Abstract
In natural systems, pre-adult stages of some insect herbivores are known to be attacked by several species of parasitoids. Under certain conditions, hosts may be simultaneously parasitised by more than one parasitoid species (= multiparasitism), even though only one parasitoid species can successfully develop in an individual host. Here, we compared development, survival, and intrinsic competitive interactions amongst three species of solitary larval endoparasitoids, Campoletis sonorensis (Cameron) (Hymenoptera: Ichneumonidae), Microplitis demolitor Wilkinson, and Microplitis croceipes (Cresson) (Hymenoptera: Braconidae), in singly parasitised and multiparasitised hosts. The three species differed in certain traits, such as in host usage strategies and adult body size. Campoletis sonorensis and M. demolitor survived equally well to eclosion in two host species that differed profoundly in size, Pseudoplusia includens (Walker) and the larger Heliothis virescens (Fabricius) (both Lepidoptera: Noctuidae). Egg-to-adult development time in C. sonorensis and M. demolitor also differed in the two hosts. Moreover, adult body mass in C. sonorensis (and not M. demolitor) was greater when developing in H. virescens larvae. We then monitored the outcome of competitive interactions in host larvae that were parasitised by one parasitoid species and subsequently multiparasitised by another species at various time intervals (0, 6, 24, and 48 h) after the initial parasitism. These experiments revealed that M. croceipes was generally a superior competitor to the other two species, whereas M. demolitor was the poorest competitor, with C. sonorensis being intermediate in this capacity. However, competition sometimes incurred fitness costs in M. croceipes and C. sonorensis, with longer development time and/or smaller adult mass observed in surviving wasps emerging from multiparasitised hosts. Our results suggest that rapid growth and large size relative to competitors of a similar age may be beneficial in aggressive intrinsic competition.
Keywords: Campoletis sonorensis, Heliothis virescens, Microplitis croceipes, Microplitis demolitor, multiparasitism, Pseudoplusia includens, Hymenoptera, Braconidae, Ichneumonide, Lepidoptera, Noctuidae, Pseudoplusia includens, Heliothis virescens
Introduction
In studying the evolution or development strategies in arthropods, parasitoid wasps have proven to be model organisms (Harvey, 2005). The development of parasitoids is known to vary with host quality, which describes differences in the state or the condition of the host and how this affects the performance of the parasitoid offspring. For instance, immature parasitoid growth, development, and survival has been shown to be influenced by such factors as the size, age, or stage of the host parasitised, host species, and on the nutritional status of the host during the course of parasitism (Sequeira & Mackauer, 1992; Pettit & Wieslebach, 1993; Harvey et al., 1994; Harvey & Vet, 1997; Harvey, 2000; Ode, 2006). Host quality-related effects on parasitoid development will ultimately influence adult fitness, based on the ability of parasitoids to secure mates and/or to locate host patches, and most importantly on their lifetime reproductive success (see reviews by Godfray, 1994; Strand, 2000; Harvey, 2005).
Some insect herbivores are hosts for several species of parasitoids (Hawkins, 1994). In order to diffuse competition for host resources, most parasitoids have evolved highly specialized behavioral and physiological strategies for exploiting different host stages. This has resulted in the formation of guilds of parasitoids each of which is restricted to attacking a specific stage in the host's life cycle, for example eggs, larvae, or pupae (Godfray, 1994). Parasitoids in each guild exhibit a suite of adaptations that most effectively enable them to exploit their hosts (Price, 1970, 1972). However, even though interspecific competition amongst parasitoids may have been diffused by host-stage specialization, many parasitoid guilds remain species-rich (Hawkins, 1994; Elzinga et al., 2007). Consequently, it is possible that under certain conditions, such as when suitable hosts are scarce, different parasitoid species may compete both extrinsically (e.g., during the host selection process by adult females) and intrinsically (i.e., during development by immature stages) for access to and control of host resources.
In nature, two or more parasitoid species may attack and oviposit in the same individual host, a process known as multiparasitism. In multiparasitised hosts, the progeny of only one species survives with the superior competitor destroying the inferior competitor (Godfray, 1994). Intrinsic interspecific competition in parasitoids has received considerable attention over the years (e.g., Fisher, 1963; Wen & Brower, 1995; De Moraes et al., 1999; Cusson et al., 2002; Wang & Messing, 2003; Bajpai et al., 2006; Tian et al., 2008). The two main mechanisms by which one parasitoid species excludes the other are physical attack and physiological suppression. In the former, the first instars of many solitary parasitoids possess sickle-like mandibles that are used to kill competitors (Quicke, 1997; Tian et al., 2008), whereas the latter involves monopolization of dissolved oxygen in the host or the production of toxic factors by the dominant species (Strand & Vinson, 1984).
Examples of multiparasitism have often been reported in koinobiont endoparasitoids attacking young larvae of lepidopterous hosts (Fisher, 1963; Laing & Corrigan, 1987; De Moraes et al., 1999; Cusson et al., 2002; Marktl et al., 2002; Tian et al., 2008). Koinobionts attack hosts that continue feeding and growing during the course of parasitism, and thus hosts represent dynamic resources that can change dramatically in size between parasitism and death (Askew & Shaw, 1986; Mackauer, 1986; Harvey et al., 1994). However, the size (or stage) of the host when it is developmentally arrested by the parasitoid depends on several factors, including the size of the host at parasitism and the nutritional requirements of the parasitoid progeny (Mackauer & Sequeira, 1993). Most importantly, these characteristics are often association-specific, with smaller parasitoid species arresting host growth earlier than larger parasitoid species.
In this study we compare development and intrinsic competitive interactions amongst three species of solitary larval endoparasitoids. We first examined development in Campoletis sonorensis (Cameron) (Hymeoptera: Ichneumonidae) and Microplitis demolitor Wilkinson (Hymenoptera: Braconidae) in two species of hosts that vary considerably in their growth potential: Pseudoplusia includens (Walker) and the larger Heliothis virescens (Fabricius) (both Lepidoptera: Noctuidae). The development of Microplitis croceipes (Cresson) (Hymenoptera: Braconidae) was also examined in larvae of H. virescens. We then monitored the outcome of competitive interactions in host larvae that were parasitised by one parasitoid species and subsequently multiparasitised by another species at various time intervals. Finally, we compared development time and adult body mass in parasitoids developing in singly parasitised and multiparasitised hosts. Our results reveal that large size may play an important role in resolving intrinsic conflicts amongst competing parasitoids. Furthermore, even for the winning competitor, we report that there may be significant costs on adult fitness correlates such as development time and adult size, processes which have been rarely examined in other empirical studies.
