Abstract
Exposure to tobacco smoke, through both active and passive measures, has a significant impact on women's health, including effects on the cardiovascular, pulmonary and reproductive systems. Of particular interest is the effect of smoking on pregnancy outcomes. One crucial outcome that has been linked to the subsequent development of both neonatal and adult disease is intrauterine or fetal growth restriction. In this article, we will summarize the effects of smoking on newborn size and fetal growth. We will review evidence showing that tobacco consumption during pregnancy leads to a reduction in birthweight, largely through affecting specific anthropometric measures and newborn body composition. We will highlight the role of genetic susceptibility to these effects and discuss how smoking cessation prior to the third trimester results in a reduction in the risk of fetal growth restriction.
Keywords: birthweight, body composition, cotinine, fetal growth, growth restriction, pregnancy, smoking, smoking cessation, ultrasound
In pregnancy, maternal smoking is associated with multiple outcomes that result in increased perinatal morbidity and mortality. Understanding the relationship of smoking to pregnancy, and thereby pregnancy outcome, allows for potentially important insights into a preventable cause of adverse pregnancy outcome. In many countries, the rate of smoking has recently decreased [1–5], perhaps due to increased knowledge of its harmful effects; nevertheless, 22% of reproductive-age women continue to smoke in the USA [6]. It appears that despite increased information regarding the health risks of cigarette smoking, women continue to smoke during pregnancy and most find it difficult to quit. In Germany, 13% of women smoke during pregnancy, and there appears to be an inverse association of smoking frequency with maternal age, where 34% of pregnant women under 20 years of age smoke [7]. Rates of smoking during pregnancy in Norway are similar to those in Germany at 13.2% [8]. A Canadian study showed that 70% of women who smoked prior to pregnancy continued to do so at the time of delivery [9]. In the USA, under 26% of female smokers will abstain from smoking during pregnancy, and as many as 10.7% of all pregnancies are exposed to maternal tobacco consumption [1,10]. This is a high number of women, and it poses significant risk to developing fetuses. When compared with nonsmokers, mothers who are heavy smokers have an odds ratio (OR) of 1.35 for preterm delivery at less than 37 weeks [11].In a prospective cohort analysis, pregnancies exposed to maternal smoking were at a twofold risk of placental abruption. Other placentation abnormalities are increased in smoking-exposed pregnancies, where mothers who smoke have a relative risk of 1.36 for placenta previa [12]. Smoking shifts the infant mortality curve so that at any measure of birthweight, infant mortality is higher. This points to the fact that the etiology of poor outcomes in smoking might be more dependent upon its pathophysiology than on low birthweight alone [13,14]. In addition to uterine bleeding, preterm delivery and increased mortality, smoking has other marked effects on fetal growth. Most notably, it has been associated with reductions in birthweight and an increased incidence of intrauterine growth restriction (IUGR).
A fetus or neonate will be identified as small for gestational age (SGA) when its estimated fetal or birthweight is below a specified percentile on a standardized growth curve. The percentile that best predicts both short- and long-term outcomes is a matter of debate. Often, the 10th percentile on a fetal growth standard is used as a threshold for identifying a `small' fetus. However, this has been criticized because it includes many infants who are constitutionally small and not at risk for poor perinatal outcome [15]. Infants that are not constitutionally small but who fail to achieve this growth potential are often alternatively identified as having IUGR [16]. There is considerable overlap between these two terms in the literature, and determining how an infant or fetus can be identified as IUGR as opposed to SGA is beyond the scope of this article. For a review on this topic, please see the article published by Gardosi [17]. However, it is important to identify fetuses with IUGR because they have a perinatal mortality rate that is six- to ten-times that of the normally grown population [18]. Intrauterine fetal demise is often associated with IUGR, where 53% of preterm stillbirths and 26% of term stillbirths are growth restricted. In addition, intrapartum asphyxia has been reported to complicate 50% of pregnancies identified as having IUGR [19]. In addition to increased mortality, IUGR has been associated with poor perinatal outcome, including preterm birth, necrotizing enterocolitis and respiratory distress syndrome [20,21]. The risk of poor outcome extends beyond the perinatal period, and children born with IUGR are at an increased risk for short stature, cognitive delay and cerebral palsy [22]. At age 12 years, children born with IUGR are shorter and have higher cholesterol levels than children born at a normal weight [23]. There is accumulating evidence which shows that infants who are growth restricted are at an increased risk for hypertension, coronary heart disease, stroke and Type 2 diabetes mellitus in adult life [24]. This has lead to the hypothesis that environmental exposures to the fetus during development have profound effects on chronic disease in adulthood [24]. One modifiable environmental exposure that leads to IUGR is smoking. In the USA, 13.7% of IUGR infants can be attributed to maternal tobacco consumption in pregnancy [25]. In this review, the effects of maternal smoking on neonatal size and fetal growth will be discussed.
Effect of smoking on newborn size
Smoking & birthweight
Following World War II, the percentage of women who smoked increased substantially [26]. In 1957, Simpson was the first to report an association between low birthweight and maternal smoking [27]. An analysis of the 1958 British Perinatal Mortality Survey showed that smoking during pregnancy was associated with a 28% increase in the perinatal mortality rate and a 170-g reduction in birthweight, even after balancing other socioeconomic factors [28]. This lead to a number of studies investigating the effects of smoking in pregnancy on birthweight. In an Alabaman study, a survey of 1545 women showed a relationship between smoking in thin women and IUGR [29]. Another study confirmed these findings and found an association between SGA and smoking in both teenage and adult mothers [30]. Age may influence the smoking-related risk of IUGR; a study by Cnattingius showed that the relative risk of IUGR in mothers aged 40–44 years who smoked compared with nonsmokers was 3.4. In teenage mothers, the relative risk was only 1.9 [31]. The synergistic effect of smoking and maternal age was confirmed in a Norwegian study that showed mothers over the age of 35 years who smoked had a relative risk of 3.8 for SGA, while smoking mothers below 25 years did not show a significant risk for SGA [32]. Overall, these and many additional studies clearly demonstrate that maternal smoking is associated with reductions in fetal growth and birthweight. Maternal age may well confound this association and there is a suggestion that the extremes of maternal age interact with maternal smoking to influence fetal growth.
Many have investigated other factors associated with IUGR to see if smoking had an independent effect on fetal growth. Maternal BMI is an important factor, where an increased BMI is associated with larger birthweight [33]. However, a study in Austria showed that a higher prepregnancy BMI did not mitigate the effects of smoking. Perhaps more concerning, under-weight mothers who smoked had infants with the lowest birthweight [34]. A recent study investigated the effects of maternal smoking on newborn size in 424,912 newborns in Utah. The authors found that the mean birthweight was lower in newborns whose mothers reported smoking during pregnancy. This was true across all maternal BMI strata. Additionally, smoking was associated with SGA in mothers with other medical illnesses including hypertension and diabetes. After correcting for maternal disease in a multivariable logistic regression model, tobacco exposure remained the most significant associative factor for SGA (OR 3.53; 95% confidence interval [CI]: 2.61–4.79) [35]. In mothers with moderate to severe preeclampsia, smoking during pregnancy will double the risk of IUGR when compared with nonsmokers [36]. The relationship of maternal smoking to preeclampsia is complex, in that multiple studies have demonstrated maternal smoking to be associated with a reduction in the incidence of preeclampsia [37–39]. When gestational hypertension occurs with a background of maternal smoking, the relative contributions of smoking and maternal hypertension on fetal growth is a matter of debate. A recent large study using the Medical Birth Registry of Norway showed that smoking and pregnancy-induced hypertension were not synergistic for the risk of IUGR. In fact, in preterm gestations, as the severity of hypertension increased, the OR for having an IUGR infant in smoking mothers decreased [40]. Although the effect of smoking on birthweight may not be synergistic with BMI or hypertension in pregnancy, smoking is clearly an independent risk factor for IUGR. The above studies also indicate that it is a primary contributor to fetal growth abnormalities of mothers with other medical diseases. Therefore, multiple studies confirm that tobacco consumption is associated with reduced birthweights in healthy, as well as medically complicated, pregnancies.
