Abstract
This study determined immune activities in the brain of ASD patients and matched normal subjects by examining cytokines in the brain tissue. Our results showed that proinflammatory cytokines (TNF-α, IL-6 and GM-CSF), Th1 cytokine (IFN-γ) and chemokine (IL-8) were significantly increased in the brains of ASD patients compared with the controls. However the Th2 cytokines (IL-4, IL-5 and IL-10) showed no significant difference. The Th1/Th2 ratio was also significantly increased in ASD patients. Conclusion: ASD patients displayed an increased innate and adaptive immune response through the Th1 pathway, suggesting that localized brain inflammation and autoimmune disorder may be involved in the pathogenesis of ASD.
Keywords: Autistic spectrum disorders (ASD), Cytokines, Inflammation, Immune response. Flow cytometry method, Multiplexed bead analysis
1. Introduction
Autistic spectrum disorders (ASD) are complex, pervasive developmental disorders of childhood characterized by impairments in social interaction, deficits in verbal and non-verbal communication, and restricted repetitive and stereotyped patterns of behavior and interests (DSMIV criteria, American Psychiatric Association, 1994). The prevalence of ASD is currently estimated to be 5 per 1000, affecting 4 times more boys than girls (Bertrand et al, 2001). Susceptibility to ASD is clearly attributable to genetic factors (Folstein and Rosen-Sheidley, 2001), but the etiology of the disorder is unknown, and no biomarkers have yet been identified as characteristic of ASD. Recent reports suggest that a combination of environmental or perhaps in utero risk factors, autoimmune risk factors and localized inflammation of the central nervous system may contribute to the pathogenesis of ASD (Nelson et al., 2001; Vargas et al., 2005; Zimmerman et al., 2005; Chez et al., 2007). Jyonouchi et al demonstrated that elevated levels of the proinflammatory cytokines tumor necrosis factor (TNF)-α and IL-1β were produced by peripheral blood mononuclear cells from children with ASD (Jyonouchi et al., 2001, 2002). Singh reported increased plasma levels of the Th1 cytokines IL-12 and IFN-γ (Singh, 1996), and Croonenberghs et al found increased levels of IFN- γ in the supernatant of whole blood cultures from children with ASD (Croonenberghs et al., 2002a). In addition, Molloy et al reported that children with ASD had increased activation of both Th2 and Th1 arms of the adaptive immune response in blood mononuclear cells, with a Th2 predominance, and without the compensatory increase in the regulatory cytokine IL-10 (Molloy et al, 2006). In both peripheral blood and intestinal mucosa, Ashwood et al found that CD3+ TNFα and CD3+ IFNγ were increased and CD3+ IL-10 were markedly decreased in ASD children with GI symptoms (Ashwood and Wakefield, 2006). However, it is difficult to interpret these findings with respect to the pathogenesis of autism since it is not clear that the immune findings in peripheral blood mononuclear cells in autistic children correlate with immune-mediated pathology within the central nervous system. Recently one study investigated the nature of inflammatory responses in the brain of autistic patients by examining 79 proteins including cytokines, chemokines and growth factors using protein array methods (Vagars et al, 2005). In this study, Vagars et al demonstrated that tumor growth factor (TGF)-β1, derived from neuroglia, was significantly increased in the middle frontal gurus (MFG) of autistic patients, while macrophage chemoattractant protein (MCP)–1, IL-6 and IL-10 were increased in the anterior cingulated gurus (ACG). In addition, using protein array approach, Vagars et al also found that MCP-1, IL-6, IL-8 and IFNγ were significantly increased in the cerebrospinal fluid (CSF). (Vargas et al, 2005). One of the advantage of protein array is the ability to detect a whole category of proteins in a very efficient way using very small quantity of tissues. But it is also less specific comparing with enzyme-linked immunosorbent assay (Elisa). (Simpson, 2003). To further investigate whether immune-mediated mechanisms are involved in the pathogenesis of autism and gain a clearer picture of cytokine activities in the brain of autistic patients, we carried out the current study to fully investigate both innate and adaptive immune responses in the brain of ASD patients using Multiplex Bead Immunoassays. In this study, we examined the activation levels of cytokines including the proinflammatory cytokines (IL-6, IL-1 β, TNF-α and GM-CSF), Th1 cytokines (IL-2 and IFNγ), Th2 cytokines (IL-4, IL-5 and IL-10 ) and the chemokine IL-8 in the brains of well characterized ASD patients and compare the immune responses with age and gender matched normal subjects. Our results showed that TNF-α, IL-6, GM-CSF, IFN-γ and IL-8 were significantly increased in the brains of ASD patients compared with the control subjects. Th1/Th2 ratio was also significantly elevated in ASD patients. In addition, we found that the regulatory IL-10 was not compensatory increased in response to the Th1 cytokines increase in ASD patients.
