Abstract
Recent reports from Japan implicated wild Sika deer (Cervus nippon) in the zoonotic transmission of hepatitis E to humans. Seroprevalence studies were performed to determine if imported feral populations of Sika deer in Maryland and Virginia posed a similar risk of transmitting hepatitis E virus (HEV). Hunters collected blood on filter paper disks from freshly killed deer. The disks were desiccated and delivered to a collection point. The dried filters were weighed to estimate the amount of blood absorbed and were eluted and collected in one tube via a novel extraction system. The procedure was quantified and validated with negative and positive serum and blood samples obtained from domestic Sika deer before and after immunization with HEV recombinant capsid protein, respectively. None of the 155 tested samples contained antibody to HEV, suggesting that Sika deer in these populations, unlike those in Japan, do not pose a significant zoonotic threat for hepatitis E. However, the new method developed for collecting and eluting the samples should prove useful for field studies of many other pathogens.
Keywords: HEV, Sika deer, filter paper discs, ELISA
1. Introduction
Hepatitis E virus (HEV) is a small, non-enveloped virus that is enterically transmitted. In this respect, it is similar to hepatitis A virus (HAV), although the two viruses belong to different virus families: HEV has recently been reclassified in its own genus (Hepevirus) and its own family (Hepeviridae), whereas, HAV is classified in the genus Hepatovirus in the family Picornaviridae (Emerson et al., 2004; Stanway et al., 2004). Although both viruses are enterically transmitted, their epidemiology is quite different: HAV, like many other enterically transmitted viruses, almost universally infects infants and children in developing countries and causes little disease but infects older children and adults in more “sanitized” industrialized countries and causes significant morbidity in this age group. In contrast, HEV causes disease principally in older children and young adults in developing countries whereas the few clinical cases of hepatitis E in industrialized countries occur mostly in older adults. Also unlike the situation with HAV, in which virtually 100% of the populations of developing countries have antibody to HAV (anti-HAV) by age 5 years, the prevalence of antibody to HEV (anti-HEV) in developing countries is generally quite low in infants and young children, with peak infection rates in older children and young adults. Furthermore, the prevalence of anti-HEV seldom exceeds 50% in most developing countries where HEV is endemic and is generally 10–20% in industrialized countries (Purcell and Emerson, 2001). While some of these differences may be ascribed to differences in fecal shedding and resistance to inactivation by environmental factors, it is likely that other epidemiologic factors are responsible.
One of these epidemiologic factors may be modes of transmission. Whereas HAV is transmitted only from humans to other humans (with the possible exception of transmission from certain monkey species where humans and wild monkeys overlap), at least 2 of the 4 recognized genotypes of human HEV naturally infect swine and, on occasion other species (Meng, 2000). Zoonotic spread of HEV has been proposed and, indeed, documented in a few cases. Thus, several cases of hepatitis E following ingestion of raw or under-cooked pork were reported from Japan and one instance of a small outbreak of hepatitis E following ingestion of Sika deer meat was also reported from Japan (Masuda et al., 2005; Matsuda et al., 2003; Takahashi et al., 2004; Tamada et al., 2004; Tei et al., 2003). Furthermore, anti-HEV was found in 9% of wild boars and 2% of wild Sika deer in Japan (Sonoda et al., 2004).
HEV is highly endemic in domestic swine herds in North America and transmission from swine to humans has been suspected on epidemiologic grounds, but not proven in the U.S. (Meng et al., 2002). Although not native to the United States, Sika deer were introduced into the country during the past century and local populations have expanded to the point that their numbers are now controlled by hunting. To determine whether U.S. Sika deer had serologic evidence of HEV infection and therefore posed a risk of HEV infection to deer hunters during the annual deer season, hunters were asked to collect blood from freshly killed Sika deer at a hunting site in the Maryland portion of Assateague Island (Assateague Island National Seashore) and from another on the Eastern Shore of Maryland (Blackwater National Wildlife Refuge). Hunters were supplied with kits for the blood collection and the resultant samples were tested for anti-HEV with a sensitive enzyme-linked solid immunosorbent assay (ELISA) that could detect antibodies to all recognized mammalian strains of HEV.
2. Materials and Method
2.1 Sample Collection
Each hunter was given a blood collection kit, which consisted of a 2.3 cm filter paper disc (Whatman 1003323), plastic forceps (TradeWinds Direct DF8088N) and a desiccant packet (Control Company 3151) inside a closed zip-lock bag (SKS Plastics 1304M08). Each hunter was instructed to collect as much fresh blood as the filter paper disc would absorb and to drop the filter paper disc and the forceps back into the zip-lock plastic bag. Bags were labeled with the hunter’s registration number and turned into authorities upon hunter check-out. Bags were collected from both sites and stored at room temperature. Upon receipt in the laboratory, each filter was logged in and weighed.
