Abstract
Few studies in community settings have evaluated predictors, mediators, and moderators of treatment success for medically supervised opioid withdrawal treatment. This report presents new findings about these factors from a study of 344 opioid dependent men and women prospectively randomized to either buprenorphine-naloxone or clonidine in an open-label 13-day medically-supervised withdrawal study. Subjects were either inpatient or outpatient in community treatment settings; however not randomized by treatment setting. Medication type (buprenorphine-naloxone versus clonidine) was the single best predictor of treatment retention and treatment success, regardless of treatment setting. Compared to the outpatient setting, the inpatient setting was associated with higher abstinence rates but similar retention rates when adjusting for medication type. Early opioid withdrawal severity mediated the relationship between medication type and treatment outcome with buprenorphine-naloxone being superior to clonidine at relieving early withdrawal symptoms. Inpatient subjects on clonidine with lower withdrawal scores at baseline did better than those with higher withdrawal scores; inpatient subjects receiving buprenorphine-naloxone did better with higher withdrawal scores at baseline than those with lower withdrawal scores. No relationship was found between treatment outcome and age, gender, race, education, employment, marital status, legal problems, baseline depression, or length/severity of drug use. Tobacco use was associated with worse opioid treatment outcomes. Severe baseline anxiety symptoms doubled treatment success. Medication type (buprenorphine-naloxone) was the most important predictor of positive outcome; however the paper also considers other clinical and policy implications of other results, including that inpatient setting predicted better outcomes and moderated medication outcomes.
1. Introduction
Heroin dependence continues to be a serious public health issue throughout many parts of the world, and there is a need for better access to effective treatments (SAMHSA NSDUH, 2004). Buprenorphine-naloxone (Suboxone®) has been found to be effective in many diverse treatment settings (Bickel et al., 1988; Bickel and Amass, 1995; Boatwright 2002; Johnson et al., 2003; Amass et al., 2004; Ling et al., 2005). However, there have been few studies of predictors, mediators, and moderators of outcome for medication treatment for opioid dependence (Morral et al., 1997), and these studies have focused on the inpatient setting and clonidine medication. In the inpatient setting, factors such as gender, age, duration of drug use, education, psychopathology, and employment did not predict better or worse treatment outcome in some studies (San et al., 1989; Armenia et al., 1999); however other reports have found worse outcomes for younger age, (Jeremiah et al., 1995; Armenia et al., 1999; Gossling et al., 2001; Backmund et al., 2001; Ghodse et al., 2002) being single, (Armenia et al., 1995; Perez de los Cobos et al., 1997) and having more severe drug and medical problems (Franken and Hendriks, 1999). One study found worse outcomes amongst those with less education, less regular contact with a counselor, no aftercare plans, history of imprisonment, and not being on probation (Backmund et al., 2001). Another inpatient study found that the intensity of withdrawal symptoms and craving was not related to premature termination from treatment (Scherbaum et al., 2004). In the outpatient setting, worse clonidine treatment outcomes have been found for heroin addicts versus other opioids (Strobbe et al, 2003; McCann et al, 1997), higher levels of subjective withdrawal symptoms (Strobbe et al., 2003; Rounsaville et al., 1985), intravenous users (McCann et al, 199&), benzodiazepine use pre-detoxification (McCann et al., 1997), and depression (Ziedonis and Kosten, 1991).
This report focuses on predictors, moderators, and mediators for buprenorphine/naloxone and clonidine medication treatment and expands on an earlier report of the primary finding that buprenorphine-naloxone had better clinical outcomes compared to clonidine (Ling et al, 2005). This report presents new findings about how predictors, mediators, and moderators are the same (or different) for the two medications. Two prior reports provide more detail about the field experience of using these medications in community-based addiction treatment settings and more details regarding the study design, procedures, medications and settings (Amass et al., 2004; Ling et al., 2005). This study evaluates new variables and the nature of these variables (predictors, moderators, and mediators).
2. Methods
2.1. Overview
Study subjects were treatment seeking opioid-dependent men and women who were at least 15 years of age and in general good health. They were randomly assigned in a 2:1 ratio to either buprenorphine-naloxone or clonidine for 13-days. Ancillary medications were available as needed. Doses of study medication were lowered gradually over 13-days. The treatment setting was self-selected or clinically selected, and not randomized (six inpatient; six outpatient sites). “Setting” was categorized at the beginning of treatment, although some inpatients became outpatients. A diverse group of community-based treatment programs participated from the National Institute on Drug Abuse’s Clinical Trials Network.
2.2. Measurements
Key baseline measures that were used as predictors of outcome included opioid withdrawal severity (interviewer rated and subject rated), urine drug testing, and items from the Addiction Severity Index 5th Edition (McLellan et al., 1992). Urine drug testing was done at baseline, four times during the 13-day treatment period and at day 13 or 14.
Subject withdrawal was based on observer (Wesson and Ling, 2003) and self-report scales of opioid withdrawal (Bickel et al., 1988a; Bickel et al., 1988b; Amass et al., 2000). Baseline withdrawal measures were performed prior to receiving study medication. The Clinical Opiate Withdrawal Scale (COWS) is an 11-item interviewer administered questionnaire of observable signs and symptoms (resting pulse rate, sweating, restlessness, pupil size, bone or joint ache, runny nose or tearing, gastro-intestinal upset, tremor, yawning, anxiety or irritability, and gooseflesh skin). The Adjective Rating Scale for Withdrawal (ARSW) is a self-report scale of 16 signs and symptoms (Bickel et al., 1988a; Bickel et al., 1988b; Amass et al., 2000). Subjects rated themselves on a scale of 0 (none) to 9 (severe) on the following withdrawal symptoms: muscle cramps, depressed or sad, painful joints, excessive yawning, hot or cold flashes, trouble getting to sleep, sick to stomach, irritable, runny nose, poor appetite, weak knees, excessive sneezing, tense and jittery, watery eyes, abdominal cramps, and fitful sleep.
