Abstract
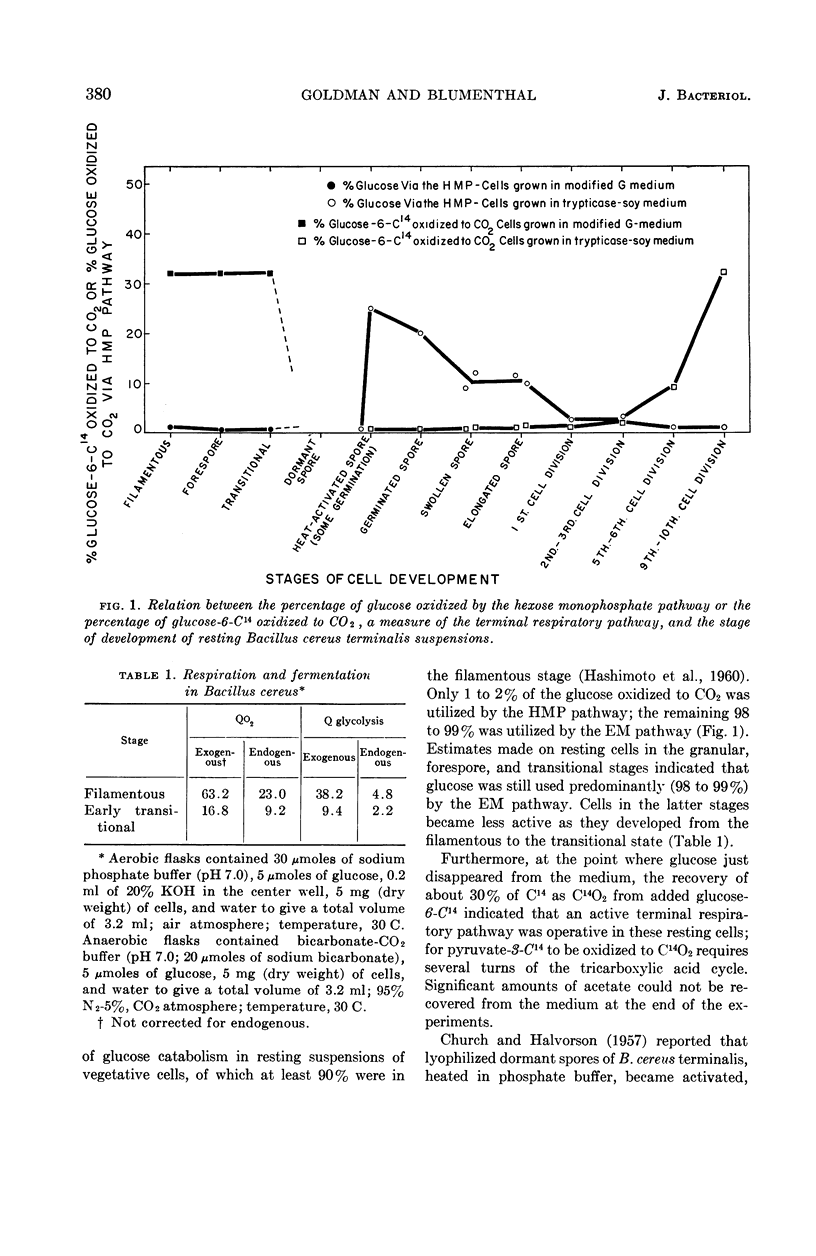
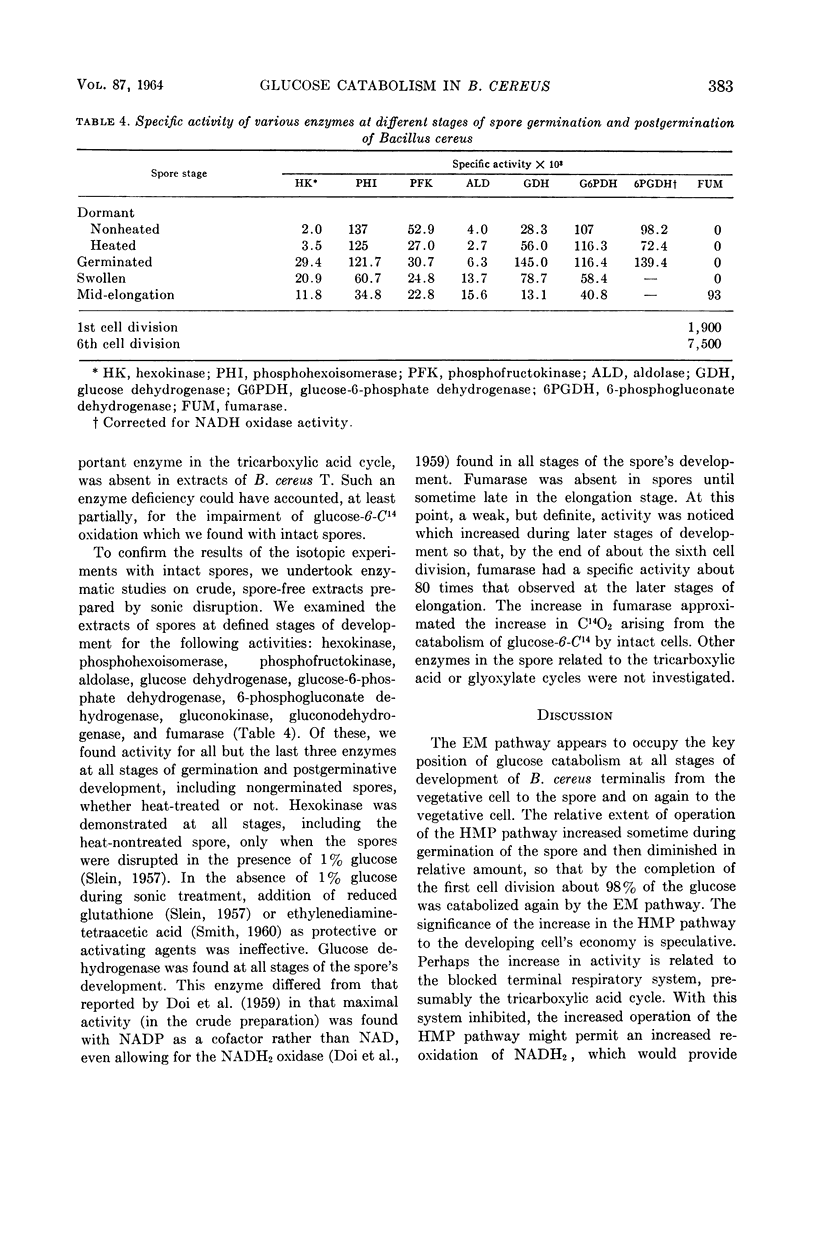
Goldman, Manuel (The University of Michigan, Ann Arbor), and Harold J. Blumenthal. Pathways of glucose catabolism in Bacillus cereus. J. Bacteriol. 87:377–386. 1964.—Estimates by a radiorespirometric method of the pathways of glucose catabolism of resting-cell suspensions of Bacillus cereus strain terminalis indicate that the Embden-Meyerhof pathway predominates at every stage of development, including the sporogenic and germinative phases. At the filamentous, granular, forespore, and transitional stages, 98% of the glucose was catabolized by the Embden-Meyerhof pathway, and the remainder by the hexose monophosphate oxidative pathway. Estimates of the pathways in resting spore-suspensions arrested at defined stages of development indicate that 20% of the glucose was catabolized through the hexose monophosphate pathway in germinated spores, and 10% in the swollen and elongated stages of postgermination. In cells which had completed the first cell division, the figure fell to about 2%, a level similar to that found for vegetative cells at later stages of development. The key Embden-Meyerhof enzymes, hexokinase, phosphohexoisomerase, phosphofructokinase, and aldolase, as well as several other enzymes, were present at all stages of germination and postgerminative development, supporting the radioisotopic data obtained with whole cells. As indicated by the release of C14O2 from glucose-6-C14, terminal respiration of resting-cell suspensions operates maximally in vegetative cells at the granular, fore-spore, and transitional stages. There was marked inhibition of terminal respiration during the development of spores into vegetative cells. Only slight activity occurred in the earliest vegetative stages, and maximal operation developed after about ten cell divisions. Fumarase was absent in