2.2 Control samples
Two domestically raised Sika deer were immunized with 20 μg of purified HEV capsid protein emulsified in Freund’s incomplete adjuvant (Pierce 77145). The immunization was repeated four weeks later. Blood was collected before immunization and weekly thereafter. The same antigen was used in the ELISA for antibody to HEV. The National Institutes of Health guidelines for the humane use of animals were followed during the study.
2.3 Calibration of weight of dried filter paper discs with volume of blood
Control filter paper discs were inoculated with 25–300 μL (in 25 μL increments) of whole blood from a seronegative human volunteer with a hematocrit of 45% or with 25– 400 μL (in 25 μL increments) of Sika deer blood (hematocrit 43–45%) collected before the immunization and two weeks after the booster immunization, respectively. Each control filter paper disc was dried in a zip-lock bag with desiccant for at least five days. Dried filter paper discs were weighed and an equation was derived from the relationship between the filter’s dry weight and the amount of whole blood inoculated on the filter paper. This equation was used to estimate the amount of deer blood absorbed by the sample filter paper discs.
2.4 Elution procedure
Elution of antibody from the dried filter paper discs was accomplished by adding 500 μL of gelatin solution (0.5% gelatin [BIO-RAD 170-6537], 1% bovine serum albumin (BSA) [KPL 50-61-01], and 0.05% Tween 20 [BIO-RAD 170-6531] in phosphate-buffered saline) to the dried filter paper discs in the inner compartment of an Ultra Free-CL centrifugal filter device (Millipore UFC40GV00). The centrifugal filter device was placed in a roller tube drum and rotated horizontally at room temperature for two hours at a speed of 20 rpm. The tube was then centrifuged at 3 000 rpm for 10 min. to extract the blood and gelatin solution from the filter paper.
2.5 ELISA plate preparation
The wells of a polystyrene 96-well plate (Falcon 353228) were coated with 100 μL of HEV SAR-55 (recombinant truncated (56 kDa) capsid protein, expressed in insect cells from ORF2 of the SAR-55 strain of HEV and purified as described previously (Robinson et al., 1998) at a concentration of 0.25 μg/mL. Alternatively, the wells of the plate were coated with rabbit anti-deer IgG (heavy plus light chain specific) (KPL 01-31-06) at a concentration of 1 μg/mL in carbonate-bicarbonate buffer (Sigma C-3041). The plates were incubated overnight (18–22 hours) at room temperature, washed twice with KPL wash solution (0.02% Tween 20 in 0.002 M imidazole-buffered saline [KPL 50-63-00]), and then blocked with 120 μL of gelatin solution in PBS before incubation at 37°C for one hour. The blocked plate was washed twice with KPL wash solution before use.
2.6 ELISA for deer IgG anti-HEV
Eluates were diluted 1:50 and 1:100 with the gelatin solution and tested against the HEV SAR-55 antigen. A 1:1 000 dilution of the deer blood eluates was tested against the anti-deer IgG. One hundred μL of diluted deer serum or eluate was inoculated into assigned wells, the plate was incubated at 37°C for 30 min., washed four times with the KPL wash solution, and in wells with diluted deer eluate or serum samples, 100 μL of peroxidase-labeled rabbit anti-deer IgG was added at a concentration of 1 μg/mL. The plate was incubated at 37°C for 30 min. and washed four times with the KPL wash solution. Color development was initiated by addition of 100 μL of ABTS substrate (0.3 g of 2,2′-azino-di [2-ethyl-benzthiazoline-6-sulfonate] per liter with 0.1% H2O2 in glycine-citric acid buffer [KPL 50-66-18]) to each well. The optical density (OD) was read at 405 nm for forty min. at five-minute intervals.
Four dilutions of an anti-HEV positive secondary standard which were comparable to 0.25, 0.05, 0.01 and 0.002 U/mL of the WHO human anti-HEV standard (Ferguson et al., 2002) served as a plate control. These controls were tested with peroxidase-labeled goat anti-human IgG (heavy plus light chain specific) (KPL 074-1006), at a concentration of 1μg/mL instead of the deer-specific conjugate. Sika deer controls consisted of Sika deer serum and eluates extracted from filter papers that had been inoculated with 50 μL or 400 μL of blood from an experimentally immunized Sika deer. These samples were serially diluted in 10-fold increments and tested in parallel with the field samples.
2.7 Blocking ELISA for deer IgG anti-HEV
A blocking procedure was performed on samples with optical densities greater than 0.200 at the 1:50 dilution. Equal amounts of HEV SAR-55 antigen (1 μg/mL) or gelatin solution were incubated with the diluted eluate at 37°C for one hour and tested for anti-HEV. Pre- and post-immunization eluates and sera from a Sika deer served as negative and positive controls, respectively, in the blocking test.
