Abstract
Background
Prospective data on the association between common mental disorders and obesity are scarce, and the impact of ageing on this association is poorly understood.
Aims
To examine the association between common mental disorders and obesity (body mass index ⩾30 kg/m2) across the adult life course.
Method
The participants, 6820 men and 3346 women, aged 35–55 were screened four times during a 19-year follow-up (the Whitehall II study). Each screening included measurements of mental disorders (the General Health Questionnaire), weight and height.
Results
The excess risk of obesity in the presence of mental disorders increased with age (P = 0.004). The estimated proportion of people who were obese was 5.7% at age 40 both in the presence and absence of mental disorders, but the corresponding figures were 34.6% and 27.1% at age 70. The excess risk did not vary by gender or according to ethnic group or socioeconomic position.
Conclusions
The association between common mental disorders and obesity becomes stronger at older ages.
Owing to their increasing prevalence, common mental disorders (such as symptoms of anxiety and depression) and obesity are widely regarded as major public health issues.1–3 Mental disorders are associated with considerable disease burden4,5 and obesity is a leading cause of preventable death and chronic disease.6 There is also some evidence to suggest that common mental disorders and obesity may be related to one another, such that men and women with common mental disorders experience a higher risk of obesity than those free of such conditions,7–9 although this is not a universal finding.10,11
It remains unclear whether ageing affects the association between mental disorders and obesity, because the majority of previous studies are based on cross-sectional study design and the few published longitudinal studies do not include repeated measurements of both common mental disorders and obesity.12,13 Strengthening of the association between the two disorders by increasing age is plausible as there is a continuity in both experiencing mental health problems and obesity, potentially leading to cumulated effects.14 Furthermore, chronic pain and disabling physical conditions are more prevalent at older ages and may lead to both obesity and mental health problems.15–17
In the present study, multiple measurements of common mental disorders and obesity were taken over the adult life course from 35 to 74 years of age. These data therefore provide us with the opportunity to examine the manner in which the association between common mental disorders and obesity develops with ageing in more detail than has previously been possible.
Method
Participants
Data are drawn from the British Whitehall II study. The target population was all London-based office staff, aged 35–55, working in 20 civil service departments.18 With a response of 73%, the baseline cohort consisted of 10 308 employees (6895 men and 3413 women). The true response proportion was, in fact, higher because around 4% of those invited were not eligible for inclusion. Ethical approval for the Whitehall II study was obtained from the University College London Medical School committee on the ethics of human research.
Design
After the first medical examination (phase 1, 1985–1988), screenings by trained research staff were repeated three times over a 19-year period: phase 3 (1991–1993), phase 5 (1997–1999) and phase 7 (2003–2004). All these phases included a standardised assessment of common mental disorders and a direct measurement of weight and height to determine obesity.
Measurements
We assessed common mental disorders using the self-administered 30-item General Health Questionnaire (GHQ), which focuses on self-reported symptoms of anxiety and depression, and associated psychosocial dysfunction.19 This device is designed as a screening instrument for use in community settings. It has been validated against standardised clinical interviews and has shown high reliability.20 In each GHQ item an enquiry is made about a specific symptom; the response categories are scored either 1 or 0 to indicate whether the symptom is present or not. On the basis of receiver operating characteristics analysis and previous studies, we defined people with a sum score 5 or more in GHQ as cases and those scoring 0–4 as non-cases.21 In the present study in which GHQ scores were validated against a Clinical Interview Schedule, the sensitivity (73%) and specificity (78%) using this measure of `caseness' was acceptable.21 General Health Questionnaire caseness showed temporal stability as the odds of being a case at phase 3 were 3.77 (95% CI 3.38–4.20) times higher for those who were cases already at phase 1 than for those who were not. The corresponding odds ratio (OR) was 4.11 (95% CI 3.62–4.67) for GHQ-caseness at phases 3 and 5, and 4.7 (95% CI 4.18–5.50) at phases 5 and 7 (all P<0.0001).