2. Material and methods
2.1. Study material
Frozen human brain tissue (front cerebral cortex) of 8 autistic patients and 8 age and gender matched normal subjects were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders. Donors with autism fit the diagnostic criteria of the Diagnostic and Statistical Manual-IV, as confirmed by the Autism Diagnostic Interview-Revised. This study was approved by the Institutional Review Board of the NY State Institute of Basic Research and the subjects' information were summarized in Table I
Table 1. Study subjects information.
| Groups | n | Ages | Sex (M/F) | PMF(h) | Diagnoses |
|---|---|---|---|---|---|
| Autism Group | 8 | 12.8 (4-37) | 5/3 | 23(9-50) | Autism, moderate to severe |
| Control Group | 8 | 12.5 (4-37) | 5/3 | 18(12-20) | Asthma, injuries, heart disease |
2.2. Preparation of brain tissue lysate
Frozen frontal cortex tissue was homogenized (10% W/V) in cold buffer containing 50 mM Tris-HCl (pH 7.4), 8.5% sucrose, 2 mM EDTA, 10 mM β-mercaptoethanol and protease inhibitor cocktail (Sigma-Aldrich). The protein concentration was assayed by the Bradford method (Bradford M, 1976).
2.3. Multiplexed analyses of cytokines in brain cortex with the Bio-Plex system
A standard capture sandwich assay was used to determine the levels of different cytokines in brain cortex. Each captured antibody was coupled to a different bead set (Invitrogen's Multiplex Bead Immunoassays). The system used a liquid suspension array of 10 sets of beads (Invitrogen, Human Cytokine 10-plex) internally dyed with different ratios of two spectrally distinct fluorochromes to assign a unique spectral address. Each set of beads was combined with a monoclonal antibody raised against GM-CSF, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IFN-γ, and TNF-α. Beads were incubated first (2 h, at room temperature) with diluted standards (serial dilutions from 1.95 to 32 000 pg/ml) or the brain cortex sample, and then with biotinylated detector antibodies (30 min, at room temperature). They were washed twice in phosphate-buffered saline, and incubated for 30 min at room temperature with phycoerythrin-conjugated streptavidin. Cytokine levels were measured with a Luminex 200 ™ system (Bio-Rad Laboratories). Each measurement was taken in duplicate. Standard curves were generated by using the reference cytokine concentrations supplied by the manufacturer. Raw data (mean fluorescent intensities) were analyzed by Bio-Plex Manager Software 4.1 version (Bio-Rad Laboratories) to obtain concentration values.
2.4. Statistical Analysis
In accordance with the matched study design, paired t-tests were conducted to compare the mean differences in cytokine levels produced from the brain of ASD patients to those of their matched controls. P <0.05 is considered significantly different.