Dose–response effects of tobacco consumption as determined by cotinine or self-report
There seems to be a dose–response effect of tobacco consumption in pregnancy. A prospective analysis at the University of Vermont was performed on 160 pregnant women and included an analysis of prepregnancy cigarette consumption. Third trimester cigarette use demonstrated the strongest relationship with birthweight, and for each additional cigarette per day consumed in the third trimester, there is an estimated 27-g reduction in birthweight (Figure 1). This effect was consistent across maternal parity [41]. Another recent study in the UK has also shown a significant dose–response effect of reported maternal smoking habits and birthweight. Compared with nonsmoking mothers, women who smoked 1–10 cigarettes per day produced an adjusted mean decrease in newborn birthweight of 86 g, while those who smoked 11–20 cigarettes per day produced an adjusted mean decrease of 190 g. If a mother smoked more than 20 cigarettes per day, there was an adjusted mean decrease of 277 g [42]. Both of these studies relied on maternal report of tobacco consumption, and the authors point to a relationship between this report and decreased birthweight.
Figure 1. The relationship between third trimester cigarette consumption and newborn birthweight is illustrated for both primiparous and multiparous women.
The effect of each added cigarette consumed in the third trimester on newborn birthweight is independent of parity.
Reproduced with permission from [41].
Using an objective measure of smoking volume, Bardy et al. in 1993 measured maternal and umbilical cord serum levels of cotinine in 1237 pregnancies. A total of 300 pregnancies were found to be exposed to cotinine and, after correction for parity, gender and gestational age, the exposed newborns were, on average, 188 g lighter and 10 mm shorter than the nonexposed newborns. In maternal serum 1 μg/ml of cotinine was associated with a mean decrease of 1.29 g in birthweight and a mean decrease of 0.059 mm in birth length. Additionally, maternal cotinine concentration showed a closer correlation with neonatal findings than the self-reported smoking habits of the mother [43]. Using data collected in the Collaborative Perinatal Project (1959–1966), Klebanoff et al. assessed the accuracy of self-reported smoking behavior compared with serum cotinine levels. Assuming that a serum cotinine concentration of over 10 ng/ml was representative of active smoking, 94.9% of women who denied smoking and 87.0% of women who reported smoking had cotinine levels that confirmed their reports (κ = 0.83). In this study, the serum cotinine concentration correlated more strongly than self-report to infant birthweight (r = 0.246 vs 0.200) [44]. The Collaborative Perinatal Project is an older database, and some have questioned whether its data can be generalized to women of today. In a more recent trial, George et al. looked at mothers who self-reported smoking in the first trimester and in late pregnancy. The authors compared the results to serum cotinine levels. In patients who reported continued smoking, the validity was high. However, in mothers who reported that they quit prior to pregnancy, 13% had cotinine levels that were inconsistent. In addition, in mothers who reported quitting smoking in pregnancy, 25% had mis-reported smoking habits [45]. Other than inaccurate reporting, variation between the maternal report and measurable levels of cotinine may, in part, be due to passive exposures or variation in maternal metabolism of cotinine. In different prospective cohort analyses, investigators have shown a similar birthweight relationship with urine cotinine levels as has been observed in serum levels. That is, as urine cotinine levels increase, neonatal birthweight decreases [46,47]. In addition to cotinine, increases in urinary nicotine also negatively correlate with birthweight, and this measurement has been more accurate in predicting the deficit in growth than the patients' report [48]. Multiple studies therefore confirm that increased exposure to tobacco is associated with an increased degree of growth restriction. The degree to which this dose–response is specifically linear is uncertain. Patient self-report is less predictive of birthweight abnormalities than other objective measures of exposure; however, there is a correlation between reported amount of smoking and measurable levels of cotinine. Although maternal report may be less accurate than measured levels of cotinine, particularly in patients who report quitting during pregnancy, it is an inexpensive tool that can be used to gauge the risk of smoke exposure on the fetus.
Passive exposure & birthweight
Evidence suggests that `second-hand' smoke, or passive maternal exposure to smoke, also affects birthweight. In the study by Bardy, 38% of mothers with positive cotinine levels did not report smoking [43]. This could be secondary to inaccurate reporting by the mother or second-hand exposure. Another study investigated the effect of passive exposure to paternal smoking on birthweight in mothers who denied tobacco consumption. In this study, umbilical cord blood cotinine levels were measured in 175 newborns. There was a mean birthweight deficit of 88 g in newborns exposed to passive smoke whose fathers smoked more than 20 cigarettes per day during maternal pregnancy. Only two of the 138 babies who had measurable levels of cotinine had levels that were indicative of maternal smoking (>10 ng/ml). The amount of cotinine directly correlated with the reported amount of paternal smoking in infants of nonsmoking mothers [49]. A more recent study in Denmark showed that nonsmoking mothers who reported passive exposure to smoke inside and outside the home gave birth to children with a mean birthweight approximately 79 g lighter than unexposed women [50]. Another study showed that passive tobacco exposure during pregnancy was significantly associated with a higher risk of SGA babies (OR: 2.10; 95% CI: 1.27–23.48) [51]. Recent USA data examined serum levels of cotinine in 3000 women and compared the results of pregnancy outcome. Passive exposure was assumed in nonsmoking women with cotinine levels between 0.236 and 10 ng/ml. In these women, the OR for term low birthweight was 1.8, and there was a linear, dose-dependent effect of log cotinine on mean birthweight [52]. These studies indicate that passive exposure can lead to measurable levels of cotinine in the mother, and this can affect newborn weight.
Genetic susceptibility to tobacco smoke
Maternal tobacco smoke, either through direct or passive exposure, is associated with decreased birthweight. However, not all mothers who smoke will have small children. There is evidence that the risk of having an SGA infant may be determined by maternal metabolic susceptibility. Tobacco smoke contains more than 4000 chemicals, the most toxic of which include polycyclic aromatic hydrocarbons (PAHs), N-nitro samines and arylamines [53,54]. Metabolism of these compounds is largely accomplished by cytochrome (CY) P450 enzymes and glutathione S-transferases (GST) [55]. Recent studies have investigated the role of CYP1A1 and GSTT1, two genes coding enzymes involved in detoxifying the chemicals in tobacco smoke, in mothers who smoke and have infants who are SGA. In a 2002 case–control study, 207 cases of preterm or low-birthweight neonates were identified and compared with 534 normal-weight, full-term infants. The mothers were then investigated for a polymorphism in the CYP1A1 gene called CYP1A1 MspI. There are three different genotypes of the polymorphism: AA (homozygous wild type), Aa (heterozygous variant type) and aa (homozygous variant type). They also looked for a gene deletion polymorphism in GSTT1. In mothers who smoked, the investigators found a significant relationship between genetic polymorphism in CYP1A1 and low birthweight (<2500 g). The AA genotype was not associated with a reduction in birthweight, but having either the Aa or aa genotype was associated with a 520 g decrease in birthweight (OR: 3.2; 95% CI: 1.6–6.4). Similarly, the presence of a GSTT1 deletion of both alleles was associated with a 642 g decrease in birthweight (OR: 3.5; 95% CI: 1.5–8.3). In nonsmoking mothers, these genotypes did not confer risk of having a low-birthweight infant [56]. Another group looked at the CYP1A1 MspI polymorphism in mothers exposed to passive smoke. Again, genetic polymorphism was significantly associated with smaller birthweight in passively exposed mothers and not in nonexposed mothers [57]. In addition to the CYP1A1 gene, other gene polymorphisms have been investigated. In Japan, 293 women were prospectively followed for infant birthweight, and they had genotypes performed for the aryl hydrocarbon receptor (AhR), the CYP1A1 polymorphism and the GSTM1 enzyme. This group found that the AhR wild-type genotype, CYP1A1 variant (Aa or aa) genotype and the GSTM1 null genotype were all associated with lower birthweight in smoking mothers but not in nonsmoking mothers [58]. These studies indicate that maternal susceptibility to tobacco smoke, through direct or passive exposure, is largely determined by genetic polymorphisms. Also, it gives credence to the concept of a gene–environment interaction that leads to SGA neonates in exposed pregnancies.