spores until sometime late in the elongation stage. At this point, a weak but definite activity appeared which increased during later stages of development so that, by the end of about the sixth cell division, fumarase had a specific activity about 80 times that observed at elongation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHURCH B. D., HALVORSON H. Intermediate metabolism of aerobic spores. I. Activation of glucose oxidation in spores of Bacillus cereus var terminalis. J Bacteriol. 1957 Apr;73(4):470–476. doi: 10.1128/jb.73.4.470-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R. H., HALVORSON H. Comparison of electron transport systems in vegetative cells and spores of Bacillus cereus. J Bacteriol. 1961 Jan;81:51–58. doi: 10.1128/jb.81.1.51-58.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOI R., HALVORSON H., CHURCH B. Intermediate metabolism of aerobic spores. III. The mechanism of glucose and hexose phosphate oxidation in extracts of Bacillus cereus spores. J Bacteriol. 1959 Jan;77(1):43–54. doi: 10.1128/jb.77.1.43-54.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. The phosphorus fractions of Bacillus cereus and Bacillus megaterium. I. A comparison of spores and vegetative cells. Can J Microbiol. 1955 Aug;1(7):502–519. doi: 10.1139/m55-064. [DOI] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. The phosphorus fractions of Bacillus cereus and Bacillus megaterium. II. A correlation of the chemical with the cytological changes occurring during spore germination. Can J Microbiol. 1955 Aug;1(7):525–548. doi: 10.1139/m55-066. [DOI] [PubMed] [Google Scholar]
- GOLDMAN M., BLUMENTHAL H. J. Arrest of bacterial spores in stages of postgerminative development. Can J Microbiol. 1961 Aug;7:677–679. doi: 10.1139/m61-080. [DOI] [PubMed] [Google Scholar]