Methods and materials
Insects
All hosts and parasitoids were reared at 27 ± 2 °C, with 65 ± 5% r.h., and a L16:D8 photocycle. Larvae of P. includens were originally obtained from a culture maintained at Clemson University (Clemson, SC, USA). Host larvae were reared in 30-ml plastic cups on artificial diet (Strand, 1990). Moth pupae were placed in 4–1 glass jars covered by netting that was secured by elastic bands. Newly emerged male and female adult moths were allowed to mate in the jars and were fed with a 20% (wt/vol)sucrose solution. Female moths oviposited directly onto the cotton netting. When they are fully grown, healthy late fifth instars (L5) of P. includens typically attain fresh body masses of 200–300 mg. The complete biology of P. includens is described in Strand (1990).
Heliothis virescens larvae were originally obtained from a culture kept at the University of Kentucky (Lexington, KY, USA). Larvae and adults of H. virescens were reared using the same methods as described above for P. includens. Various instars and fully-grown L5 larvae of H. virescens are significantly larger than corresponding larval instars of P. includens. Fully-grown larvae, for example, can grow to between 500 and 600 mg. The full biology of H. virescens is described by Neunzig (1969).
Campoletis sonorensis was obtained from a culture maintained for many generations at Texas A & M University (College Station, TX, USA). This Nearctic species reproduces sexually and is known to attack ~30 species of Macrolepidoptera in the large family Noctuidae (Lingren et al., 1970). Female wasps locate hosts both visually and with their antennae and then rapidly oviposit into the host haemocoel. The parasitoid larvae develop by initially feeding on host haemolymph and fat body but as it approaches maturity it begins to attack other tissues indiscriminately, eventually consuming virtually the entire host except for the larval head capsule (Harvey & Strand, 2002). The parasitoid pupates adjacent to the host carcass on the food plant and adults emerge about a week later.
Microplitis demolitor was obtained from a culture maintained at the University of Georgia (Athens, GA, USA). This sexually reproducing parasitoid originates from Africa and Asia where it attacks a number of hosts in the Noctuidae, and was introduced into the USA in the 1980's as a biological control agent against P. includens (Shepard et al., 1983). During a single oviposition event adult wasps typically lay 1–3 eggs into the host haemocoel, although only one wasp generally survives to eclosion (Strand et al., 1988; Harvey et al., 2004). Mature parasitoid larvae perforate the host cuticle with their mandibles and emerge through the hole where they spin a cocoon and pupate adjacent to the host caterpillar, usually on the host food plant. Adult wasps emerge within several
Microplitis croceipes was obtained from a culture maintained at the University of Kentucky (Lexington, KY, USA). This sexually reproducing species is native to the Nearctic and attacks the caterpillars of several species in the Noctuidae (Lewis, 1970). Wasps oviposit into the hemoceol of host larvae, and the parasitoid larvae feed primarily on host haemolymph and fat body during their development. Towards the end of parasitoid development, the host crawls into leaf litter or loose soil in order to prepare a pupal cell. During this time the mature parasitoid larva chews a hole in the side of the host, egresses, and pupates. Adult wasps emerge several days later.
Experimental protocol
Comparison of body masses in L2 larvae of Pseudoplusia includens and Heliothis virescens.
Based on their head capsule dimensions, L2 larvae of P. includens and H. virescens were removed from artificial diet and individually weighed (accuracy 1 μg) on a Cahn 29 Electronic Microbalance (Cahn Instruments, Cerritos, CA, USA).
Development of Campoletis sonorensis, Microplitis demolitor, and Microplitis croceipes in L2 larvae of Pseudoplusia includens and/or Heliothis virescens. Larvae of P. includens and H. virescens were parasitised as L2 by 5–10-day-old females of C. sonorensis and M. demolitor by presenting individual larvae to parasitoids at the end of a brush in plastic vials. Parasitism was verified by allowing wasps to insert and remove the ovipositor. Parasitised caterpillars were placed immediately in plastic cups containing artificial diet as described by Strand (1990). Because the survival of M. croceipes in P. includens larvae is very low (K Kadash, pers. comm.) the development of this parasitoid was only monitored in H. virescens. The protocol for parasitism of H. virescens by M. croceipes was the same as described above for the other host-parasitoid combinations. Parasitised larvae were then monitored until (a) parasitoid eclosion, (b) host eclosion, or (c) neither species emerged, recorded as `precocious host death'. Upon eclosion, newly emerged parasitoid adults were anesthetized using C02 and weighed on the Cahn 29 microbalance. Offspring sex was also determined. Oviposition-to-adult development time was recorded in days.
Multiparasitism by Campoletis sonorensis, Microplitis demolitor, and Microplitis croceipes in pairwise contests. Multiparasitism was studied by determining the outcome of time-lapse contests between C. sonorensis and M. demolitor in larvae of P. includens and H. virescens, and between these two parasitoids individually and M. croceipes in larvae of H. virescens only. Individual hosts were singly parasitised by one parasitoid species (as described earlier) and then subsequently multiparasitised by a second parasitoid species at various time intervals following the initial parasitism event. These intervals were: 0, 6, 24, and 48 h. Dissections of L2 P. includens larvae revealed that both C. sonorensis and M. demolitor readily superparasitised hosts, even when they already contained several eggs from conspecific females (see also Strand et al., 1988). Because C. sonorensis and M. demolitor (but not M. croceipes) could develop to eclosion in the small (P. includens) and the large (H. virescens) host species, intrinsic competition in these two parasitoids was compared in both of these hosts. Therefore, the following host-parasitoid + parasitoid combinations were compared, in L2 larvae of P. includens: C. sonorensis +0 h, +6 h, +24 h, +48 h versus M. demolitor, and reciprocally, M. demolitor +0 h, +6 h, +24 h, +48 h versus M. demolitor. Multiparasitism was also tested between the same two parasitoid species in L2 H. virescens larvae, but the +6 h treatment was excluded because of insufficient numbers of replicates. This process was repeated with M. croceipes in competition with either C. sonorensis or M. demolitor in L2 H. virescens larvae only. All parasitised (and subsequently multiparasitised) hosts were reared in artificial diet in plastic cups. The fate of multiparasitised hosts was determined as (1) winning parasitoid species `a', (2) winning parasitoid species `b', (3) successful herbivore pupation, or (4) precocious host larval death. The winning parasitoid was that species which managed to egress from the host and successfully construct a cocoon. Upon adult eclosion, the egg-to-adult development time, in days, and adult body mass, in mg, of the adult parasitoid was determined. These data were compared with data on these same fitness correlates in wasps emerging from singly parasitised hosts (= controls). However, an insufficient number of male and/or female wasps emerged from multiparasitised hosts in some treatments to compare with controls. Therefore, data on development time and adult body mass of emerging adult parasitoids that had oviposited first in the four time intervals (0, 6, 24, and 48 h) were pooled.