Smoking & other measures of newborn size
It has been well-shown that smoking affects infant birthweight, especially in genetically susceptible individuals. Studies have confirmed that smoking is associated with a negative effect on multiple newborn anthropometric measures including femur length, limb length, total length, head circumference, chest circumference and abdominal circumference. In addition, specific effects on newborn body composition have been identified [59–61]. In order to better characterize the effects of smoking on birthweight, D'Souza et al. looked at 452 mother–baby pairs and stratified them according to the amount of tobacco consumed during pregnancy. They showed that heavy smokers gained significantly less weight during pregnancy than nonsmokers, but maternal skin fold thickness was not different between the strata. Babies born to smokers had lower birthweights, smaller head circumferences and shorter lengths, but their skin fold thicknesses were similar to babies born to nonsmokers. This is one of the first reports to suggest that maternal smoking is likely to reduce fetal lean body mass to a greater extent than fetal fat mass [62]. Other studies corroborated these findings and pointed to a predominant effect of decreasing weight, head circumference and newborn limb length, while leaving measures of subcutaneous fat relatively unchanged [43,61,63]. These studies point to a specific and unique effect of tobacco smoke on birthweight, where overall body weight is reduced primarily due to decreases in indices of lean body mass.
A direct contributor to these changes in newborn size may be the effect of maternal cigarette smoking on plasma volume expansion in pregnancy. In nonsmoking mothers, maternal plasma volume in the third trimester is significantly and positively associated with newborn size [64,65]. This relationship has often been examined through the inverse association of circulating hemoglobin with plasma volume [66–70]. There is evidence that plasma volume in the third trimester of pregnancy is reduced in mothers who smoke, compared with nonsmoking mothers [71,72]. Within the group of smoking mothers, as is observed in nonsmoking mothers, lower hemoglobin concentrations are associated with increased birthweights of infants [73].
The studies mentioned previously, and many others, confirm the negative effect of maternal smoking on birthweight. There is a significant dose–response relationship, and maternal exposure, even if passive, can produce infants at risk for perinatal complications associated with growth restriction. Of importance, there appears to be a genetic susceptibility to the untoward fetal effects of tobacco smoke.
Intrauterine effect of smoking
Smoking & fetal growth measures
It is clear that maternal smoking in pregnancy is associated with a reduction in birthweight, and with the advent of modern ultrasound technology, the effects of maternal smoking can be further evaluated in utero. In a Scandinavian study, 547 women were evaluated by ultrasound at 17 weeks, and 31.9% were smokers. They found no statistically significant differences between the estimated fetal weight, biparietal diameter or femur lengths between the ultrasounds of smokers versus nonsmokers [74]. Other investigators have used ultrasound to see if the differences between fetal measures occur at later gestational ages. In the National Institute of Child Health and Human Development Study of Successive Small for Gestational Age Births, 1349 women were followed with serial ultrasound measurements. IUGR was identified if a fetus had an estimated weight less than the 10th percentile on the Hadlock growth curve [75]. In smokers, low birthweight was best predicted by third trimester ultrasonographic detection of IUGR. First and second trimester ultrasound did not predict which infants would have low birthweight [76]. This suggests that the primary direct effects of smoking do not occur until the third trimester, and ultrasound may be a useful tool to identify fetuses at risk of low birthweight in the early third trimester.
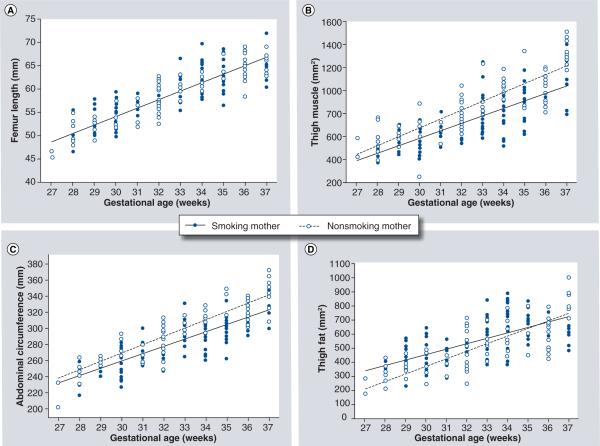
Further investigation of smoking on fetal growth can be achieved by utilizing ultrasound technology to measure other anthropometric data. A 1987 study by Jeanty et al. was one of the first to use ultrasound to longitudinally assess fetal parameters in smoking mothers. They looked at 40 fetuses, ten of whom were exposed to maternal smoking, and were followed with serial ultrasound measurements throughout pregnancy. Examining the fetal head, they found that biparietal diameter and occipito-frontal diameters were decreased in fetuses exposed to smoking. Likewise, renal volume, abdominal circumference and cardiac volume and length were decreased in exposed fetuses. These fetuses also had shortened long bones, including the humerus, ulna, femur and tibia. Surprisingly, the estimated fetal weight was not different between smokers and nonsmokers, but this may be due to the small number of patients in the study [77]. This report was one of the first to show ultrasonographic measures that matched neonatal anthropometric data. Another longitudinal study with larger numbers was published confirming the above findings [78]. As noted earlier, newborn data has indicated that lean body mass was preferentially affected by smoking. A longitudinal study at the University of Vermont was performed where multiple ultrasound measures in unexposed fetuses were compared with those of exposed fetuses. The authors found that the growth rates of the femur length were not different between the two groups. However, the rate of growth of the thigh muscle area and the abdominal circumference were significantly reduced in the fetuses exposed to smoking. The growth rate of the thigh fat area in smoking mothers was slower than that of the non-smoking mothers; however, the absolute amount of fat measured in the thigh did not differ between the two groups (Figure 2) [79]. This adds credence to the hypothesis that smoking has a selective effect within lean body mass compartments.
Figure 2.
(A) Growth of fetal femur length is demonstrated for fetuses of smoking and nonsmoking mothers. There was no difference between groups in estimated rate of femur growth. (B) Growth of fetal thigh muscle area is demonstrated for fetuses of smoking and nonsmoking mothers. There was a significantly slower growth of thigh muscle mass in fetuses of smoking mothers. (C) Growth of fetal abdominal circumference is demonstrated for fetuses of smoking and nonsmoking mothers. There was a significantly slower growth of abdominal circumference in fetuses of smoking mothers. (D) Growth of fetal thigh fat area is demonstrated for fetuses of smoking and nonsmoking mothers. There was a significantly slower growth of thigh fat in fetuses of smoking mothers; however, there was no absolute difference between the groups at 33–37 weeks' gestation.