Weight was measured in underwear to the nearest 0.1 kg on Soehnle electronic scales. Height was measured in bare feet to the nearest 1 mm using a stadiometer with the participant standing erect with their head in the Frankfurt plane. Reproducibility of the weight and height measurements over 1 month (i.e. between-participant variability/total (between + within participant) variability), undertaken on 306 participants, was 0.99 at phase 7 screening. We calculated body mass index (BMI) by dividing weight (in kilograms) by height (in metres squared). Following the World Health Organization definition, participants with BMI ⩾30 kg/m2 were considered obese and those with BMI <30 kg/m2 non-obese.22
Other variables in this study were gender, ethnicity (White v. Black and minority ethnic) and, at each phase, age, marital status (married or cohabiting v. single, divorced or widow), socioeconomic position, derived from the civil service employment grade, classified into high (upper administrator categories combined), intermediate (executive officer categories combined) and low (clerical and office support staff), and use of psychotropic drugs (antidepressants, tranquillisers, sleeping pills, antipsychotics).
Statistical analysis
At each phase, the analytic sample included participants with complete data on GHQ-caseness and obesity. To examine the cross-sectional association between GHQ-caseness and obesity at each study phase we used logistic regression analysis, from which we report odds ratios to summarise this relationship for the total cohort and separately for men and women. For the analyses of the prospective data, which are structured such that measurement times (observations) are nested within individuals, we used multilevel logistic regression analysis based on generalised estimating equations (GEE) to model the association between GHQ-caseness and obesity across study phases. The status of GHQ-caseness and obesity was allowed to change within participants over time, i.e. these variables were modelled as time variant, and the analysis used all available measurements from every participant at all phases. Repeated measurements within individuals constitute a cluster and the calculation of standard errors takes into account the non-independence of the measurements; that is, the same individual contributes more than one observation in the dataset and these observations are of course related. Odds ratios to summarise associations between GHQ-caseness and obesity were adjusted for age, gender, ethnicity, marital status, socioeconomic position and use of psychotropic drugs. To test the association between GHQ-caseness and obesity as a function of age, we included an interaction term `GHQ-caseness age' in a model including main effects. This model was used to develop growth curves estimating obesity prevalence for GHQ-cases and non-cases at each age with 95% confidence intervals. We also ran a series of sensitivity analyses. We compared the strength of the age-dependent association between GHQ and obesity before and after adjustment for birth year and study phase to examine whether this association was attributable to cohort effects or the impact of historical trends. As increased sample attrition at later study phases could introduce a healthy survivor bias, we repeated the analyses of age-dependent associations in a subcohort with no missing data for GHQ or obesity at any study phase. If results in these analyses were similar to those from the main analyses with all available data, this would provide evidence against a healthy survivor bias. To study whether the age-dependent association was specific to the BMI cut-off point used in defining obesity, we repeated the interaction test with continuous BMI as the outcome. All analyses were performed with Stata 9.0 statistical software for Windows, StataCorp LP, Texas, USA.
Results
Of all 10 308 baseline cohort members, 10 166 (99%) had complete data on GHQ-caseness and obesity at phase 1. Their mean age was 44.5 years (s.d. = 6.0), approximately two-thirds were men, around 10% of the study sample were Black and minority ethnic and 3.5% reported being treated by psychotropic drugs. Men were slightly older, less likely to be treated with psychotropic drugs and were more often White, married and from high socioeconomic positions than women (Table 1). At subsequent data collection phases, complete data on GHQ and obesity were obtained for 54% (the lowest, phase 5) to 77% (the highest, phase 3) of all baseline participants. General Health Questionnaire caseness at phase 1 was not associated with having missing data on these measures at phase 5 (OR = 1.00, 95% CI 0.92–1.09, P = 0.96), but baseline obesity was associated with greater missing values (OR = 1.76, 95% CI 1.51–2.06, P<0.001).
Table 1.
Baseline characteristics of the study population
|
Total, n |
|||||
|---|---|---|---|---|---|
| Characteristic | Men | Women | Men | Women | P |
|
Age, years: mean (s.d.)
|
44.0 (6.0)
|
45.3 (6.1)
|
6820
|
3346
|
<0.0001
|
| Ethnicity, % | |||||
| White | 92.1 | 86.4 | 6246 | 2848 | <0.0001 |
|
Black and minority
|
7.9
|
13.6
|
537
|
447
|
|
| Marital status, % | |||||
| Married or cohabiting | 80.5 | 61.2 | 5475 | 2038 | <0.0001 |
|
Single/divorced/widowed
|
19.5
|
38.8
|
1326
|
1290
|
|
| Socioeconomic position, % | |||||
| High | 38.3 | 11.2 | 2614 | 375 | <0.0001 |
| Intermediate | 52.5 | 39.3 | 3577 | 1316 | |
|
Low
|
9.2
|
49.5
|
629
|
1655
|
|
| Use of psychotropic drug, % | |||||
| No | 97.2 | 95.0 | 6628 | 3180 | <0.0001 |
|
Yes
|
2.8
|
5.0
|
191
|
166
|
|
The prevalence of GHQ-caseness was 27% at phase 1, but declined to 22% at phases 3 and 5, and was 20% at phase 7. In contrast, there was a gradual increase in obesity prevalence from 7% at phase 1 to 19% at phase 7. These trends in GHQ-caseness and obesity were broadly similar for men and women (Table 2). They were also replicated in a subcohort of participants with no missing data on GHQ or obesity at any of the four study phases (n = 4364).