2.8 Fractionation of deer IgG
Quantification of deer IgG was preceded by the removal of excessive amounts of hemoglobin in the filter eluate. Deer IgG was removed from eluates of filters inoculated with 100, 200, 300 or 400 μL of deer blood by passage through ImmunoPure ® Immobilized Protein A/G (Pierce 20422) gel slurry equilibrated with binding buffer (Pierce 54200) in a Handee™ Spin Cup Column (Pierce 69702). After incubation at room temperature for 30 min., the column was washed four times with binding buffer. IgG bound to protein A/G was eluted with 400 μL of the elution buffer (Pierce 21004), and immediately neutralized with 40 μL of 1 M Trizma®-HCl (Sigma T-2819). The elution step was repeated four times, and electrophoresis was performed on the protein A/G eluate to assess the performance of the procedure and to quantify the amount of IgG.
2.9 Electrophoresis
One to five microliters of the protein A/G eluate was added to the reducing sample buffer (60 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5 mM DTT and 0.01% bromophenol blue) to obtain a final volume of 15 μL. In addition, a human IgG standard (Sigma I4506) was tested at 0.25 to 1 μg per 15 μL volume. Samples were heated at 58°C for 30 min., cooled to room temperature and centrifuged briefly.
Next, the samples were applied to 8–16% Tris-HCl polyacrylamide gradient gels (BioRad 161-1222). The running buffer consisted of 25 mM Tris, 192 mM glycine, pH 8.3, 0.1% SDS. Electrophoresis was conducted at 50 V for 30 min. followed by 125 V for 90 min.. The gel was rinsed in 200 mL of deionized water and stained for approximately 15 hr. in colloidal Coomassie Blue (20% methanol, 2% phosphoric acid, 10% ammonium sulfate and 0.1% Coomassie Brilliant Blue G-250). The gels were de-stained over 6 hours with four 200 mL changes of de-ionized water. Heavy and light chain bands were quantified, based on the human IgG standard, in a Molecular Dynamics Personal Densitometer SI.
3. Results
3.1 Sika Deer
Samples from 174 deer were collected for the study. Sixty-nine were from the Assateague Island National Seashore and 105 were from the Blackwater National Wildlife Refuge. Among the deer collected from the Assateague Island National Seashore, 31 were males and 38 females. The males’ estimated field-dressed weights ranged from 16–102 pounds; the females’ estimated field-dressed weights were 16–66 pounds. This information was not available for the Blackwater deer.
3.2 Evaluation of filter paper discs
Of the 174 filters collected, 9 (all from Assateague) were placed in bags without desiccant packets; these were deleted from the study. The weights of usable filters ranged from 0.080–0.216 grams with a mean, median and mode of 0.112, 0.110 and 0.093 grams, respectively, (data not shown).
3.3 Determination of the amount of blood absorbed by the filters
An equation was derived from the dry weights of filters that were inoculated with known volumes of whole blood from a human volunteer. The equation was linear with an intercept close to the mean weight of the clean filter paper discs (0.107 ± 0.0212): y equals 0.000222 x plus 0.0730, where y is the weight (grams) of the dried filter paper disc and x is the amount (μL) of blood absorbed by the filter. The hematocrit of all the deer in this study was assumed to be 45%. From this equation, the volume of Sika deer blood absorbed by the filter paper discs ranged from 32–643 μL, with a mean, median and mode of 175, 167 and 91 μL, respectively (data not shown).
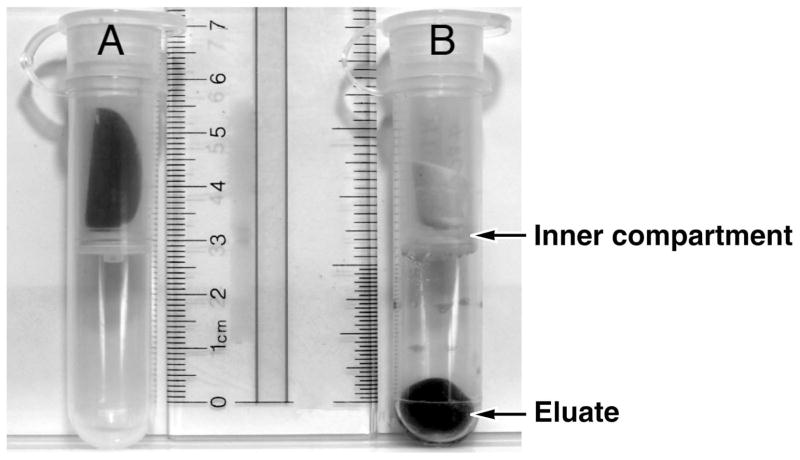
On average, the percent of fluid recovered from the rehydrated filter paper discs was over 80% (greater than 410 μL) and, with a few exceptions, the color of the filter paper disc after elution was white to light red, indicating successful removal of blood from the dried filter. The eluate was grossly hemolyzed (Figure 1).