2.3. Treatment Success
Treatment success was defined as being “present” to complete the study medication on day 13 and the absence of opioids according to the urine toxicology assessment on the last day of research clinic attendance (day 13 or 14). This definition of treatment success was the primary outcome (Ling et al., 2006) and includes two important factors: retention and abstinence. Retention and abstinence were examined separately to determine the factors influencing each component. Treatment “completion” (versus “success”) was defined as being present to complete day 13 study medication (with or without opioid abstinence).
3. Hypotheses
3.1. Primary Hypotheses
Three primary hypotheses were tested: (1) Subjects receiving buprenorphine-naloxone will have better outcomes than those receiving clonidine; (2) Subjects treated in the inpatient setting would have better outcomes than in the outpatient; and (3) Subjects with more severe withdrawal symptoms, regardless of which medication they received, would drop out of treatment earlier and have worse outcomes than subjects with lower levels of withdrawal. Hypothesis one was also a primary hypothesis of the primary outcome paper for this study (Ling et al., 2006); however we included this hypothesis again in the context of assessing for predictors, mediators, and moderators of outcome, especially given the proposed mediational model and the need to further explore the relationship of medication type, opioid severity, and treatment outcomes. A mediational model was proposed whereby early opioid withdrawal (day 2 COWS withdrawal scores) was hypothesized to mediate the relationship between medication type (buprenorphine-naloxone or clonidine) and treatment outcome. Day 2 withdrawal scores were selected because day 2 is commonly a time-point of peak withdrawal symptoms during unmedicated withdrawal.
3.2. Secondary Hypotheses
Subjects who were younger, male, less educated, injected heroin intravenously, had higher levels of anxiety and/or depression, used other substances (alcohol, cocaine, or tobacco), used more ancillary medications, and/or had legal problems were hypothesized to have worse treatment outcomes.
3.3. Data Analyses
Data analyses were organized to evaluate predictor, moderator, and mediator factors in this study, including dichotomous and continuous variables. Logistic regression analyses were performed when dependent variables were dichotomous. These results are presented as odds ratios. The results of all analyses where medication type (buprenorphine-naloxone versus clonidine) was not the independent variable of primary interest are reported as adjusted odds ratios with medication type in the proposed mediational model. Additionally, the results of all analyses where treatment setting (inpatient versus outpatient) was not the independent variable of primary interest are reported as adjusted odds ratios with treatment setting in the proposed mediational model. Independent sample t-tests were used when one dependent variable was continuous and the independent variable was dichotomous. Analyses of variance were conducted when there were multiple dichotomous independent variables and one continuous dependent variable (such as when evaluating interaction effects).
This study evaluated predictor, moderator, and mediator variables. The moderator variables affect the direction and/or strength of the relationship between independent and dependent variables (such as medication type and treatment response), while a mediator variable actually accounts for the relationship between said variables and the mediator variable occurs during the treatment and helps to better explain the relationship between the independent and dependent variable. Identifying mediators can be an early step to identifying causal mechanisms.
Moderator factors analyzed included level of care and opioid withdrawal severity. In the moderator analysis it is a difference score from baseline to day 3 on the opioid withdrawal severity scores. The proposed mediational model was tested based on the recommendations of Baron and Kenney (Baron and Kenny, 1986). Mediator factors considered included severity of opioid withdrawal and setting. To examine mediators of treatment abstinence, the two opioid withdrawal scales (the COWS and ARSW scores) were dichotomized into participants experiencing higher withdrawal symptoms on day two (those in the top 25% of symptom severity scores) versus all the other participants who were experiencing lower withdrawal symptoms on day two. Early withdrawal scores (day 2 COWS and ARSW scores) were used in the mediator analysis.
For all analyses, an alpha level of 0.05 was used. In regards to missing data and dropouts, if subjects were not present and opioid abstinent according to the urine toxicology assessment on the last day of research clinic attendance (day 13 or 14), then they were not counted as abstinent, including missing data and dropouts.
4. Results
4.1. Sample
Of the 344 opioid-dependent men and women, 234 (68%) were randomized to buprenorphine-naloxone and 110 (32%) were randomized to clonidine. The median age was 39.5 years (range 19–65), and subjects were mostly male (68%), white (48.5%) or African-American (31.4%) and unemployed (50.8%). On the average, patients attended two prior drug treatments with 19.3% having no prior treatment and 36.8% having three or more. Patients used heroin a mean of 25.2 (9.7) of 30 days prior to enrollment and 66.5% were daily heroin users. Most subjects used intravenously (63.3%) or nasally (30.8%). According to the ASI, 43.3% reported serious anxiety symptoms and 32.7% reported serious depression symptoms. Alcohol (52%), cocaine (44.6%), marijuana (36.7%), and tobacco (81.6%) were the most frequent types of others substances used with 19.2% drinking alcohol to intoxication. Daily smokers were 81.6% of the sample; 13.1% were non-smokers and 5.3% smoked cigarettes on at least one day, but on less than 30 days in the past month. This 5.3% were dropped from tobacco analyses because it was uncertain if they had recently quit smoking or were only occasional smokers.