Table 2.
Common mental disorders and obesity by study phase
| Characteristic | Phase 1 (1985–88) | Phase 3 (1991–93) | Phase 5 (1997–99) | Phase 7 (2003–04) |
|---|---|---|---|---|
| Men | ||||
| n | 6820 | 5473 | 3857 | 4484 |
| Age, mean (range) | 44.0 (35–56) | 49.3 (39–63) | 55.6 (45–69) | 60.9 (51–74) |
| GHQ category,an (%) | ||||
| Non-case | 5098 (74.8) | 4361 (79.7) | 3085 (80.0) | 3651 (81.4) |
| Case | 1722 (25.3) | 1112 (20.3) | 772 (20.0) | 833 (18.6) |
| GHQ score, mean (s.d.) | 3.4 (5.4) | 2.8 (4.9) | 2.8 (5.3) | 2.7 (5.3) |
| BMI category,an (%) | ||||
| Non-obese | 6470 (94.9) | 5090 (93.0) | 3403 (88.2) | 3760 (83.9) |
| Obese | 350 (5.1) | 383 (7.0) | 454 (11.8) | 724 (16.2) |
|
BMI, kg/m2: mean (s.d.)
|
24.6 (3.1)
|
25.1 (3.2)
|
26.0 (3.5)
|
26.6 (3.8)
|
| Women | ||||
| n | 3346 | 2424 | 1602 | 1823 |
| Age, mean (range) | 45.3 (35–56) | 50.2 (39–62) | 56.1 (45–68) | 61.3 (50–74) |
| GHQ category,an (%) | ||||
| Non-case | 2331 (69.7) | 1813 (74.8) | 1175 (73.4) | 1373 (75.3) |
| Case | 1015 (30.3) | 611 (25.2) | 427 (26.7) | 450 (24.7) |
| GHQ score, mean (s.d.) | 4.1 (5.8) | 3.4 (5.5) | 3.9 (6.3) | 3.6 (5.3) |
| BMI category,an (%) | ||||
| Non-obese | 2985 (89.2) | 2049 (84.5) | 1286 (80.3) | 1361 (74.7) |
| Obese | 361 (10.8) | 375 (15.5) | 316 (19.7) | 462 (25.3) |
|
BMI, kg/m2: mean (s.d.)
|
24.8 (4.3)
|
25.7 (4.7)
|
26.4 (4.9)
|
27.2 (5.4)
|
GHQ, General Health Questionnaire; BMI, body mass index
At every phase, prevalence of GHQ-caseness and obesity is significantly lower among men than women (P < 0.0001)
The association between GHQ-caseness and obesity strengthened at each successive study phase with the age- and gender-adjusted odds ratio being 1.10 (95% CI 0.93–1.31) at phase 1, 1.12 (95% CI 0.94–1.34) at phase 3, 1.18 (95% CI 0.99–1.42) at phase 5 and 1.34 (95% CI 1.15–1.56) at phase 7. As shown in Table 3, there were no large differences in the strength of the association between men and women, and in both genders the association was stronger at the last follow-up than at baseline. Adjustment for ethnicity, marital status, socioeconomic position and treatment with psychotropic drugs had little effect on these associations.
Table 3.