3. Results
3.2. Pro-inflammatory cytokines profile in the brain cortex of ASD patients
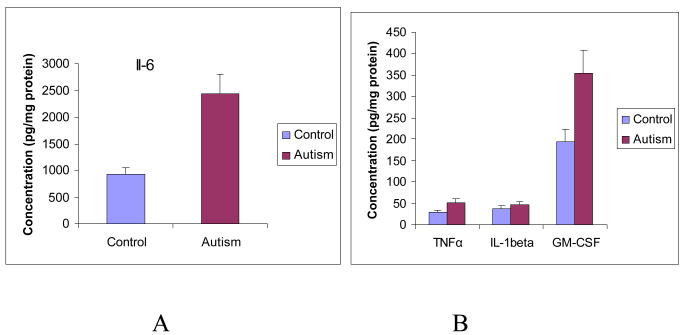
Pro-inflammatory cytokines (IL-6, IL-1β, TNFα) are predominantly produced by activated immune cells and involved in the amplification of inflammatory reactions. This set of cytokines was analyzed in the brain cortex from both ASD patients and the control subjects. The mean values (ASD versus control) of IL-6 and IL-1β were 2441.89 ± 267.34 pg/mg protein versus 920.81 ± 107.92 pg/mg protein (P<0.001), and 44.21 ± 21.33 pg/mg protein versus 18.54 ± 15.8 pg/mg protein (P=0.11) respectively. The mean values (ASD versus control) of TNF-α and GM-CSF were 51.81 ± 6.72 pg/mg protein versus 29.85 ± 4.31 pg/mg protein (P<0.05), and 354.04 ± 46.38 pg/mg protein versus 193.64 ± 26.43 pg/mg protein (P<0.01) (Figure 1). Our results indicate that pro-inflammatory cytokines TNF-α, IL-6 and GCSF were significantly increased in the brain of ASD patients compared with the control, although IL-1β was not significantly different in ASD patients.
Figure 1A and 1B. Pro-inflammatory cytokines profile in the brain cortex of ASD patients.
3.3. Th-1 cytokines profile in the brain cortex of ASD patients
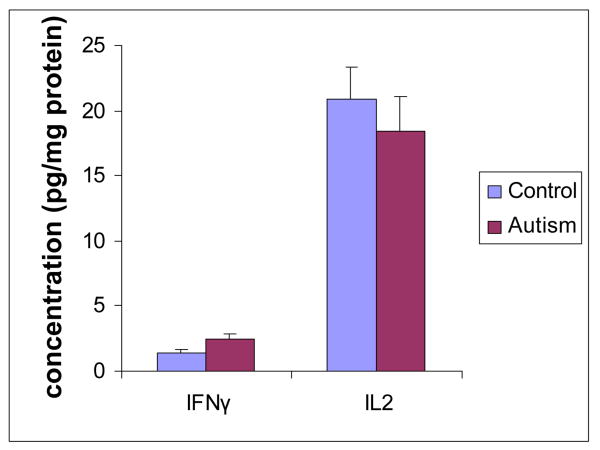
IL-2 and IFN-γ, secreted by Th-1 cells, were analyzed in the brain cortex from both ASD patients and normal subjects. The mean value of IL-2 (ASD versus control) was 18.34 ± 4.28 pg/mg protein versus 20.87 ± 4.13 pg/mg protein (P>0.05). The mean value of IFN-γ (ASD versus control) was 2.51± 0.34 pg/mg protein versus 1.45 ± 0.18 pg/mg protein (P<0.05) (Figure 2). Our results showed that IFN-γ was significantly increased in the brains of ASD patients.
Figure 2. Th-1 cytokines profile in the brain cortex of ASD patients.
3.4. Th-2 type cytokines profile in the brain cortex of ASD patients
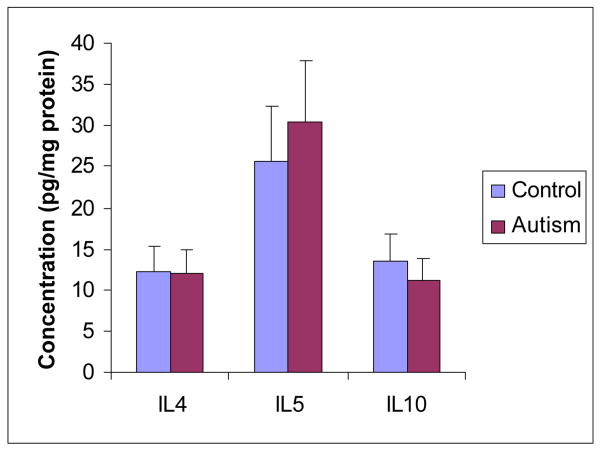
IL-4, IL-5 and IL-10, secreted by Th-2 cells, were analyzed in the brain cortex from both ASD patients and normal subjects. The mean values of IL-4 and IL-5 (ASD versus control) were 11.98 ± 1.59 pg/mg protein versus 12.33 ± 1.87 pg/mg protein (P>0.05), and 30.51 ± 4.13 pg/mg protein versus 25.8 ± 3.46 pg/mg protein (P>0.05) respectively. The mean value of IL-10 (ASD versus control) was 11.13 ± 2.18 pg/mg protein versus 13.45 ± 2.59 pg/mg protein (P>0.05) (Figure 3). Our results indicated that Th-2 type cytokines (IL-4, IL-5 and IL-10) were not significantly changed in the brain of ASD patients in comparison with their matched control subjects.
