Abstract
Human septic shock involves multiple genome-level perturbations. We have conducted microarray analyses in children with septic shock within 24 hours of intensive care unit admission, using whole-blood derived RNA. Based on sequential statistical and expression filters, there were 2,482 differentially regulated gene probes (1,081 upregulated and 1,401 downregulated) between patients with septic shock (n = 42) and controls (n = 15). Both gene lists encompassed several biologically relevant gene ontologies and canonical pathways. Notably, many of the genes downregulated in the patients with septic shock, relative to the controls, participate in gene ontologies related to metal or zinc homeostasis. Comparison of septic shock survivors (n = 33) and nonsurvivors (n = 9) demonstrated differential regulation of 63 gene probes. Among the 63 gene probes differentially regulated between septic shock survivors and nonsurvivors, two isoforms of metallothionein (MT) demonstrated increased expression in the nonsurvivors. Consistent with the ability of MT to sequester zinc in the intracellular compartment, nonsurvivors had lower serum zinc levels compared to survivors. In a corroborating study of murine sepsis, MT-null mice demonstrated a survival advantage compared to wild-type mice. These data represent the largest reported cohort of pediatric patients with septic shock, which has undergone genome-level expression profiling based on microarray. The data are biologically plausible and demonstrate that genome-level alterations of zinc homeostasis may be prevalent in clinical pediatric septic shock.
Introduction
Pediatric septic shock continues to be an important public health problem despite potent antibiotics and the development of pediatric intensive care units (34). There are approximately 42,000 cases per year of pediatric septic shock in the United States, with a mortality rate of approximately 10%, and higher mortality rates in children with co-morbidities such as cancer and prematurity (39). A great deal of basic research efforts have focused on the biological processes that occur in septic shock. While highly informative, a relative paucity of this information has been readily translated to the bedside in the form of meaningful therapeutic advances for children (12, 34). For example, multiple trials focused on immune modulation strategies have been conducted in adults with septic shock (1). Despite strong preclinical data, as well as strong phase I and II data, the majority of these strategies have failed when subjected to large scale, randomized placebo-controlled trials. Consequently, the majority of these strategies have not been effectively tested in the pediatric population. One notable exception is activated protein C, which recently received Food and Drug Administration approval for use in adults with septic shock (2, 4). Unfortunately, a phase III trial of activated protein C in children with septic shock was recently terminated early due to lack of efficacy (14). Current care for pediatric septic shock remains fundamentally based on antibiotics and supportive care (7, 34).
Zinc homeostasis appears to be required for normal function of both the innate and acquired immune systems. For example, King and colleagues have demonstrated that zinc deficiency causes a cumulative loss of T and B cell maturation, which subsequently leads to lymphopenia (16, 23). Natural killer cell function and phagocytic cell function are impaired by zinc deficiency (20), as is expression of specific cytokines that modulate the immune system (15, 33). In clinical states associated with immune suppression (e.g. sickle cell disease, human immunodeficiency virus infection, Down Syndrome, and the elderly), zinc supplementation has been shown to restore natural killer cell activity, lymphocyte production, mitogen responses, wound healing, and resistance to infection (33). Finally, in clinical trials involving children in developing countries or in rural communities, zinc supplementation has been demonstrated to reduce the incidence and severity of gastroenteritis and upper respiratory tract infections (5, 32). Thus, the established literature would suggest that zinc homeostasis may be an important research paradigm in the context of pediatric septic shock.
The clinical problem presented by pediatric septic shock, coupled with the relative lack of specific therapies, warrant a more comprehensive understanding of this condition at the translational level. This is a complex task given the inherent complexity of clinical septic shock and patient heterogeneity. We are addressing this complexity through genome-level expression profiling based on microarray technology. This approach has led to advances in specific forms of cancer (11, 31, 36), and is beginning to show promise in the more heterogeneous condition of septic shock (6, 8, 9, 26, 29). Our goal for these studies is to define the genome level expression profiles that occur in pediatric septic shock as a means of substantially advancing our understanding of this condition.
METHODS
Patients
The study protocol was approved by the individual Institutional Review Boards of each participating institution. Children < 10 years of age admitted to the pediatric intensive care unit (PICU) and meeting criteria for septic shock were eligible for the study. Septic shock was defined using pediatric-specific criteria (18). Control patients were recruited from the participating institutions using the following exclusion criteria: any acute illness, a recent febrile illness (within 2 weeks), recent use of anti-inflammatory medications (within 2 weeks), or any history of chronic or acute disease associated with inflammation.
Sample and data collection
After obtaining informed consent, blood samples (for RNA and serum isolation) were obtained within 24 hours of admission to the PICU, heretofore referred to as “Day 1” of septic shock. Severity of illness was calculated using the PRISM III score (27), and organ failure was defined using pediatric-specific criteria (18, 28, 40). Annotated clinical and laboratory data were collected daily while in the PICU. All study patients were followed for 28 days to determine survival. Clinical, laboratory, and biological data were entered and stored using a web-based database developed locally.
RNA extraction, microarray hybridization, and microarray analysis
The data and protocols described in this manuscript have been deposited in the NCBI Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE4607.
Total RNA was isolated from whole blood samples using the PaxGene™ Blood RNA System (PreAnalytiX, Qiagen/Becton Dickson, Valencia, CA) according the manufacturer’s specifications. Microarray hybridization was performed by the Affymetrix Gene Chip Core facility at Cincinnati Children’s Hospital Research Foundation as previously described using the Human Genome U133 Plus 2.0 GeneChip (Affymetrix, Santa Clara, CA) (42).
Analyses were performed using one patient sample per chip. Image files were captured using an Affymetrix GeneChip Scanner 3000. .CEL files were subsequently preprocessed using Robust Multiple-array Average (RMA) normalization (21) using GeneSpring GX 7.3 software (Agilent Technologies, Palo Alto, CA). All signal intensity-based data was used after RMA normalization, which specifically suppresses all but significant variation among lower intensity probe sets (21). All chips were then normalized to the respective median values of controls. Differences in mRNA abundance between patient samples were determined using GeneSpring GX 7.3. All statistical analyses used corrections for multiple comparisons. The specific statistical and filtering approaches are provided in the Results section because of their relevance to data interpretation.
Two-dimensional cluster maps were constructed using GeneSpring GX 7.3. Gene trees are represented in the vertical dimension and condition trees are represented in the horizontal dimension. Both the gene trees and condition trees are based on the Pearson similarity algorithm. The coloring conventions for all maps are as follows: red intensity correlates with increased gene expression, blue intensity correlates with decreased gene expression, and yellow intensity correlates with no change in gene expression relative to the median of controls.
Ontology analyses were performed by uploading specific gene expression lists to the web-based application D.A.V.I.D. (Database for Annotation, Visualization and Integrated Discovery) that allows public access to a relational database of functional gene annotations (13). Canonical pathway analyses were performed by uploading specific gene lists to the Ingenuity Systems Pathways Knowledge Base (Ingenuity Systems, Redwood City, CA) that provides a tool for discovery of canonical pathways within the uploaded gene lists (6). Both applications use specific approaches to estimate significance based on non-redundant representations of the microarray chip and to convert the uploaded gene lists to gene lists containing a single value for each gene.