4.2. Settings/Level of Care
The same study medication treatment protocol was used in the outpatient (n = 231; 67%) and inpatient settings (n = 113; 33%). Subjects were not randomized by these two levels of care, but were randomized within setting to either buprenorphine-naloxone or clonidine. While we did not detect differences between groups on days of any opioid use, inpatients used heroin on fewer days than outpatients (22.57 vs. 26.53 days; t (179.930) = −3.317, p = .001). Additionally, as compared to outpatients, inpatients were more likely to be injecting heroin than using via other routes [76.2% vs. 57.1%; χ2 (1, N=313) = 10.808, p = .001] at baseline. Clinical, personal, and administrative factors other than opioid dependence severity may have contributed to the final level of care placement, including simply the need for housing; however, these items were not systematically assessed or evaluated.
Inpatient services were hypothesized to produce better outcomes than outpatient services because of increased psychosocial treatment, reduced access to substances, and reduced cues for substance use. Inpatients were seven times more likely to have treatment success (OR = 7.267, 95% CI: 4.128 – 12.791, p < .001) with a trend towards higher completion rates for inpatients (56.6%) than for outpatients (45.5%), χ2 (1, N=344) = .3797, p = .051). Level of care moderated the relationship between medication type and treatment retention. A significant treatment modality X medication type interaction, F (1, 344) = 4.134, p = .043, suggested that while buprenorphine-naloxone administration was associated with more days of treatment attendance in general, this was especially true in the inpatient setting. Outpatients on buprenorphine-naloxone had similar treatment success (29%) as those inpatients on clonidine (22%, p = .487).
4.3. Medication Type
Adjusting for level of care (inpatient versus outpatient), subjects receiving buprenorphine-naloxone were nine times more likely to have achieved treatment success than those receiving clonidine (OR = 9.503, 95% CI: 4.604 – 19.614, p < .001), and 22 times more likely to complete treatment (OR = 22, 95% CI: 11 – 46 p<.001). 69.1% receiving clonidine dropped out by day four versus 12% of patients receiving buprenorphine-naloxone, χ2 (1, N = 344) = 115.765, p < .001.
4,4. Opioid Withdrawal Severity
Opioid withdrawal severity is an important variable in this study and used in many ways in the data analysis. Reduction in opioid withdrawal severity during treatment can be seen as an important short-term outcome measure. Baseline opioid withdrawal severity was a predictor variable, early reduction from baseline to day 3 score was a moderator, and day 2 COWS score was a mediator in the proposed mediational model.
Contrary to expectations, adjusted odds ratios adjusting for study medication, treatment modality, and their interaction term found subjects with high baseline withdrawal scores were four times more likely to have treatment success (OR = 4.397, 95% CI: 1.605 – 12.051, p = .004) than those with lower early withdrawal on the COWS but not on the ARSW (p = .773). While we did not detect an effect of treatment modality on early ARSW score reduction (p = .403), we detected a significant main effect for medication type, F(1,235) = 8.979, p = .003 with buprenorphine-naloxone being associated with significantly greater reductions than clonidine. We detected a significant 2-way interaction, F(1,235) = 4.144, p = .043, indicating that the medication effect was especially potent for individuals in inpatient treatment. We detected significant main effects for medication type, F(1,190) = 4.619, p = .033, and for treatment modality, F(1,190) = 4.588, p = .033 on early COWS score reduction. These main effects indicated a greater effect for buprenorphine-naloxone than clonidine and a greater effect for inpatient than for outpatient treatment on early COWS score reduction. A significant interaction term, F(1,190) = 4.456, p = .036, indicates that those receiving buprenorphine-naloxone and attending inpatient treatment had a large early reduction of withdrawal symptoms based on the COWS.
In the moderator analysis, logistic regression analyses examined the relationship between early changes in withdrawal scores on the COWS and ARSW (from baseline severity to day three severity) after adjusting for treatment modality, medication type, and their interaction term. After adjusting for these factors, greater early decreases in COWS scores were significantly correlated to treatment success (OR = .913, 95% CI: .849 - .981, p = .013). The significant interaction effect suggests a moderating effect of withdrawal severity at baseline, F (3,309) = 17.03, p<.001. Of note, the relationship between treatment success and changes in withdrawal was dependent on the instrument used since this finding was significant with the COWS score, but not significant with ARSW score.
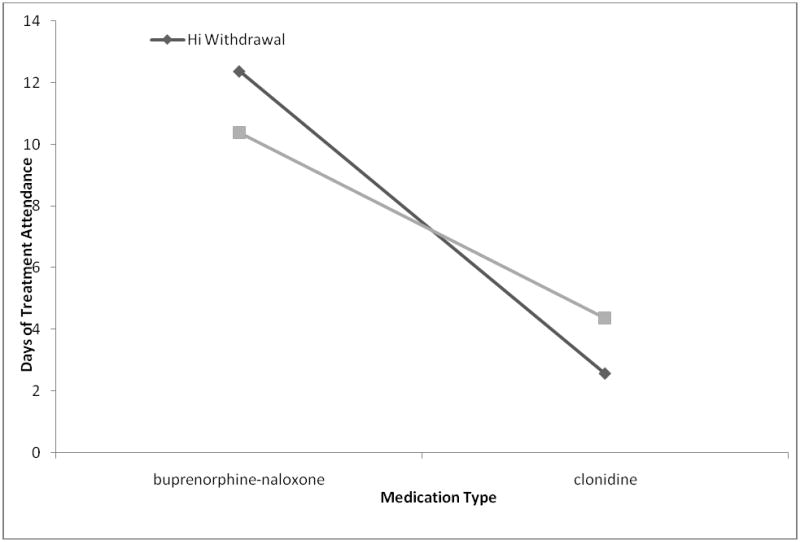
In addition, number of days of treatment attendance was considered as an outcome. Subjects receiving buprenorphine-naloxone with high COWS withdrawal severity at baseline attended more treatment days versus those subjects receiving buprenorphine-naloxone with low withdrawal symptoms. Importantly, both of the withdrawal severity groups who received buprenorphine-naloxone had more days of treatment attendance than those receiving clonidine. In contrast to subjects receiving buprenorphine-naloxone, the opposite pattern was seen with subjects receiving clonidine, in which better outcomes were associated with lower withdrawal severity versus high withdrawal severity (see Figure 1).