Smoking & measures of vascular resistance
The pathogenesis of IUGR in smoking is complex. A variety of factors has been considered, including poor nutritional state of the mother, toxins and carbon monoxide disruption of oxygen binding. For a review on the topic, please see the references by Jauniaux and Pastrakuljic [80,81]. A full description of the pathogenesis is beyond the scope of this article, but we would like to focus on the effects of tobacco smoke on altered vascular physiology, as this is an area of some controversy. In a study by Newnham et al., growth measurements were lagging in exposed fetuses. Interestingly, umbilical artery systolic/diastolic (S/D) ratios were not different between exposed and unexposed fetuses, suggesting that umbilical–placental vascular resistance was either not effected by smoking, or that it was only intermittently effected [78]. Of interest, evaluation of the uterine artery in nonpregnant women found that after smoking two cigarettes, the S/D ratio decreased from 2.33 to 2.02, and the resistance index decreased from 0.55 to 0.49 (p < 0.001). Alternatively, the maternal heart rate increased 27%, the systolic blood pressure increased 8%, and the diastolic blood pressure increased 19% with smoking, indicating that smoking increases systemic vascular resistance while decreasing uterine vascular resistance [82]. In contrast to these data, another study was performed in pregnant patients where two cohorts were examined: a control group of nonsmoking mothers, and a study group of chronically smoking mothers. Ultrasonographic assessment of the uterine and umbilical arteries was performed at baseline in the two groups and then after smoking one cigarette in the study group. There was no significant change in the uterine or umbilical artery S/D ratio, resistance index or pulsatility index in the study group after smoking the cigarette. However, all uterine artery indices and the umbilical artery S/D ratio were higher in the study group than the control group both before and after the cigarette exposure [83]. Corroborating this study, Albuquerque et al. compared ultrasonographic assessment of vascular resistance between chronically smoking pregnant mothers and nonsmoking ones. They found that a diastolic notch, an index of elevated vascular resistance, was more frequently observed in the uterine artery waveform of smoking mothers. Also, umbilical artery and middle cerebral artery S/D ratios were higher in the fetuses of smokers, indicative of increased vascular resistance in both umbilical–placental and cerebrovascular blood flow [84]. Overall, these data suggest an increased placental and uterine vascular resistance in chronically smoking mothers. Kalinka et al. measured serum cotinine and found that levels positively correlated with elevated S/D ratios [85]. Owing to conflicting data, further studies are needed to clarify the acute and chronic vascular effects of smoking in pregnancy.
Ultrasound in the third trimester, which identifies fetuses of smoking mothers as having IUGR, has close correlations to infant birthweight data. Anthropometric neonatal data and antenatal ultrasound measurements show consistent findings, all pointing to a loss in lean body mass with maternal smoking mothers. Ultrasound has the added benefit of being able to identify IUGR in infants prior to delivery. Early identification should prompt further evaluation with fetal monitoring and Doppler assessment, which has been shown to reduce perinatal morbidity and mortality [16]. Assessment of early third trimester fetal growth should be considered routine in mothers who smoke in pregnancy.
Smoking cessation during pregnancy
It is well-documented that smoking affects fetal growth as demonstrated by in utero ultrasound measurements and neonatal anthropometric data. As outlined earlier, there is some evidence that chronic smoking leads to hemodynamic effects that change the blood flow to the uterus and placenta. Some have argued that smoking is merely a marker for women who are predisposed to a particular type of reproductive outcome, and other differences that exist between smokers and nonsmokers account for growth variation [86]. This line of thinking would suggest that intervention during pregnancy would not result in improved pregnancy outcome.
In order to refute the belief that smoking cessation would not be beneficial, one of the first randomized trials of smoking cessation in pregnancy was published in 1984. In this study, 935 pregnant women were randomized to either a smoking intervention arm or a control group. By 8 months of pregnancy, salivary thiocynate levels were different between the groups, indicating a degree of successful cessation in the intervention group. The treatment group had infants with birthweights that were 92 g heavier and 0.6 cm longer than the infants of the control group. These data demonstrate a small but real benefit of smoking cessation in pregnancy [87]. Another study randomized 982 smoking pregnant women to a cessation program or routine care. In the cessation program, people received a pamphlet outlining the untoward effects of smoking in pregnancy, and they were advised to stop smoking. Consistent with the previous trial, infants of the treatment group were 68 g heavier and 0.75 cm longer than infants of the control group [88]. Women were randomized to smoking cessation or routine care in one trial but added the interpretation of serum cotinine levels at various intervals in pregnancy to help motivate women to quit smoking. Again the treatment group had a 66 g increase in mean birthweight and a 30% reduction in the rate of low-birthweight pregnancies [89]. A meta-analysis of 11 randomized controlled trials showed that intervention programs can improve smoking cessation by 50%. This resulted in a significant decrease in the number of low-birthweight infants [90]. The data from these randomized trials indicate that women can decrease tobacco consumption in pregnancy, and it will result in increasing newborn birthweight.
In addition to affecting newborn weight, one trial has shown that smoking cessation in pregnancy can affect fetal growth parameters. In this study, 82 smoking mothers were randomized to a treatment arm or control arm. In the treatment arm, the mother received vouchers for retail merchandise contingent upon a urine cotinine level over or equal to 80 ng/ml. In the control arm, the mothers received vouchers regardless of their cotinine levels. Both groups were instructed on the benefits of smoking cessation in pregnancy and given pamphlets outlining how to quit. Contingent vouchers significantly increased the proportion of women abstaining from smoking at the end of pregnancy (41 versus 10%) and at the 12-week postpartum evaluation (24 versus 3%). Ultrasonographic evaluation showed that the members of the contingent arm had significantly greater growth of the estimated fetal weight, femur lengths and abdominal circumferences. In the treatment arm, the mean birthweight was 3355 ± 96 g, while in the control arm, the mean birthweight was 3102 ± 89 g. This difference of 253 g did not reach statistical significance due to the relatively small sample size (p = 0.06) [91]. Nevertheless, not only did this study show a novel method to assist in smoking cessation, it demonstrated that quitting smoking in pregnancy can benefit fetal growth as measured by ultrasound.
The timing of maternal exposure to smoking is important to the development of growth restriction. Women who quit smoking by the third trimester are not at an increased risk for having a newborn with a low birthweight, but women who begin smoking in the late second or third trimester have a risk of low birthweight equal to women who smoke during the entire pregnancy [92]. In fact, stopping during the first trimester, especially before 16 weeks of pregnancy, produces neonates with anthropometric measures similar to women who never smoked [29,93]. Gestational age-dependent effects were also shown in a study by Bernstein et al. In this study, 160 pregnant women were enrolled in a prospective study where cigarette consumption was determined by self-report and urinary cotinine levels. Third-trimester consumption was the strongest predictor of birthweight percentile [41]. Decreased birthweight is therefore determined mostly by exposure to smoking during the later half of pregnancy, and cessation prior to the late second trimester is most beneficial.
There is a dose–response effect of tobacco exposure and birth-weight, especially in the third trimester. As mentioned previously, smoking cessation can lead to improved outcomes, but many women reduce their intake of cigarettes in pregnancy rather than quit entirely. Some have questioned the benefits of smoking reduction instead of smoking cessation. England et al. looked at self-reported use of cigarettes in pregnancy and urine cotinine concentrations in 1583 pregnant smokers. Reducing smoking by 50% resulted in an increase in mean birthweight, but this did not reach statistical significance (p = 0.33). In addition, as third-trimester cigarette use increased, birthweight declined sharply but leveled off at eight cigarettes per day, suggesting a nonlinear relationship. Women may need to decrease their consumption to less than eight cigarettes per day to see benefits in birthweight [94]. A study by Li et al. showed that quitting smoking in pregnancy produced mean birthweights that were 167 g heavier than those of mothers who had reduced smoking based on urinary cotinine levels. The patients who reduced intake had infants that were 92 g heavier than the infants of mothers who continued to smoke, but this did not reach statistical significance (p = 0.08) [95]. Reduction in smoking may be encouraged in patients who cannot quit, but cessation is clearly more beneficial to birthweight than that of smoking reduction.