Odds ratios (95% CIs) for the cross-sectional associations between General Health Questionnaire-caseness (GHQ-caseness) and obesity by study phase (logistic regression analysis)
|
Men: odds ratio (95% CI) for obesity
|
Women: odds ratio (95% CI) for obesity
|
|||
|---|---|---|---|---|
| GHQ category | Age adjusted | Multiply adjusteda | Age adjusted | Multiply adjusteda |
| Phase 1 | ||||
| Non-case | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) |
|
Case
|
1.12 (0.88–1.43)
|
1.11 (0.86–1.42)
|
1.09 (0.86–1.39)
|
1.19 (0.93–1.52)
|
| Phase 3 | ||||
| Non-case | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) |
|
Case
|
1.32 (1.03–1.68)
|
1.33 (1.04–1.70)
|
0.96 (0.74–1.24)
|
1.00 (0.76–1.30)
|
| Phase 5 | ||||
| Non-case | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) |
|
Case
|
1.26 (1.00–1.59)
|
1.26 (0.99–1.60)
|
1.10 (0.83–1.45)
|
1.05 (0.78–1.40)
|
| Phase 7 | ||||
| Non-case | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) |
|
Case
|
1.33 (1.09–1.61)
|
1.31 (1.08–1.60)
|
1.33 (1.05–1.69)
|
1.30 (1.02–1.67)
|
Ref., reference
Adjusted for age, ethnicity, marital status, socioeconomic position and use of psychotropic drugs
Multilevel analysis
The age-adjusted odds of being obese were 13% higher for GHQ-cases than non-cases in men (OR = 1.13, 95% CI 1.03–1.25) and 11% higher in women (OR = 1.11, 95% CI 1.00–1.23) across all of the study phases. There was no evidence to suggest that this association differed between men and women (P = 0.92 for GHQ gender interaction) or according to ethnicity (P = 0.67), marital status (P = 0.72), or socioeconomic position (P = 0.82).
Table 4 shows multivariably adjusted analyses of the GHQ obesity association for men and women analysed together. The age- and gender-adjusted odds ratio for this association was 1.12 (model A) and further adjustment for ethnicity, marital status, socioeconomic position and use of psychotropic drugs had little effect on this odds ratio (model B). However, a statistically significant GHQ age interaction term shows that the association between GHQ and obesity was dependent on age (model C). There was no evidence to suggest that this age-dependent effect differed between men and women (P = 0.33 for `GHQ age gender' term in a model including main effects and two-way interactions).
Table 4.
Odds ratios (95% CIs) for the cross-sectional associations between General Health Questionnaire-caseness (GHQ-caseness) and obesity across the study phases (multilevel general estimation equation logistic regression analysis)a
|
Odds ratio (95% CI)
|
|||
|---|---|---|---|
| GHQ category | Model A | Model B | Model C |
|
Age (per year)
|
1.06 (1.06–1.07)
|
1.07 (1.06–1.07)
|
1.06 (1.06–1.07)
|
| Gender | |||
| Men | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) |
|
Women
|
1.83 (1.65–2.02)
|
1.47 (1.30–1.65)
|
1.47 (1.30–1.66)
|
| Ethnicity | |||
| White | 1.0 (ref.) | 1.0 (ref.) | |
|
Black and minority ethnic
|
1.01 (0.85–1.19)
|
1.00 (0.85–1.19)
|
|
| Marital status | |||
| Married | 1.0 (ref.) | 1.0 (ref.) | |
|
Single/divorced/widow
|
1.07 (0.98–1.18)
|
1.07 (0.98–1.18)
|
|
| Socioeconomic status | |||
| High | 1.0 (ref.) | 1.0 (ref.) | |
| Intermediate | 1.28 (1.12–1.45) | 1.27 (1.12–1.45) | |
|
Low
|
1.72 (1.46–2.03)
|
1.71 (1.46–2.02)
|
|
| Use of psychotropic drugs | |||
| No | 1.0 (ref.) | 1.0 (ref.) | |
|
Yes
|
1.08 (0.93–1.26)
|
1.07 (0.92–1.25)
|
|
| GHQ | |||
| Non-case | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) |
|
case
|
1.12 (1.04–1.20)
|
1.12 (1.04–1.20)
|
0.63 (0.41–0.97)
|
|
GHQ age interaction term
|
1.01
(1.003–1.02)* |
||
ref., reference
This analysis is based on 29 829 observations among 10 256 individuals with at least one measurement of GHQ and obesity
P = 0.008
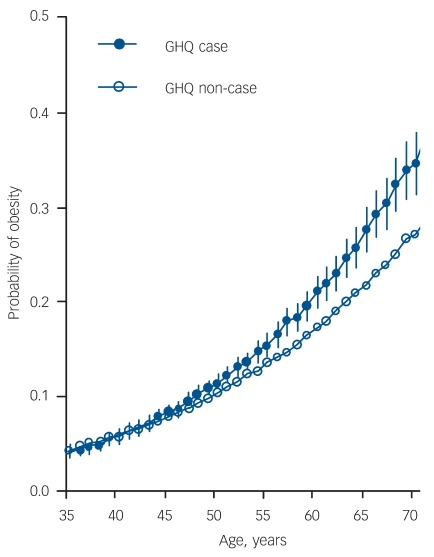
To illustrate the interaction between GHQ and age on obesity, Fig. 1 shows the proportion of individuals who were obese by GHQ-caseness as a function of age (proportions are estimated based on a model including age, gender, GHQ-caseness and GHQ age interaction term as independent variables). There was a general rising trend in obesity by age. Within this general trend there was also a growing divergence in obesity prevalence between GHQ-cases and non-cases. For example, the estimated proportion of people who were obese was 5.7% at age 40 both in GHQ-cases and non-cases (OR = 1.00); however, by age 70, this had risen to 34.6% of GHQ-cases and 27.1% of non-cases (OR = 1.42). In men, the estimated obesity prevalence was 4.4% among the GHQ-cases and 4.8% among the non-cases at age 40 (OR = 0.91), but 30.8% and 23.4% at age 70 (OR = 1.46). The corresponding figures for women were 8.8% v. 8.7% at age 40 (OR = 1.01) and 41.0% v. 35.8% at age 70 (OR = 1.25).