Figure 1. Interaction between Medication Type, Withdrawal Severity, and Attendance Outcome.
Interaction between medication type and withdrawal symptoms. Compared to clonidine, subjects on buprenorphine-naloxone had more days of treatment abstinence regardless of baseline COWS severity. For the clonidine group, individuals with low withdrawal symptoms had more days of treatment abstinence than those with high withdrawal symptoms.
4.5 Mediational Model Relationship
Because opioid withdrawal predicted treatment success and medication type predicted symptom reduction and treatment success, a mediational model was hypothesized and tested. Analyses used the COWS because the ARSW did not predict opiate abstinence. Figure 2 graphically displays this partial mediational model. According to Baron and Kenny (Baron and Kenny, 1986), testing a mediational relationship requires several regression equations.
Figure 2. Mediational model.
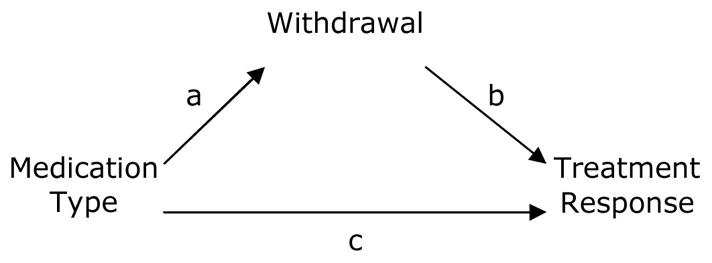
Mediational Model based on Baron and Kenny. Establishing mediation requires a) medication type to affect opioid withdrawal (i.e., path a), b) opioid withdrawal to affect classification as a treatment responder (i.e., path b), and c) medication type to affect treatment response (i.e., path c). When adjusting for the proposed mediator (opioid withdrawal), the regression coefficient associated with medication type in path c should be non-significant, or substantially reduced.
The data supported the mediational model proposed in Figure 2 with respect to both opiate abstinence and total drug abstinence (see Tables 1 and 2). Paths a, b, and c displayed in Figure 2 were statistically significant (all p values < .05) when measuring withdrawal with the COWS. When adjusting for the mediator (i.e., early opioid withdrawal), path c was still significant, but standardized beta weights were reduced.
Table 1.
Mediational Model Analysis (Withdrawal Measured with COWS) Opiate Abstinence.
| Wald | β | SE | p | |
|---|---|---|---|---|
| Path A | - | .214 | .476 | .001** |
| Path B | 4.314 | 1.074 | .034 | .038* |
| Path C | 37.085 | 9.503 | .370 | .001** |
| Path C1 | 19.818 | 9.270 | .500 | .001** |
Re-calculated controlling for mediator, COWS score at day 2.
p<.05;
p<.001
Table 2.
Mediational Model Analysis (COWS measured withdrawal) Total Drug Abstinence.
| Wald | β | SE | p | |
|---|---|---|---|---|
| Path A | - | .214 | .476 | .001** |
| Path B | 4.630 | 1.077 | .034 | .031* |
| Path C | 26.496 | 8.432 | .414 | .001** |
| Path C1 | 10.369 | 4.834 | .489 | .001** |
Re-calculated controlling for proposed mediator, COWS score at day 2.
p<.05;
p<.001
Two separate repeated measures ANOVAs were computed to examine differences in withdrawal scores early in treatment. The main effect of medication type on COWS scores [F(1,186) = 3.226, p = .074] was weak but a significant main effect for treatment modality was observed, F(1,186) = 303.737, p < .001. Finally, a significant medication type X treatment modality interaction suggested that buprenorphine-naloxone was associated with greater decreases in early COWS scores for inpatients than outpatients.
4.6. Outcome of complete drug abstinence
The primary predictors for complete drug abstinence (i.e., opioid, cocaine and marijuana abstinence) were similar to the findings for opioid abstinence alone, including better outcomes for inpatients (OR = 20.857, 95% CI: 2.493 – 174.464, p = .005), those receiving buprenorphine-naloxone (OR = 14.489, 95% CI: 1.926 – 108.970, p < 0.009), those with greater changes in early withdrawal (baseline to day 3) as measured by the COWS (OR = .914, 95% CI: .855 – .977, p = .008), and those with more severe baseline withdrawal (OR = 3.893, 95% CI: 1.496 – 10.130, p = .005). Odds ratios are adjusted for medication type, treatment modality, and their interaction term.
5. Secondary Predictors of Outcome
5.1. Socio-demographic Predictors
Logistic regression adjusting for medication type suggested that none of the socio-demographic factors predicted better treatment success: age, gender, race, years of education, employment status (employed versus not employed), and legal status (being on probation or having a history of imprisonment).
