Abstract
The electrical activity in the electroencephalogram (EEG) and the event-related potentials extracted from the EEG provide the greatest temporal resolution for examining brain function. When coupled with the high spatial resolution of structural magnetic resonance imaging (sMRI), the combined techniques provide a powerful tool for neuroscience in the examination of brain abnormalities in major psychiatric illnesses. Over the last 20 years, our work has examined brain structure and function in schizophrenia. Both EEG and MRI measures have indicated profound abnormalities in schizophrenia within the temporal lobe, particularly marked over the left hemisphere. Our studies of patients first hospitalized due to psychosis revealed the early course of the disease to be characterized by progressive impairment and cortical gray matter reduction, most intense near the time of first hospitalization. Knowledge of those locations and brain signals affected early should help understand the basic physiological defect underlying this progression, with potential implications for new therapeutic interventions.
Keywords: Bipolar Disorder, Event-related Potential, Magnetic Resonance Imaging, Multimodal Imaging, Schizophrenia
INTRODUCTION
The electroencephalogram (EEG) was the first physiological technique used to examine brain activity in schizophrenia and has evolved into a powerful method for studying brain information processing activity. In today’s world of multi-modal imaging, the EEG is still unsurpassed in providing real-time, millisecond resolution of normal and pathological brain processing, literally at the speed of thought. In general, the EEG derives from summated dendritic inhibitory and excitatory post-synaptic activity in neurons, primarily pyramidal cells in the neocortex of the brain. It is important to emphasize that EEG does not typically reflect neuronal discharges, since they are usually too brief and too asynchronous. (As an aside, we note Blood Oxygenation Level Dependent (BOLD) fMRI “activation” also mainly reflects post synaptic potential (PSP) activity, which is metabolically most demanding and necessitates the increased blood flow; action potential activity is metabolically much less demanding.1)
The EEG primarily reflects the activity generated in the large dendritic trees of pyramidal cells, with an especially strong representation of activity in dendrites oriented in parallel with one another and perpendicular to the plane of the scalp surface. One of the main limitations of the EEG technique is difficulty in determining the source of the recorded activity, since generators in different brain locations can produce the same EEG pattern recorded distally at the scalp.
This temporal sensitivity and spatial insensitivity of the EEG is nicely complemented by the exquisitely detailed spatial information from the structural MRI. Structural MRI provides detailed information on which brain regions show pathological reduction in gray matter in many diseases, including schizophrenia. To combine EEG and MRI information we determine the association of structural volumetric abnormalities in particular regions with particular evoked potentials. This resembles the classical “lesion” approach to behavior and disturbed physiology, where disease-caused lesions are associated with disturbed behavior or physiological processing. Historically, this method has been productively used by many investigators dating back to Dax, Broca and Wernicke localizing language and speech functions to the left hemisphere on the basis of postmortem lesion data.
This methodology has advantages over some other source localization methods in that the associations are not subject to the caveat that dipole source localization techniques do not have unique solutions. We illustrate this method using the P300 and the Mismatch Negativity event-related potentials (ERPs) in schizophrenia.
P300 IN FIRST EPISODE SCHIZOPHRENIA SUBJECTS
Association of left temporal amplitude with gray matter volume of left superior temporal gyrus volume
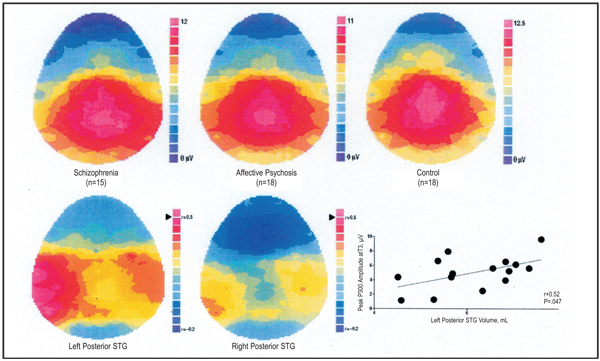
Following our demonstration in chronic schizophrenia of an association between left posterior superior temporal gyrus (STG) gray matter volumes and left-sided reductions in P3002 and with thought disorder,3 we began to examine P300 in patients at, or within 1 year of, their first hospitalization. Like chronic patients, P300 is reduced in both schizophrenia and affective disorder at first hospitalization along the middle of the scalp, but schizophrenia patients selectively show a left temporal area P300 reduction.4 In a subset of patients, we5 recorded P300 and performed MRI. First-episode subjects with schizophrenia (n = 15) or affective psychosis (n = 18) and control subjects (n = 18) silently counted infrequent target tones amid standard tones (oddball task). Spoiled gradient-recalled acquisition MRI images on a 1.5 T GE scanner provided quantitative measures of temporal lobe gray matter regions of interest. Although both psychotic groups show relatively healthy P300 over the midline sites (no group differences, P > 0.7), the first episode schizophrenia group was significantly asymmetrical with selective left-sided reductions compared with the affective psychosis group (group × side: F1,31 = 11.0, P = .002) and the control group (group × side: F1,31 = 17.01, P<.001, Figure 1). MRI revealed first hospitalized schizophrenia patients to also show reduced gray matter volumes of left posterior STG relative to control subjects and patients with affective psychosis (15.4% and 11.0%, respectively). Within STG, they showed smaller gray matter volumes of left planum temporale (21.0% relative to both) and a smaller total Heschl’s gyrus volume (14.6% and 21.1%, respectively). Left posterior STG and the left planum temporale, but not other regions of interest, were specifically and positively correlated (r>0.5) with left temporal P300 voltage in patients with schizophrenia but not in patients with affective psychosis or in control subjects.