Fig. 1.
Growth curves for obesity risk by age and General Health Questionnaire (GHQ)-caseness (estimated based on multilevel general estimation equation logistic regression analysis).
Sensitivity analysis
The interaction term of `GHQ-caseness age' with obesity as the outcome was little affected by adjustment for birth year and study phase, and remained statistically significant (P<0.02 after both adjustments) suggesting that cohort and historical effects are unlikely to explain our results. There was no evidence of a `healthy survivor' bias as the statistically significant interaction between GHQ and age on obesity was also found in a subcohort with no missing data in GHQ or obesity at any of the study phases (P = 0.005, n = 4363). Furthermore, the growth curves were very similar to those in the main analysis with all available data. Finally, repeating multilevel analysis with continuous BMI as the outcome showed a significant GHQ age interaction term both in men (P<0.0001) and women (P = 0.05), confirming that the age-dependent association between GHQ-caseness and obesity was not sensitive to the specific cut-off for BMI used to define obesity.
Discussion
Although ageing is a complex phenomenon involving a range of psychological and physiological changes, the evidence from this 19-year four-wave study suggests a relatively simple overall effect of age on the association between common mental disorders and obesity. We found that people with common mental disorders have an excess risk of obesity and there was no strong evidence of gender or ethnic differences in this association. The excess obesity risk strengthened with age: irrespective of mental health status, about 6% were obese at age 40, but by age 70 the corresponding prevalence rose to 35% in people with common mental disorders and to 27% in others. This finding emphasises the role of common mental disorders in the risk of obesity at older ages.
Comparison with previous studies
Our study is probably the largest longitudinal investigation of the effect of age on the association between common mental disorders and obesity. Previous studies on this issue are based mostly on cross-sectional comparisons between age groups; they do not include multiple measurements of both common mental disorders and obesity, and they show mixed findings.7 Such data may not accurately reflect longitudinal trends. We did not find strong evidence of gender, ethnic or socioeconomic differences in the association between common mental disorders and obesity risk. Although gender and ethnic differences have been reported in some previous studies,11 a large survey of a nationally representative sample of US adults found, in agreement with our findings, an association between psychiatric disorders and obesity that was equally strong for men and women.23
Our target group was an occupational cohort and therefore likely to be healthier than a general population. We adopted the view that common mental disorders in such samples are validly represented as a single dimension combining symptoms of anxiety and depression.24–26 Measuring common mental disorders is particularly relevant in community-based samples such as ours, as mental disorders in the community are frequently characterised by comorbidity between the disorders and by shifting patterns of symptoms that resist precise clinical classification.24,25,27 The use of such a composite outcome, such as ours, is supported by studies showing anxiety disorders and mood disorders (depression) to be equally related to excess risk of obesity.23
Across age groups, the odds of being obese was 1.12 times higher for people with common mental disorders than for others. This estimate is smaller than the odds ratios of over 1.20 typically found for obesity in individuals with specific diagnosed mental disorders in general populations.23,28 Factors that can cause this discrepancy in risk estimates between our cohort and the previous studies include differences in the target group (occupational cohort study v. general population sample), the definition of mental disorders (GHQ symptoms scale v. other screening measures and clinical interview), and the assessment of obesity (measured v. self-reported weight and height).