5.2. Heroin use, severity, and route of administration
As compared to daily heroin users, non-daily heroin users were three times more likely to have treatment success (OR = 3.091, 95% CI: 1.709 – 5.590, p < .001) after adjusting for medication type, treatment modality, and their interaction term. No differences were detected between users of IV opiates and those using other routes on opiate abstinence; nor length of drug use.
5.3. Other Substance Usage
Poly-substance use was common, but only tobacco use was associated with worse clinical outcomes. No differences in clinical outcomes were found between users and non-users of alcohol to intoxication, of cocaine, or of marijuana adjusting for medication type, treatment modality, and their interaction term.
5.4. Tobacco Use Status
Non-smokers were almost four times more likely than daily smokers to have treatment success (OR = 3.956, 95% CI: 1.821 – 8.591, p = .001) (when adjusted for daily heroin use, treatment medication, treatment modality, and the medication X modality interaction term). Because non-smokers were significantly less likely to be daily heroin users than daily smokers (OR = .298, 95% CI: .147 – .604, p = .001), daily heroin use was added to the logistic regression when the adjusted odds ratios were calculated. There was a trend suggesting that smokers experienced less withdrawal reduction than non-smokers (OR = 1.085, 95% CI: .988 – 1.192, p = .089). Of note, non-smoking status predicted more likelihood to have opioid or complete drug abstinence (OR = 3.128, 95% CI: 1.357 – 7.213, p = .007).
5.6. Anxiety and Depression
Depression and anxiety problems for the past 30 days were determined by the subject’s self-report on the ASI (present or absent). Based on logistic regression analyses adjusting for medication type and treatment modality, individuals with baseline anxiety problems were almost twice as likely to have treatment success (OR = 1.861, 95% CI: 1.092 – 3.169, p = .022) as those without baseline anxiety. Baseline depression problems were unrelated to treatment success.
5.7. Ancillary Medications
Most subjects (84.6%) received some ancillary medications to treat specific withdrawal symptoms of anxiety, restlessness, insomnia, nausea, vomiting, diarrhea, and muscle, bone, or joint pain. Less ancillary medications were used with buprenorphine-naloxone versus clonidine [t(109) = −3.1, p < 0.01], and more ancillary medications were used during inpatient treatment (95.6%) versus outpatient (79.2%), [χ2(1, N=344) = 15.572, p < .001]. Ancillary medication use was not a significant factor predicting opioid treatment success.
6. Discussion
This paper reports predictors, mediators and moderators of treatment success for buprenorphine-naloxone versus clonidine for medically supervised opioid withdrawal in either the inpatient or outpatient setting. Identification of factors that predict relapse and treatment success during acute withdrawal illuminates the early recovery process and may help inform clinical recommendations for a difficult to treat population.
As expected, medication type (buprenorphine-naloxone versus clonidine) was the strongest predictor of treatment success. The ability of buprenorphine-naloxone to reduce symptoms and retain subjects in the early phase of treatment may be a critical factor in understanding the better outcomes compared to clonidine (only a 12% drop out by day four of treatment with buprenorphine-naloxone versus 69.1% with clonidine). This paper expands on this finding by examining moderators and mediators of this important primary outcome, including the interactive role of level of care, severity of withdrawal symptoms, and other secondary predictors.
Level of care (inpatient versus outpatient) was both a strong predictor of treatment success and a moderator of medication treatment outcomes. This finding has important potential policy implications and requires additional study. Overall subjects in inpatient treatment were seven times more likely to have treatment success than those in outpatient treatment. Surprisingly the level of care differences for treatment retention were relatively small (56.6% inpatient versus 45.5% outpatient, p = .051), and most of the enhanced treatment success was secondary to enhancing abstinence rates. Because of the expense of inpatient treatment, level of care is important in evaluating treatment efficacy and cost effectiveness. Based on our findings, inpatient care may be more appropriate if a clinical program is limited to providing only clonidine medication (perhaps due to staff prescribing limitations, formulary restrictions, or the higher cost of buprenorphine-naloxone). If a program is able to offer buprenorphine-naloxone medication, then treatment outcomes will likely be better for those receiving inpatient treatment versus outpatient. Based on our finding that outpatient buprenorphine-naloxone is similar in outcomes to inpatient clonidine, buprenorphine-naloxone would be the preferred medication option if outpatient treatment is the only level of care available.
The third primary hypothesis, that participants with more severe baseline withdrawal symptoms, regardless of medication condition, would drop out of treatment and have worse outcomes compared to participants with lower levels of withdrawal, was not supported except among outpatients receiving clonidine. This finding is consistent with the Rounsaville et al., 1985 findings in an outpatient study that participants with more severe withdrawal who received clonidine (or methadone) had worse clinical outcomes in opioid dependence treatment than those with less severe withdrawal. Another prior study did not find that intensity of withdrawal predicted early drop-out from treatment (Scherbaum et al., 2004). Unexpectedly, subjects on buprenorphine-naloxone with higher baseline opioid withdrawal symptoms had better treatment success than those with lower severity withdrawal symptoms. A speculative explanation is that the low severity withdrawal patients on buprenorphine-naloxone may not have needed 13 days of opioid detoxification, and dropped out because they were improving.
Future medical withdrawal treatment studies might focus on the first few days of opioid withdrawal and examine higher dosages of buprenorphine-naloxone or clonidine, especially for patients with high severity withdrawal symptoms. Of note, any decision to evaluate higher doses of medication would have to consider safety issues and risks of additional side effects. Interestingly, there were differences between the two opioid withdrawal severity instruments (COWS versus ARSW). The COWS predicted differences in outcome based on severity, while the ARSW, a self-report measure, did not. This finding suggests that patient self-report alone may not be as useful a measure for evaluating withdrawal symptom severity compared to clinician rated instrument assessments.