Figure 1.
Ultra Free-Cl centrifugal filter device. A. Each sample filter was incubated with gelatin solution in the inner compartment of the filter device. B. Eluate after extraction and centrifugation.
3.4 Sika deer controls
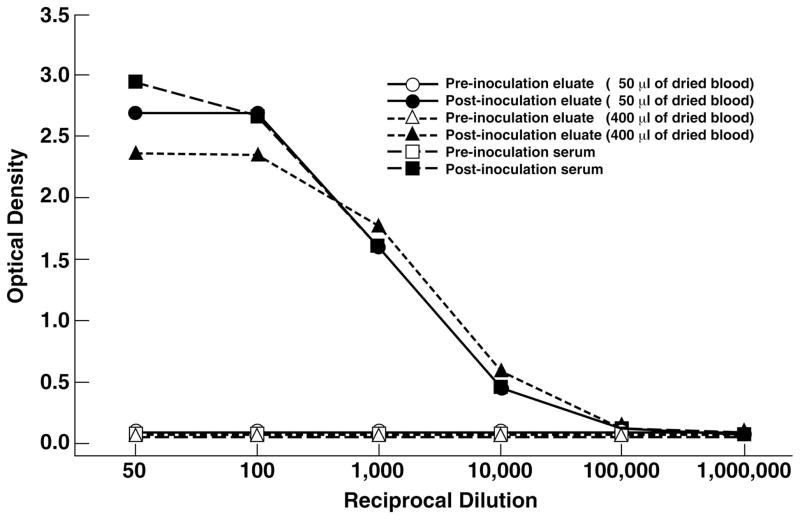
The two immunized Sika deer seroconverted 2 weeks after the first immunization and, following a booster immunization at week 4, blood and sera were collected at week 6, when both deer had anti-HEV titers of approximately 1:10 000. Filter paper discs inoculated with pre-immunization and post-immunization blood from the Sika deer had comparable weights. Based on the equation for conversion of filter paper weights to volume of blood, the average calculated blood volume was greater than 90% of the actual volume. In addition, serial dilutions of post-immunization deer serum and eluate (from a filter that had been inoculated with 50 μL of post-immunization deer blood) had comparable and parallel optical densities that were linear from approximately OD 0.5–2.6 (Figure 2). The linear range of an eluate from a filter that had been inoculated with 400 μL of post-immunization deer blood had a linear OD range that was slightly less but the differences were not significant (Figure 2).
Figure 2.
Serial dilution of pre-immunization and post-immunization samples from immunized Sika deer. Two filters, inoculated with 50 μL and 400 μL of deer blood, respectively and a serum sample were diluted and tested for deer IgG anti-hepatitis E virus in an ELISA as described in the text.
3.5 Primate Controls
Since the ELISA for IgG anti-HEV was optimized for primate samples, the same optimization criteria were used to evaluate the performance of the assay when comparing serum with eluates of blood-impregnated filters. The slope and the intercept of the secondary standards (corresponding to 0.25, 0.05, 0.01 and 0.002 U/mL of the WHO standard (Ferguson et al., 2002)) were used to determine the optimal reading time within the 40 minute kinetic-reading timeframe, as described previously (Engle et al., 2002). Optical density readings after 15 and 20 min. were used in this study.
3.6 Quantification of total deer IgG
The amount of deer IgG on four filters with different amounts of deer blood was measured by fractionating the IgG on a protein A/G affinity matrix. After washing and eluting, the IgG was electrophoretically separated into its heavy and light chain components, which were well separated from residual hemoglobin and other proteins such as transferrin that non-specifically bound to protein A/G; hemoglobin migrated in parallel with the 15 kDa marker, while the deer IgG heavy and light chains migrated near the 50 kDa and 25 kDa markers, respectively. In addition, both heavy and light chains migrated similarly to human IgG heavy and light chains, and the bands were quantified based on the human IgG standard; filters inoculated with 100, 200, 300 and 400 μL of deer whole blood contained 320, 779, 1139, and 1638 μg of total deer IgG, respectively (Figure 3). The same four eluates that were used for electrophoretic quantification of deer IgG were tested by ELISA for total deer IgG and the OD of each was 1.015, 1.144, 1.327 and 1.544, respectively. Thus, the ELISA for deer IgG was linear over the range of 320–1638 ng per 100 μL (Figure 3).
Figure 3.
Optical density based on total deer IgG. Eluates from four filters with different amounts of deer blood were tested at dilutions of 1:1 000 for total deer IgG by ELISA. The amount of total deer IgG was determined by extrapolating from known concentrations of human IgG standards run in parallel on a (8–16%) SDS gel.
