Abstract
Control of sexually transmitted infections (STIs) is feasible, leads to improved sexual and reproductive health and contributes to preventing HIV transmission. The most advanced HIV epidemics have developed under conditions of poor STI control, particularly where ulcerative STIs were prevalent. Several countries that have successfully controlled STIs have documented stabilization or reversal of their HIV epidemics.
STI control is a public health outcome measured by reduced incidence and prevalence. The means to achieve this include: (i) targeting and outreach to populations at greatest risk; (ii) promoting and providing condoms and other means of prevention; (iii) effective clinical interventions; (iv) an enabling environment; and (v) reliable data.
Clinical services include STI case management, screening and management of STIs in sex partners. Syndromic case management is effective for most symptomatic curable STIs and screening strategies exist to detect some asymptomatic infections. Presumptive epidemiologic treatment of sex partners and sex workers complement efforts to interrupt transmission and reduce prevalence. Clinical services alone are insufficient for control since many people with STIs do not attend clinics. Outreach and peer education have been effectively used to reach such populations.
STI control requires effective interventions with core populations whose rates of partner change are high enough to sustain transmission. Effective, appropriate targeting is thus necessary and often sufficient to reduce prevalence in the general population. Such efforts are most effective when combined with structural interventions to ensure an enabling environment for prevention. Reliable surveillance and related data are critical for designing and evaluating interventions and for assessing control efforts.
Résumé
L’endiguement des infections sexuellement transmissibles (IST) est réalisable, permet d’améliorer la santé sexuelle et génésique et contribue à prévenir la transmission du VIH. Les plus fortes progressions de l’épidémie de VIH/sida ont été relevées dans des conditions de maîtrise insuffisante des IST, et notamment dans des situations de prévalence importante des IST ulcérantes. Plusieurs pays ayant réussi à endiguer les IST ont enregistré aussi sur leur territoire une stabilisation de l’épidémie de VIH/sida, voire une inversion de ses tendances.
L’endiguement des IST est un résultat de santé publique, qui se mesure par la réduction de l’incidence et de la prévalence de ces infections. Pour parvenir à ce résultat, il faut notamment : (i) cibler les populations les plus à risque et leur offrir des services de proximité ; (ii) promouvoir l’usage des préservatifs et d’autres moyens de prévention et fournir ces moyens ; (iii) mettre en œuvre des interventions cliniques efficaces ; (iv) créer un environnement favorable ; et (v) disposer de données fiables.
Les services cliniques couvrent la prise en charge des cas d’IST, ainsi que le dépistage des partenaires sexuels et leur prise en charge s’ils sont contaminés. Une prise en charge syndromique est efficace pour la plupart des cas curables et symptomatiques d’IST et il existe des stratégies pour dépister certaines infections asymptomatiques. Le traitement épidémiologique présomptif des partenaires sexuels et des professionnels du sexe complète les efforts pour interrompre la transmission et réduire la prévalence. Néanmoins, les services cliniques ne peuvent à eux seuls endiguer les IST dans la mesure où de nombreuses personnes infectées ne vont pas consulter. L’éducation sanitaire sur le terrain et auprès des personnes ayant le même mode de vie a été employée avec succès pour atteindre ces populations.
L’endiguement des IST nécessite d’intervenir efficacement sur un cœur de cible composé d’individus chez lesquels la fréquence des changements de partenaire est assez élevée pour entretenir la transmission. Un ciblage efficace et approprié est ainsi nécessaire et souvent suffisant pour réduire la prévalence des IST dans la population générale. Ces efforts gagnent en efficacité lorsqu’ils sont combinés à des interventions structurelles pour garantir un environnement favorable à la prévention. Il est essentiel de disposer de données de surveillance et d’informations connexes fiables pour concevoir et jauger les interventions et pour évaluer les efforts de lutte.
Resumen
El control de las infecciones de transmisión sexual (ITS) es una medida factible, que propicia una mejor salud sexual y reproductiva y que ayuda a prevenir la transmisión del VIH. Las epidemias más avanzadas de infección por VIH se han desarrollado en condiciones de bajo control de las ITS, sobre todo en los lugares donde abundan los casos de ITS ulcerativas. Varios países que han conseguido controlar las ITS han documentado la estabilización o incluso reversión de sus epidemias de VIH.
El control de las ITS es un resultado de salud pública medido por la disminución de su incidencia y prevalencia. Entre los medios aplicados para lograr ese control cabe citar: (i) la focalización de las medidas en las poblaciones en mayor riesgo y las actividades de extensión a éstas; (ii) la promoción y el suministro de preservativos y otras formas de prevención; (iii) unas intervenciones clínicas eficaces; (iv) un entorno favorable; y (v) datos fiables.