Ancillary validation studies
Real time quantitative PCR was performed for selected genes by a standard approach involving the Superscript First Strand Synthesis kit (Invitrogen, Carlsbad, CA), SYBR green (BioRad, Hercules, CA) and the iCycler Thermal Cycler (BioRad). Serum interleukin-8 was measured using an ELISA kit, as specified by the manufacturer (Biosource, Camarillo, CA). Metallothionein-null mice having mutations for metallothionein-1 and -2 were purchased from The Jackson Laboratory (Bar Harbor, ME, Stock #002211, Strain Name: 129S7/SvEvBrd-Mt1tm1Bri Mt2tm1Bri/J). The appropriate control mice were also purchased from The Jackson Laboratory (Strain # 002448, Strain Name: 129S1/SvImJ mice). Mice were subjected to cecal ligation and puncture as previously described (35). All experiments involving animals conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23 revised 1996) and with the approval of the Institutional Animal Care and Use Committee.
RESULTS
General study subject data
Summary demographic, clinical, laboratory, and microbiologic data for the study subjects are provided in Tables 1 and 2. A total of 57 individual microarray chips, representing 15 individual controls and 42 individual patients with septic shock, were used for analysis. The controls were comparable to the patients with septic shock with regard to age, race, and gender. Among the patients with septic shock there were 28 having positive identification of an infecting organism (67%) and 9 deaths (21% mortality). Overall, the patients with septic shock were heterogeneous with respect to age, illness severity, gender, race, infectious organisms, and sites of infection. The annotated clinical data provided in Table 1 were not significantly different between septic shock survivors and septic shock nonsurvivors, except that the nonsurvivors had a significantly higher (p < 0.05) severity of illness score (PRISM score) than the survivors.
Table 1.
Clinical, laboratory, and demographic data for all subjects.
| Controls | S.S. (survivors) | S.S. (nonsurvivors) | |
|---|---|---|---|
| No. of subjects | 15 | 33 | 9 |
| Mean age (yrs) | 3.2 ± 2.9 | 3.8 ± 3.3 | 3.9 ± 4.1 |
| Median Age, Range (yrs) | 2.4, (0 to 9.7) | 2.7, (0 to 10) | 1.6, (0 to 10) |
| Mean PRISM Score | n/a | 16.2 ± 7.0 | 28.0 ± 15.4a |
| Fluid resuscitation (ml/kg)b | n/a | 132 ± 66 | 153 ± 83 |
| % blood transfusionc | n/a | 12 | 22 |
| Total WBC (103)d | n/a | 14.3 ± 12.6 | 7.6 ± 6.1 |
| Total Neutrophils (103)e | n/a | 10.4 ± 10.9 | 5.4 ± 5.6 |
| Gender (Male/Female) | 8/7 | 19/14 | 7/2 |
| Race (no.) | A.A./Black (6) | A.A./Black (8) | A.A./Black (2) |
| Asian (4) | American Indian (1) | Unreported (1) | |
| White (5) | Asian (2) | White (6) | |
| Unreported (1) | |||
| White (21) |
p < 0.05 versus survivors, t-test.
Total fluid resuscitation during first 24 hours of admission.
Percent of patients receiving packed red blood cell transfusion during the first 24 hours of admission.
Total peripheral white blood cell count during the first 24 hours of admission.
Peripheral neutrophil count during the first 24 hours of admission.
Table 2A.
Microbiology data for patients with septic shock (survivors).
| Organism (no.) | Primary source of positive culture (no.) |
|---|---|
| Candida albicans (2) | Blood (15) |
| Enterococcus faecalis (1) | Lung (4) |
| Escherichia coli (1) | Urine (1) |
| Streptococcus pyogenes (1) | Retropharyngeal abscess (1) |
| Streptococcus agalactiae (1) | |
| Herpes simplex I (1) | |
| Klebsiella pneumoniae (2) | |
| Neisseria meningitidis (2) | |
| Ricketssia ricketsii (1) | |
| Staphylococcus aureus (3) | |
| Staphylococcus epidermidis (1) | |
| Streptococcus pneumoniae (2) | |
| Streptococcus viridans (2) | |
| Varicella (1) |
Differential gene expression in pediatric septic shock
To begin testing the hypothesis that pediatric septic shock is characterized by broad alterations in gene expression, we conducted a two group ANOVA (Benjamini-Hochberg false discovery rate of 5%) using controls and patients with septic shock as the comparison groups, and all gene probes within the microarray (54,681 gene probes). This statistical filter yielded a working list of 17,601 gene probes that were differentially regulated between controls and patients with septic shock. To further refine this 17,601 gene list, we next applied an expression filter that selected only the genes, within the above 17,601 gene list, having at least 2-fold expression difference in at least 50% of the patients with septic shock, compared to the median of the controls. This expression filter yielded a final working list of 2,482 gene probes that were differentially regulated between patients with septic shock and controls.
These 2,482 gene probes were then subjected to two-dimensional cluster analysis as depicted in Figure 1. All of the patients with septic shock cluster together at the center of the map in a homogenous manner, thus demonstrating a relative commonality of gene regulation on Day 1 of pediatric septic shock. This homogenous clustering is dependent on a group of genes in the upper portion of the map having increased expression (1,081 genes) and a group of genes in the lower portion of the map having decreased expression (1,401 genes).
Figure 1.
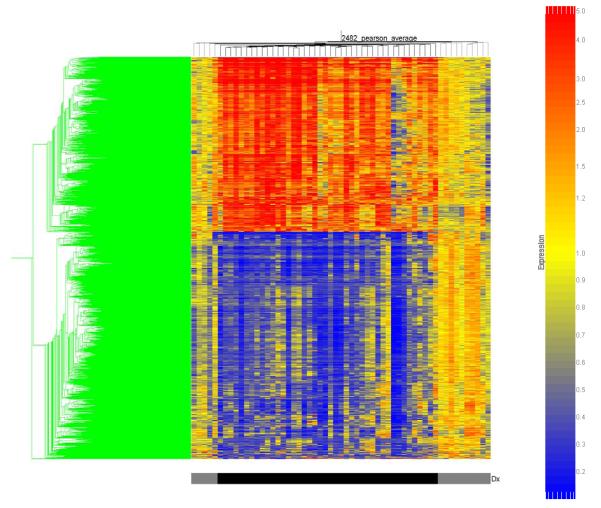
Two dimensional gene cluster map representing 2,482 genes differentially regulated between patients with septic shock and control patients (see text for filtering strategy). Individual patients are oriented horizontally and individual genes are oriented vertically. The color coded bar at the bottom of the map represents controls in grey and the patients with septic shock in black.
In order to begin deriving biological meaning from these 2,482 differentially regulated genes, we uploaded the individual list of genes with increased expression and the individual list of genes with decreased expression to both the D.A.V.I.D. database (13) and the Ingenuity Systems Pathways Knowledge Base (6). As shown in Tables 3 and 4, the D.A.V.I.D.-dependent analyses yielded several relevant functional annotations within both gene lists. The functional annotations derived from the list of genes with increased expression (Table 3) are consistent with the current literature focused on septic shock (3, 34). The functional annotations derived from the list of genes with decreased expression (Table 4) are notable for the prevalence of zinc- and metal binding-related ontologies.