Plausible mechanisms
Common mental disorders can be a cause and a consequence of obesity and a number of plausible mechanisms may underlie these bidirectional associations, presenting cumulated effects with increasing age. First, common mental disorders are associated with eating disorders, both over- and underconsumption, which could influence future changes in adiposity. Exercise has been found to improve depressive symptoms among those with a diagnosis of depression,29 but physical inactivity, a major contributing factor to obesity, is more prevalent among people with mental health problems. Furthermore, commonly used pharmacological treatments for depression have known side-effects that may result in weight gain (tricyclic antidepressants), weight loss (selective serotonin reuptake inhibitors, SSRIs) or both (short- and long-term effects of SSRIs).30–32
Second, it is plausible that the direction of the association may also be from obesity to increased future risk of common mental disorders, with adverse effects being more likely in societies where obesity is stigmatised.33 Internalisation of negative obesity-related stereotypes, negative self-body image and unsuccessful weight control by dieting are related to increased risk of mental ill health among individuals who are obese.34–36 Biological factors, such as dysregulation of the hypothalamic–pituitary–adrenocortical (HPA) system, may further strengthen the link beween obesity and depression.37–39 There is some evidence of abnormal hormone concentrations of the HPA axis among people who are obese with and without coexisting depressive symptoms,40,41 and among people who are obese with binge eating disorder.42 Furthermore, studies show remission in depressive symptoms following surgically induced weight loss among individuals who are obese.43,44
Third, `common cause' may contribute to the age-dependent association between mental disorders and obesity as chronic bodily pain and disabling sensory and physical conditions are increasingly common at older ages and contribute to both obesity prevalence and common psychiatric disorders.15–17 Underlying disability that accompanies ageing may therefore reinforce the association between mental health and obesity at older ages.
Strengths and limitations
Our study is unique in having four measurements of both common mental disorders and obesity across the adult life course. An advantage of such a design compared with cross-sectional studies is the possibility of conducting multilevel analyses that not only use information about differences between participants, but also exploit data from the same individuals at different ages. The large sample size and direct measurements of height and weight are also particular strengths of the present study. However, several limitations should be taken into account when interpreting the findings. First, our cohort of civil servants do not include blue collar workers or unemployed people and is therefore not representative of the general population, which potentially limits the generalisability of our findings. Nevertheless, we are not aware of reasons why the age-related strengthening of the association between mental health problems and obesity would be specific to employed people. Second, sample loss as a result of missing data varied between 1 and 47% depending on study phase and obesity at baseline predicted subsequent non-participation. However, increasing non-participation across successive study phases is not a plausible explanation of the observed age-dependent associations because the findings were reproducible in a subcohort with full data at every study phase. Third, common mental disorders were measured using a validated symptom scale, the GHQ, that is not a measure of clinically recognised psychiatric disorder.19 Although the symptom scale is reliable we cannot be certain that our findings would be directly transferable to individuals meeting the DSM–IV45 or ICD–1046 criteria for specific mental disorders such as major depressive disorder or anxiety disorders. Furthermore, the symptom scale does not measure severity. Fourth, waist circumference and waist–hip ratio were not assessed at all the four phases. Thus, it remains unclear whether the age-dependent association of common mental disorders is specific to general obesity or also observable in relation to central obesity and related HPA axis disturbances.40,47 Further research on these issues is needed.
Implications
Evidence from a well-characterised occupational cohort shows that the association between common mental disorders and obesity strengthens with age. Given that people aged 65 plus is the fastest growing age group worldwide,48 we recommend that this finding is taken into account both in the prevention of obesity and treatment of mental disorders in ageing populations. Diet and physical activity are central to weight management emphasising the relevance of health policies that improve the opportunities to control weight for older people (e.g. provision of nutrition guidance, availability of exercise places, reimbursement of weight control treatments and effective prevention of physical impairments and pain that may increase risk of obesity and distress). There is also a need for more detailed clinical guidelines to help physicians prevent and treat obesity among adults with mental disorders, and promote mental health among individuals who are obese and of older age.