Smoking cessation can be achieved in pregnancy. Improvement in birthweight refutes the notion that smoking is a marker for other factors that lead to IUGR, and it supports the claim that smoking inhibits fetal growth. Randomized controlled trials demonstrate a clear benefit to smoking cessation and birthweight, but less is known about potential benefits with regard to other perinatal outcomes. Regardless, the benefit of smoking cessation on fetal weight should encourage the creation of smoking intervention programs. The goal of these programs should be smoking cessation, because reduction alone has not been shown to significantly improve birthweight.
Expert commentary
Maternal smoking in pregnancy has been shown to decrease neonatal birthweight. This is dose-dependent, where increased exposure leads to a greater reduction in weight. This dose–response relationship is most specific to third trimester maternal smoking volume. Evidence is mounting that supports a gene–environment interaction where women who have polymorphisms in genes encoding proteins involved in toxin metabolism are at the greatest risk for developing infants with IUGR. Anthropometric measures including head circumference, abdominal circumference and limb length are also affected by smoking. All of this occurs without significant changes in neonatal subcutaneous fat measures, indicating a predilection for lean body mass effects. Fetal growth, as determined by antenatal ultrasound assessment, is also affected by smoking. Since fetal exposure in the second half of pregnancy produces the most notable variation, assessment of fetal growth in a smoking mother through ultrasound in the third trimester is paramount to identifying fetuses with IUGR. In addition to increased surveillance for growth abnormalities in the smoking mother, there is level I evidence showing that intervention programs targeting smoking cessation will improve growth measures. The smoking patient has a high-risk pregnancy, and as the risk factor is modifiable, measures should be taken to decrease the possibility of perinatal morbidity and mortality.
Five-year view
As mentioned previously, maternal tobacco consumption in pregnancy is associated with growth restriction. This outcome can be relatively easily identified through ultrasonographic surveillance of an ongoing pregnancy. With the advancement of ultrasound technology, we predict that there will be further investigation into the effects of tobacco smoke on specific fetal growth parameters. With the advent of 3D imaging, evaluation of the effects of smoking on volumetric assessments of fetal organs and lean body mass compartments can be performed. This may be able to identify specific parameters that best predict infants at highest risk for outcomes associated with growth restriction and help to segregate the morbidities that may attend specific anthropometric phenotypes.
In addition to the improvement in ultrasound technology, the development of new methods for rapidly and thoroughly investigating genetic polymorphisms may add to the molecular understanding of how tobacco consumption affects fetal growth. Understanding the genes involved in regulating and performing the metabolism of tobacco toxins is important. Genetic polymorphisms have been shown to directly affect the mother's risk of having a growth restricted fetus when exposed to tobacco smoke. Possibly through the use of technologies, such as micro-array assays, polymorphisms that place a mother at risk for poor pregnancy outcomes can be quickly identified. This will allow for better stratification of the mother's risk, and it may prompt future surveillance and intervention strategies when resources are limited. A clearer understanding of the gene–environment interaction that places certain mothers and babies at risk will be crucial to developing patient-specific medical care.
In addition to improvement in technology, there needs to be a focus on developing more effective preventative programs for smoking. Clearly, smoking has multiple effects on pregnancy, and there is sufficient knowledge to support the prevention of smoking in reproductive-age women. Further studies on the most effective program for promoting smoking prevention and cessation must be performed in the future.
Key issues.
In pregnancy, maternal tobacco consumption is associated with decreased infant birthweight.
There is a dose–response effect of tobacco smoke on newborn weight, where a higher degree of antenatal tobacco exposure is associated with a lower birthweight.
Both active and passive exposures to tobacco smoke while pregnant increase a mother's risk of having a growth-restricted newborn.
Genetic polymorphisms in genes encoding enzymes responsible for metabolizing tobacco smoke will directly modify a mother's risk of having a growth restricted infant.
Neonatal anthropometric measurements and antenatal ultrasonographic parameters both demonstrate a decrease in lean body mass in infants and fetuses that are exposed to tobacco smoke.
Exposure to tobacco smoke in the third trimester has the greatest affect on fetal growth, and quitting prior to the late second trimester will significantly reduce the chances of having a growth-restricted fetus.
Smoking cessation programs that actively encourage patients are successful in getting women to quit, and these result in improvement in both antenatal growth parameters and neonatal birthweight.
Learning Objectives.
Upon completion of this activity, participants should be able to:
Identify the interactions between smoking and other maternal variables on birthweight
List measures of newborn size that are adversely affected by maternal smoking
Describe ultrasound findings and practice among maternal smokers
Specify outcomes associated with smoking cessation during pregnancy
Footnotes
Financial & competing interests disclosure
Authors Shane Reeves and Ira Bernstein; Department of Obstetrics and Gynecology, University of Vermont College of Medicine, VT, USA. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this review manuscript.
Editor Elisa Manzotti, Editorial Director, Future Science Group, London, UK. Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME Author Charles P Vega, MD, Associate Professor; Residency Director, Department of Family Medicine, University of California, Irvine, CA, USA. Disclosure: CP Vega has served as an advisor or consultant to Novartis, Inc.; CP Vega has no other relevant financial relationships.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Martin JA, Hamilton BE, Sutton P, et al. Births: final data for 2005. Natl Vital Stat. Rep. 2007;56(6):1–104. [PubMed] [Google Scholar]
- 2.Dodds L. Prevalence of smoking among pregnant women in Nova Scotia from 1988 to 1992. CMAJ. 1995;152:185–190. [PMC free article] [PubMed] [Google Scholar]
- 3.Egebjerg Jensen K, Jensen A, Nohr B, Kruger Kjaer S. Do pregnant women still smoke? A study of smoking patterns among 261,029 primiparous women in Denmark 1997–2005. Acta Obstet. Gynecol. Scand. 2008;87(7):760–767. doi: 10.1080/00016340802179814. [DOI] [PubMed] [Google Scholar]
- 4.Centers for disease control and prevention Cigarette use among high school students – United States, 1991–2007. Morb. Mortal. Wkly Rep. 2008;57(25):686–688. [PubMed] [Google Scholar]
- 5.Sigfusdottir ID, Kristjansson AL, Thorlindsson T, Allegrante JP. Trends in prevalence of substance use among Icelandic adolescents, 1995–2006. Subst. Abuse Treat. Prev. Policy. 2008;3:12. doi: 10.1186/1747-597X-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for disease control and prevention (CDC) Smoking prevalence among women of reproductive age – United States, 2006. Morb. Mortal. Wkly Rep. 2008;57(31):849–852. [PubMed] [Google Scholar]