Table 3.
Top 20 functional annotations among 1081 upregulated genes in patients with septic shock (based on p value). The analysis is based on the default parameters in D.A.V.I.D.
| Category | Term | # of genes | p-value |
|---|---|---|---|
| SP_PIR_KEYWORDS | direct protein sequencing | 150 | 1.2E-27 |
| GOTERM_BP_ALL | response to other organism | 80 | 2.3E-24 |
| GOTERM_BP_ALL | response to pest/pathogen/parasite | 77 | 4.6E-24 |
| SP_PIR_KEYWORDS | glycoprotein | 178 | 7.5E-18 |
| GOTERM_BP_ALL | response to stress | 98 | 4.2E-17 |
| SP_PIR_KEYWORDS | membrane | 190 | 6.3E-17 |
| GOTERM_BP_ALL | response to biotic stimulus | 108 | 2.8E-15 |
| GOTERM_BP_ALL | response to wounding | 52 | 1.8E-15 |
| SP_PIR_KEYWORDS | signal | 140 | 2.8E-15 |
| GOTERM_BP_ALL | inflammatory response | 37 | 5.5E-15 |
| GOTERM_BP_ALL | response to external stimulus | 59 | 9.4E-15 |
| GOTERM_BP_ALL | defense response | 101 | 1.6E-14 |
| GOTERM_BP_ALL | immune response | 93 | 9.7E-14 |
| GOTERM_MF_ALL | protein binding | 224 | 2.5E-12 |
| SP_PIR_KEYWORDS | lipoprotein | 49 | 2.5E-12 |
| SP_PIR_KEYWORDS | phosphorylation | 107 | 8.2E-12 |
| GOTERM_BP_ALL | intracellular signaling cascade | 86 | 2.7E-11 |
| GOTERM_CC_ALL | plasma membrane | 107 | 1.7E-8 |
| GOTERM_BP_ALL | signal transduction | 170 | 2.7E-8 |
| GOTERM_BP_ALL | protein kinase cascade | 32 | 3.2E-8 |
Table 4.
Top 20 functional annotations among 1401 downregulated genes in patients with septic shock (based on p value). The analysis is based on the default parameters in D.A.V.I.D.
| Category | Term | # of genes | p-value |
|---|---|---|---|
| SP_PIR_KEYWORDS | nuclear protein | 270 | 4.0E-41 |
| SP_PIR_KEYWORDS | zinc | 151 | 8.0E-20 |
| SP_PIR_KEYWORDS | zinc-finger | 132 | 9.9E-20 |
| SP_PIR_KEYWORDS | transcription | 131 | 2.0E-19 |
| GOTERM_CC_ALL | nucleus | 303 | 5.2E-18 |
| SP_PIR_KEYWORDS | transcription regulation | 129 | 6.8E-18 |
| SP_PIR_KEYWORDS | dna-binding | 125 | 1.0E-15 |
| SP_PIR_KEYWORDS | metal-binding | 163 | 4.3E-15 |
| SP_PIR_KEYWORDS | t-cell | 17 | 7.5E-15 |
| GOTERM_MF_ALL | nucleic acid binding | 251 | 1.4E-14 |
| GOTERM_MF_ALL | zinc ion binding | 166 | 3.8E-13 |
| GOTERM_CC_ALL | membrane-bound organelle | 380 | 3.9E-13 |
| GOTERM_BP_ALL | regulation of intracellular physiological process |
237 | 1.0E-11 |
| GOTERM_BP_ALL | regulation of cellular process | 244 | 2.9E-11 |
| GOTERM_BP_ALL | defense response | 113 | 3.6E-11 |
| GOTERM_BP_ALL | nucleobase, nucleoside, nucleo- tide, and nucleic acid metabolism |
246 | 4.1E-11 |
| GOTERM_BP_ALL | regulation of physiological process | 240 | 4.3E-11 |
| GOTERM_BP_ALL | immune response | 105 | 5.1E-11 |
| KEGG_PATHWAY | antigen processing/presentation | 22 | 7.8E-11 |
| GOTERM_BP_ALL | regulation of biological process | 255 | 7.9E-11 |
As shown in Tables 5 and 6, the Ingenuity Systems Pathways Knowledge Base-dependent analysis also yielded several relevant canonical pathways within both gene lists. Similar to the functional annotations listed in Table 3, the majority of canonical pathways derived from these gene lists are consistent with the current experimental literature focused on septic shock (3, 34).
Table 5.
Canonical pathways among 1081 upregulated genes in patients with septic shock. The analysis is derived from the Ingenuity Pathways Analysis default parameters and the canonical pathways represent the top 10 most significant p values.
| Canonical Pathway | # of genes | p-value |
|---|---|---|
| Interleukin-6 signaling | 16 | 7.1E-7 |
| Interleukin-10 signaling | 13 | 1.8E-5 |
| Toll-like receptor signaling | 11 | 3.0E-5 |
| B-cell receptor signaling | 18 | 5.2E-5 |
| Integrin signaling | 20 | 8.5E-4 |
| Complement and coagulation cascades | 11 | 1.7E-3 |
| Granulocyte/macrophage-colony stimulation factor signaling | 9 | 1.7E-3 |
| p38 MAP kinase signaling | 10 | 2.2E-3 |
| Leukocyte extravasation signaling | 16 | 2.5E-3 |
| NF-κB signaling | 14 | 2.9E-3 |
Table 6.
Canonical pathways among 1401 downregulated genes in patients with septic shock. The analysis is derived from the Ingenuity Pathways Analysis default parameters and the canonical pathways represent the top 5 most significant p values.
| Canonical Pathway | # of genes | p-value |
|---|---|---|
| T-cell receptor signaling | 16 | 3.4E-8 |
| Antigen presentation pathway | 10 | 4.6E-6 |
| Natural killer cell signaling | 12 | 3.2E-4 |
| Cell Cycle: G1/S checkpoint regulation | 6 | 1.7E-2 |
| N-glycan biosynthesis | 5 | 1.8E-2 |
In summary, these data demonstrate that the pattern of differential gene expression depicted in Figure 1 is biologically plausible, and therefore provide an important level of confidence in the feasibility of studying clinical pediatric septic shock using a genomic approach based on microarray analysis. Most importantly, the data indicate that within 24 hours of presentation to the PICU, children with septic shock are characterized by decreased expression of a large number of genes that are either dependent on zinc homeostasis or play a direct role in zinc homeostasis, an observation that is indirectly supported by the experimental literature (17, 24, 25, 30, 37, 41), but has not been previously reported at the genomic level and in the setting of clinical pediatric septic shock.