In regards to the mediational model proposed, the reduction in significance of path C was modest, rather than robust, which suggests a limitation of the proposed model of medication type to affect opioid withdrawal (path a), opioid withdrawal to affect treatment response (path b), and medication type to affect treatment response (path c). There is a need for additional exploration of this model in future studies with larger sample sizes.
Consistent with previous research, most demographic and baseline characteristics failed to predict treatment outcome (San et al., 1989; Armenia et al., 1999). Previous inpatient and outpatient studies evaluated clonidine (not buprenorphine-naloxone) and were not in different settings (only inpatient or outpatient). Findings across studies have not been consistent (Gossup, 1988; Endicott & Watson, 1994; Morral et al., 1997; Franken and Hendriks, 1999; Backmund et al., 2001; Ghodse et al., 2002; Scherbaum et al., 2004). Significant predictors of failure have included younger age, (Jeremiah et al., 1995; Armenia et al., 1999; Backmund et al., 2001) being single, (Perez de los Cobos et al., 1997; Armenia et al., 1999) having more severe drug problems, (Franken and Hendriks, 1999) and having less education and not being on probation (Backmund et al., 2001). In our study no clearly significant demographic predictors emerged.
Multiple substance use was common in this study as with many other studies of heroin dependence (Oliveto et al., 1994; Perez de los Cobos et al., 1997; SAMHSA NSDUH 2004). Concurrent alcohol, cocaine, or marijuana use was not predictive of worse clinical outcomes. Interestingly tobacco use was associated with worse opioid treatment outcomes. Daily smoking may be a marker for worse addiction severity (Krejci et al., 2003) and/or tobacco use may increase other substance craving (Taylor et al., 2000; Frosch et al., 2002) and worsen withdrawal symptoms. Changes in smoking status can affect the metabolism of some medications (those metabolized through the P450 1A2 isoenzyme); however this issue was unlikely to be a factor in this study since smoking cessation was not an effort of this protocol.
Concurrent tobacco use is not a factor reported in previous predictor studies for medications in opioid withdrawal and is often ignored in addiction treatment settings; however other studies have reported tobacco use as a trigger for other substance relapse, including smokers who are opiate addicts have a harder time maintaining abstinence than non-smokers (Frosch et al., 2000) There is a need to better understand the impact of tobacco use/dependence on opioid withdrawal and longer-term outcomes.
Severe baseline anxiety symptoms doubled treatment success in this study. The literature suggests that depression and anxiety is common in addiction treatment populations and tends to be associated with worse treatment outcome; however this literature is complicated and differences vary by current versus past symptoms versus disorders (APA 2006; Arujo et al 1996). One explanation of the current data is that subjects with severe anxiety symptoms might have felt relief for their anxiety symptoms from the study medications (buprenorphine-naloxone, clonidine or ancillary medications); however longer outcomes and ongoing monitoring of these symptoms might help better understand the possible explanations of these findings. There is a need for more studies of buprenorphine-naloxone outcomes with patients with psychiatric symptoms and disorders.
While a major strength of this study is being a multi-site randomized clinical trial with a fairly large sample size, the study had limitations. The diversity of treatment sites adds to the external validity of the findings, but reduces the internal validity. While the sample size is appropriate for the analyses described in this report, the sample sizes from the individual sites are inadequate to evaluate differences between sites. In regards to comparing outcomes by level of care, both setting used the same protocol, however, subjects were not randomized by level of care, but were either self-selected or clinically-selected. The low retention rate for clonidine subjects precluded making a comparison with buprenorphine-naloxone subjects who received a full course of treatment. Other weaknesses include the fact that this was an open-label study where the subjects knew the medication they were receiving and this introduces bias on both the subject’s expectations about the medication and potentially the observers’ ratings of withdrawal. Another limitation was that treatment setting (inpatient or outpatient) was not randomized and some of the subjects changed treatment setting status during the study. Another potential limitation is the definition of “treatment success” including the two components of being “present” to complete the study medication on day 13 and “absent” of opioids on the urine toxicology assessment on the last day of research clinic attendance (day 13 or 14). Although grounded in clinical experience, being somewhat conservative, and selected by a group of experts in this topic; another choice of definition could have resulted in different findings and conclusions.
In the future, other potential predictors of treatment outcomes may be considered during the design of randomized clinical trials, including genotype markers, personality disorder traits, neuropsychological testing, and brain imaging differences. Future studies should consider other methods for assessing causal mechanisms that include biological, psychological, and social variables.
In addition, we appreciate that future studies must focus on the long-term outcomes of individuals after the detoxification time period. Ultimately, the most important clinical outcomes will be long-term and detoxification is only one of the key beginning phases of treatment, including the prior engagement into treatment phase and the next management of protracted withdrawal phase. However, the findings from this study do suggest robust differences based on medication type and level of care.
In summary, medication was the best predictor of treatment outcome for opiate detoxification regardless of treatment setting, and inpatient treatment was a strong predictor of treatment success and a moderator of medication outcomes. Reduction in opioid withdrawal severity from baseline to day three was a moderating factor in treatment outcomes. Those who did the best with clonidine had low severity withdrawal symptoms at baseline. The apparent demand for ambulatory medical withdrawal services supports the need for additional research aimed at identifying patients and/or approaches that might be best suited for this treatment approach (Westreich et al., 1997; Broers et al., 2000). There is limited research to guide clinicians; however this study provides some additional information from community based treatment programs.