Los servicios clínicos incluyen el tratamiento de los casos de ITS, y el cribado y tratamiento de las ITS de las parejas. El tratamiento sindrómico de los casos es eficaz en la mayoría de las ITS sintomáticas curables, y existen estrategias de cribado para detectar algunas infecciones asintomáticas. El tratamiento epidemiológico de sospecha de las parejas sexuales y de las profesionales del sexo complementa las actividades de interrupción de la transmisión y reducción de la prevalencia. Los servicios clínicos son insuficientes por sí solos para controlar esas infecciones, pues muchas de las personas afectadas por las ITS no acuden a los consultorios. Para llegar a esas poblaciones se ha recurrido con éxito a la proyección exterior y la educación entre compañeros.
Si se quiere controlar las ITS, se requieren intervenciones eficaces centradas en ese núcleo de personas cuya frecuencia de cambio de pareja es lo bastante elevada para sostener la transmisión. Una focalización adecuada y eficaz es por tanto necesaria y a menudo suficiente para reducir la prevalencia en la población general. Esos esfuerzos revisten la máxima eficacia cuando se combinan con intervenciones estructurales tendentes a garantizar un entorno favorable para la prevención. Una vigilancia fiable y los datos por ella aportados son elementos fundamentales para diseñar y evaluar las intervenciones, así como para evaluar las medidas de control.
ملخص
إن مكافحة العدوى المنقولة جنسياً ممكنة التحقيق، وتؤدي إلى تحسين الصحة الجنسية والإنجابية، وتساهم في توقّي انتقال العدوى بفيروس الإيدز. وقد وقعت أشد أوبئة فيروس الإيدز سوءاً في الحالات التي ساءت فيها مكافحة العدوى المنقولة جنسياً، ولاسيّما عند انتشار العدوى التقرحية المنقولة جنسياً. ولقد وثـّقت عديد من البلدان التي نجحت في مكافحة العدوى المنقولة جنسياً ثبات أو تراجع أوبئة فيروس الإيدز لديها.
وتعتبر مكافحة العدوى المنقولة جنسياً نتيجة صحية عمومية من الممكن قياسها من انخفاض معدلات الوقوع والانتشار. وتتضمن طرق تحقيق ذلك: (أ) استهداف الفئات السكانية الأكثر عرضة للخطر والوصول إليها؛ (ب) نشر وتقديم العازل الجنسي وسائر وسائل الوقاية؛ (ج) التدخلات الإكلينيكية الفعالة؛ (د) البيئة المشجعة؛ (هـ) البيانات الموثوق بها.
وتتضمن الخدمات السريرية التدبير العلاجي للمرضى المصابين بالعدوى المنقولة جنسياً، والتحري والتدبير العلاجي للعدوى المنقولة جنسياً بين الشركاء الجنسيين ويكون التدبير العلاجي لحالات المتلازمات فعالاً في أغلب حالات العدوى المنقولة جنسياً ذات الأعراض والممكن شفاؤها. وتوجد استراتيجيات التحري للكشف عن بعض حالات العدوى عديمة الأعراض. ويستكمل العلاج الوبائي المفترض للشركاء الجنسيين والمشتغلين بالجنس الجهود المبذولة لقطع انتقال العدوى والحد من انتشارها. والخدمات الإكلينيكية وحدها غير كافية للمكافحة حيث إن كثيراً من المصابين بالعدوى المنقولة جنسياً لا يراجعون العيادات. وقد استخدمت برامج الإيصال والتوعية بين الأقران بفعالية للوصول إلى هذه الفئات السكانية.
وتتطلب مكافحة العدوى المنقولة جنسياً تدخلات فعالة بين الفئات السكانية الرئيسية التي تتغير فيها معدلات الإصابة بين الشركاء الجنسيين على نحو عالٍ وكافٍ لاستمرار انتقال العدوى. ومن الضروري إذن الاستهداف الفعال والملائم، الذي غالباً ما يكون كافياً للحد من انتشار المرض بين الفئات السكانية العامة. وهذه الجهود تكون أكثر فعالية عندما تدمج مع التدخلات الهيكلية لضمان توفير بيئة مشجعة للوقاية، وإن الترصُّد الذي يعتد به والبيانات ذات العلاقة مهمان لتخطيط وتقييم التدخلات ولتقييم جهود المكافحة.
Introduction
In the history of sexually transmitted infection (STI) control, as with other communicable diseases, the pendulum swings between vertical disease-specific and broader horizontal approaches, from a narrow focus on pathogens and their treatment to the wider needs of populations who host and transmit them.