Differential gene expression between survivors and nonsurvivors of pediatric septic shock
Since our initial attempt at elucidating the genome level response of children with septic shock yielded biologically plausible data, we next tested the hypothesis that there is a differential pattern of gene expression between survivors and nonsurvivors of pediatric septic shock. To this end, we conducted a three group ANOVA (Benjamini-Hochberg false discovery rate of 5%) using controls, septic shock survivors, and septic shock nonsurvivors as the comparison groups, and all gene probes within the microarray (54,681 gene probes). This statistical filter yielded a working list of 13,054 gene probes that were differentially regulated between the three groups. A post hoc Tukey test indicated that 589 of these 13,054 gene probes were differentially regulated between the survivors and the nonsurvivors. To further refine this 589 gene list, we applied an expression filter that selected only the genes, within the above 589 gene list, having at least 2-fold expression difference in at least 50% of the survivors, compared to the median of the nonsurvivors. This expression filter yielded a final working list of 63 gene probes that were differentially regulated between survivors and nonsurvivors.
These 63 gene probes were then subjected to two-dimensional cluster analysis as depicted in Figure 2. All of the nonsurvivors cluster to the left side of the map, thus demonstrating a relative commonality of gene regulation in nonsurvivors and survivors, respectively. As shown in Table 7, D.A.V.I.D.-based analysis of the 63 gene probes depicted in Figure 2 yielded several biologically relevant functional annotations, including the “metal-binding” functional annotation previously noted in Table 4. Among the 63 gene probes depicted in Figure 2, 36 gene probes (corresponding to 34 individual genes, Table 8) were upregulated and 27 gene probes (corresponding to 26 individual genes, Table 9) were downregulated in the nonsurvivors relative to survivors, respectively. The two gene lists provided in Tables 8 and 9 represent potential biomarkers for poor outcome and/or potential novel therapeutic targets in the context of pediatric septic shock.
Figure 2.
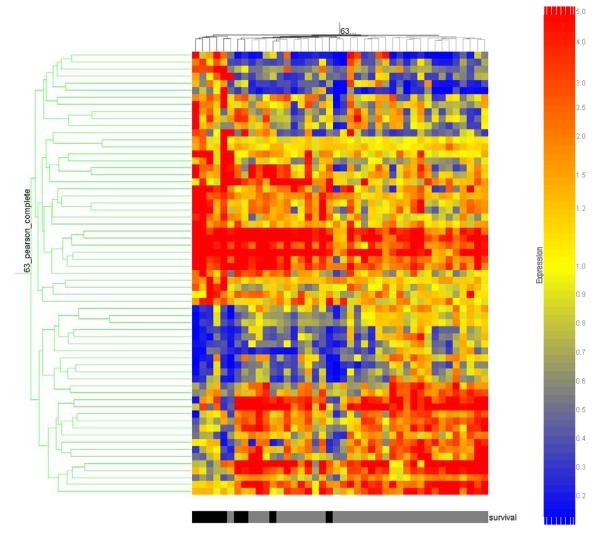
Two dimensional gene cluster map representing 63 genes differentially regulated between septic shock survivors and nonsurvivors (see text for filtering strategy). Individual patients are oriented horizontally and individual genes are oriented vertically. The color coded bar at the bottom of the map represents survivors in grey and nonsurvivors in black.
Table 7.
Top 20 functional annotations among 63 gene probes differentially regulated between survivors and nonsurvivors with septic shock (based on p value). The analysis is based on the default parameters in D.A.V.I.D.
| Category | Term | # of genes | p-value |
|---|---|---|---|
| GOTERM_BP_ALL | response to external stimulus | 10 | 6.0E-6 |
| SP_PIR_KEYWORDS | phosphoprotein | 9 | 1.1E-5 |
| SP_PIR_KEYWORDS | glycoprotein | 20 | 3.7E-5 |
| SP_PIR_KEYWORDS | signal | 17 | 4.8E-5 |
| GOTERM_BP_ALL | cell communication | 20 | 5.3E-4 |
| GOTERM_BP_ALL | response to wounding | 7 | 5.5E-4 |
| GOTERM_BP_ALL | negative regulation of signal transduction |
4 | 5.6E-4 |
| SP_PIR_KEYWORDS | metal-binding | 3 | 9.0E-4 |
| SP_PIR_KEYWORDS | direct protein sequencing | 13 | 1.3E-3 |
| SP_PIR_KEYWORDS | signal transduction inhibitor | 3 | 1.7E-3 |
| GOTERM_BP_ALL | response to stress | 10 | 1.9E-3 |
| GOTERM_MF_ALL | protein kinase inhibitor activity | 3 | 2.3E-3 |
| GOTERM_BP_ALL | regulation of body fluids | 4 | 2.8E-3 |
| GOTERM_MF_ALL | kinase inhibitor activity | 3 | 2.9E-3 |
| GOTERM_BP_ALL | response to pest/pathogen/parasite | 7 | 3.4E-3 |
| GOTERM_BP_ALL | cell motility | 5 | 3.4E-3 |
| GOTERM_BP_ALL | locomotion | 5 | 3.4E-3 |
| GOTERM_BP_ALL | localization of cell | 5 | 3.4E-3 |
| UP_SEQ_FEATURE | signal peptide | 17 | 3.7E-3 |
| SP_PIR_KEYWORDS | transmembrane | 17 | 3.9E-3 |
Table 8.
Thirty four upregulated genes in nonsurvivors compared to survivors with septic shock.
| Symbol | GenBank # | Description |
|---|---|---|
| *** | AK057572 | Homo sapiens, clone IMAGE:5106451, mRNA |
| ALB | NM_000477 | Albumin |
| ANKRD22 | NM_144590 | Ankyrin repeat domain 22 |
| CCL4 | NM_002984 | Chemokine (C-C motif) ligand 4 |
| CCRL2 | NM_003965 | Chemokine (C-C motif) receptor-like 2 |
| CD69 | NM_001781 | CD69 antigen (p60, early T-cell activation antigen) |
| CEACAM1 | NM_001024912 | Carcinoembryonic antigen-related cell adhesion molecule 1 |
| CLEC5A | NM_013252 | C-type lectin domain family 5, member A |
| DDIT4 | NM_019058 | DNA-damage-inducible transcript 4 |
| EMP1 | NM_001423 | Epithelial membrane protein 1 |
| ENPP2 | NM_006209 | Ectonucleotide pyrophosphatase/phosphodiesterase 2 (autotaxin) |
| FER1L3 | NM_013451 | Fer-1-like 3, myoferlin (C. elegans) |
| FGG | NM_000509 | Fibrinogen gamma chain |
| G0S2 | NM_015714 | Putative lymphocyte G0/G1 switch gene |
| GPR171 | NM_013308 | G protein-coupled receptor 171 |
| GZMB | NM_004131 | Granzyme B |
| IL8 | NM_000584 | Interleukin 8 |
| LOC200230 | XM_114166 | Similar to KIAA0386 |
| MAFF | NM_012323 | V-maf musculoaponeurotic fibrosarcoma oncogene homolog F |
| MT1E | NM_175617 | Metallothionein 1E (functional) |
| MT1M | NM_176870 | Metallothionein 1K |
| NMES1 | NM_032413 | Normal mucosa of esophagus specific 1 |
| PDE4D | CD245404 | Phosphodiesterase 4D, cAMP-specific |
| RAB20 | NM_017817 | RAB20, member RAS oncogene family |
| RARRES3 | NM_004585 | Retinoic acid receptor responder (tazarotene induced) 3 |
| RGS1 | NM_002922 | Regulator of G-protein signaling 1 |
| ROCK1 | NM_005406 | Rho-associated, coiled-coil containing protein kinase 1 |
| SLAMF8 | NM_020125 | SLAM family member 8 |
| SLC39A8 | NM_022154 | Solute carrier family 39 (zinc transporter), member 8 |
| SOCS1 | NM_003745 | Suppressor of cytokine signaling 1 |
| TF | NM_001063 | Transferrin |
| THBS1 | NM_003246 | Thrombospondin 1 |
| TNFAIP3 | NM_006290 | Tumor necrosis factor, alpha-induced protein 3 |
| TRIB1 | NM_025195 | Tribbles homolog 1 (Drosophila) |
Table 9.