- 7.Schneider S, Maul H, Freerksen N, Potsche-Langer M. Who smokes during pregnancy? An analysis of the German Perinatal Quality Survey 2005. Public Health. 2008 doi: 10.1016/j.puhe.2008.02.011. DOI:10.1016/j.puhe.2008.02.011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Kvalvik LG, Skjaerven R, Haug K. Smoking during pregnancy from 1999 to 2004: a study from the Medical Birth Registry of Norway. Acta Obstet. Gynecol. Scand. 2008;87(3):280–285. doi: 10.1080/00016340701837801. [DOI] [PubMed] [Google Scholar]
- 9.Kirkland SA, Dodds LA, Brosky G. The natural history of smoking during pregnancy among women in Nova Scotia. CMAJ. 2000;163:281–282. [PMC free article] [PubMed] [Google Scholar]
- 10.Castrucci BC, Culhane JF, Chung EK, Bennett I, McCollum KF. Smoking in pregnancy: patient and provider risk reduction behavior. J. Public Health Manag. Pract. 2006;12(1):68–76. doi: 10.1097/00124784-200601000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Nabet C, Ancel PY, Burguet A, Kaminski M. Smoking during pregnancy and preterm birth according to obstetric history: French national perinatal surveys. Paediatr. Perinat. Epidemiol. 2005;19:88–96. doi: 10.1111/j.1365-3016.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 12.Ananth CV, Savitz DA, Luther ER. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am. J. Epidemiol. 1996;144:881–889. doi: 10.1093/oxfordjournals.aje.a009022. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox AJ. On the importance – and the unimportance – of birthweight. Int. J. Epidemiol. 2001;30(6):1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 14.Basso O, Wilxox AJ, Weinberg CR. Birthweight and mortality: causality or confounding? Am. J. Epidemiology. 2004;164(4):301–311. doi: 10.1093/aje/kwj237. [DOI] [PubMed] [Google Scholar]
- 15.Favre R, Nisand G, Bettahar K, et al. Measurement of limb circumferences with three-dimensional ultrasound for fetal weight estimation. Ultrasound Obstet. Gynecol. 1993;3:176–179. doi: 10.1046/j.1469-0705.1993.03030176.x. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein I, Gabbe SG, Reed KL. Intrauterine growth restriction. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics, normal and problem pregnancies. Churchill Livingstone, NY, USA: 2002. pp. 869–889. [Google Scholar]
- 17.Gardosi J. New definition of small for gestational age based on fetal growth potential. Horm. Res. 2006;65(Suppl 3):15–18. doi: 10.1159/000091501. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe HM, Gross TL. Increased risk to the growth retarded fetus. In: Gross TM, Sokol RJ, editors. Intrauterine Growth Retardation. Year Book Medical Publishers; Chicago, USA: 1989. [Google Scholar]
- 19.Hepburn M, Rosenburg K. An audit of the detection and management of small-forgestational age babies. Br. J. Obstet. Gynaecol. 1986;93:212–216. doi: 10.1111/j.1471-0528.1986.tb07895.x. [DOI] [PubMed] [Google Scholar]
- 20.Lackman F, Capewell V, Richardson B, daSilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am. J. Obstet. Gynecol. 2001;184:946–953. doi: 10.1067/mob.2001.111719. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein I, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birthweight neonates with intrauterine growth restriction. Am. J. Obstet. Gynecol. 1999;182(1):198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- 22.Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clin. Obstet. Gynecol. 2006;49(2):257–269. doi: 10.1097/00003081-200606000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Tenhola S, Martikainen A, Rahiala E, Herrgârd E, Halonen P, Voutilainen R. Serum lipid concentrations and growth characteristics in 12-year-old children born small for gestational age. Pediatr. Res. 2000;48(5):623–628. doi: 10.1203/00006450-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Barker D. Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 2006;49(2):270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Bada HS, Das A, Bauer CR, et al. Low birthweight and preterm births: etiologic fraction attributable to prenatal drug exposure. J. Perinatol. 2005;25(10):631–637. doi: 10.1038/sj.jp.7211378. [DOI] [PubMed] [Google Scholar]
- 26.Greenberger P. News from the Society for Women's Health Research: women and tobacco use. J. Womens Health Gend. Based Med. 2001;10(3):221–222. doi: 10.1089/152460901300139952. [DOI] [PubMed] [Google Scholar]
- 27.Simpson WJ. A preliminary report on cigarette smoking and the incidence of prematurity. Am. J. Obstet. Gynecol. 1957;73(4):807–815. [PubMed] [Google Scholar]
- 28.Butler NR, Goldstein H, Ross EM. Cigarette smoking in pregnancy: its influence on birthweight and perinatal mortality. BMJ. 1972;15:127–130. doi: 10.1136/bmj.2.5806.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cliver SP, Goldenberg RL, Cutter GR, et al. The relationships among psychosocial profile, maternal size, and smoking in predicting fetal growth retardation. Obstet. Gynecol. 1992;80(2):262–267. [PubMed] [Google Scholar]
- 30.Dewan N, Brabin B, Wood L, Dramond S, Cooper C. The effects of smoking on birthweight-for-gestational-age curves in teenage and adult primigravidae. Public Health. 2003;117(1):31–35. doi: 10.1016/s0033-3506(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 31.Cnattingius S. Does age potentiate the smoking-related risk of fetal growth retardation? Early Hum. Dev. 1989;20(3–4):203–211. doi: 10.1016/0378-3782(89)90006-6. [DOI] [PubMed] [Google Scholar]
- 32.Backe B. Maternal smoking and age. Effect on birthweight and risk for small-forgestational age births. Acta Obstet. Gynecol. Scand. 1993;72(3):172–176. doi: 10.3109/00016349309013367. [DOI] [PubMed] [Google Scholar]
- 33.Shepard MJ, Bakketeig LS, Jacobsen G, O'Connor T, Bracken MB. Maternal body mass, proportional weight gain, and fetal growth in parous women. Paediatr. Perinat. Epidemiol. 1996;10(2):207–219. doi: 10.1111/j.1365-3016.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 34.Laml T, Hartmann BW, Kirchengast S, Preyer O, Albrect AH, Husslein PW. Impact of maternal anthropometry and smoking on neonatal birthweight. Gynecol. Obstet. Invest. 2000;50(4):231–236. doi: 10.1159/000010322. [DOI] [PubMed] [Google Scholar]
- 35.Aagaard-Tillery KM, Porter TF, Lane RH, et al. In utero tobacco exposure is associated with modified effects of maternal factors on fetal growth. Am. J. Obstet. Gynecol. 2008;198:66.E1–66.E6. doi: 10.1016/j.ajog.2007.06.078. [DOI] [PubMed] [Google Scholar]
- 36.Pipkin FB, Genetics of Preeclampsia Consortium Smoking in moderate/severe preeclampsia worsens pregnancy outcome, but smoking cessations limits the damage. Hypertension. 2008;51(4):1042–1046. doi: 10.1161/HYPERTENSIONAHA.107.106559. [DOI] [PubMed] [Google Scholar]
- 37.Lindqvist PG, Marsal K. Moderate smoking during pregnancy is associated with a reduced risk of preeclampsia. Acta Obstet. Gynecol. Scand. 1999;78:693–697. [PubMed] [Google Scholar]
- 38.Zhang J, Klebanoff MA, Levine RJ, Puri M, Moyer P. The puzzling association between smoking and hypertension during pregnancy. Am. J. Obstet. Gynecol. 1999;181:1407–1413. doi: 10.1016/s0002-9378(99)70384-4. [DOI] [PubMed] [Google Scholar]
- 39.Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am. J. Obstet. Gynecol. 1999;4:1026–1035. doi: 10.1016/s0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen S, Irgens LM. The effects of smoking and hypertensive disorders on fetal growth. BMC Pregnancy Childbirth. 2006;6:16. doi: 10.1186/1471-2393-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernstein IM, Mongeon JA, Badger GJ, Solomon L, Heil SH, Higgins ST. Maternal smoking and its association with birthweight. Obstet. Gynecol. 2005;106(5–1):986–991. doi: 10.1097/01.AOG.0000182580.78402.d2. [DOI] [PubMed] [Google Scholar]
- • Prospective study that demonstrated that for each additional cigarette per day consumed in the third trimester, there is an estimated 27-g reduction in birthweight.