Since the emergence of HIV in the 1980s, STI control efforts have increasingly been defined in relation to HIV programme priorities.1 Although HIV is itself an STI, efforts to prevent its transmission are largely managed through programmes that are funded, implemented and evaluated independently of other STI control efforts. Such a fractured paradigm has had unfortunate consequences. Too often, neglected STI programmes – the foundation upon which HIV prevention efforts were built – collapse due to reduced funding. As a result, STI clinics and services are understaffed, understocked or disappearing altogether; pregnant women may be offered HIV tests but are no longer screened for syphilis; and STI reporting, an important marker of sexual transmission trends, has largely collapsed.2,3
In other areas of communicable disease control, the pendulum is moving in a different direction towards strategies that aim for broad public health benefit while pursuing disease-specific control objectives. Examples include attention to general lung health within the Stop TB partnership and integrated vector management in malaria efforts. The rationale is that sustainable disease control requires coordinated efforts to address common conditions that may facilitate transmission or impede access to prevention, case detection, diagnosis and treatment.4,5
This paper describes a unified paradigm of STI control where HIV is an important focus. The approach is analytical and programme-oriented, with attention to public health outcomes and means. We start by reviewing definitions and outlining basic components of STI control, and then examine empirical evidence of the feasibility and benefits of STI control under different conditions. We also consider what happens to HIV under different scenarios and look at the overlap and potential synergies between HIV prevention and STI control efforts.
Defining STI control
STI control is a public health outcome, measured as reduced incidence and prevalence, achieved by implementing strategies composed of multiple synergistic interventions. In the literature, the term “STI control” is frequently used interchangeably with “STI treatment”, yet these are quite different things.6,7 Control of any communicable disease is a public health outcome, measured as reduced prevalence (total infections) or incidence (new infections) in a population. Treatment is a biomedical intervention that, unless part of a broader control strategy, usually does not result in lower transmission rates or disease burden.
STI control can be measured in absolute or relative terms, for example, as elimination of chancroid or 50% reduction of the prevalence of gonorrhoea. Monitoring trends of common curable STIs, etiologically or syndromically, can provide evidence of changing incidence. Where STI surveillance is supported and functioning (often it is not), these data also reflect general sexual transmission trends and can be used to assess the adequacy of overall STI/HIV prevention efforts.8 Since HIV shares many aspects with other STIs – including modes of transmission, behavioural and other cofactors and potential control measures – HIV prevention can logically be situated within the larger, encompassing domain of STI control.
Back to basics
A comprehensive STI control strategy includes targeted community-based interventions, promotion and provision of the means of prevention and effective clinical services within an enabling environment, as well as reliable data to guide the response.
The science and methods of STI control build on several centuries’ experience backed by evidence of progressively declining incidence and prevalence, particularly in developed countries.9 Over the past three decades, these methods have been adapted and are proving valid in some less-developed countries with limited resources. They have also been adapted to better address chronic viral STIs such as herpes simplex virus type 2 and HIV. In many countries, however, such proven control methods have not been implemented consistently or at sufficient scale to have public health impact.
The effectiveness of standard STI control interventions and strategies – from condom promotion to epidemiologic targeting and partner treatment – is supported by extensive empirical evidence. Despite limited data from randomized controlled trials, most have been adapted to form the basis for HIV prevention work. Recent calls for “combination HIV prevention” and “back to basics” approaches to HIV emphasize the use of combinations of feasible and proven interventions.10,11
A recent review listed priority STI control interventions to include “STI treatment of high-risk sub-populations, comprehensive case management of symptomatic STIs, antenatal syphilis screening and treatment and ophthalmia neonatorum prophylaxis, condom promotion and risk reduction counselling” with increased emphasis on the role of STI clinics in identifying and counselling HIV-infected persons and in diagnosing and managing their STIs.12 The review pragmatically provides evidence for the effectiveness of individual intervention components within the fragmented domains of STI control and HIV prevention, while making a case for better alignment of efforts.
What would such aligned control efforts include? Historical experience argues for coordinated effort in five main areas: (i) appropriate epidemiologic targeting; (ii) primary prevention and access to means of prevention; (iii) provision of effective clinical services to shorten the duration of infectivity; (iv) an “enabling environment” for prevention; and (v) reliable data to guide decision-making.
Clinical services
Clinical interventions can be broadly categorized as STI management approaches for symptomatic patients, screening for asymptomatic infections and partner strategies. All should be supported by appropriate efforts to educate, counsel and provide the means, such as condoms, to prevent infection.