Figure 1.
Top. P300 to target tones. Note the lateralized amplitude reduction at over left temporal scalp areas in schizophrenia. Bottom. Pearson correlation r-values between posterior superior temporal gyrus (STG) and P300 in schizophrenia. The gray matter volume of the left posterior STG shows a regionally selective association with P300 amplitude, but the gray matter volume of the right posterior STG does not. (After McCarley et al. 20025).
Thus, both chronic and first hospitalized schizophrenia patients show reduced P300, particularly marked over the left hemisphere, that correlates with reductions in left posterior STG. These combined functional and structural measures home in on an area with important roles in language, memory, and attention, in which pathophysiology is central to the symptoms and course of schizophrenia.
MISMATCH NEGATIVITY
Initial and longitudinal association of amplitude with left Heschl’s gyrus volume and volume reduction over time
Whereas P300 indexes the active detection of “oddball” stimuli, another brainwave is elicited automatically by small to moderately different oddballs, even when the subject is not actively attending the tones. This small negativity, termed the mismatch negativity (MMN), was generated by tones presented 3/sec as subjects performed a visual distractor task. MMN amplitude was measured from the midline anterior site (Fz), where it is typically largest. Amplitude was quantified as the mean voltage from 100 to 200 msec in the subtraction waveform constructed by removing the brain activity to standard, repetitive stimuli (1 kHz, 95%) from the brain response to rare, deviant stimuli (1.2 kHz, 5%). Unlike chronic patients who show MMN reductions, patients at first hospitalization do not show MMN reductions.6
To examine brain function and structure relationships, we7 acquired MRI from a subset of patients, obtained as described above and measured in particular for left and right Heschl’s gyrus, containing primary and portions of secondary auditory cortex. These areas are likely to contain MMN generators. Patients were tested 6 months or less from their first hospitalization (median <1 week), and comprised 20 subjects with first-episode schizophrenia and 21 subjects with first-episode psychotic bipolar disorder in a manic phase. A group of 32 psychiatrically-well subjects served as controls. Samples were matched on age, parental socio-economic status, and handedness. Like the original report,6 patient and control subjects did not differ in MMN amplitude at initial testing. However, schizophrenia patients had reduced Heschl’s gray matter volumes compared with bipolar and control subjects. Within the schizophrenia group there was a significant correlation between their left hemisphere Heschl’s gyrus gray matter volume (r =−.51, p =.02) and MMN amplitude at the mid-frontal site (where it is largest).
To further explore this abnormal brain function-brain structure relationship, subjects were reassessed approximately a year and a half after protocol entrance. The first longitudinal comparison is for MMN alone and included 16 subjects with schizophrenia, 17 subjects with psychotic bipolar disorder, and 20 psychiatrically-well control subjects. Although all groups were similar in MMN at protocol entrance schizophrenia patients showed progressive reductions of MMN amplitude (group × time, F2,50 =4.97, p =.011, t15 =3.4, p=.004 within schizophrenia).
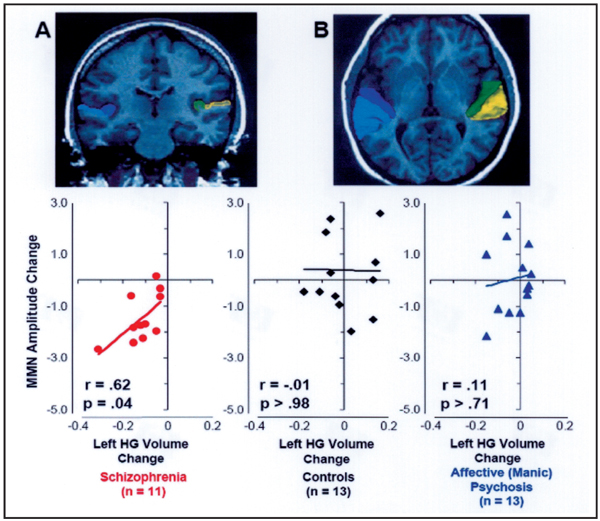
The combined longitudinal retesting MMN and MRI subjects comprised 11 subjects with schizophrenia, 13 subjects with psychotic bipolar disorder, and 13 psychiatrically-well control subjects. As in the MMN only group described above, the MMN/MRI group showed significant reductions in MMN only for the schizophrenia patients (t10 =5.2, p<.001). Groups did not differ in overall relative Heschl’s gyrus gray matter volumes (F2,34 =0.97, p >.39), but there were significantly different changes in gray matter over time in the groups, restricted to one hemisphere (group × hemisphere × time, F2,34 = 4.88, p = .014). The changes occurred in the left hemisphere of schizophrenia subjects (t10 =4.5, p<.001). Both MMN and gray matter volumes in left Heschl’s gyrus, its putative generator site, showed progressive reductions in schizophrenia in the first year and a half after first hospitalization.
