental Reference, April 2008 Update. Vol. 511. Thompson Healthcare; Montvale, NJ, USA: 2008. [Google Scholar]
- 42.Bartlett JG, Gallant JE. 2007 Medical Management of HIV Infection. Vol. 193. Johns Hopkins Medicine/Health Publishing Business Grou.; Baltimore, MD, USA: 2007. p. 272. [Google Scholar]
Websites
- 101.British HIV Association recommendations. [November 17 2008]; www.bhiva.org/files/file1030835.pdf.
- 102.Panel on Antiretroviral Guidelines for Adults and Adolescents. U.S. Department of Health and Human Services; Jan 17, 2008. [November 17 2008]. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents; pp. 1–128. http://aidsinfo.nih.gov/contentfiles/AA_Tables.pdf. [Google Scholar]
- 103.US Food and Drug Administration. [November 17 2008]; http://www.fda.gov/cder/rdmt/InternetNDA08.htm.