STI case management aims to provide rapid and effective treatment to patients presenting with symptoms to break the chain of infection. Shortening the duration of infectivity is an important objective in the control of STI epidemics. There is strong evidence that syndromic case management is an effective approach for patients with urethral discharge and genital ulcers. It has advantages over previous approaches (i.e. etiologic and clinical diagnosis) in most service delivery settings.12,13 Syndromic case management also performs well for common vaginal infections although it is not designed to detect asymptomatic cervical infections. STI screening and case finding are time-tested approaches for identifying asymptomatic infections. Although feasible, screening to detect cervical infection remains problematic since sensitive tests for detecting gonorrhoea and chlamydial infection remain too expensive for widespread use.
Breaking the chain of infection also involves treating as many sexual partners of people with STIs as can be identified. Several partner treatment strategies have been described with success rates as high as 30–50% (of index patients).14 Due to frequent uncertainty about STI diagnoses in women and potentially serious social consequences of notification, partner strategies should focus on identifying symptomatic men who should then be offered counselling and assistance with notifying their partners.
Other interventions aim to interrupt transmission through epidemiologic targeting and presumptive treatment. Asking STI patients about the location of recent contacts can help direct prevention efforts to epidemiologically important “hot-spots” where incidence may be high. Presumptive treatment has been used to rapidly reduce STI prevalence among populations at highest risk, such as sex workers.15
A relatively new area for STI clinical services is identification and early intervention with people living with HIV, particularly those recently infected. Promising interventions include early HIV testing and counselling of STI patients, detection of acute HIV infection and regular STI screening and treatment to reduce genital viral load.16 It is important that clinical interventions be seen as an extension of prevention work in the community and, as such, reinforce prevention messages and promote condoms.
Primary prevention
STI control cannot be achieved by means of clinical interventions alone. Primary prevention interventions at the clinic and outside, where transmission takes place, are required. Such interventions emphasize the means of prevention, information and referrals to clinical services.11,17 There is strong evidence that male latex condoms reduce transmission of HIV by at least 80–85%, are effective against most other STIs and reduce the risk of unintended pregnancy.18 Other barrier methods, such as the female condom, may have advantages over the male condom in some situations, or as backup methods.19,20
From the perspective of the STI control programme, the challenge is to make condoms and other means of prevention available and affordable, promote their use and reduce barriers to utilization. Social marketing has proven effective in increasing supply and demand.21 Maximizing the public health benefit of the condom component of the programme is not simply a question of promotion and distribution in the community at large, however. What matters most is that condoms are used in situations where STIs are most likely to spread.22
There is some evidence that other behavioural interventions can be synergistic to targeted condom promotion. In Thailand, explicit messages to men about the risks of STIs from unprotected commercial sex resulted in higher reported condom use, lower reported numbers of sex worker visits and lower infection rates.22 Behaviour change in the general population, such as delaying sexual debut, may also serve to reduce transmission but there is little evidence to support abstinence or monogamy as stand-alone strategies.
Targeting high-risk populations
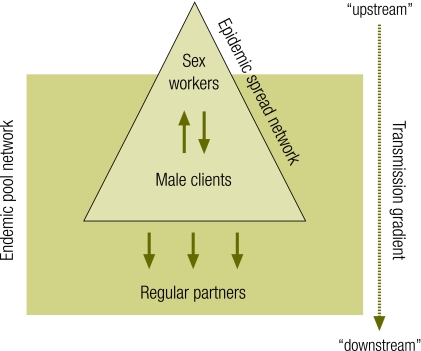
Primary prevention and clinical services contribute synergistically to STI control. The success of these efforts depends not on reaching all people but on reaching the right people with effective interventions. If there is a fundamental tenet of STI control, it is that transmission depends on high rates of sexual partner change. Epidemics are sustained “upstream” in relatively small sub-groups of the population where rates of sexual partner change are sufficient to sustain high incidence (Fig. 1). Secondary or “downstream” transmission accounts for infections among people at lower risk. An important corollary is that prevention efforts that effectively reduce transmission in the high-partner “core” population are necessary, and often sufficient, to reduce transmission in the population at large.
Fig. 1.
Dynamics of STI and HIV transmission
STI, sexually transmitted infection.
This principle is implemented through appropriate targeting of STI control interventions. In Asia, for example, STI control programmes increasingly aim for “saturation coverage” of high-risk populations of sex workers, men who have sex with men, and persons injecting drugs. Other interventions target bridge populations – such as clients and partners of high-risk individuals – through STI clinics or workplace interventions.