Figure 3. Th-2 type cytokines profile in the brain cortex of ASD patients.
3.5. Th1/Th2 cytokine ratio increases in the brains of ASD patients
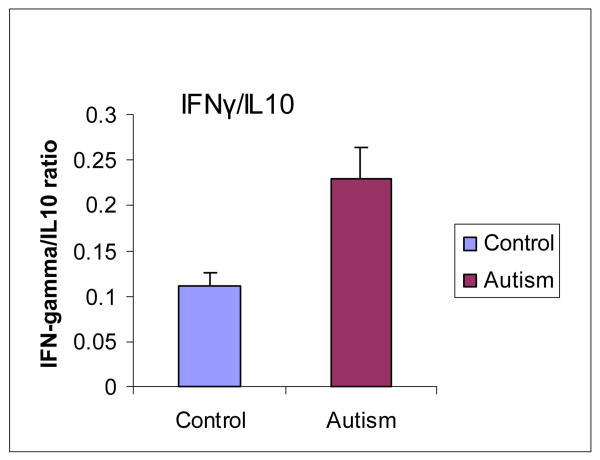
The Th1/Th2 ratio determined by IFNγ/IL-10 was also analyzed in the brain cortex from both ASD patients and normal subjects. The mean value of Th1/Th2 was 0.23 ± 0.03 in ASD patients versus 0.11 ± 0.02 in normal subjects (P<0.05). This result demonstrated that the Th1/Th2 ratio was significantly increased in the brain of ASD patients. (Figure 4).
Figure 4. Th1/Th2 cytokine ratio increases in the brain of ASD patients.
3.6. Chemokine profile in the brain cortex of ASD patients
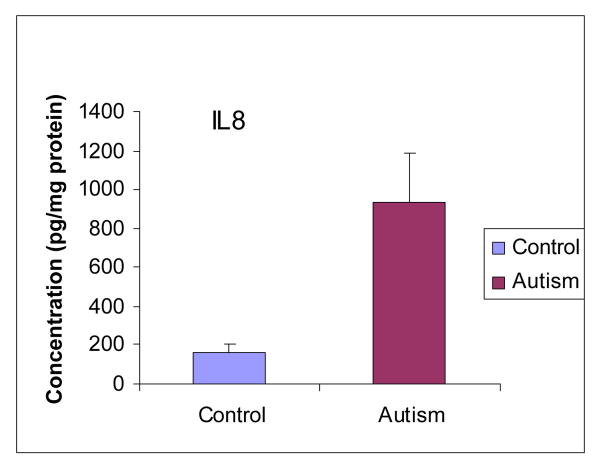
Chemokine IL-8 was also analyzed in the brain cortex from both ASD patients and normal subjects. The mean value of IL-8 was 936.42 ± 111.33 pg/ml in ASD patients versus 157.71 ± 21.43 pg/ml in normal subjects (P<0.01), demonstrating a dramatic increase of IL-8 in ASD patients compared with the control (Figure 5).
Figure 5. Chemokine profile in the brain cortex of ASD patients.