3.7 Sika Deer Field Samples
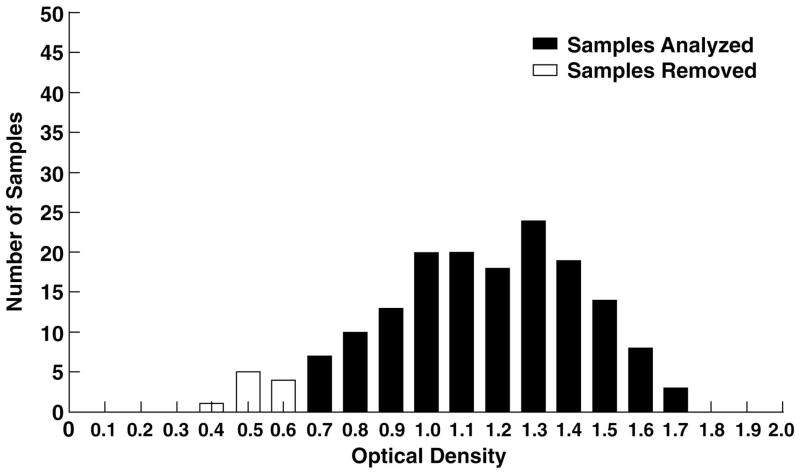
Because the volume of blood absorbed to the individual filters could be estimated from weight as described above, filter paper eluates from the wild Sika deer were adjusted to standard dilutions of 1:50 and 1:100 for determinations of IgG-HEV and to 1:1 000 for determinations of total deer IgG. As seen in Table 1 and Figure 4, the range of OD values for total deer IgG, measured at a 1:1 000 dilution, was 0.401–1.787. Ten outlying samples with OD less than 0.700 were deleted from the study because the total deer IgG in these samples appeared to be abnormally low. When the remaining 155 samples were tested for Sika deer IgG anti-HEV at dilutions of 1:50 and 1:100, the range of OD values was 0.548–0.061 and 0.339–0.057, respectively (Table 1). The average OD at both dilutions was approximately 0.100 and all ODs were below 0.200 except for five samples, which had ODs of 0.215, 0.223, 0.223, 0.252 and 0.548 at the 1:50 dilution. For these samples, the total deer IgG OD values at the 1:1 000 dilution were 1.733, 1.429, 1.145, 1.627 and 1.611, respectively. Thus, all but one of these had total IgG OD values greater than the mean, median and mode for all of the samples (Table 1).
Table 1.
Optical density values of Sika deer sample eluatesa
| Deer IgG anti-hepatitis E virus | Total Deer IgG Control | ||
|---|---|---|---|
| 1:50 dilution | 1:100 dilution | 1:1000 dilution | |
| MIN | 0.061 | 0.057 | 0.701 |
| MAX | 0.548 | 0.339 | 1.787 |
| MEAN | 0.116 | 0.092 | 1.233 |
| MEDIAN | 0.109 | 0.087 | 1.238 |
| MODE | 0.086 | 0.076 | 1.222 |
155 sample filters were included in the analysis.
Figure 4.
The distribution of total deer IgG in the Sika deer sample eluates based on optical density. Each eluate was tested for total deer IgG at a 1:1 000 dilution by ELISA (n = 165). Ten samples with OD values that were less than 0.700 were removed from the study.
3.8 Competition Assay
To determine if the above samples with high (but negative) values for Sika deer anti-HEV were the result of a high background of OD or whether they contained low levels of authentic anti-HEV, sera and filter eluates from one of the immunized Sika deer and the five eluates from wild Sika deer that had OD values greater than 0.200 were diluted to 1:50 and incubated with either 1 μg/mL of the hepatitis E recombinant antigen used for the anti-HEV assay or with the gelatin dilution solution before testing in the standard ELISA for Sika deer anti-HEV. Serum and eluate collected from the immunized Sika deer before immunization showed less than 5% reduction in OD when tested at a 1:50 dilution. In contrast, the OD of serum and eluate obtained from the Sika deer after immunization, and diluted (1:10 000) to an OD comparable to that of the eluates from the five wild Sika deer was reduced by greater than 60% (Table 2). The eluates from all five Sika deer with higher than average OD values demonstrated less than 10% reduction in OD when they were incubated with HEV antigen and compared with results obtained after incubation with diluent (Table 2). Thus, there was no evidence that any of the 155 Sika deer examined in this study had been exposed to HEV (less than 0.6% prevalence of antibody).
Table 2.