Twenty six downregulated genes in nonsurvivors compared to survivors with septic shock.
| Symbol | GenBank # | Description |
|---|---|---|
| *** | BF510602 | Transcribed locus, similar to XP_517083.1 |
| *** | AK091419 | CDNA FLJ34100 fis, clone FCBBF3007597 |
| ABHD2 | NM_007011 | Abhydrolase domain containing 2 |
| ANP32A | AW978849 | Acidic (leucine-rich) nuclear phosphoprotein 32 family, member A |
| ANPEP | NM_001150 | Alanyl (membrane) aminopeptidase (microsomal aminopeptidase) |
| C1QR1 | NM_012072 | Complement component 1, q subcomponent, receptor 1 |
| C1QR1 | NM_012072 | Complement component 1, q subcomponent, receptor 1 |
| C6orf155 | AL137346 | Chromosome 6 open reading frame 155 |
| CD86 | NM_006889 | CD86 antigen (CD28 antigen ligand 2, B7-2 antigen) |
| CPVL | NM_019029 | Carboxypeptidase, vitellogenic-like |
| CRTAP | NM_006371 | Cartilage associated protein |
| DNAJC3 | NM_006260 | Hypothetical protein LOC144871 |
| DPEP2 | NM_022355 | Dipeptidase 2 |
| DSC2 | NM_004949 | Desmocollin 2 |
| FLJ10357 | NM_018071 | Hypothetical protein FLJ10357 |
| GLIPR1 | NM_006851 | GLI pathogenesis-related 1 (glioma) |
| HRB2 | NM_007043 | HIV-1 rev binding protein 2 |
| KRT23 | NM_015515 | Keratin 23 (histone deacetylase inducible) |
| LOC389734 | XM_372097 | Similar to cell recognition molecule CASPR3 |
| MANSC1 | NM_018050 | MANSC domain containing 1 |
| PDK3 | NM_005391 | Pyruvate dehydrogenase kinase, isoenzyme 3 |
| PYGL | NM_002863 | Phosphorylase, glycogen; liver |
| RGS2 | NM_002923 | Regulator of G-protein signaling 2, 24kDa |
| SLC8A1 | BF223010 | Solute carrier family 8 (sodium/calcium exchanger), member 1 |
| SRPK2 | NM_182691 | SFRS protein kinase 2 |
| SULF2 | NM_018837 | Sulfatase 2 |
Validation of interleukin-8 microarray data
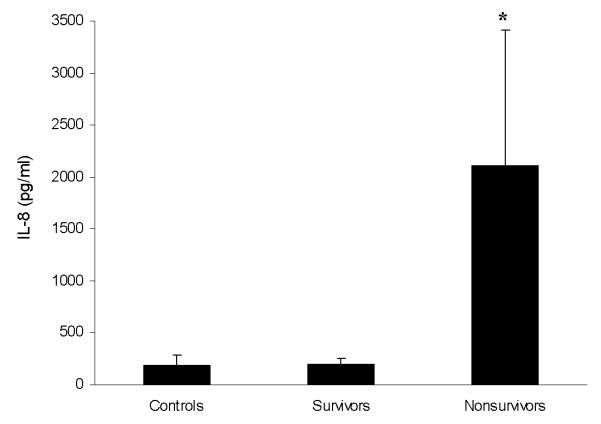
Our initial approach to validating our microarray data involved two complementary approaches: expression validation and functional validation. Table 8 indicates that interleukin-8 (IL-8) mRNA expression is increased in nonsurvivors of pediatric septic shock, compared to survivors. Since IL-8 protein is readily detectable in serum samples, we assayed parallel serum samples from the same patient cohort by way of ELISA. As shown in Figure 3, nonsurvivors had increased serum levels of IL-8 protein compared to survivors, thereby corroborating the IL-8-specific microarray data.
Figure 3.
ELISA data (mean ± SEM) verifying that nonsurvivors with septic shock have increased serum levels of IL-8 protein compared to survivors and control patients. * p < 0.05 versus controls and survivors.
Increased expression of metallothionein and mortality in pediatric septic shock
Among the other group of genes that were increased in nonsurvivors of pediatric septic shock (Table 8), there were two isoforms of metallothionein (MT) that were increased at least 2-fold in the nonsurvivors: MT-1E (2.0 fold versus both survivors and controls) and MT-1M (2.5 fold versus survivors, and 3.1 fold versus controls). Using a lower cutoff fold change of 1.5, we also detected increased expression of the MT-1G isoform (1.8 fold) and MT-1H isoform (1.7 fold) in the nonsurvivors relative to the survivors. Increased expression of MT isoforms in nonsurvivors, compared to survivors, was corroborated by real time quantitative PCR (data not shown).
MTs are cysteine-rich, low-molecular weight, intracellular metal-binding proteins (10). In particular, MTs are capable of avidly binding zinc in the intracellular compartment. Given this biochemical property of MT, the recurrence of zinc-related ontologies in our microarray data, and the demonstration that nonsurvivors had increased expression of MT, we hypothesized that nonsurvivors would have decreased serum (extracellular) zinc levels, compared to survivors. Accordingly, we measured zinc levels in the parallel serum samples from the same patient cohort by way of atomic absorption.
The 50th percentile range of normal serum zinc concentrations in children < 10 years of age is between 75 and 80 μg/dL, and the 2.5th percentile range is between 50 and 55 μg/dL (19). As shown in Figure 4, survivors had normal serum zinc concentrations. In contrast, nonsurvivors had serum zinc concentrations that were significantly lower than the survivors, and at the 2.5th percentile range. These data indirectly indicate that increased MT expression in nonsurvivors of pediatric shock may have a functional consequence on zinc homeostasis.
Figure 4.
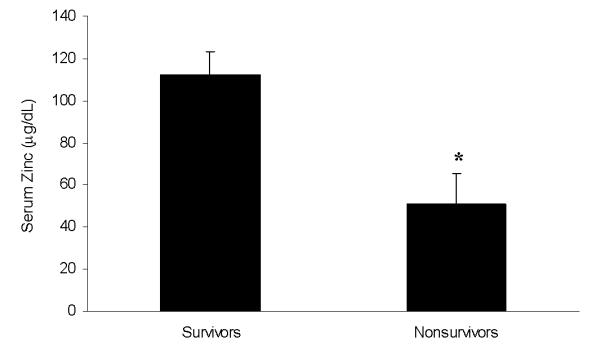
Serum zinc levels (mean ± SEM) demonstrating that nonsurvivors with septic shock have decreased serum zinc levels compared to survivors. * p < 0.05 versus survivors.