Targeting generally builds on two methods – outreach and peer interventions. Programmes begin with mapping to locate and estimate sizes of target populations.23 Contacts made during this formative stage provide the initial building blocks for peer-based interventions. Peer workers have been shown to be most effective in catalysing change in their communities, from condom use to clinic attendance to structural changes.24
Related and important concepts are structural interventions and creating an enabling environment. Structural interventions address root causes of problems, such as the difficulties of individual sex workers to negotiate condom use. By shifting responsibility from the individual to the establishment – in this case by requiring sex work establishments to enforce 100% condom use – better compliance can be achieved and monitored.22 Other examples of structural interventions include collective action by sex workers to change social conditions that put them at risk.25
Such efforts aim to create an enabling environment for prevention. STIs spread most easily among marginalized populations that live with daily risk, have little power to negotiate safer conditions and have poor access to health services. Recent successful examples of STI control have integrated basic prevention components – targeted provision of condoms and STI services – into broader community-based efforts for enabling structural change.26
Measuring STI control
STIs are reliable markers of HIV transmission that should be monitored to assess effectiveness of combined prevention efforts. Feasible methods, based on case reporting and periodic surveys, can identify areas where STI control is poor and provide outcome data needed to monitor programme performance. Surveillance should be based on routine STI case reporting, supplemented with special surveys of STI and HIV prevalence, assessment of STI syndrome etiologies, antimicrobial resistance monitoring and risk behaviour prevalence. It is also important to monitor coverage of STI services, particularly for priority population groups.
STI surveillance is a recommended component of second-generation HIV surveillance. Trends of short-duration STIs are more sensitive indicators of high-risk sexual activity than those based on HIV prevalence and can be monitored widely, even in underserved areas where STI control is often poor. Yet few countries maintain systems to collect and use such data.27
Empirical evidence
STI control outcomes
STI control has been shown to be feasible in a wide range of countries at different levels of development. STI trends have been on the decline since the early twentieth century in many developed countries and increasingly in resource-constrained countries. For example, China, Cuba and Sri Lanka documented large reductions of STIs in the 1950s and 1960s. STI control is relative and dynamic, however, and sensitive to social and economic change. STI rates surged in western Europe and North America following introduction of hormonal contraception and in China following economic liberalization.9,28,29
More recently, several countries in Asia have documented large reductions in common STIs. Thailand measured a 95% drop in common curable STIs during the 1990s following introduction of the 100% condom use programme implemented by STI clinic staff working with sex work establishments. Chancroid was quickly eliminated, congenital syphilis has become rare and maternal syphilis is stable at about two cases per 1000 pregnant women.30 Cambodia measured large decreases in both ulcerative and non-ulcerative STIs over five years following a similar intervention.22,31
In Africa, STI control efforts have been relatively neglected and STI surveillance is generally inadequate to reliably depict trends. However, several exceptions, where STI control programmes and surveillance have been maintained, provide examples of the feasibility of improving STI control even where resources are limited. Senegal, where sex work is decriminalized and STI services are accessible to sex workers, has reported moderately low and stable STI prevalence.32,33 In Nairobi, Kenya, targeted interventions with sex workers and improved STI case management in health centres preceded declines in ulcerative and other STIs and local disappearance of chancroid.34,35
The scale of decline in STI rates in these examples is noteworthy. Declines of 90% or more in incidence of one or more common curable STIs have been documented in China, Kenya (Nairobi), Sri Lanka and Thailand with rapid elimination of chancroid and congenital syphilis in most settings.28,30,35 In comparison, intervention trials evaluating STI treatment approaches or limited STI control interventions, have reported much lower STI reductions.36–38
HIV prevention outcomes
Evidence supporting the role of STIs as HIV cofactors is extensive and indisputable. Reducing STI prevalence removes cofactors, making HIV transmission less efficient. Countries with poor STI control have been most vulnerable to HIV epidemics, while improvements in STI control parallel or precede declines in HIV incidence and prevalence. There is growing evidence that countries that control STIs are more likely to halt and reverse their HIV epidemics than those that do not. This observation builds on an extensive body of observational and historical evidence which documented: (i) strong associations between several individual STIs and HIV; and (ii) susceptibility of countries with poor STI control to developing sizable HIV epidemics.1
More recently, several countries have reported large changes in STI incidence and/or prevalence. Cambodia, Kenya (data from Nairobi) and Thailand show HIV epidemics stabilizing and reversing in both high-risk and general population groups coincident with or following STI reductions. Sri Lanka had established good STI control before the introduction of HIV and its HIV epidemic has remained low-level for more than two decades. Despite a long-standing civil war and an uncircumcised male population, there is little evidence of transmission within the population and most cases of HIV are found among migrant labourers returning from abroad. The experience of China illustrates how deteriorating STI control since economic liberalization in the 1980s preceded a rapidly increasing HIV epidemic.28,29
Implications for programmes
What are the implications for STI control and HIV prevention programmes? If broader STI control is important for HIV prevention, how can this be achieved? What are the conditions where investment in STI control is most likely to contribute to slowing HIV epidemics? Table 1 follows standard epidemiological parameters of disease control to address these questions.