Funding
The Whitehall II study has been supported by grants from the Medical Research Council; British Heart Foundation; Health and Safety Executive; Department of Health; National Heart Lung and Blood Institute (HL36310), US, NIH: National Institute on Aging (AG13196), US, NIH; Agency for Health Care Policy Research (HS06516); and the John D and Catherine T MacArthur Foundation Research Networks on Successful Midlife Development and Socioeconomic Status and Health. M.K. and J.V. are supported by the Academy of Finland (Projects no. 117604, 124322); G.D.B. is a Wellcome Trust Research Fellow; A.S.-M. is supported by a `EURYI' award from the European Science Foundation; and M.G.M. is supported by a MRC Research Professorship.
Declaration of interest
None.
References
- 1.Kopelman PG. Obesity as a medical problem. Nature 2000; 404: 635-43. [DOI] [PubMed] [Google Scholar]
- 2.Compton WM, Conway KP, Stinson FS, Grant BF. Changes in the prevalence of major depression and comorbid substance use disorders in the United States between 1991–1992 and 2001–2002. Am J Psychiatry 2006; 163: 2141-7. [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3: e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penninx BW, Beekman AT, Honig A, Deeg DJ, Schoevers RA, van Eijk JT, et al. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry 2001; 58: 221-7. [DOI] [PubMed] [Google Scholar]
- 5.Everson SA, Roberts RE, Goldberg DE, Kaplan GA. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med 1998; 158: 1133-8. [DOI] [PubMed] [Google Scholar]
- 6.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999; 282: 1523-9. [DOI] [PubMed] [Google Scholar]
- 7.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry 2004; 65: 634-51. [DOI] [PubMed] [Google Scholar]
- 8.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 2006; 63: 824-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer A, Korszun A, Owen MJ, Craddock N, Jones L, Jones I, et al. Medical disorders in people with recurrent depression. Br J Psychiatry 2008; 192: 351-5. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM–IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health 2000; 90: 251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo M, Pietrobelli A, Fontaine KR, Sirey JA, Faith MS. Depressive mood and obesity in US adults: comparison and moderation by sex, age, and race. Int J Obes (Lond) 2006; 30: 513-9. [DOI] [PubMed] [Google Scholar]
- 12.Pine DS, Cohen P, Brook J, Coplan JD. Psychiatric symptoms in adolescence as predictors of obesity in early adulthood: a longitudinal study. Am J Public Health 1997; 87: 1303-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics 2001; 107: 1049-56. [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM–IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 593-602. [DOI] [PubMed] [Google Scholar]
- 15.Weil E, Wachterman M, McCarthy EP, Davis RB, O'Day B, Iezzoni LI, et al. Obesity among adults with disabling conditions. JAMA 2002; 288: 1265-8. [DOI] [PubMed] [Google Scholar]
- 16.Heim N, Snijder MB, Deeg DJ, Seidell JC, Visser M. Obesity in older adults is associated with an increased prevalence and incidence of pain. Obesity (Silver Spring) 2008; 16: 2510-7. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Devine A, Dick IM, Dhaliwal SS, Prince RL. Prevalence of lower extremity pain and its association with functionality and quality of life in elderly women in Australia. J Rheumatol 2003; 30: 2689-93. [PubMed] [Google Scholar]
- 18.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol 2005; 34: 251-6. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg DP. Detecting Pychiatric Illness by Questionnaire. Oxford University Press, 1972.