Blocking EIA for deer IgG anti-hepatitis E virus (anti-HEV)
| ODa | ||||
|---|---|---|---|---|
| Sample Eluate | Blocked with HEVb | Blocked with gelatin | Percent reduction in OD | Dilution of blocking |
| HTL 55 | 0.801 | 0.700 | −14 | 1:50 |
| HTL 58 | 0.366 | 0.353 | −4 | 1:50 |
| HTL 29 | 0.368 | 0.406 | 9 | 1:50 |
| HTL 7 | 0.173 | 0.142 | −22 | 1:50 |
| HTL 128 | 0.196 | 0.141 | −39 | 1:50 |
| Negative eluate controlc,d | 0.087 | 0.091 | 4 | 1:50 |
| Positive eluate controlc,e | 0.199 | 0.594 | 66 | 1:104 |
| Negative serum controld | 0.075 | 0.077 | 3 | 1:50 |
| Positive serum controle | 0.201 | 0.848 | 76 | 1:104 |
OD is the optical density at 405 nm.
Samples and controls were blocked with 1 μg/mL of HEV Sar-55.
Filters inoculated with 400 μL of deer blood were used for this study.
Baseline eluate and sera were used as negative controls.
Positive controls were collected six weeks after the immunization.
Discussion
Sika deer (Cervus nippon) are native to Japan, Taiwan and Eastern Asia. In the early 1900s, Clement Henry, a resident of Cambridge, Maryland, released five or six imported Sika deer onto Assateague Island, and the deer reproduced and expanded into contiguous parts of Maryland and Virginia (Eyler, 2003). Sika deer now inhabit much of the lower Eastern Shore, including Assateaque Island National Seashore, in Maryland, and Chincoteague National Wildlife Refuge in the Virginia part of the island, which is approximately 57 kilometers long and approximately 2.5 kilometers wide. They live mostly in the western forested and salt marsh regions of the island and, on occasion, they forage east into the dunes bordering the Atlantic Ocean. They share the island with numerous wildlife, including the native white-tailed deer and the famous Chincoteague ponies. To control the burgeoning Sika deer population, annual hunts are scheduled. Following the 2004 hunting season in the Maryland portion of the island, the Sika deer population was estimated to be approximately 10–15 animals per square kilometer. There are campgrounds on the island and the deer come into relatively close contact with humans but they would be unlikely to have contact with HEV through exposure to human waste.
Sika deer also inhabit approximately 1/3 of the Blackwater National Wildlife Refuge on the Chesapeake side of the Eastern Shore of Maryland. The 26 000 acre refuge is composed of approximately 1/3 forest land, 1/3 marsh lands and 1/3 water. Population studies of Sika deer in the refuge have not been performed. There are no campgrounds in the Blackwater National Wildlife Refuge and little contact between humans and deer.
The current study was designed to evaluate the prevalence of anti-HEV in the two Sika deer populations residing on opposite sides of the Eastern Shore of Maryland. Hunters participating in the controlled hunts at the two sites cooperated by collecting blood from freshly-killed Sika deer and returning the collection kits to officials when registering their kills.
Published procedures for extracting dried blood from filter paper have differed greatly and often have not included quantification of the amount of blood absorbed or retained by the filter paper (De Swart et al., 2001; Helfand et al., 2001; Jafri et al., 1998; Oppelaar, 1966). Because of the lack of a standardized procedure for processing blood-impregnated filter paper discs, a simplified collection and elution procedure was developed for this study. A gelatin solution was used both for extraction and dilution of samples and reagents. Another component of this procedure was the use of Millipore Ultrafree-CL centrifugal filter tubes. This tube simplified the elution procedure because the elution and extraction steps were both carried out in the same device. Similarly, the HEV-specific antigen used in the ELISA assay was the same antigen that has been shown to detect all four mammalian genotypes of HEV with a high degree of sensitivity and to stimulate antibodies that neutralize at least three of the four HEV genotypes when incorporated into a vaccine (Emerson et al., 2006; Engle et al., 2002; Purcell et al., 2003; Zhou et al., 2004). Finally, to confirm that the commercial rabbit anti-white-tail deer IgG secondary antibody employed for this study was sufficiently cross-reactive to detect anti-HEV in Sika deer, two Sika deer were immunized with HEV antigen and an ELISA was standardized with pre-immunization and post-immunization sera and filter eluates from these animals. Serial dilutions of serum and blood eluate (corrected for volume of the dried blood) from one of the control deer yielded a linear assay over a 100 fold dilution of serum and eluate (Figure 2). Slight differences were seen in the OD values of one eluate (50 μl) but these represented less than a two-fold difference in the titer in the linear range of the assay and less than a four-fold difference in the nonlinear range. Thus these differences were not significant.