- 42.Ward C, Lewis S, Coleman T. Prevalence of maternal smoking and environmental tobacco smoke exposure during pregnancy and impact on birthweight: retrospective study using Millenium Cohort. BMC Public Health. 2007;7:81. doi: 10.1186/1471-2458-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bardy AH, Seppala T, Lillsunde P, et al. Objectively measured tobacco exposure during pregnancy: neonatal effects and relation to maternal smoking. Br. J. Obstet. Gynaecol. 1993;100:721–726. doi: 10.1111/j.1471-0528.1993.tb14262.x. [DOI] [PubMed] [Google Scholar]
- 44.Klebanoff MA, Levine RJ, Clemens JD, DerSimonian R, Wilkins DG. Serum cotinine concentration and self-reported smoking during pregnancy. Am. J. Epidemiol. 1998;148(3):259–262. doi: 10.1093/oxfordjournals.aje.a009633. [DOI] [PubMed] [Google Scholar]
- 45.George L, Granath F, Johansson AL, Cnattingius S. Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy. Acta Obstet. Gynecol. Scand. 2006;85(11):1331–1337. doi: 10.1080/00016340600935433. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Tager IB, Van Vunakis H, Speizer FE, Hanrahan JP. Maternal smoking during pregnancy, urine cotinine concentrations and birth outcomes. A prospective cohort study. Int. J. Epidemiol. 1997;26(5):978–988. doi: 10.1093/ije/26.5.978. [DOI] [PubMed] [Google Scholar]
- 47.England LJ, Kendrick JS, Gargiullo PM, Zahniser C, Hannon WH. Measures of maternal tobacco exposure and infant birthweight at term. Am. J. Epidemiol. 2001;153(10):954–960. doi: 10.1093/aje/153.10.954. [DOI] [PubMed] [Google Scholar]
- 48.Ellard GA, Johnstone FD, Prescott RJ, Ji-Xian W, Jian-Hua M. Smoking during pregnancy: the dose dependence of birthweight deficits. Br. J. Obstet. Gynaecol. 1996;103:806–813. doi: 10.1111/j.1471-0528.1996.tb09878.x. [DOI] [PubMed] [Google Scholar]
- 49.Martinez FD, Wright AL, Taussig LM. The effect of paternal smoking on birthweight of newborns whose mothers did not smoke. Am. J. Public Health. 1994;84(9):1489–1491. doi: 10.2105/ajph.84.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hegaard HK, Kjaergaard H, Møller LF, Wachmann H, Ottesen B. The effect of environmental tobacco smoke during pregnancy on birthweight. Acta Obstet. Gynecol. Scand. 2006;85(6):675–681. doi: 10.1080/00016340600607032. [DOI] [PubMed] [Google Scholar]
- 51.Goel P, Radotra A, Singh I, Aggarwal A, Dua D. Effects of passive smoking on outcome in pregnancy. J. Postgrad. Med. 2004;50(1):12–16. [PubMed] [Google Scholar]
- 52.Kharrazi M, DeLorenze GN, Kaufman FL, et al. Environmental tobacco smoke and pregnancy outcome. Epidemiology. 2004;15(6):660–670. doi: 10.1097/01.ede.0000142137.39619.60. [DOI] [PubMed] [Google Scholar]
- • Recent study that demonstrated the effects of passive exposure to smoke on birthweight.
- 53.Brunnemann K, Hoffmann D. Analytical studies on tobacco-specific N-nitrosamines in tobacco and tobacco smoke. Crit. Rev. Toxicol. 1991;21:235–240. doi: 10.3109/10408449109017910. [DOI] [PubMed] [Google Scholar]
- 54.Bartsch H, Nair U, Rish A, et al. Genetic polymorphism of CYP genes, alone or in combination as a risk modifier of tobacco-related cancers. Cancer Epidemiol. Biomarkers Prev. 2000;9:3–28. [PubMed] [Google Scholar]
- 55.Timbrell J. Principles of Biochemical Toxicology. 2nd Edition Taylor and Francis; Washington, DC, USA: 1991. [Google Scholar]
- 56.Wang Z, Zuckerman B, Pearson C, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birthweight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- • Case-control study that demonstrated a relationship between genetic polymorphism, environmental exposure and birthweight.
- 57.Wu T, Hu Y, Chen C. 2007;166(3):313–322. doi: 10.1093/aje/kwm090. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki S, Kondo T, Sata F, et al. Maternal smoking during pregnancy and genetic polymorphisms in the Ah receptor, CYP1A1 and GSTM1 affect infant birth size in Japanese subjects. Mol. Hum. Reprod. 2006;12(2):77–83. doi: 10.1093/molehr/gal013. [DOI] [PubMed] [Google Scholar]
- 59.Cliver SP, Goldenberg RL, Cutter GR, Hoffman HJ, Davis RO, Nelson KG. The effect of cigarette smoking on neonatal anthropometric measurements. Obstet. Gynecol. 1995;84(4):625–630. doi: 10.1016/0029-7844(94)00437-I. [DOI] [PubMed] [Google Scholar]
- 60.Zaren B, Lindmark G, Gebre-Medhin M. Maternal smoking and body composition of the newborn. Acta Paediatr. 1996;85:213–219. doi: 10.1111/j.1651-2227.1996.tb13995.x. [DOI] [PubMed] [Google Scholar]
- 61.Lindsay CA, Thomas AJ, Catalano PM. The effect of smoking tobacco on neonatal body composition. Am. J. Obstet. Gynecol. 1997;177(5):1124–1128. doi: 10.1016/s0002-9378(97)70027-9. [DOI] [PubMed] [Google Scholar]
- 62.D'Souza SW, Black P, Richards B. Smoking in pregnancy: associations with skinfold thickness, maternal weight gain, and fetal size at birth. BMJ. 2821981:1661–1663. doi: 10.1136/bmj.282.6277.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison GG, Branson RS, Vaucher YE. Association of maternal smoking with body composition of the newborn. Am. J. Clin. Nutr. 1983;38:757–762. doi: 10.1093/ajcn/38.5.757. [DOI] [PubMed] [Google Scholar]
- 64.Salas SP, Rosso P, Espinoza R, Robert JA, Valdes G, Donoso E. Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation. Obstet. Gynecol. 1993;81:1029–1033. [PubMed] [Google Scholar]
- 65.Duvekot JJ, Cheriex EC, Pieters FAA, Menherre PPCA, Schouten HJA, Peeters LLH. Maternal volume homeostasis in early pregnancy in relation to fetal growth restriction. Obstet. Gynecol. 1995;85:361–367. doi: 10.1016/0029-7844(94)00417-C. [DOI] [PubMed] [Google Scholar]
- 66.Steer PJ. Maternal hemoglobin concentration and birthweight. Am. J. Clin. Nutr. 2000;71:1285–1287. doi: 10.1093/ajcn/71.5.1285s. [DOI] [PubMed] [Google Scholar]
- 67.Scanlon KS, Yip R, Schieve LA, et al. High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet. Gyncol. 2000;5:741–748. doi: 10.1016/s0029-7844(00)00982-0. [DOI] [PubMed] [Google Scholar]
- 68.Mau G. Hemoglobin changes during pregnancy and growth disturbances in the neonate. J. Perinat. Med. 1977;5:172–177. doi: 10.1515/jpme.1977.5.4.172. [DOI] [PubMed] [Google Scholar]
- 69.Yazdani M, Tadbiri M, Shakeri S. Maternal hemoglobin level, prematurity, and low birthweight. Int. J. Gyncol Obstet. 2004;85:163–164. doi: 10.1016/j.ijgo.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Murphy JF, O'Riordan J, Newcombe RG, et al. Relation of haemoglobin levels in first and second trimesters to outcome of pregnancy. Lancet. 1986;1(8488):992–995. doi: 10.1016/s0140-6736(86)91269-9. [DOI] [PubMed] [Google Scholar]
- 71.Pirani BBK, MacGillivray I. Smoking during pregnancy: its effect on maternal metabolism and fetoplacental function. Obstet. Gynecol. 1978;52:257–263. [PubMed] [Google Scholar]
- 72.Bruinse HW, van den Berg H, Haspels AA. Smoking and its effect on maternal plasma volume during and after normal pregnancy. Eur. J. Obstet. Gynecol. 1985:215–219. doi: 10.1016/0028-2243(85)90067-x. [DOI] [PubMed] [Google Scholar]
- 73.Nilsen ST, Sagen N, Kim HC, Bergsjo P. Smoking hemoglobin levels and birthweights in normal pregnancies. Am. J. Obstet. Gynecol. 1984;148:752–758. doi: 10.1016/0002-9378(84)90561-1. [DOI] [PubMed] [Google Scholar]
- 74.Bergsjø P, Bakketeig L, Lindmark G. Maternal smoking does not affect fetal size as measured in the mid-second trimester. Acta Obstet. Gynecol. Scand. 2007;86(2):156–160. doi: 10.1080/00016340600984696. [DOI] [PubMed] [Google Scholar]
- 75.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements – a prospective study. Am. J. Obstet. Gynecol. 1985;151:333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 76.Hemachandra AH, Klebanoff MA. Use of serial ultrasound to identify periods of fetal growth restriction in relation to neonatal anthropometry. Am. J. Hum. Biol. 2006;18(6):791–797. doi: 10.1002/ajhb.20552. [DOI] [PubMed] [Google Scholar]
- • Demonstrates that in smokers, low birthweight is best predicted by third trimester ultrasonographic detection of intrauterine growth restriction.