- 20.Pevalin DJ. Multiple applications of the GHQ–12 in a general population sample: an investigation of long-term retest effects. Soc Psychiatry Psychiatr Epidemiol 2000; 35: 508-12. [DOI] [PubMed] [Google Scholar]
- 21.Stansfeld SA, Marmot MG. Social class and minor psychiatric disorder in British Civil Servants: a validated screening survey using the General Health Questionnaire. Psychol Med 1992; 22: 739-49. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. WHO, 1995. [PubMed]
- 23.Simon GE, Fleck M, Lucas R, Bushnell DM. Prevalence and predictors of depression treatment in an international primary care study. Am J Psychiatry 2004; 161: 1626-34. [DOI] [PubMed] [Google Scholar]
- 24.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry 1999; 56: 921-6. [DOI] [PubMed] [Google Scholar]
- 25.Vollebergh WA, Iedema J, Bijl RV, de Graaf R, Smit F, Ormel J. The structure and stability of common mental disorders: the NEMESIS study. Arch Gen Psychiatry 2001; 58: 597-603. [DOI] [PubMed] [Google Scholar]
- 26.Kendell R, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry 2003; 160: 4-12. [DOI] [PubMed] [Google Scholar]
- 27.Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, et al. No health without mental health. Lancet 2007; 370: 859-77. [DOI] [PubMed] [Google Scholar]
- 28.Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med 2008; 70: 288-97. [DOI] [PubMed] [Google Scholar]
- 29.Mead GE, Morley W, Campbell P, Greig CA, McMurdo M, Lawlor DA. Exercise for depression. Cochrane Database Syst Rev 2008: CD004366. [DOI] [PubMed]
- 30.Rapaport MH, Thase ME. Translating the evidence on atypical depression into clinical practice. J Clin Psychiatry 2007; 68: e11. [DOI] [PubMed] [Google Scholar]
- 31.Sussman N, Ginsberg DL, Bikoff J. Effects of nefazodone on body weight: a pooled analysis of selective serotonin reuptake inhibitor- and imipramine-controlled trials. J Clin Psychiatry 2001; 62: 256-60. [PubMed] [Google Scholar]
- 32.Demyttenaere K, Jaspers L. Review: bupropion and SSRI-induced side effects. J Psychopharmacol 2008; 22: 792-804. [DOI] [PubMed] [Google Scholar]
- 33.Andreyeva T, Puhl RM, Brownell KD. Changes in perceived weight discrimination among Americans, 1995–1996 through 2004–2006. Obesity (Silver Spring) 2008; 16: 1129-34. [DOI] [PubMed] [Google Scholar]
- 34.Puhl RM, Moss-Racusin CA, Schwartz MB. Internalization of weight bias: Implications for binge eating and emotional well-being. Obesity (Silver Spring) 2007; 15: 19-23. [DOI] [PubMed] [Google Scholar]
- 35.Friedman KE, Reichmann SK, Costanzo PR, Musante GJ. Body image partially mediates the relationship between obesity and psychological distress. Obes Res 2002; 10: 33-41. [DOI] [PubMed] [Google Scholar]
- 36.Ross CE. Overweight and depression. J Health Soc Behav 1994; 35: 63-79. [PubMed] [Google Scholar]
- 37.Bornstein SR, Schuppenies A, Wong ML, Licinio J. Approaching the shared biology of obesity and depression: the stress axis as the locus of gene–environment interactions. Mol Psychiatry 2006; 11: 892-902. [DOI] [PubMed] [Google Scholar]
- 38.Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry 2003; 54: 330-7. [DOI] [PubMed] [Google Scholar]
- 39.Atlantis E, Baker M. Obesity effects on depression: systematic review of epidemiological studies. Int J Obes (Lond) 2008; 32: 881-91. [DOI] [PubMed] [Google Scholar]
- 40.Ahlberg AC, Ljung T, Rosmond R, McEwen B, Holm G, Akesson HO, et al. Depression and anxiety symptoms in relation to anthropometry and metabolism in men. Psychiatry Res 2002; 112: 101-10. [DOI] [PubMed] [Google Scholar]
- 41.Ljung T, Holm G, Friberg P, Andersson B, Bengtsson BA, Svensson J, et al. The activity of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in relation to waist/hip circumference ratio in men. Obes Res 2000; 8: 487-95. [DOI] [PubMed] [Google Scholar]
- 42.Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med 2004; 66: 876-81. [DOI] [PubMed] [Google Scholar]
- 43.Dixon JB, Dixon AF, O'Brien PE. Improvements in insulin sensitivity and beta-cell function (HOMA) with weight loss in the severely obese. Homeostatic model assessment. Diabet Med 2003; 20: 127-34. [DOI] [PubMed] [Google Scholar]
- 44.Dixon JB, Dixon ME, O'Brien PE. Depression in association with severe obesity: changes with weight loss. Arch Intern Med 2003; 163: 2058-65. [DOI] [PubMed] [Google Scholar]
- 45.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder (4th edn) (DSM–IV). APA, 1994.
- 46.World Health Organization. The ICD–10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. WHO, 1992.
- 47.Rivenes AC, Harvey SB, Mykletun A. The relationship between abdominal fat, obesity, and common mental disorders: results from the HUNT study. J Psychosom Res 2009; 66: 269-75. [DOI] [PubMed] [Google Scholar]
- 48.Kirkwood TB. A systematic look at an old problem. Nature 2008; 451: 644-7. [DOI] [PubMed] [Google Scholar]