Statistical analysis
Within each instar, larval mass in L1–L4 P. includens and H. virescens larvae were compared using Student's t-tests. Data on the fate of C. sonorensis and M. demolitor, respectively, when developing in two different host species were analysed using a χ2-test with H0: fate of the parasitoid (dead or alive) did not differ between the two host species. The outcome of competition in multiparasitised hosts was compared using a binomial test with H0: both species have equal probability to win the competition (p = q = 0.5, two tailed). Hosts that did not produce a parasitoid were excluded from the analyisis. When developing in the two host species data on development time and adult body mass in C. sonorensis and M. demolitor were separately compared using two-way analysis of variance (ANOVA) with host species, offspring sex, and their interaction as factors. Post-hoc comparisons were made using Tukey-Kramer tests for data with unequal sample sizes. Data comparing development time and body size in male and female M. croceipes was made using Student's t-tests. Development time and adult body mass data for parasitoids emerging from singly and multiparasitised hosts were compared using two-way ANOVA's with treatment (single or multiparasitism), offspring sex, and their interaction as factors. Post-hoc comparisons were made using Tukey-Kramer for data with unequal sample sizes. All data on development time were log-transformed to meet assumptions on normality and homoschedasticity. All statistical analyses were made using Minitab Statistical Software version 15, State College, PA, USA.
Results
Comparison of L1–L4 larval body masses in Pseudoplusia includens and Heliothis virescens larvae
Within-instar comparisons of the mass of L2 larvae of both herbivores revealed that L2 H. virescens larvae were significantly heavier (L2: t32 = 3.96, P<0.001; L3: t53 = 4.14, P<0.001; L4: t65 = 6.34, P<0.0001)
Development of Campoletis sonorensis and Microplitis demolitor in larvae of Pseudoplusia includens and Heliothis virescens
The fate of C. sonorensis (χ21 = 1.45, P = 0.23) and M. demolitor (χ21 = 1.45, P = 0.10) did not vary with host species. Slightly (but not significantly) more C. sonorensis wasps survived to pupation in the smaller host, P. includens, whereas the opposite was true for M. demolitor (data not shown). Furthermore, of all L2 hosts parasitised by the two parasitoids, only a single P. includens attacked by C. sonorensis was able to successfully pupate.
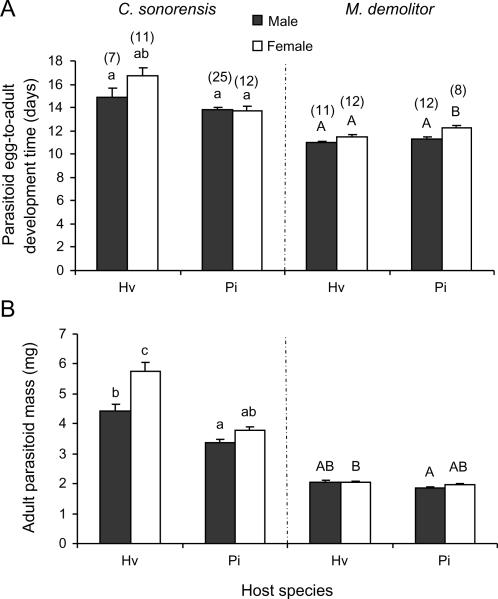
Egg-to-adult development time of C. sonorensis varied significantly with host species (ANOVA: F1,51 = 18.87, P<0.001). The interaction between host and sex was also significant (F1,51 = 4.16, P = 0.05). Development was extended in H. virescens larvae and took longer in female than in male wasps but only when reared on this host (Figure 1A). Adult body mass also varied significantly with host species in C. sonorensis (F1,51 = 55.89, P<0.001) and offspring sex (F1,51 = 18.46, P<0.001) with a significant interactive effect between these parameters also found on mass (F1,51 = 4.62, P = 0.03). Parasitoids were much larger when developing in H. virescens larvae, and female wasps were larger than male wasps, but this was only significant when the wasps developed in H. virenscens (Figure 1B).
Figure 1.
Development of Campoletis sonorensis (left panel) and Microplitis demolitor (right panel) of males (black bars) and females (open bars) in L2 caterpillars of Heliothis virescens (Hv) and Pseudoplusia includens (Pi). (A) Mean egg-to-adult development time (days) and (B) mean adult fresh body mass (mg). Line bars represent standard errors of the mean with the sample size between brackets. Bars with different letters are significantly different (Tukey-Kramer test: P<0.05). Data were analysed separately for the two parasitoid species as indicated by small and capital letters.
Egg-to-adult development time of M. demolitor varied significantly with host species (F1,39 = 10.44, P = 0.003) and offspring sex (F1,39 = 18.01, P<0.001). The interactive effect between these parameters on development time was not significant (F1,39 = 1.32, P = 0.26). In contrast with the pattern observed in C. sonorensis, development took somewhat longer in P. includens larvae (Figure 1A). Adult body mass also varied significantly with host species in M. demolitor (F1,39= 5.74, P = 0.02) but not with offspring sex (F1,39 = 1.30; P = 0.26), nor was there a significant interactive effect between these parameters on mass (F1,39 = 0.43, P = 0.52). Parasitoids were larger when developing in H. virescens larvae (Figure 1B).
Development time (t30 = 2.84, P<0.01) but not adult mass (t30 = 0.33, P = 0.75) differed significantly between male and females of M. croceipes when developing in L2 larvae of H. virescens. Females took almost a full day longer to complete their development than males. On the other hand, body mass of newly emerged female and male parasitoids was almost identical.