Because a small number of Sika deer blood eluates had a relatively high (but negative) OD value when compared to controls, a blocking assay was developed to determine if they contained low levels of specific anti-HEV. Although the blocking assay confirmed the specificity of the anti-HEV in the immunized Sika deer, it demonstrated that the field samples were, in fact, negative for anti-HEV. A similar blocking assay was used previously to confirm the specificity of anti-HEV in sera from Japanese Sika deer (Sonoda et al., 2004)
Previous studies have shown that the prevalence of anti-HEV in several species (humans, swine and rats) is age-specific and that the peak acquisition of such antibody occurs in juveniles and young adults (Arankalle et al., 1995; Kabrane-Lazizi et al., 1999; Meng et al., 1997). In order to determine if the populations of Sika deer that were surveyed had sufficient older animals, i.e., those most likely to have been exposed to HEV, to draw a valid conclusion about anti-HEV prevalence, the age of the Sika deer was determined based on their weight. Sika deer are more closely related to elk than to white-tailed deer and are smaller than the native deer (Eyler, 2003). Data collected by the Department of Natural Resources indicated that the average weight of a 1.5 year old male Sika deer is 53 pounds, while male deer over 3.5 years of age weigh 80 or more pounds (Hutton, 2002–2003). Females are smaller and a 1.5 year old female has an average weight of 45 pounds; females older than 3.5 years weigh 60 pounds or more. Sika deer reach sexual maturity at about 1.3 years and breed during their second year (Eyler, 2003). Hunters from the Assateague Island National Seashore provided the deer’s field-dressed weight, which does not include the internal organs of the animal. Thirty percent of the field-dressed weight was added to the recorded weight to estimate the live weight of the animal. Among the male deer killed, 17% were estimated to be less than 1.5 years of age, 63% had an estimated age of 1.5–3.5 years and 20% were estimated to be over 3.5 years of age. Among the female deer killed, 45% were estimated to be less than 1.5 years of age, 37% between 1.5 and 3.5 years of age and 18% greater than 3.5 years of age. Thus, 83% of the bucks and 55% of the does were of an age that would be expected to have been exposed to HEV if natural infection of Sika deer is similar to that of the other animal species studied to date.
A larger sample size can further substantiate the absence of hepatitis E antibodies in the Sika deer populations at the Assateague Island National Seashore and the Blackwater National Wildlife Refuge, especially if the expected seroprevalence of anti-HEV was only 2 percent, as seen in Japan (Sonoda, et al., 2004), and presumed specific to certain age groups as described above. The quantity of filters available for this study was limited by the number of hunters who agreed to collect the filters and the number of samples that were collected properly. The failure to detect anti-HEV in the two populations of deer, however, strongly suggests that HEV is not ecologically important in these populations and that infection of hunters or other wildlife, such as white-tailed deer in both ecological settings and the feral Chincoteague ponies in the National Seashore, from Sika deer is very low.
The negative finding was not unexpected since Assateague Island National Seashore and the Blackwater National Wildlife Refuge are geographically isolated and environmentally protected and conserved by the National Park Service and the U.S. Fish and Wildlife Service, respectively. In addition, the marshes and thick forested wetlands in these two regions have limited the geographic distribution of the Sika deer (Eyler, 2003). In both of these regions, food and water are available to the animals and their need to leave the area is minimal. In contrast, Sika deer living in Japan live in close proximity not only to human populations, but to swine (especially wild boars) that are known to harbor HEV (Sonoda et al., 2004; Takahashi et al., 2004). It will therefore be useful to study Sika deer (and white-tailed deer) that live in close proximity to populations of swine that might be infected with HEV. One such diverse habitat is in Texas, where Sika deer, white-tailed deer, peccaries and possibly other feral swine live in close proximity. Such studies might provide important insights to understanding of the ecology of HEV.
Acknowledgments
We are grateful to Jim Sears who obtained the Sika deer that were used as controls, Dale C. Streams, D.V.M. who performed the immunization and the sample collection, Michael Hsu and Steve Gable, who housed and cared for the control deer.
This research was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arankalle VA, Tsarev SA, Chadha MS, Alling DW, Emerson SU, Banerjee K, Purcell RH. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–450. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- De Swart RL, Nur Y, Abdallah A, Kruining H, El Mubarak HS, Ibrahim SA, Van Den Hoogen B, Groen J, Osterhaus AD. Combination of reverse transcriptase PCR analysis and immunoglobulin M detection on filter paper blood samples allows diagnostic and epidemiological studies of measles. J Clin Microbiol. 2001;39:270–273. doi: 10.1128/JCM.39.1.270-273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson SU, Anderson D, Arankalle A, Meng X-J, Purdy M, Schlauder GG, Tsarev SA. Herpesvirus. Virus taxonomy, VIIth Report of the ICTV. 2004:851–855. [Google Scholar]
- Emerson SU, Clemente-Casares P, Moiduddin N, Arankalle VA, Torian U, Purcell RH. Putative neutralization epitopes and broad cross-genotype neutralization of Hepatitis E virus confirmed by a quantitative cell-culture assay. J Gen Virol. 2006;87:697–704. doi: 10.1099/vir.0.81545-0. [DOI] [PubMed] [Google Scholar]
- Engle RE, Yu C, Emerson SU, Meng XJ, Purcell RH. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J Clin Microbiol. 2002;40:4576–4580. doi: 10.1128/JCM.40.12.4576-4580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler B. Sika: Maryland’s exotic little elk. 2003 http://www.dnr.state.md.us/naturalresource/summer2003/sika.html.