MT-null mice have increased survival in polymicrobial sepsis
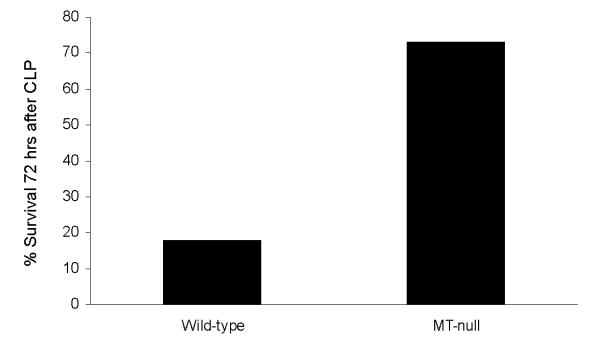
The above MT-and zinc-related data provide the foundation for a novel hypothesis involving MT and poor outcome in the context of septic shock. To begin testing this hypothesis, we subjected MT null mice and their wild-type counterparts to cecal ligation and puncture (CLP), a well accepted murine model of polymicrobial sepsis. As shown in Figure 5, MT-null mice had a higher survival rate at 72 hours after CLP compared to their wild-type counterparts. These data indicate that MT ablation confers a survival advantage in the context of murine polymicrobial sepsis and are consistent with our microarray data demonstrating that increased MT expression is associated with mortality in the context of pediatric septic shock.
Figure 5.
Survival study demonstrating that MT-null mice have a survival advantage compared to wild-type mice 72 hours after CLP (p = 0.03, Fisher’s exact test, n = 11 animals per group).
DISCUSSION
These data represent the largest reported cohort of patients with septic shock (pediatric or adult), to date, which has undergone genome-level expression profiling based on microarray technology. The patients in this cohort are heterogeneous at several levels and received clinical care at multiple centers. Despite this heterogeneity and the potential confounding variables, the data are coherent and biologically plausible.
The data demonstrate that Day 1 of pediatric septic shock is characterized by broad alterations of gene expression and that these broad alterations can be identified through genome-level expression profiles generated from accessible, clinically relevant biological samples. Importantly, the coordinately regulated genes fit well within biologically relevant gene ontologies and canonical pathways. The gene ontologies (Table 3) and canonical pathways (Table 5) detected within the 1,081 upregulated genes in this patient cohort do not necessarily represent novel concepts. The existing experimental and clinical literature has well established that inflammation-, immunity-, and stress response-related genes are highly regulated in the context of septic shock (3, 34). Nevertheless, the current demonstration of these ontologies and pathways provide confidence that the overall data are biologically relevant, rather than being artifacts of this high throughput approach.
Given that some elements of our microarray data are well substantiated in the established literature, we were intrigued to find that a large number of genes that are either dependent on zinc homeostasis or play a direct role in zinc homeostasis, were downregulated in this cohort of patients. The biological plausibility of these observations is supported by the literature discussed above regarding zinc homeostasis, zinc supplementation, and immunity (5, 15, 16, 20, 22, 32, 33). In addition, a recent review focused on the link between zinc homeostasis and immunity (30), and number of previous reports involving experimental endotoxemia/sepsis indicate that altered zinc homeostasis may indeed play a role in pediatric septic shock. For example, Kitamura et al recently established a link between zinc homeostasis and lipopolysaccharide (LPS)-mediated signaling through Toll-like receptor-4 in dendritic cells (24). Liuzzi et al demonstrated that systemic administration of LPS or turpentine in mice induced liver expression of the zinc transporter protein, Zip14, in an interleukin-6-dependent manner (25). The induction of Zip14 in the liver was postulated to represent a mechanism by which the acute phase response/sepsis induces hypozincenemia. In another study involving systemic LPS injection in mice, Zhou et al demonstrated that zinc administration protected mice from LPS-mediated liver injury in an NF-κB-dependent manner (41). Finally, von Bulow et al demonstrated that zinc inhibits in vitro, LPS-mediated release of tumor necrosis factor-α and interleukin-1β in primary human monocytes (37). Collectively, these data involving LPS-mediated signaling and injury support a role for altered zinc homeostasis in sepsis and septic shock. Our current data significantly add to this body of literature by demonstrating an analogous principle at the level of the entire genome, and in human children with clinical septic shock, a far more complex process than experimental endotoxemia.
We are ultimately interested in determining whether or not there exist biologically significant gene expression profiles that distinguish survivors and nonsurvivors of pediatric septic shock. The rationale for addressing this question is to discover novel biomarkers of poor outcome and novel therapeutic targets as means for developing more effective therapeutic strategies. The current data represent an initial approach to this question given the relatively small number of nonsurvivors in the current patient cohort. The data demonstrate, however, that our microarray-based approach is a feasible means of addressing this question. Furthermore, the validity of these data is suggested by the demonstration that increased IL-8 mRNA expression in nonsurvivors (microarray) correlated with increased IL-8 protein levels (ELISA).
Within the current patient cohort, we have elucidated a relatively small group of genes that are differentially regulated between survivors and nonsurvivors of pediatric septic shock. The veracity of this particular gene expression profile to effectively predict survival versus nonsurvival is the subject of ongoing studies in which the current data set will serve as a learning data set and a separate, future group of patients will serve as a validation data set. Nevertheless, the current data provide a foundation to begin formulating testable, novel hypotheses regarding the pathophysiology of poor outcome in pediatric septic shock.
The current cohort of nonsurvivors was characterized by increased expression of MT. One interpretation of this finding is that increased MT expression in nonsurvivors is simply an epiphenomenon of illness severity, rather than being directly involved in the pathobiology of septic shock. As such, MT expression could still potentially serve as a robust biomarker for poor outcome in pediatric septic shock. The development of this type of biomarker would be a powerful clinical tool for the selective implementation of high risk, experimental forms of therapy for pediatric patients with septic shock (e.g. plasmapheresis, extracorporeal membrane oxygenation, etc.) or for patient stratification in future therapeutic trials.
More intriguing is the alternative interpretation that increased MT expression plays a direct role in the pathobiology of septic shock. We have begun to address this possibility by conducting initial experiments directed toward understanding the functional role of MT in septic shock. Using a well established murine model of polymicrobial sepsis, we have demonstrated that MT ablation confers a survival advantage in mice subjected to CLP, thus supporting the concept that MT expression plays a direct role in the pathobiology of septic shock. This assertion is further supported by two notable reports in the literature. Using a rat model of polymicrobial sepsis, Chinnaiyan et al assessed multi-organ gene expression profiles (microarray) and found that there was differential gene expression of a variety of genes, including MT (8). In support of our clinical data, MT was part of the gene cluster defining a “systemic sepsis signature.” Waelput and colleagues evaluated the role of MT in the context of tumor necrosis factor (TNF) lethality, a model of severe systemic inflammation having many common biological and physiological features with septic shock (38). Based on the assumption that MT would be protective against TNF-mediated oxidant stress, these investigators sought to demonstrate that MT-null mice would be more sensitive to TNF-mediated lethality, whereas MT overexpressing mice would be more resistant to MT-mediated lethality. Their results were completely opposite of what they expected: MT-null animals were the most resistant to TNF lethality, MT overexpressing mice were the most sensitive to TNF-mediated lethality, and wild-type mice had an intermediate phenotype between these two extremes. Interestingly, however, Waelput et al demonstrated that zinc supplementation was protective against this model of TNF-lethality, but the protective effect of zinc seemed to be independent of MT expression (38).