The outcome of competitive interactions in multiparasitised hosts
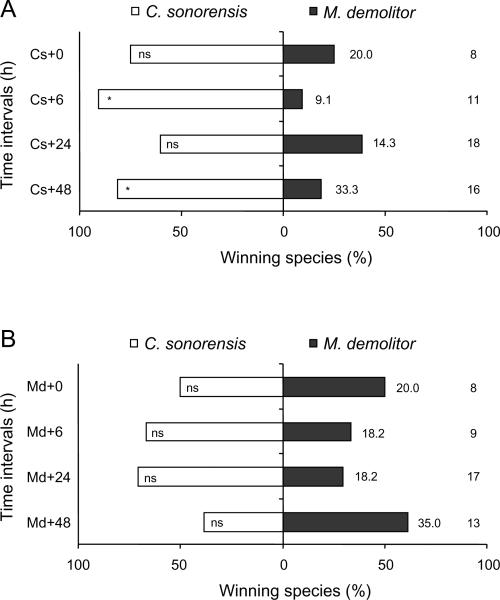
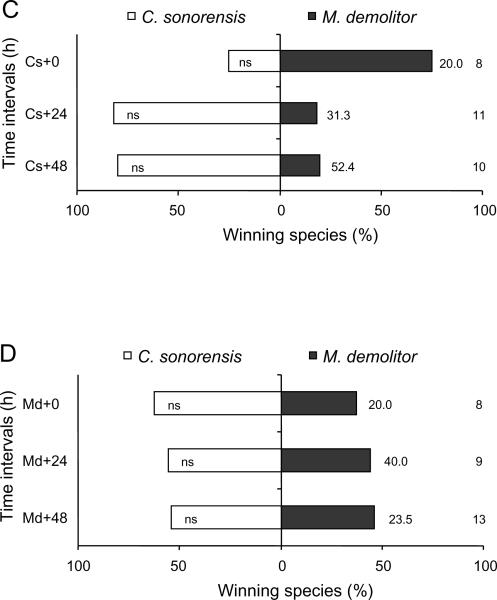
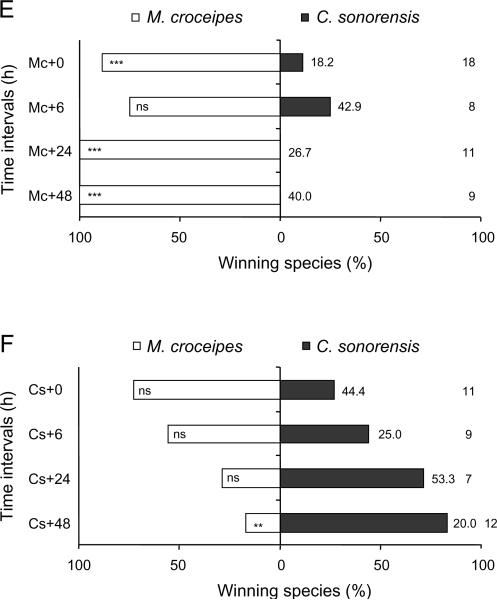
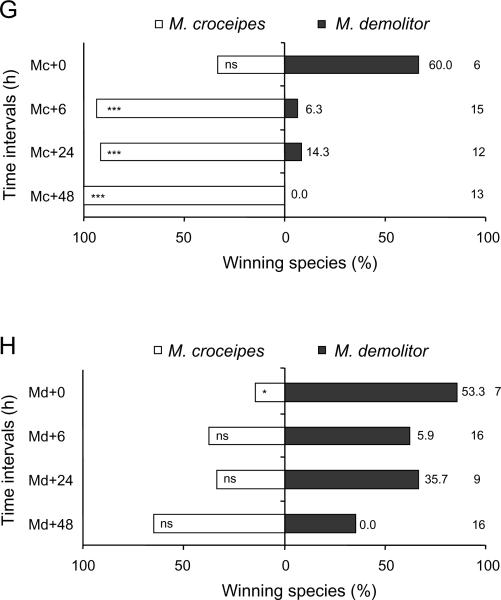
From a total of 452 multiparasitised P. includens and H. virescens, 311 (68.8%) yielded a parasitoid and none survived to produce a moth pupa. The outcome of competition among parasitoids, however, varied with order of oviposition and wasp species (Figure 2). Consider first wasp survival. In P. includens, we detected no difference in survival of C. sonorensis and M. demolitor when wasps parasitised hosts simultaneously (0 h) or when C. sonorensis parasitised a host 6 h earlier than M. demolitor. Significantly more C. sonorensis survived, however, when this parasitoid species had a 6 h (binomial test: P = 0.02) or 48 h (P = 0.02) temporal advantage over M. demolitor (Figure 2A). Neither wasp species preferentially survived when M. demolitor oviposited first in P. includens (Figure 2B). The outcome of competition between C. sonorensis and M. demolitor in H. virescens was very similar: C. sonorensis was only a marginally superior competitor when ovipositing 24 h (P = 0.07) or 48 h (P = 0.07) ahead of M. demolitor (Figure 2C) and neither wasp preferentially survived when M. demolitor oviposited first in H. virescens (Figure 2D). In the case of M. croceipes, this species preferentially survived in competition with C. sonorensis when H. virescens were parasitised simultaneously (0 h) (P<0.001) and when M. croceipes had a 24 h (P<0.001) or 48 h (P<0.001) temporal advantage (Figure 2E). Croceipes sonorensis in contrast only out-competed M. croceipes when it had a 48 h advantage (P = 0.04; Figure 2F). Microplitis croceipes similarly outcompeted M. demolitor when given a 6 h (P<0.001), 24 h (t11 = 5.00, P<0.01), or 48 h (P<0.001) temporal advantage.
Figure 2.
Percentage emergence and pupation by Campoletis sonorensis (Cs), Microplitis demolitor (Md), and M. croceipes (Mc) in pairwise contests at different time intervals (indicated in hours by the symbol `+'). 0, 6, 24, and 48 h refer to the time interval (in hours) between the initial parasitism and subsequent multiparasitism. (A, B) Croceipes sonorensis vs. M. demolitor in larvae of Pseudoplusia includens, (C, D) C. sonorensis vs. M. demolitor in larvae of Heliothis virescens, (E, F) M. croceipes vs. C. sonorensis in larvae of H. virescens, and (G, H) M. croceipes vs. M. demolitor in larvae of H. virescens. Sample sizes are as indicated at far right column; percentage mortality as indicated adjacent to bar on right hand side. Asterisks indicate significant difference within each time interval (binomial test: * P<0.05, ** P<0.01, *** P<0.001).
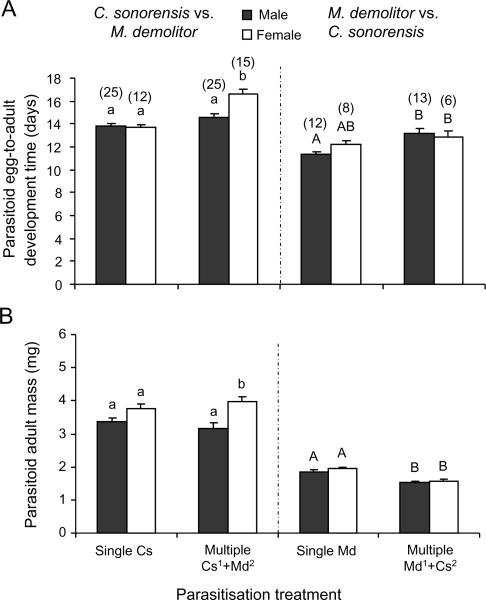
Comparisons between development time and adult size of the emerging parasitoids in singly and multiparasitised hosts
In P. includens, the development time of C. sonorensis that survived competition with M. demolitor was significantly longer than for C. sonorensis that developed in singly parasitised hosts (F1,73 = 31.97, P<0.001). Development times also differed between multiparasitised and singly parasitised hosts as a function of offspring sex (F1.73 = 8.85; P = 0.004) with a significant interactive effect (F1,73 = 9.46, P = 0.003) that resulted in females from multiparasitised hosts taking longer to develop into adults than males (Figure 3A). Adult body mass in C. sonorensis did not differ significantly between singly and multiparasitised hosts (F1,73 = 0.00, P = 0.97). However, as found for singly parasitised hosts, female C. sonorensis from multiparasitised hosts were larger than males (F1,73 = 14.19, P<0.001). Microplitis demolitor that survived competition with C. sonorensis similarly exhibited longer development times (F1,35 = 11.47, P = 0.002) and smaller body masses (F1,35 = 32.50, P<0.001) than M. demolitor from singly parasitised hosts with no differences detected in relation to sex (development time: F1,35 = 0.71, P = 0.41; body mass: F1,35 = 1.58, P = 0.22) (Figure 3A, B).