Acknowledgments
The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIDA.
Role of funding source. This publication was supported by a series of grants from NIDA as part of the Cooperative Agreement on CTN: University of California, Los Angeles: U10 DA13045; Oregon Health & Science University: U10 DA13036; New York University School of Medicine: U10 DA13046; University of Pennsylvania: U10 DA13043, K05-DA17009 and the Department of Veterans Affairs; Wayne State University: U10 DA13710; University of Cincinnati: U10 DA13732; University of Miami Center for Family Studies: U10 DA13720; Research Foundation for Mental Hygiene, Inc., New York State Psychiatric Institute Division: U10 DA13035; grant NIH/NCRR M01RR00096 to NYU GCRC; and KO5 DA-17009 to G.W. The NIH and NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors. A.J.C., M.S.R. and W.L. designed the study, L.S.B. participated in protocol development. L.A. and W.L. wrote the main protocol. D.M.Z., G.W., N.W.-O., D.M., L.S.B., D.B., G.B., J.M., and B.J.B. conducted the study and managed study sites for the study; J.K., A.J.C., S.M.S., M.S.R., L.S.B., R.M., D.B., G.B., D.O., and B.J.B. recruited patients. B.J.B. managed the literature searches and summaries of previous related work. J.J.A. and B.J.B. provided data for the analysis. M.S. undertook the statistical analysis for the study. D.M.Z. oversaw data analysis. G.B. contributed to interpretation of study results. D.M.Z., L.A., M.S., J.K., and A.J.C. participated in writing the first draft of the manuscript. D.M.Z., M.S., G.W., J.K., A.J.C., S.M.S., D.M., M.S.R., L.S.B., T.W., D.B., G.B., J.M., T.H. and W.L. participated in editing of the manuscript. D.M.Z. oversaw and coordinated all aspects of manuscript preparation. All authors contributed to and have approved the final manuscript.
Conflict of Interest. The following is a list of potential conflicts of interest within three years of beginning the work submitted.
D.M.Z. has consulted for Pfizer, Bristol-Myers Squibb, Janssen, Eli Lilly, and Alkermes/Cephalon. D.M.Z. has received grant support from Bristol-Myers Squibb, Janssen, and Eli Lilly. L.A. is currently employed by Schering-Plough, a distributor of buprenorphine. G.W. has consulted for Denver Health. S.M.S. has received grant support from Purdue Pharma, US WorldMeds LLC, and Titan Pharmaceuticals. L.A. and T.H. have received honoraria from Reckitt Benckiser and Schering Plough.
All other authors declare they have no conflicts of interest.
References
- Araujo L, Goldberg P, Eyma J, et al. The effect of anxiety and depression on completion and withdrawal status in patients admitted to substance abuse detoxification program. Journal of Substance Abuse Treatment. 1996;13:61–66. doi: 10.1016/0740-5472(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Amass L, Kamien JB, Mikulich SK. Efficacy of daily and alternate-day dosing regimens with the combination buprenorphine-naloxone tablet. Drug Alcohol Depend. 2000;58(1–2):143–52. doi: 10.1016/s0376-8716(99)00074-5. [DOI] [PubMed] [Google Scholar]
- Amass L, Ling W, Freese TE, Reiber C, Annon JJ, Cohen AJ, et al. Bringing Buprenorphine-Naloxone Detoxification to Community Treatment Providers: The NIDA Clinical Trials Network Field Experience. Am J Addict. 2004;13(Suppl 1):S42–66. doi: 10.1080/10550490490440807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Practice Guidelines for the Treatment of Patients With Substance Use Disorders. American Psychiatric Press Inc; Washington DC: 2006. [Google Scholar]
- Armenia SH, Chutuape MA, Stitzer ML. Predictors of discharge against medical advice from a short-term hospital detoxification unit. Drug Alcohol Depend. 1999;56(1):1–8. doi: 10.1016/s0376-8716(99)00027-7. [DOI] [PubMed] [Google Scholar]
- Backmund M, Meyer K, Eichenlaub D, Schutz CG. Predictors for completing an inpatient detoxification program among intravenous heroin users, methadone substituted and codeine substituted patients. Drug Alcohol Depend. 2001;64(2):73–80. doi: 10.1016/s0376-8716(01)00122-3. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L. Buprenorphine treatment of opiate dependence: a review. Exp Clin Psychopharmacol. 1995;3:477–89. [Google Scholar]
- Bickel WK, Stitzer MI, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. Buprenorphine: dose-related blockade of opioid challenge effects in opioid dependent humans. J Pharmacol Exp Ther. 1998a;247(1):47–53. [PubMed] [Google Scholar]
- Bickel WK, Stitzer MI, Bigelow GE, Liebson IA, Jasinski DR, Johnson RE. A clinical trial of buprenorphine: comparison with methadone in the detoxification of heroin addicts. Clin Pharmacol Ther. 1988b;43(1):72–8. doi: 10.1038/clpt.1988.13. [DOI] [PubMed] [Google Scholar]
- Boatwright DE. Buprenorphine and addiction: challenges for the pharmacist. J Am Pharm Assoc (Wash) 2002;42(3):432–8. doi: 10.1331/108658002763316860. [DOI] [PubMed] [Google Scholar]