4. Discussion
Although the pathogenesis of ASD is not well understood, recent studies have suggested that localized inflammation of the central nervous system may contribute to the pathogenesis of ASD. A number of studies have shown that TNFα, IFNγ, IL-1β and IL-12 were increased in the peripheral blood of ASD patients (Zimmerman et al., 2005; Molloy et al, 2006; Ashwood et al, 2006). TNFα was also shown to be increased in the cerebral spinal fluid of autistic patients (Vargas et al, 2005; Chez MG, 2007). However, there is only one study that has been conducted to investigate the inflammatory cytokine profile in the brain of autistic patients using protein arrays (Vargas et al, 2005). To further characterize the nature of the inflammatory responses in autistic brains, we used a recently developed flow cytometry method (multiplexed bead analysis) to measure cytokine levels in the brain (cerebral cortex) extracts. This method provides many advantages over the traditional ELISA methods, and with better results for characterization of cytokines (Khan et al., 2007; DuPont et al., 2005). This method also makes it possible to measure several cytokines in a single sample to establish a profile of the inflammatory reaction. Our results showed that pro-inflammatory cytokines (TNFα, IL-6 and GM-CSF) were significantly increased in the brains of ASD patients in comparison with the control subjects. TNF-α and IL-6 are cytokines involved in cell-mediated immune response and their production has been shown to be associated with tissue inflammation and necrosis (Beutler and Cerami, 1989). The role of TNF-α as a neuromodulating agent has also been described in brain development, and it may play a role in neurons and neuroglial cells modulating glutaminergic transmission (Pickering et al., 2005). Excessive glutamate excitotoxic effects acting on NMDA receptors could occur in the presence of excess TNF-α (Pickering et al, 2005). This occurrence can lead to effects on microglial activation, as well as on nuclear factor kappa-B (NF-kB). Such changes have also been seen in models of inflammation inducing epileptic activity in which neuroglial inflammation has caused epileptic spikes (Pickering et al, 2005). Thus elevations of TNF-α, IL-6 and GM-CSF in the brain of ASD patients suggest that children with ASD have a heightened immune response that may be associated with localized brain inflammation and tissue necrosis.
Immune responses that are stimulated by exposure to specific antigens are considered adaptive responses and are often driven by CD4+ T helper (Th) cells. Th cell clones have been classified into distinct functional types on the basis of the cytokines they secrete (Abbas et al, 1996). The most clearly defined of these subsets are Th1, which is characterized by the production of interferon (IFN)-γ and IL-2, and Th2, which is characterized by the production of IL-4, IL-5 and IL-10. The main effect or function of Th1 cytokines is phagocyte mediated defense, especially against intracellular microbes, while Th2 cytokines function to affect IgE and eosinophil/mast cell-mediated immune responses. An imbalance of these cytokines, skewed toward Th2, is seen in allergic responses (Ngoc et al, 2005) and some systemic autoimmune responses such as systemic lupus erythematosus (Spadaro et al, 2003). A skewing toward Th1 cytokines is seen in some organ specific autoimmune disorders such as insulin-dependent diabetes mellitus and multiple sclerosis (Liblau et al, 1995). In our study, we detected significant elevations in IFN-γ (Th1 origin), but not in IL-4, IL-5 and IL-10 (Th2 origin), suggesting that the brain of ASD patients have an excess adaptive response through activation of the Th1 pathway, rather than activation of the Th2 pathway. The increased activation of Th1 cytokines, but not Th2 cytokines in the ASD brain also suggests that an autoimmune disorder may be involved in the pathogenesis of ASD, but not an allergic response. Moore et al (2001) reported that children with ASD had increased activation of both Th2 and Th1 immune response in blood mononuclear cells, with Th2 predominance. This finding is different from our results, suggesting that immune responses in the brain and blood mononuclear cells in autistic patients could be different. In addition, the ratio of IFN-γ to IL-10 was significantly higher in ASD patients than in controls, showing an absence of a compensatory increase in IL-10. This supports the concept of a dysregulation of the adaptive immune responses in ASD patients. These results are also consistent with those reported by Jyonouchi et al (2002) involving heightened and dysregulated innate immune responses in children with ASD as evidenced by elevated proinflammatory cytokine production from their peripheral blood mononuclear cells.