Figure 3.
Development of Campoletis sonorensis and Microplitis demolitor males (black bars) and females (open bars) developing in singly and multiparasitised L2 larvae of Pseudoplusia includens. Individual hosts were singly parasitised by C. sonorensis (left panel, first two bars) or M. demolitor (right panel, first two bars) or subsequently multiparasitised by the other species (last two bars in a panel) at various time intervals (0, 6, 24, or 48 h) following the initial parasitism event. Data were combined for the different time intervals. (A) Mean egg-to-adult development time (days) and (B) mean adult fresh body mass (mg). Line bars represent standard errors of the mean with the sample size between brackets. Bars with different letters are significantly different (Tukey-Kramer test: P<0.05). Data were analysed separately for the two competition experiments as indicated by small and capital letters.
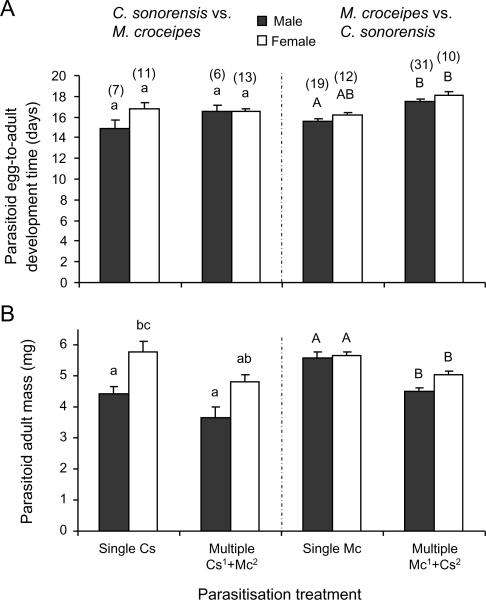
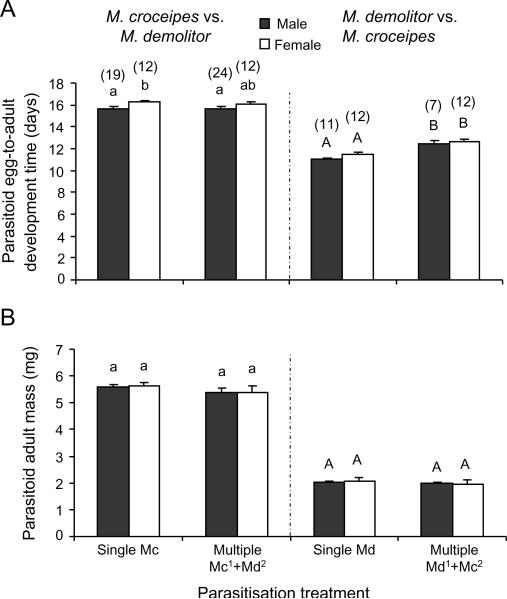
Similar patterns were found when comparing the mass and development times of C. sonorensis and M. croceipes that survived competition in multiparasitised H. virescens (Figure 4). Surviving wasps from multiparasitised hosts were significantly smaller than wasps from singly parasitised hosts (C. sonorensis vs. M. croceipes: F1,33 = 7.47, P = 0.01; M. croceipes vs. C. sonorensis: F1,68 = 26.45, P<0.001; Figure 4B). Wasps also developed slower when M. croceipes parasitized the host first (F1,68 = 39.83, P<0.001; Figure 4A), but not when C. sonerensis had a head start (F1,33 = 1.51, P = 0.23). The effects of multiparasitism on development time and biomass of the surviving parasitoid were less pronounced when M. croceipes and D. demolitors were competing in H. virescens hosts (Figure 5). When M. croceipes parasitised the host first, development time and biomass of the surviving M. croceipes wasps did not differ between multiparasitised and singly parasitised hosts (development time: F1,63 = 0.16, P = 0.69; biomass: F1,63 = 1.76, P = 0.19). However, development times did differ as function of offspring sex (F1,63 = 10.65, P = 0.002), with female wasps in multiparasitised hosts taking longer to develop than male wasps. In the reciprocal experiment in which M. demolitor had a head start, M. demolitor took longer to develop (F1,38 = 47.98, P<0.001) in multiparasitised than in singly parasitised host, whereas the size of M. demolitor was similar in multi- and singly parasitised hosts (F1,38 = 1.61, P = 0.21).
Figure 4.
Development of Campoletis sonorensis and Microplitis croceipes males (black bars) and females (open bars) developing in singly and multiparasitised L2 larvae of Heliothis virescens. Individual hosts were singly parasitised by C. sonorensis (left panel, first two bars) or M. croceipes (right panel, first two bars) or subsequently multiparasitised by the other species (last two bars in a panel) at various time intervals (0, 6, 24, or 48 h) following the initial parasitism event. Data were combined for the different time intervals. (A) Mean egg-to-adult development time (days) and (B) mean adult fresh body mass (mg). Line bars represent standard errors of the mean with the sample size between brackets. Bars with different letters are significantly different (Tukey-Kramer test: P<0.05). Data were analysed separately for the two competition experiments as indicated by small and capital letters.
Figure 5.
Development of Microplitis croceipes and Microplitis demolitor males (black bars) and females (open bars) developing in singly and multiparasitised L2 larvae of Heliothis virescens. Individual hosts were singly parasitised by M. croceipes (left panel, first two bars) or M. demolitor (right panel, first two bars) or subsequently multiparasitised by the other species (last two bars in a panel) at various time intervals (0, 6, 24, or 48 h) following the initial parasitism event. Data were combined for the different time intervals. (A) Mean egg-to-adult development time (days) and (B) mean adult fresh body mass (mg). Line bars represent standard errors of the mean with the sample size between brackets. Bars with different letters are significantly different (Tukey-Kramer test: P<0.05). Data were analysed separately for the two competition experiments as indicated by small and capital letters.