To begin assessing the possibility that increased MT expression has a functional effect in clinical septic shock, we measured serum zinc levels in our patient cohort. Based on the ability of MT to avidly bind zinc in the intracellular compartment, we predicted that nonsurvivors would have decreased serum zinc levels and found that this was indeed the case. It should be noted that these data are semi-quantitative in that serum samples were not originally collected with the specific intent of measuring heavy metal concentrations (i.e. not collected in metal free specimen tubes). Thus, while it is possible that the samples are contaminated with exogenous heavy metals, all samples were collected in a similar manner and we have demonstrated a clear trend in that nonsurvivors had substantially lower serum zinc levels compared to survivors.
As previously discussed, zinc is an essential trace element required for normal function of multiple biological processes and the clinical manifestations of zinc deficiency have been well described in the medical literature and in the context of immunity (22, 30, 33). It is unlikely that the decreased serum zinc levels described in the current cohort of patients represent “classic” zinc deficiency. Rather, they are more likely to represent an acute redistribution of tissue zinc levels, which may nonetheless represent an acute disruption of zinc homeostasis. This assertion is supported by the work of Gaetke et al involving systemic LPS administration to healthy adult volunteers (17). These investigators demonstrated acute decreases of serum zinc levels in response to LPS administration. In addition, they found no acute alterations of serum albumin-zinc binding or of urinary excretion of zinc, leading the investigators to postulate that the decreased zinc levels found in their study subjects was a reflection of acute zinc redistribution.
Given the importance of zinc homeostasis to a myriad of biological processes, including immunity, the data presented herein provide a strong rationale for pursuing further research efforts focused on MT expression and zinc homeostasis in clinical septic shock. This assertion is further supported by the demonstration that Day 1 of pediatric septic shock is characterized by broad alterations in gene programs that are either dependent on zinc/metal homeostasis or play a direct role in regulating zinc/metal homeostasis.
Several study limitations deserve discussion. The patient population in this study is heterogeneous. Patient heterogeneity is an intrinsic problem to clinical investigations involving septic shock, which is more accurately characterized as a syndrome rather than a disease. Despite this heterogeneity, we have been able to demonstrate coordinately expressed patterns of gene expression that correspond to multiple biologically relevant gene ontologies and canonical pathways, thus indicating that genomic-based approaches are effective means of approaching this problem of heterogeneity. We expect to have the opportunity to study more homogenous populations in the future (e.g. patients affected by the same pathogen). Another limitation is a common criticism of microarray-based experiments: exclusively measuring mRNA abundance. We have been able to validate some of our key findings by standard gene expression assays (PCR and ELISA). More importantly, we have validated some of our key findings at the functional level. Finally, another limitation is our reliance on whole blood-derived RNA, which is not necessarily reflective of organ-specific gene expression and contains a mixed population of white blood cells. Whole blood, however, is a readily accessible clinical sample that appears to provide a broad window of systemic gene expression. In addition, we have not found any correlation between differential white blood cell counts and the reported gene expression patterns in our cohort.
These limitations not withstanding, the current data represent an unprecedented first approximation of whole genome expression profiles in children with septic shock. As an initial approximation the data are highly plausible based on the established literature and potentially direct the field of clinical pediatric septic shock into new realms supported by the experimental literature.
Table 2B.
Microbiology data for patients with septic shock (nonsurvivors).
| Organism (no.) | Primary source of positive culture (no.) |
|---|---|
| Cytomegalovirus (1) | Blood (6) |
| Streptococcus pyogenes (2) | Lung (1) |
| Streptococcus agalactiae (1) | |
| Influenza B (2) | |
| Neisseria meningitidis (1) |
ACKNOWLEDGMENTS
The Genomics of Pediatric SIRS/Septic Shock Investigators: Julie Simon, R.N. (Children’s Hospital and Research Center Oakland, Oakland, CA); Carey Roth Bayer, Ed.D., R.N. (The Children’s Hospital of Philadelphia, Philadelphia, PA); Joseph Hess, R.N. (Penn State Children’s Hospital, Hershey, PA); Margaret Winkler, M.D. (The University of Alabama at Birmingham, Birmingham, AL); Robert Fitzgerald, M.D. (Devos Children’s Hospital, Grand Rapids, MI); Gwenn McLaughlin, M.D. (Jackson Memorial Hospital, Miami, FL); Cheri Landers, M.D. (Kentucky Children’s Hospital, Lexington, KY); Vicki Whitehead, R.N., C.C.R.C. (Kentucky Children’s Hospital, Lexington, KY); Gary Kohn, M.D. (Morristown Memorial Hospital, Morristown, NJ); Paul Checchia, M.D. (St. Louis Children’s Hospital, St. Louis, MO); Jose Gutierrez, M.D. (Pediatric Critical Care of Arizona, Phoenix, AZ); Steve Shane, M.D. (Washoe Medical Center, Reno, NV); Douglas Willson, M.D. (University of Virginia Medical Center, Charlottesville, VA); Stephanie Lowenhaupt, R.N., M.B.A. (University of Virginia Medical Center, Charlottesville, VA); Yi Zhu, M.D. (DuPont Hospital for Children, Wilmington, DE); Keith Meyer, M.D. (Miami Children’s Hospital, Miami, FL); Mercedes Galera, B.S., C.R.C. (Miami Children’s Hospital, Miami, FL), Nick Anas, M.D. (Children’s Hospital of Orange County, Orange, CA); Stephanie Wronski, B.S., R.N. (Children’s Hospital of Orange County, Orange, CA); Robert Freishtat, M.D., M.P.H. (Children’s National Medical Center, Washington, DC).
Supported by a grant from the National Institute of General Medical Sciences (RO1 GM064619), and The Amanda Kanowitz Foundation (http://www.amandakfoundation.org).