Table 1. Epidemiologic parameters for control of STIs including HIV.
| Focus | Methods | Notes | |||
|---|---|---|---|---|---|
| Who? | High coverage of “core” populations of sex workers and men who have sex with men is the first priority. Drug users, also often at high risk through sexual transmission, should also be targeted. | Targeted interventions linked to outreach and clinical services. Several countries have committed to scaling-up targeted interventions to reach saturation coverage of these populations.39,40 | Targeting is highly efficient. Population-level impact is feasible with interventions directed to core populations who generally comprise less than 5% of the sexually active population. | ||
| Male bridge populations. Efforts should also be made to reach actual or likely clients of sex workers and other bridge populations who disseminate STIs from core networks to the general population. | STI clinic patients are men with recent exposure. Workplace interventions particularly in settings of migrant labour or mobility. Outreach, peer education and STI services in red light districts where transmission potential is high.40 | Bridge populations may account for 20% or more of the sexually active male population. Interventions likely to reach men at high probability of having STIs and/or acute HIV infection; many report recent sex worker contact. | |||
| STI patients and people living with HIV. A high proportion of STI clinic patients may have acute HIV infection | Provider initiated testing and counselling, STI screening, treatment and counselling for people living with HIV under care.16,41,42 | Strengthening STI services offers opportunities to treat STIs and offer risk reduction counselling and HIV testing. | |||
| What? | Curable ulcerative STIs. Control of curable genital ulcers is highly feasible. Control of these infections correlates well with stabilization of HIV.43–45 | Effective antibiotic treatment of chancroid and syphilis results in rapid cure. Combined with targeted prevention efforts, control or elimination is feasible. | Data and modelling have established that ulcerative STIs are the most important STI cofactors for HIV transmission.44 | ||
| Viral ulcerative STIs. HSV-2 being an incurable viral infection requires different control strategies. | Studies have demonstrated the feasibility of suppressing HSV-2 and reducing HSV-2 and HIV concentrations in genital secretions.46,47 | Ongoing research is exploring optimal regimens for HIV prevention. | |||
| Curable non-ulcerative STIs. Non-ulcerative STIs are prevalent and increase HIV transmission 2–4 times. | Effective antibiotic treatment of gonorrhoea or chlamydial infection reduces HIV viral load to normal levels.48 | Reductions in gonorrhoea and chlamydial infection have been reported in high and lower risk populations. | |||
| Where? | Effective targeting requires a 2-stage process: (i) identifying epidemiologic “hot-spots” where risk is present and/or transmission is believed to be taking place; and (ii) mapping of populations in those areas. | STI surveillance helps identify “hot-spots” in the first stage of mapping. STI case reports from sentinel STI clinics can be used to monitor trends of new male STIs at district levels. | Demonstrated in Thailand and Sri Lanka. Builds on historical experience with contact tracing and STI outbreak control in developed countries.49,50 | ||
| When? | STI control is most effective in preventing HIV transmission: (i) when STIs, particularly ulcers are poorly controlled; and (ii) early in HIV epidemics. | STI surveillance. STI case reports and STI prevalence surveys among high-risk populations can be used to assess impact and monitor trends. | Modelling has shown the potential contribution of STI control to HIV prevention at different phases of STI and HIV epidemics. | ||
HSV-2, herpes simplex virus type 2; STI, sexually transmitted infection.
Who?
STI control efforts should focus on core and bridge populations, symptomatic patients and persons living with HIV. High coverage of such key populations as sex workers and men who have sex with men is the first priority.39,40 Efforts should also be made to reach actual or likely clients of sex workers and other bridge populations who disseminate STIs from core networks to the general population. Clinics providing STI treatment are a good entry point to screen and identify persons living with HIV, and additional effort is required to screen persons living with HIV under care to ensure that any STIs are detected and treated.16,41,42
What?
Interventions that reduce STI prevalence will reduce the respective cofactor effect and blunt the efficiency of HIV transmission. Data and modelling have established that ulcerative STIs, particularly chancroid, herpes simplex virus type 2 and syphilis, are the most important STI cofactors for HIV transmission.43,44 Control of curable genital ulcers – chancroid and syphilis – is highly feasible and correlates well with stabilization of HIV epidemics.45
Where?