Discussion
The results of the initial experiment revealed that the two host species, P. includens and H. virescens, differed in terms of quality for the development of C. sonorensis and M. demolitor. Although the survival of both parasitoids to pupation did not differ between the two host species, other fitness-related traits did vary. For instance, C. sonorensis took longer to develop in larvae of H. virescens but emerging adult wasps were also significantly larger when developing in this host than in P. includens. By contrast, development time in female (but not male) M. demolitor was longer on P. includens larvae, although there was (as in C. sonorensis) a tendency for emerging parasitoids to be larger when developing in H. virescens. Because it is a much more specialised parasitoid, it was only possible to measure development of another haemolymph feeder, M. croceipes, in one host species (H. virescens). The main differences with its congener, M. demolitor, is that M. croceipes adults are much larger (e.g., similar in size with C. sonorensis).
Variation in the quality of the two hosts for the development of M. demolitor and C. sonorensis may reflect different feeding and development strategies of the two parasitoids as this relates to species- and instar-specific differences in the biology and in the growth of P. includens and H. virescens larvae during parasitism. Because C. sonorensis larvae devour virtually the entire host before pupation, the longer development time of this parasitoid in H. virescens is presumably due to the fact that this host was larger than P. includens when it was destroyed by the parasitoid, and thus took longer to consume. This also accounts for the larger size of newly emerged adult wasps from this host. For M. demolitor, the observed differences in development patterns between the two hosts might be more strongly determined by variation in the quality than the quantity of available resources.
In multiparasitised H. virescens larvae, M. croceipes was generally found to dominate in competition with C. sonorensis and M. demolitor when stinging the host first. This was particularly evident in hosts where M. croceipes had a 24- or a 48-h head start over both of the other species. In contrast, when the temporal parasitism-multiparasitism sequence was reversed, the outcome of competition was less clear. In multiparasitised larvae of both P. includens and H. virescens, C. sonorensis was generally dominant over M. demolitor when it oviposited first. Of the three parasitoid species, M. demolitor appeared to be the worst competitor, and lost many contests even when it parasitised the host first.
For the winning competitor significant costs on other developmental traits were also frequently observed. In all three parasitoid species, egg-to-adult development time of the winning parasitoid was usually longer compared to controls. Furthermore, the mass of newly emerged adult parasitoids was frequently much lower in multiparasitised hosts. A similar reduction in the fitness of the winning parasitoids has been recorded in studies with both super- and multiparasitised hosts (Vinson & Sroka, 1978; Wylie, 1983; Harvey et al., 1993; but see Zaviezo & Mills, 2001).
The mechanisms responsible for a reduction in fitness of the winning parasitoid in super- and/or multiparasitised hosts are poorly understood. These costs may be related to changes in the physiological synchrony between the developing parasitoid and its host, leading to suboptimal conditions for the development of the parasitoid progeny. For example, Harvey et al. (1993) suggested that an increase in development time could be due to the extra time expended by parasitoid larvae in finding and excluding other intra-interspecific competitors, which would otherwise be allocated to feeding and growth. Furthermore, in order to compensate for an increase in development time, parasitoid larvae may reduce efficient allocation of host resources to body size in order to grow faster, which would account for the smaller wasps emerging from super- or multiparasitised hosts.
Another factor that may reduce the quality of multiparasitised hosts is that there may be physiological conflicts initiated by the multiple doses of regulatory factors injected by different female wasps into the host during the oviposition sequence. These factors include venoms and polydnaviruses that are known to mediate changes in the developmental program of hosts in accordance with the physiological and nutritional requirements of the parasitoid progeny in an association-specific manner (Fleming, 1993; Beckage & Gelman, 2004). Consequently, if a host is multiparasitised by two or more parasitoid species that differ greatly in traits such as growth rate and adult body mass, the expression of regulatory factors from each parasitoid species may have very different effects on host growth patterns (Kadash et al., 2003). This may desynchronize the finely tuned physiological relationship between the dominant competitor and its host (discussed above), such that although the parasitoid is able to survive in the host, it experiences a reduction in fitness through extended development time and reduced adult size. This area certainly merits further investigation.
Our results suggest that large body size, possibly in combination with rapid embryonic development and early larval growth that is associated with the haemolymph feeding habit of the larvae, may account for the dominance of M. croceipes over the other two parasitoids. A previous study (De Moraes et al., 1999) studied competition between M. croceipes and another solitary braconid, Cardiochiles (= Toxoneuron) nigriceps Viereck in larvae of H. virescens. They found that M. croceipes also dominated in intrinsic competition with C. nigriceps, and attributed this to the fact that M. croceipes had a much shorter hatching time of about 8 h. Once this time-threshold was exceeded, however, C. nigriceps out-competed M. croceipes. In our study we did not measure hatching time, although it may have been more important in resolving conflicts between M. croceipes and C. sonorensis, given the overall competitive inferiority of M. demolitor. Another potentially important factor is that adults of both C. sonorensis and M. croceipes are some 2–3 × larger, in terms of body mass, than adults of M. demolitor. If we assume that larval masses of corresponding instars of these parasitoids are similarly different in size, this may explain why the larger parasitoids dominated in competition with M. demolitor.
Tian et al. (2008) also examined intrinsic competition between two species of endoparasitoids in their host, Helicoverpa armigera (Hübner), when there were variable time lags between the initial parasitism and multiparasitism. The authors found that each parasitoid dominated competition when they oviposited first with a time lag of 12 h or more. However, one major difference between the two studies was that larvae of H. armigera were older (late L2 or L3) when they were initially parasitised than the larvae of both P. includens and H. virescens (early L2) in this study. Although this has not been investigated, it is possible that the competitive ability of parasitoid larvae of different species varies with host instar. Previous studies have reported that host quality for the development of M. demolitor and C. sonorensis changes dramatically from one host instar to another (Gunasena et al., 1989; Harvey et al., 2004). Both of these parasitoids may also pass through several host instars during their development, and are thus confronted with instar-specific alterations in the host's biochemical environment (Lawrence, 1990; Strand & Pech, 1995). Little is known about the competitive abilities of immature parasitoids in different host stages. However, if the physiological requirements of different species of larval endoparasitoids sharing a common host differ in an instar-specific manner, then we may expect the outcome of conflicts to be resolved asymmetrically.