- 77.Jeanty P, Cousaert E, de Maertelaer V, Cantaine F. Sonographic detection of smoking-related decreased fetal growth. J. Ultrasound Med. 1987;6:13–18. doi: 10.7863/jum.1987.6.1.13. [DOI] [PubMed] [Google Scholar]
- 78.Newnham JP, Patterson L, James I, Reid SE. Effects of maternal cigarette smoking on fetal growth and on Doppler flow velocity waveforms. Early Hum. Dev. 1990;24:23–36. doi: 10.1016/0378-3782(90)90003-2. [DOI] [PubMed] [Google Scholar]
- 79.Bernstein IM, Plociennic K, Stahle S, Badger GJ, Secker-Walker R. Impact of maternal cigarette smoking on fetal growth and body composition. Am. J. Obstet. Gynecol. 2000;183:883–886. doi: 10.1067/mob.2000.109103. [DOI] [PubMed] [Google Scholar]
- 80.Jauniax E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum. Dev. 2007;83(11):699–706. doi: 10.1016/j.earlhumdev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 81.Pastrakuljic A, Derewlany LO, Koren G. Maternal cocaine use and cigarette smoking in pregnancy in relation to amino acid transport and fetal growth. Placenta. 1999;20(7):499–512. doi: 10.1053/plac.1999.0418. [DOI] [PubMed] [Google Scholar]
- 82.Castro LC, Allen R, Ogenyemi D, Roll K, Platt LD. Cigarette smoking during pregnancy: acute effects on uterine flow velocity waveforms. Obstet. Gynecol. 1993;81(4):551–555. [PubMed] [Google Scholar]
- 83.Kimya Y, Cengiz C, Ozan H, Kolsal N. Acute effects of maternal smoking on the uterine and umbilical artery blood velocity waveforms. J. Matern. Fetal Investig. 1998;8(2):79–81. [PubMed] [Google Scholar]
- 84.Albuquerque CA, Smith KR, Johnson C, Chao R, Harding R. Influence of maternal tobacco smoking during pregnancy on uterine, umbilical and fetal cerebral artery blood flows. Early Hum. Dev. 2004;80(1):31–42. doi: 10.1016/j.earlhumdev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 85.Kalinka J, Hanke W, Sobala W. Impact of prenatal tobacco smoke exposure, as measured by midgestation serum cotinine levels, on fetal biometry and umbilical flow velocity waveforms. Am. J. Perinatol. 2005;22(1):41–47. doi: 10.1055/s-2004-837266. [DOI] [PubMed] [Google Scholar]
- 86.Yerushalmy J. The relationship of parents' cigarette smoking to outcome of pregnancy: implications as to the problem of inferring causation from observed associations. Am. J. Epidemiol. 1971;93:443–446. doi: 10.1093/oxfordjournals.aje.a121278. [DOI] [PubMed] [Google Scholar]
- 87.Sexton M, Hebel JR. A clinical trial of change in maternal smoking and its effect on birthweight. JAMA. 1984;251:911–915. [PubMed] [Google Scholar]
- 88.MacArthur C, Newton JR, Knox EG. Effect of anti-smoking health education on infant size at birth: a randomized controlled trial. Br. J. Obstet. Gynaecol. 1987;94:295–300. doi: 10.1111/j.1471-0528.1987.tb03094.x. [DOI] [PubMed] [Google Scholar]
- 89.Haddow JE, Knight GJ, Kloza EM, Plamaki GE, Wald NJ. Cotinine-assisted intervention in pregnancy to reduce smoking and low birthweight delivery. Br. J. Obstet. Gynaecol. 1991;98:859–865. doi: 10.1111/j.1471-0528.1991.tb13506.x. [DOI] [PubMed] [Google Scholar]
- 90.Dolan-Mullen P, Ramirez G, Groff JY. A meta-analysis of randomized trials of prenatal smoking cessation interventions. Am. J. Obstet. Gynecol. 1994;171:1328–1334. doi: 10.1016/0002-9378(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 91.Heil SH, Higgins ST, Bernstein IM, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–1018. doi: 10.1111/j.1360-0443.2008.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• Prospective randomized controlled trial showing a significant reduction in the amount of smoking during pregnancy using vouchers given upon maternal compliance to cessation.
- 92.Lieberman E, Gremy I, Lang JM, Cohen AP. Low birthweight at term and the timing of fetal exposure to maternal smoking. Am. J. Public Health. 1994;84(7):1127–1131. doi: 10.2105/ajph.84.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Shows that quitting smoking prior to the third trimester will reduce the risk of having a newborn with low birthweight.
- 93.MacArthur C, Knox EG. Smoking in pregnancy: effects of stopping at different stages. Br. J. Obstet. Gynaecol. 1988;95(6):551–555. doi: 10.1111/j.1471-0528.1988.tb09481.x. [DOI] [PubMed] [Google Scholar]
- 94.England LJ, Kendrick JS, Wilson HG, Merritt RK, Gargiullo PM, Zahniser SC. Effects of smoking reduction during pregnancy on the birthweight of term infants. Am. J. Epidemiol. 2001;154:694–701. doi: 10.1093/aje/154.8.694. [DOI] [PubMed] [Google Scholar]
- 95.Li CQ, Windsor RA, Perkins L, Goldenberg RL, Lowe JB. The impact on infant birthweight and gestational age of cotinine-validated smoking reduction during pregnancy. JAMA. 1993;269:1519–1524. [PubMed] [Google Scholar]