- GOLDMAN M., BLUMENTHAL H. J. CHANGES IN TERMINAL RESPIRATORY PATHWAYS OF INTACT CELLS OF BACILLUS CEREUS AT VARIOUS STAGES OF DEVELOPMENT. J Bacteriol. 1964 Feb;87:387–390. doi: 10.1128/jb.87.2.387-390.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN M., BLUMENTHAL H. J. PATHWAYS OF GLUCOSE CATABOLISM IN BACILLUS SUBTILIS. J Bacteriol. 1963 Aug;86:303–311. doi: 10.1128/jb.86.2.303-311.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN M., BLUMENTHAL H. U. Pathways of glucose catabolism in intact heat-activated spores of Bacillus cereus. Biochem Biophys Res Commun. 1960 Aug;3:164–168. doi: 10.1016/0006-291x(60)90215-1. [DOI] [PubMed] [Google Scholar]
- GOTTLIEB D., CALTRIDER P. G. Synthesis of enzymes during the germination of fungus spores. Nature. 1963 Mar 2;197:916–917. doi: 10.1038/197916a0. [DOI] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDWICK W. A., FOSTER J. W. Enzymatic changes during sporogenesis in some aerobic bacteria. J Bacteriol. 1953 Apr;65(4):355–360. doi: 10.1128/jb.65.4.355-360.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO T., BLACK S. H., GERHARDT P. Development of fine structure, thermostability, and dipicolinate during sporogenesis in a bacillus. Can J Microbiol. 1960 Apr;6:203–212. doi: 10.1139/m60-022. [DOI] [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. Correlation of respiratory activity with phases of spore germination and growth in Bacillus megaterium as influenced by manganese and L-alanine. J Bacteriol. 1956 Aug;72(2):176–183. doi: 10.1128/jb.72.2.176-183.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSEY V. The crystallization of fumarase. Biochem J. 1952 Jul;51(4):490–494. doi: 10.1042/bj0510490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- Smith P. J. Carbohydrate metabolism in Spirochaeta recurrentis. 2. Enzymes associated with disintegrated cells and extracts of spirochaetes. Biochem J. 1960 Sep;76(3):500–508. doi: 10.1042/bj0760500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]