Identification of high-risk populations generally requires a two-stage process: (i) identification of epidemiologic “hot-spots” where risk is present and/or transmission is believed to be taking place; and (ii) mapping of populations in those areas. The importance of STI surveillance in this first stage of mapping has been demonstrated.49,50
When?
Modelling has helped in understanding the potential contribution of STI control to HIV prevention at different phases of STI and HIV epidemics. Two conclusions are apparent, that STI control is most effective in preventing HIV transmission: (i) when STIs, particularly ulcers, are poorly controlled; and (ii) early in HIV epidemics. It thus makes sense to strengthen STI control rapidly when prevalence is found to be high or increasing. However, monitoring STI trends requires reliable surveillance, which is lacking in many countries.
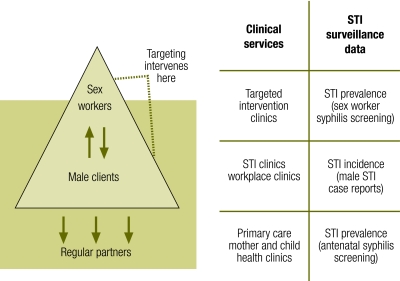
A combination of factors – led by unprotected sex work and ulcerative STIs – create ideal conditions, a “perfect storm”, for rapid spread of HIV epidemics. Such conditions are most likely to be found in settings with mobile populations and uncircumcised men. Successful STI control programmes have responded with a combination of interventions, guided by reliable mapping and surveillance, to disrupt those conditions for optimal STI control and HIV prevention outcomes (Fig. 2).
Fig. 2.
Intervention opportunities for STI and HIV transmission
STI, sexually transmitted infection.
Conclusion
In many countries, basic STI services are in disarray as programme resources are determined by decisions relating to a single disease entity. Such a fractured paradigm is as counterproductive for HIV as it is for other STIs. Major HIV epidemics emerged from and spread rapidly under conditions of poor STI control, and further weakening of STI control may well undermine other HIV prevention efforts. Yet experience of countries as diverse as Cambodia, Kenya, Senegal, Sri Lanka and Thailand demonstrate that wider STI control is feasible and that HIV prevention can be strengthened in doing so. ■
Footnotes
Competing interests: None declared.
References
- 1.Consultation on STI interventions for preventing HIV: appraisal of the evidence Geneva: World Health Organization/Joint United Nations Programme on HIV/AIDS; 2008. [Google Scholar]
- 2.Sexually transmitted infections are preventable and treatable, but the full benefit depends on the local context Washington, DC: Disease Control Priorities Project; 2007. Available from: http://www.dcp2.org/file/144/DCPP-STI.pdf [accessed on 18 September 2009].
- 3.Peeling RW, Mabey D, Fitzgerald DW, Watson-Jones D. Avoiding HIV and dying of syphilis. Lancet. 2004;364:1561–3. doi: 10.1016/S0140-6736(04)17327-3. [DOI] [PubMed] [Google Scholar]
- 4.Chanda E, Masaninga F, Coleman M, Sikaala C, Katebe C, MacDonald M, et al. Integrated vector management: the Zambian experience. Malar J. 2008;7:164. doi: 10.1186/1475-2875-7-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Practical approach to lung health Geneva: World Health Organization; 2008. Available from: http://www.who.int/tb/health_systems/pal/en/index.html [accessed on 18 September 2009].
- 6.Gray RH, Wawer MJ. Reassessing the hypothesis on STI control for HIV prevention. Lancet. 2008;371:2064–5. doi: 10.1016/S0140-6736(08)60896-X. [DOI] [PubMed] [Google Scholar]
- 7.White R, Celum C, Wasserheit J, Aral S, Hayes R. Control of sexually transmitted infections for HIV prevention. Lancet. 2008;372:1297–8. doi: 10.1016/S0140-6736(08)61541-X. [DOI] [PubMed] [Google Scholar]
- 8.Guidelines for second generation HIV surveillance Geneva: World Health Organization/Joint United Nations Programme on HIV/AIDS; 2000. [Google Scholar]
- 9.Brandt AM. No magic bullet: a social history of venereal disease in the United States since 1880 New York, NY: Oxford University Press; 1987. [Google Scholar]
- 10.Merson M, Padian N, Coates TJ, Gupta GR, Bertozzi SM, Piot P, et al. Combination HIV prevention. Lancet. 2008;372:1805–6. doi: 10.1016/S0140-6736(08)61752-3. [DOI] [PubMed] [Google Scholar]