These progressive reductions in MMN amplitude and left hemisphere Heschl’s gyrus gray matter volumes in schizophrenia were highly correlated [r =.62, p =.04, Figure 2]. Nearly all of the schizophrenic patients showed both MMN reduction and Heschl’s gyrus reduction. Assuming a chance distribution for change, neither controls nor bipolars differed significantly from a random distribution (χ2 <1 in each group, p’s >.8). By contrast, schizophrenics were extremely different from a random distribution (χ2 =23.6, p <.001), lending further support to the notion that this group shows a selective and specific progressive brain reduction.
Figure 2.
Conjoint progression of gray matter loss in Heschl’s gyrus (dark blue and green in MRI images) and reduction of Mismatch Negativity (MMN) over 1.5 years after first hospitalization in schizophrenia, but not in affective (manic) psychosis. (After Salisbury et al. 20077).
COMMENT
These data suggest that the conjoint use of structural MRI and ERPs can provide important information on the brain gray matter sources of ERP abnormalities in schizophrenia and bipolar disorder. Of interest with respect to the sensitivity of the method, the initial test of association between MMN amplitude within the schizophrenia group showed a significant correlation with reduced volume, although MMN amplitude itself did not differ across groups. We interpret this as evidence of a beginning pathological process in Heschl’s gyrus which orders MMN amplitude within the schizophrenia group according to volume, although not strong enough to affect group mean MMN values. Of particular note is the association between reduction in MMN amplitude and reduction in Heschl’s gyrus gray matter volume in schizophrenia alone, further reinforcing the value of the conjoint structural MRI/ERP analysis. Also of note is the diagnostic differentiation between schizophrenia and bipolar disorder on both the initial and longitudinal conjoint association of MMN and MRI reductions.
The combined use of high temporal resolution EEG methods and high spatial resolution MRI methods have indicated brain areas involved specifically in the brain disorder known as schizophrenia, and have shown a progressive course to the disease peri-onset. We speculate that the progressive changes are related to a deficiency in inhibition from projections of the parvalbumin-containing GABAergic neurons in the cortex to pyramidal neurons. This may lead to unchecked excitation in the pyramidal neurons which in turn may act on their targets to produce excitotoxic changes, leading to neuropil regression from the loss of dendrites and synapses. Supporting this model are the neuropathological findings of loss of soma volume in deep layer 3 of the auditory primary and secondary cortex — pyramidal cell somal volume is correlated with the extent of dendritic arborization and number of dendritic spines.8 Of note, an impaired GABAergic-glutamatergic (pyramidal neuron) interaction may also be the basis of the observed gamma band deficiency in schizophrenia, both for steady state and evoked gamma.9 The field’s growing awareness of the importance of altered glutamatergic activity in schizophrenia has recently been underscored by the promising initial clinical results of a new schizophrenia drug that activates the presynaptic metabotrophic glutamate 2/3 receptors and reduces glutamate release.10
ACKNOWLEDGMENTS
Supported by the Department of Veterans Affairs (Merit Awards to RWM & MES, Schizophrenia Center Award to RWM & MES, Middleton Award to RWM), by the National Institute of Health (R01 MH 40799, R01 MH 052807, P50MH080272 to RWM; K05 MH 01110 and R01 MH 50747 to MES; and R01 MH58704 to DFS), the MIND foundation (RWM), and NARSAD (DFS).
REFERENCES
- 1.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 2.McCarley RW, Shenton ME, O’Donnell BF, Faux SF, Kikinis R, Nestor PG, et al. Auditory P300 abnormalities and left posterior superior temporal gyrus reduction in schizophrenia. Arch Gen Psychiatry. 1993;50:190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- 3.Shenton ME, Kikinis R, McCarley RW, Jolesz FA, Pollak SD, LeMay M, et al. Left temporal lobe abnormalities in schizophrenia and thought disorder: a quantitative MRI study. N Eng J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- 4.Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First episode schizophrenic psychosis differs from first episode affective psychosis and controls in P300 amplitude over left temporal lobe. Arch Gen Psychiatry. 1998;55:173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, et al. Association between smaller left posterior superior temporal gyrus MRI volume and smaller left temporal P300 amplitude in first episode schizophrenia. Arch Gen Psychiatry. 2002;59:321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- 6.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 7.Salisbury DF, Kasai K, Kuroki N, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 9.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nature Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]