The importance of competition between parasitoids in driving the evolution of specialized host exploitation strategies and, ultimately, in shaping community structure, has long been the subject of debate (e.g., DeBach, 1966; Price, 1970, 1972; Force, 1974, 1985; Dean & Ricklefs, 1979; Hassell & Waage, 1984; Bogran et al., 2002; De Moraes & Mescher, 2004). However, it is likely that, under certain conditions, for example in simple landscapes such as in agricultural systems, or in homogeneous plant assemblages, different parasitoid species may compete for access to and control of host resources. Co-existence of two or more parasitoid species attacking the same host stage may be maintained under conditions in which the parasitoids trade-off the benefits of possessing some traits (e.g., rapid hatching time) against the costs of being poor in others (e.g., host-finding ability) (Price, 1970; De Moraes et al., 1999). Our study has reported that some traits may play a role in resolving intrinsic conflicts. Furthermore, C. sonorensis and M. croceipes are Nearctic species with sympatric distributions whereas M. demolitor is native to Africa and Australia and thus it does not have a long co-evolutionary history with the other two parasitoids. Most importantly, the ecological significance of competition amongst parasitoids is still poorly understood and thus further work will hopefully shed new light on the ways in which interspecific conflicts are resolved.
Acknowledgments
The authors wish to thank Jena Johnson for rearing the cultures of Microplitis demolitor, Pseudoplusia includens, and Heliothis virescens, Kristy Kadash for rearing the culture of Microplitis croceipes, and the Department of Entomology at the University of Wisconsin-Madison for providing facilities for this work. The research was funded by Hatch project 3929, NIH grant R01A132617, and NSF grant IBN-9514231 to M.R. Strand.
References
- Askew RR, Shaw MR. Parasitoid communities: their size, structure and development. In: Waage J, Greathead D, editors. Insect Parasitoids. Academic Press; London, UK: 1986. pp. 225–264. [Google Scholar]
- Bajpai NK, Ballal CR, Rao NS, Singh SP, Bhaskaran TV. Competitive interactions between two ichneumonid parasitoids of Spodoptera litura. BioControl. 2006;51:419–438. [Google Scholar]
- Beckage NE, Gelman DB. Wasp parasitoid disruption of host development: implications for new biologically-based strategies for insect control. Annual Review of Entomology. 2004;49:299–330. doi: 10.1146/annurev.ento.49.061802.123324. [DOI] [PubMed] [Google Scholar]
- Bográn CE, Heinz KM, Ciomperlik MA. Interspecific competition among insect parasitoids: field experiments with whiteflies as hosts in cotton. Ecology. 2002;83:653–668. [Google Scholar]
- Cusson M, Laforge M, Régnière J, Béliveau C, Trudel D, et al. Multiparasitism of Choristoneura fumiferana by the ichneumonid Tranosema rostrale and the tachinid Actia interrupta: occurrence in the field and outcome of competition under laboratory conditions. Entomologia Experimentalis et Applicata. 2002;102:125–133. [Google Scholar]
- Dean JM, Ricklefs RE. Do parasites of lepidoptera larvae compete for hosts? No! American Naturalist. 1979;113:302–306. [Google Scholar]
- DeBach P. The competitive displacement and coexistence principles. Annual Review of Entomology. 1966;11:183–212. [Google Scholar]
- De Moraes CM, Mescher MC. Biochemical crypsis in the avoidance of natural enemies by an insect herbivore. Proceedings of the National Academy of Sciences of the USA. 2004;101:8993–8997. doi: 10.1073/pnas.0403248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moraes CM, Cortesero AM, Stapel JO, Lewis WJ. Intrinsic and extrinsic competitive interactions between two larval parasitoids of Heliothis virescens. Ecological Entomology. 1999;24:402–410. [Google Scholar]
- Elzinga JA, Zwakhals K, Harvey JA, Biere A. The parasitoid complex associated with the herbivore Hadena bicruris (Lepidoptera:Noctuidae) on Silene latifolia (Caryophyllaceae) in the Netherlands. Journal of Natural History. 2007;41:101–123. [Google Scholar]
- Fisher RC. Oxygen requirements and the physiological suppression of supernumerary parasitoids. Journal of Experimental Biology. 1963;40:531–540. [Google Scholar]
- Fleming J. Polydnaviruses - Mutualists and pathogens. Annual Review of Entomology. 1992;37:401–425. doi: 10.1146/annurev.en.37.010192.002153. [DOI] [PubMed] [Google Scholar]
- Force DC. Ecology of host-parasitoid communities. Science. 1974;184:624–632. doi: 10.1126/science.184.4137.624. [DOI] [PubMed] [Google Scholar]
- Force DC. Competition among parasitoids of endophytic hosts. American Naturalist. 1985;126:440–444. [Google Scholar]
- Godfray HCJ. Parasitoids: Behavioral and Evolutionary Ecology. Princeton University Press; Princeton, NJ, USA: 1994. [Google Scholar]
- Gunasena GH, Vinson SB, Williams HJ. Interrelationships between growth of Heliothis virescens (Lepidoptera, Noctuidae) and that of its parasitoid, Campoletis sonorensis (Hymenoptera, Ichneumonidae) Annals of the Entomological Society of America. 1989;82:187–191. [Google Scholar]
- Harvey JA. Venturia canescens parasitizing Galleria mellonella and Anagasta kuehniella: is the parasitoid a conformer or a regulator? Journal of Insect Physiology. 1996;42:1017–1025. [Google Scholar]
- Harvey JA. Dynamic effects of parasitism by an endoparasitoid wasp on the development of two host species: implications for host quality and parasitoid fitness. Ecological Entomology. 2000;25:267–278. [Google Scholar]
- Harvey JA. Factors affecting the evolution of development strategies in parasitoid wasps: the importance of functional constraints and incorporating complexity. Entomologia Experimentalis et Applicata. 2005;117:1–13. [Google Scholar]
- Harvey JA, Vet LEM. Venturia canescens parasitizing Galleria mellonella and Anagasta kuehniella: differing suitability of two hosts with highly variable growth potential. Entomologia Experimentalis et Applicata. 1997;84:93–100. [Google Scholar]
- Harvey JA, Harvey IF, Thompson DJ. The effect of superparasitism on development of the solitary parasitoid wasp, Venturia canescens (Hymenoptera, Ichneumonidae) Ecological Entomology. 1993;18:203–208. [Google Scholar]
- Harvey JA, Strand MR. The developmental strategies of endoparasitoid wasps vary with host feeding ecology. Ecology. 2002;83:2439–2451. [Google Scholar]
- Harvey JA, Harvey IF, Thompson DJ. Flexible larval growth allows use of a range of host sizes by a parasitoid wasp. Ecology. 1994;75:1420–1428. [Google Scholar]