- Broers B, Giner F, Dumont P, Mino P. Inpatient opiate detoxification in Geneva: follow-up at 1 and 6 months. Drug Alcohol Depend. 2000;58(1–2):85–92. doi: 10.1016/s0376-8716(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Endicott P, Watson B. Interventions to improve the AMA discharge rate for opiate-addicted patients. J Psychosoc Nurs Ment Health Serv. 1994;32(8):36–40. doi: 10.3928/0279-3695-19940801-14. [DOI] [PubMed] [Google Scholar]
- Franken IH, Hendriks VM. Predicting outcome of inpatient detoxification of substance abusers. Psychiatr Serv. 1999;50(6):813–7. doi: 10.1176/ps.50.6.813. [DOI] [PubMed] [Google Scholar]
- Frosch DL, Stein JA, Shoptaw S. Using latent-variable models to analyze smoking cessation clinical trial data: an example among the methadone maintained. Exp Clin Psychopharmacol. 2002;10(3):258–67. doi: 10.1037//1064-1297.10.3.258. [DOI] [PubMed] [Google Scholar]
- Ghodse AH, Reynolds M, Baldacchine AM, Dunmore E, Byrne S, Oyefeso A, et al. Treating an opiate-dependent population: a one-year follow-up study of treatment completers and noncompleters. Addict Behav. 2002;27(5):765–78. doi: 10.1016/s0306-4603(01)00208-8. [DOI] [PubMed] [Google Scholar]
- Gossling HW, Gunkel S, Schneider U, Melles W. Frequency and causes of premature termination (drop-out) during inpatient opiate detoxification. Fortschr Neurol Psychiatr. 2001;69(10):474–481. doi: 10.1055/s-2001-17565. [DOI] [PubMed] [Google Scholar]
- Gossup M. Clonidine and the treatment of the opiate withdrawal syndrome. Drug Alcohol Depend. 1988;21(3):253–9. doi: 10.1016/0376-8716(88)90078-6. [DOI] [PubMed] [Google Scholar]
- Jeremiah J, O’Sullivan P, Stein MD. Who leaves against medical advice? J Gen Intern Med. 1995;10(7):403–5. doi: 10.1007/BF02599843. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 70(2):S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- Krejci J, Steinberg ML, Ziedonis D. Smoking status and substance abuse severity in an inpatient treatment sample. Drug Alcohol Depend. 2003;72(3):249–54. doi: 10.1016/j.drugalcdep.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Ling W, Amass L, Shoptaw S, Annon J, Babcock D, Brigham G, et al. A multi-center randomized trial of buprenorphine-naloxone and clonidine for opioid detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100(8):1090–100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Miotto K, Rawson RA, Huber A, Shoptaw S, Ling W. Outpatient non-opioid detoxification for opioid withdrawal: who is likely to benefit? Am J Addict. 1997;6(3):218–23. [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters F, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Morral AR, Iguchi MY, Belding MA, Lamb RJ. Natural classes of treatment response. J Consult Clin Psychol. 1997;65(4):673–85. doi: 10.1037//0022-006x.65.4.673. [DOI] [PubMed] [Google Scholar]
- Oliveto A, Kosten TR, Schottenfeld R, Ziedonis DM, Falcioni J. Cocaine use in buprenorphine versus methadone maintained patients. Am J Addict. 1994;3:43–48. [Google Scholar]
- Perez de los Cobos J, Trujols J, Ribalta E, Casas M. Cocaine use immediately prior to entry in an inpatient heroin detoxification unit as a predictor of discharges against medical advice. Am J Drug Alcohol Abuse. 1997;23(2):267–79. doi: 10.3109/00952999709040946. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Kosten T, Kleber H. Success and failure at outpatient opioid detoxification: evaluating the process of clonidine- and methadone-assisted withdrawal. J Nerv Ment Dis. 1985;173(2):103–10. doi: 10.1097/00005053-198502000-00007. [DOI] [PubMed] [Google Scholar]
- San L, Cami J, Peri JM, Mata R, Porta M. Success and failure at inpatient detoxification. B J Addict. 1989;84(1):81–7. doi: 10.1111/j.1360-0443.1989.tb00554.x. [DOI] [PubMed] [Google Scholar]
- Scherbaum N, Heppekausen K, Rist F. Is premature termination of opiate detoxification due to intensive withdrawal or craving? Fortschr Neurol Psychiatr. 2004;72(1):14–20. doi: 10.1055/s-2003-812451. [DOI] [PubMed] [Google Scholar]
- Strobbe S, Brower KJ, Galen LW. Predicting completion of outpatient opioid detoxification with clonidine. Am J Addict. 2003;12(3):260–9. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies, NSDUH Series H 24, DHHS Publication No. SMA 04 – 3963. Rockville, MD: 2004. Overview of Findings from the 2003 National Survey on Drug Use and Health. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. National Admissions to Substance Abuse Treatment Services, DASIS Series: S-22, DHHS Publication No. (SMA) 04-3946. Rockville, MD: 2004. Treatment Episode Data Set (TEDS). Highlights 2002. [Google Scholar]
- Taylor RC, Harris NA, Singleton EG, Moolchan ET, Heishman SJ. Tobacco craving: intensity-related effects of imagery scripts in drug abusers. Exp Clin Psychopharmacol. 2000;8(1):75–87. doi: 10.1037//1064-1297.8.1.75. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35(2):253–9. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Westreich L, Heitner C, Cooper M, Galanter M, Guedj P. Perceived social support and treatment retention on an inpatient treatment unit. Am J Addict. 1997;6(2):144–9. [PubMed] [Google Scholar]
- Ziedonis DM, Kosten TR. Depression as a Prognostic Factor for Pharmacological Treatment of Cocaine Dependence. Psychopharmacol Bull. 1991;27(3):337–43. [PubMed] [Google Scholar]