In addition, our study demonstrated that chemokine IL-8 was also significantly increased in the brain of ASD patients in comparison to the control group. IL-8 has powerful chemotactic effects on T cells and neutrophils. Elaboration of IL-8 by resident tissues is an important mechanism for directing leukocytes to migrate, especially through tissues without blood vessels. In recent years, increasing attention has been focused on chemokines as inflammatory mediators in the CNS. The limited number of studies that have investigated chemokine and chemokine receptor expression in Alzheimer's disease (AD) brains and in cell culture models seem to support a role for inflammation in AD pathogenesis (Grammas et al, 2006). Studies have also suggested a possible role of chemokines as communication molecules between neurons and microglia (Wolfang J, 2001). The significant increase of IL-8 in the brain of ASD patients implicated a chemokine mediated inflammation and this excess activation of IL-8 may be a potent signal to recruit T lymphocytes that leads to the damage in the brain of ASD patients.
There are certainly limitations in this investigation. The sample sizes are relatively small. In addition, only 10 cytokines were analyzed. Despite these limitations, this is the first study to our knowledge to investigate various inflammatory cytokine expression levels in the brain of ASD patients using the most recently developed cytometry method.
In summary, we found that ASD is associated with change in expression levels of various pro-inflammatory and anti-inflammatory cytokines in the brain of ASD patients. These findings serve as further evidence that inflammation may be an important part of the pathogenesis of ASD. Based on these findings, further investigation directed at cytokine modulation as a therapeutic approach to ASD is a logical next step.
Acknowledgments
This study was partially supported by NIH/NCCAM center grant # 1P01 AT002644725-01 “Center for Chinese Herbal Therapy (CHT) for Asthma” to LXM.
References
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. APA; Washington, DC: 1994. [Google Scholar]
- Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006;173:126–34. doi: 10.1016/j.jneuroim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Mars A, Boyle C, Bove F, Yeargin-Allsopp M, Decoufle P. Prevalence of autism in a United States population: the Brick Township, New Jersey, investigation. Pediatrics. 2001;108:1155–1161. doi: 10.1542/peds.108.5.1155. [DOI] [PubMed] [Google Scholar]
- Beutler B, Cerami A. The biology of cachectin/TNF—a primary mediatoer of host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Bradford M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002a;45:1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- DuPont NC, Wang K, Wadhwa PD. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66:175–91. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- Grammas P, Samany PG, Thirumangalakudi L. Thrombin and inflammatory proteins are elevated in Alzheimer's disease microvessels: implications for disease pathogenesis. J Alzheimers Dis. 2006;9:51–58. doi: 10.3233/jad-2006-9105. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Itokazu N. Innate immunity associated with inflammatory responses and cytokine production against common dietary proteins in patients with autism spectrum disorder. Neuropsychobiology. 2002;46:76–84. doi: 10.1159/000065416. [DOI] [PubMed] [Google Scholar]
- Khan SS, Smith MS, Reda D. Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytometry. 2004;61:35–9. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- Lord C, Pickles A, McLennan J. Diagnosing autism: Analyses of data from the Autism Diagnostic Interview. J Autism Dev Disord. 1997;27:501–17. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, Altaye M, Wills-Karp M. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Nelson KB, Grether JK, Croen LA. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann Neurol. 2001;49:597–606. [PubMed] [Google Scholar]
- Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–166. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- Pickering M, Cumiskey D, O'Connor JJ. Actions of TNF-_ on glutaminergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–70. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- Simpson RJ. Characterization of protein complexes, chaptor 10. In: Simpson, editor. Protein and Proteomics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor NY, USA: 2003. [Google Scholar]
- Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996;66:143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Spadaro A, Scrivo R, Bombardieri M, Riccieri V, Rinaldi T, Taccari E, Valesini G. Relationship of interleukin-12 and interleukin-13 imbalance with class-specific rheumatoid factors and anticardiolipin antibodies in systemic lupus erythematosus. Clin Rheumatol. 2003;22:107–111. doi: 10.1007/s10067-002-0685-y. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Harrison JK. Chemokines and Alzheimer's disease. Neurobiology of Aging. 2001;22:909–913. doi: 10.1016/s0197-4580(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Zimmerman A, Jyonouchi H, Comi A, Connors S, Milstien S, Varsou A, Heyes M. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol. 2005;35:195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]