- Harvey JA, Bezemer TM, Elzinga JA, Strand MR. Development of the solitary endoparasitoid Microplitis demolitor: host quality does not increase with host age and size. Ecological Entomology. 2004;29:35–43. [Google Scholar]
- Hassell MP, Waage JK. Host-parasitoid population interactions. Annual Review of Entomology. 1984;29:89–114. [Google Scholar]
- Hawkins BA. Pattern and Process in Host-Parasitoid Interactions. Cambridge University Press; Cambridge, UK: 1994. [Google Scholar]
- Kadash K, Harvey JA, Strand MR. Cross-protection experiments with parasitoids in the genus Microplitis (Hymenoptera; Braconidae) suggest a high level of specificity in their associated bracoviruses. Journal of Insect Physiology. 2003;49:473–482. doi: 10.1016/s0022-1910(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Laing JE, Corrigan JE. Intrinsic competition between the gregarious parasite, Cotesia glomeratus and the solitary parasite, Cotesia rubecula (Hymenoptera, Braconidae) for their host, Artogeia rapae (Lepidoptera, Pieridae) Entomophaga. 1987;32:493–501. [Google Scholar]
- Lawrence PO. The biochemical and physiological effects of insect hosts on the development and ecology of their insect parasites - an overview. Archives of Insect Biochemistry and Physiology. 1990;13:217–228. [Google Scholar]
- Lewis WJ. Life history and anatomy of Microplitis croceipes (Hymenoptera: Braconidae) a parasite of Heliothis spp. (Lepidoptera: Noctuidae) Annals of the Entomological Society of America. 1970;63:67–70. [Google Scholar]
- Lingren PD, Guerra RJ, Nickelsen JW, White C. Hosts and host-age preference of Campoletis perdistinctus. Journal of Economic Entomology. 1970;63:518–522. [Google Scholar]
- Mackauer M. Growth and developmental interactions in some aphids and their hymenopterous parasites. Journal of Insect Physiology. 1986;32:275–280. [Google Scholar]
- Mackauer M, Sequeira R. Patterns of development in insect parasites. In: Beckage NE, Thompson SN, Federici BA, editors. Parasites and Pathogens of Insects. Academic Press; New York, NY, USA: 1993. pp. 1–20. [Google Scholar]
- Marktl RC, Stauffer C, Schopf A. Interspecific competition between the braconid endoparasitoids Glyptapanteles porthetriae and Glyptapanteles liparidis in Lymantria dispar larvae. Entomologia Experimentalis et Applicata. 2002;105:97–109. [Google Scholar]
- Neunzig HH. The Biology of the Tobacco Budworm and the Corn Earworm in North Carolina with Particular Reference to Tobacco as a Host. North Carolina Agricultural Experiment Station Technical Bulletin 196; Raleigh, NC, USA: 1969. [Google Scholar]
- Ode PJ. Plant chemistry and natural enemy fitness: effects on herbivore and natural enemy fitness. Annual Review of Entomology. 2006;51:163–185. doi: 10.1146/annurev.ento.51.110104.151110. [DOI] [PubMed] [Google Scholar]
- Petitt FL, Wietlisbach DO. Effects of host instar and size on parasitization efficiency and life-history parameters of Opius dissitus. Entomologia Experimentalis et Applicata. 1993;66:227–236. [Google Scholar]
- Price PW. Characteristics permitting coexistence among parasitoids of a sawfly in Quebec. Ecology. 1970;51:445–454. [Google Scholar]
- Price PW. Parasitoids utilizing same host - Adaptive nature of differences in size and form. Ecology. 1972;53:190–195. [Google Scholar]
- Quicke DLJ. Parasitic Wasps. Chapman & Hall; London, UK: 1997. [Google Scholar]
- Sequeira R, Mackauer M. Nutritional ecology of an insect host-parasitoid association: The pea aphid-Aphidius ervi system. Ecology. 1992;34:27–34. [Google Scholar]
- Shepard M, Powell JE, Jones WA. Biology of Microplitis demolitor (Hymenoptera: Braconidae), an imported parasitoid of Heliothis (Lepidoptera: Noctuidae) spp. and the soybean looper Pseudoplusia includens (Lepidoptera: Noctuidae) Environmental Entomology. 1983;12:641–645. [Google Scholar]
- Slansky F. Nutritional ecology of endoparasitic insects and their hosts: an overview. Journal of Insect Physiology. 1986;32:255–261. [Google Scholar]
- Strand MR. Characterization of larval development in Pseudoplusia includens (Lepidoptera: Noctuidae) Annals of the Entomological Society of America. 1990;83:538–544. [Google Scholar]
- Strand MR. Developmental traits and life-history evoluton in parasitoids. In: Hochberg ME, Ives AR, editors. Parasitoid Population Biology. Princeton University Press; Princeton, NJ, USA: 2000. pp. 139–162. [Google Scholar]
- Strand MR, Vinson SB. Facultative hyperparasitism by the egg parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) Annals of the Entomological Society of America. 1984;77:679–686. [Google Scholar]
- Strand MR, Pech LL. Immunological basis for compatibility in parasitoid-host relationships. Annual Review of Entomology. 1995;40:31–56. doi: 10.1146/annurev.en.40.010195.000335. [DOI] [PubMed] [Google Scholar]
- Strand MR, Johnson JA, Culin JD. Developmental interactions between the parasitoid Microplitis demolitor (Hymenoptera, Braconidae) and its host Heliothis virescens (Lepidoptera, Noctuidae) Annals of the Entomological Society of America. 1988;81:822–830. [Google Scholar]
- Tian SP, Zhang JH, Yan YH, Wang CZ. Interspecific competition between the ichneumonid Campoletis chlorideae and the braconid Microplitis mediator in their host Helicoverpa armigera. Entomologia Experimentalis et Applicata. 2008;127:10–19. [Google Scholar]
- Vinson SB, Sroka P. Effects of superparasitism by a solitary parasitoid on the host, parasitoid and field samplings. Southwestern Entomologist. 1978;3:299–301. [Google Scholar]
- Wang XG, Messing RH. Intra- and interspecific competition by Fopius arisanus and Diachasmimorpha tryoni (Hymenoptera: Braconidae), parasitoids of tephritid fruit flies. Biological Control. 2003;27:251–259. [Google Scholar]
- Wen B, Brower JH. Competition between Anisopteromalus calandrae and Choetospila elegans (Hymenoptera: Pteromalidae) at different parasitoid densities on immature rice weevils (Coleoptera: Curculionidae) in wheat. Biological Control. 1995;5:151–157. [Google Scholar]
- Wylie HG. Oviposition and survival of the European parasite Microctonus bicolor (Hymenoptera: Braconidae) in crucifer-infesting flea beetles in Manitoba. Canadian Entomologist. 1983;115:55–58. [Google Scholar]
- Zaviezo T, Mills N. The response of Hyssopus pallidus to hosts previously parasitised by Ascogaster quadridentata: heterospecific discrimination and host quality. Ecological Entomology. 2000;26:91–99. [Google Scholar]