References
- 1.Abraham E. Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med. 1999;25:556–566. doi: 10.1007/s001340050903. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous FDA approves first biologic treatment for sepsis. FDA Consum. 2002;36:3. [PubMed] [Google Scholar]
- 3.Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6(Suppl 1):S27–38. [PubMed] [Google Scholar]
- 4.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 5.Bhutta ZA, Nizami SQ, Isani Z. Zinc supplementation in malnourished children with persistent diarrhea in Pakistan. Pediatrics. 1999;103:e42. doi: 10.1542/peds.103.4.e42. [DOI] [PubMed] [Google Scholar]
- 6.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 7.Carcillo JA, Fields AI. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30:1365–1378. doi: 10.1097/00003246-200206000-00040. [DOI] [PubMed] [Google Scholar]
- 8.Chinnaiyan AM, Huber-Lang M, Kumar-Sinha C, Barrette TR, Shankar-Sinha S, Sarma VJ, Padgaonkar VA, Ward PA. Molecular signatures of sepsis: multiorgan gene expression profiles of systemic inflammation. Am J Pathol. 2001;159:1199–1209. doi: 10.1016/S0002-9440(10)62505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobb JP, Mindrinos MN, Miller-Graziano C, Calvano SE, Baker HV, Xiao W, Laudanski K, Brownstein BH, Elson CM, Hayden DL, Herndon DN, Lowry SF, Maier RV, Schoenfeld DA, Moldawer LL, Davis RW, Tompkins RG, Bankey P, Billiar T, Camp D, Chaudry I, Freeman B, Gamelli R, Gibran N, Harbrecht B, Heagy W, Heimbach D, Horton J, Hunt J, Lederer J, Mannick J, McKinley B, Minei J, Moore E, Moore F, Munford R, Nathens A, O’Keefe G, Purdue G, Rahme L, Remick D, Sailors M, Shapiro M, Silver G, Smith R, Stephanopoulos G, Stormo G, Toner M, Warren S, West M, Wolfe S, Young V. Application of genome-wide expression analysis to human health and disease. Proc Natl Acad Sci U S A. 2005;102:4801–4806. doi: 10.1073/pnas.0409768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, Fisher RI, Braziel RM, Rimsza LM, Grogan TM, Miller TP, LeBlanc M, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Connors JM, Lansdorp PM, Ouyang Q, Lister TA, Davies AJ, Norton AJ, Muller-Hermelink HK, Ott G, Campo E, Montserrat E, Wilson WH, Jaffe ES, Simon R, Yang L, Powell J, Zhao H, Goldschmidt N, Chiorazzi M, Staudt LM. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 12.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 13.Dennis G, Jr., Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 14.Eisenberg P. [Accessed March 7, 2006];Discontinuation of Study F1K-MC-EVBP, Investigation of the Efficacy and Safety of Drotrecogin Alfa (Activated) in Pediatric Severe Sepsis. Available at : http://www.fda.gov/medwatch/SAFETY/2005/xigris_dearHCP_4-21-05.htm.
- 15.Failla ML. Trace elements and host defense: recent advances and continuing challenges. J Nutr. 2003;133:1443S–1447S. doi: 10.1093/jn/133.5.1443S. [DOI] [PubMed] [Google Scholar]
- 16.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 17.Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI. Effects of endotoxin on zinc metabolism in human volunteers. Am J Physiol. 1997;272:E952–956. doi: 10.1152/ajpendo.1997.272.6.E952. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 19.Hotz C, Peerson JM, Brown KH. Suggested lower cutoffs of serum zinc concentrations for assessing zinc status: reanalysis of the second National Health and Nutrition Examination Survey data (1976-1980) Am J Clin Nutr. 2003;78:756–764. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- 20.Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133:1452S–1456S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- 21.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 22.King JC, Shames DM, Woodhouse LR. Zinc homeostasis in humans. J Nutr. 2000;130:1360S–1366S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 23.King LE, Osati-Ashtiani F, Fraker PJ. Apoptosis plays a distinct role in the loss of precursor lymphocytes during zinc deficiency in mice. J Nutr. 2002;132:974–979. doi: 10.1093/jn/132.5.974. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–977. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 25.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci U S A. 2005;102:6843–6848. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pachot A, Lepape A, Vey S, Bienvenu J, Mougin B, Monneret G. Systemic transcriptional analysis in survivor and non-survivor septic shock patients: a preliminary study. Immunol Lett. 2006;106:63–71. doi: 10.1016/j.imlet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Pollack MM, Patel KM, Ruttimann UE. The Pediatric Risk of Mortality III--Acute Physiology Score (PRISM III-APS): a method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr. 1997;131:575–581. doi: 10.1016/s0022-3476(97)70065-9. [DOI] [PubMed] [Google Scholar]
- 28.Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–1037. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 29.Prucha M, Ruryk A, Boriss H, Moller E, Zazula R, Herold I, Claus RA, Reinhart KA, Deigner P, Russwurm S. Expression profiling: toward an application in sepsis diagnostics. Shock. 2004;22:29–33. doi: 10.1097/01.shk.0000129199.30965.02. [DOI] [PubMed] [Google Scholar]
- 30.Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, Hurt EM, Zhao H, Averett L, Yang L, Wilson WH, Jaffe ES, Simon R, Klausner RD, Powell J, Duffey PL, Longo DL, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Montserrat E, Lopez-Guillermo A, Grogan TM, Miller TP, LeBlanc M, Ott G, Kvaloy S, Delabie J, Holte H, Krajci P, Stokke T, Staudt LM. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 32.Ruel MT, Rivera JA, Santizo MC, Lonnerdal B, Brown KH. Impact of zinc supplementation on morbidity from diarrhea and respiratory infections among rural Guatemalan children. Pediatrics. 1997;99:808–813. doi: 10.1542/peds.99.6.808. [DOI] [PubMed] [Google Scholar]
- 33.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 34.Shanley TP, Hallstrom C, Wong HR. Sepsis. In: Fuhrman BP, Zimmerman JJ, editors. Pediatric Critical Care Medicine. 3rd ed Mosby; St. Louis, MO: 2006. pp. 1474–1493. [Google Scholar]
- 35.Sheehan M, Wong HR, Hake PW, Zingarelli B. Parthenolide improves systemic hemodynamics and decreases tissue leukosequestration in rats with polymicrobial sepsis. Crit Care Med. 2003;31:2263–2270. doi: 10.1097/01.CCM.0000085186.14867.F7. [DOI] [PubMed] [Google Scholar]
- 36.van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 37.von Bulow V, Rink L, Haase H. Zinc-mediated inhibition of cyclic nucleotide phosphodiesterase activity and expression suppresses TNF-alpha and IL-1 beta production in monocytes by elevation of guanosine 3′,5′-cyclic monophosphate. J Immunol. 2005;175:4697–4705. doi: 10.4049/jimmunol.175.7.4697. [DOI] [PubMed] [Google Scholar]
- 38.Waelput W, Broekaert D, Vandekerckhove J, Brouckaert P, Tavernier J, Libert C. A mediator role for metallothionein in tumor necrosis factor-induced lethal shock. J Exp Med. 2001;194:1617–1624. doi: 10.1084/jem.194.11.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson JD, Pollack MM, Ruttimann UE, Glass NL, Yeh TS. Outcome of pediatric patients with multiple organ system failure. Crit Care Med. 1986;14:271–274. doi: 10.1097/00003246-198604000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Abrogation of nuclear factor-kappaB activation is involved in zinc inhibition of lipopolysaccharide-induced tumor necrosis factor-alpha production and liver injury. Am J Pathol. 2004;164:1547–1556. doi: 10.1016/s0002-9440(10)63713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, Muntel EE, Witte DP, Pegg AA, Foster PS, Hamid Q, Rothenberg ME. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]