- Ferguson M, Walker D, Mast E, Fields H. Report of a collaborative study to assess the suitability of a reference reagent for antibodies to hepatitis E virus. Biologicals. 2002;30:43–48. doi: 10.1006/biol.2001.0315. [DOI] [PubMed] [Google Scholar]
- Helfand RF, Keyserling HL, Williams I, Murray A, Mei J, Moscatiello C, Icenogle J, Bellini WJ. Comparative detection of measles and rubella IgM and IgG derived from filter paper blood and serum samples. J Med Virol. 2001;65:751–757. doi: 10.1002/jmv.2100. [DOI] [PubMed] [Google Scholar]
- Hutton D. 2002–2003 Game Program Annual Report. 2002–2003 http://www.dnr.state.md.us/wildlife/gpdeer.html.
- Jafri HS, Torrico F, Noh JC, Bryan RT, Balderrama F, Pilcher JB, Tsang VC. Application of the enzyme-linked immunoelectrotransfer blot to filter paper blood spots to estimate seroprevalence of cysticercosis in Bolivia. Am J Trop Med Hyg. 1998;58:313–315. doi: 10.4269/ajtmh.1998.58.313. [DOI] [PubMed] [Google Scholar]
- Kabrane-Lazizi Y, Fine JB, Elm J, Glass GE, Higa H, Diwan A, Gibbs CJ, Jr, Meng XJ, Emerson SU, Purcell RH. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am J Trop Med Hyg. 1999;61:331–335. doi: 10.4269/ajtmh.1999.61.331. [DOI] [PubMed] [Google Scholar]
- Masuda J, Yano K, Tamada Y, Takii Y, Ito M, Omagari K, Kohno S. Acute hepatitis E of a man who consumed wild boar meat prior to the onset of illness in Nagasaki, Japan. Hepatol Res. 2005;31:178–183. doi: 10.1016/j.hepres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Okada K, Takahashi K, Mishiro S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J Infect Dis. 2003;188:944. doi: 10.1086/378074. [DOI] [PubMed] [Google Scholar]
- Meng XJ. Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J Hepatol. 2000;33:842–845. doi: 10.1016/s0168-8278(00)80319-0. [DOI] [PubMed] [Google Scholar]
- Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Wiseman B, Elvinger F, Guenette DK, Toth TE, Engle RE, Emerson SU, Purcell RH. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppelaar L. The use of filter paper as a transport medium for blood and serum. A comparative serological investigation. Trop Geogr Med. 1966;18:60–66. [PubMed] [Google Scholar]
- Purcell RH, Emerson SU. Hepatitis E Virus. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 3051–3061. [Google Scholar]
- Purcell RH, Nguyen H, Shapiro M, Engle RE, Govindarajan S, Blackwelder WC, Wong DC, Prieels JP, Emerson SU. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine. 2003;21:2607–2615. doi: 10.1016/s0264-410x(03)00100-2. [DOI] [PubMed] [Google Scholar]
- Robinson RA, Burgess WH, Emerson SU, Leibowitz RS, Sosnovtseva SA, Tsarev S, Purcell RH. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr Purif. 1998;12:75–84. doi: 10.1006/prep.1997.0817. [DOI] [PubMed] [Google Scholar]
- Sonoda H, Abe M, Sugimoto T, Sato Y, Bando M, Fukui E, Mizuo H, Takahashi M, Nishizawa T, Okamoto H. Prevalence of hepatitis E virus (HEV) Infection in wild boars and deer and genetic identification of a genotype 3 HEV from a boar in Japan. J Clin Microbiol. 2004;42:5371–5374. doi: 10.1128/JCM.42.11.5371-5374.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G, Brown F, Christian P, Hovi T, Hyypiä T, King AMQ, Knowles NJ, Lemon SM, Minor PD, Pallansch MA, Palmenberg AC, Skern T. Picornaviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy, VIIIth Report of the ICTV. Elsevier/Academic Press; London: 2004. pp. 779–782. [Google Scholar]
- Takahashi K, Kitajima N, Abe N, Mishiro S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology. 2004;330:501–505. doi: 10.1016/j.virol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Tamada Y, Yano K, Yatsuhashi H, Inoue O, Mawatari F, Ishibashi H. Consumption of wild boar linked to cases of hepatitis E. J Hepatol. 2004;40:869–870. doi: 10.1016/j.jhep.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Purcell RH, Emerson SU. An ELISA for putative neutralizing antibodies to hepatitis E virus detects antibodies to genotypes 1, 2, 3, and 4. Vaccine. 2004;22:2578–2585. doi: 10.1016/j.vaccine.2003.12.017. [DOI] [PubMed] [Google Scholar]