- 11.Pisani E, Garnett GP, Grassly NC, Brown T, Stover J, Hankins C, et al. Back to basics in HIV prevention: focus on exposure. BMJ. 2003;326:1384–7. doi: 10.1136/bmj.326.7403.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallabetta G, Wi TEC, Nielsen G. Prevention and control of STD and HIV infection in developing countries. In: Holmes KK, Sparling P, Stamm W, Piot P, Wasserheit J, Corey L, et al., eds. Sexually transmitted diseases 4th edn. New York, NY: McGraw-Hill; 2008. [Google Scholar]
- 13.Vuylsteke B. Current status of syndromic management of sexually transmitted infections in developing countries. Sex Transm Infect. 2004;80:333–4. doi: 10.1136/sti.2004.009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathews C, Coetzee N, Zwarenstein M, Lombard C, Guttmacher S, Oxman A, et al. A systematic review of strategies for partner notification for sexually transmitted diseases, including HIV/AIDS. Int J STD AIDS. 2002;13:285–300. doi: 10.1258/0956462021925081. [DOI] [PubMed] [Google Scholar]
- 15.Periodic presumptive treatment for sexually transmitted infections: experience from the field and recommendations for research Geneva: World Health Organization; 2008. [Google Scholar]
- 16.Pilcher CD, Eron JJ, Jr, Galvin S, Gay C, Cohen MS. Acute HIV revisited: new opportunities for treatment and prevention. J Clin Invest. 2004;113:937–45. doi: 10.1172/JCI21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steen R, Dallabetta G. Sexually transmitted infection control with sex workers: regular screening and presumptive treatment augment efforts to reduce risk and vulnerability. Reprod Health Matters. 2003;11:74–90. doi: 10.1016/S0968-8080(03)02295-X. [DOI] [PubMed] [Google Scholar]
- 18.Scientific evidence on condom effectiveness for sexually transmitted diseases (STD) prevention. In: Workshop, National Institutes of Allergy and Infectious Diseases, Herdon, Virginia, 20July2001
- 19.Vijayakumar G, Mabude Z, Smit J, Beksinska M, Lurie M. A review of female-condom effectiveness: patterns of use and impact on protected sex acts and STI incidence. Int J STD AIDS. 2006;17:652–9. doi: 10.1258/095646206780071036. [DOI] [PubMed] [Google Scholar]
- 20.Telles Dias PR, Souto K, Page-Shafer K. Long-term female condom use among vulnerable populations in Brazil. AIDS Behav. 2006;10(Suppl):S67–75. doi: 10.1007/s10461-006-9139-x. [DOI] [PubMed] [Google Scholar]
- 21.Eloundou-Enyegue PM, Meekers D, Calves AE. From awareness to adoption: the effect of AIDS education and condom social marketing on condom use in Tanzania (1993-1996). J Biosoc Sci. 2005;37:257–68. doi: 10.1017/S0021932004007011. [DOI] [PubMed] [Google Scholar]
- 22.Rojanapithayakorn W. The 100% condom use programme in Asia. Reprod Health Matters. 2006;14:41–52. doi: 10.1016/S0968-8080(06)28270-3. [DOI] [PubMed] [Google Scholar]
- 23.Blanchard JF, Bhattacharjee P, Kumaran S, Ramesh BM, Kumar NS, Washington RG, et al. Concepts and strategies for scaling up focused prevention for sex workers in India. Sex Transm Infect. 2008;84(Suppl 2):ii19–23. doi: 10.1136/sti.2008.033134. [DOI] [PubMed] [Google Scholar]
- 24.Ngugi EN, Wilson D, Sebstad J, Plummer FA, Moses S. Focused peer-mediated educational programs among female sex workers to reduce sexually transmitted disease and human immunodeficiency virus transmission in Kenya and Zimbabwe. J Infect Dis. 1996;174(Suppl 2):S240–7. doi: 10.1093/infdis/174.supplement_2.s240. [DOI] [PubMed] [Google Scholar]
- 25.Jana S, Basu I, Rotheram-Borus MJ, Newman PA. The Sonagachi Project: a sustainable community intervention program. AIDS Educ Prev. 2004;16:405–14. doi: 10.1521/aeap.16.5.405.48734. [DOI] [PubMed] [Google Scholar]
- 26.Reza-Paul S, Beattie T, Rahman Syed HU, Venukumar KT, Venugopal MS, Fathima MP, et al. Declines in risk behaviour and sexually transmitted infection prevalence following a community-led HIV preventive intervention among female sex workers in Mysore, India. AIDS. 2008;22(suppl 5):S91–100. doi: 10.1097/01.aids.0000343767.08197.18. [DOI] [PubMed] [Google Scholar]
- 27.Rehle T, Lazzari S, Dallabetta G, Asamoah-Odei E. Second-generation HIV surveillance: better data for decision-making. Bull World Health Organ. 2004;82:121–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen MS, Henderson GE, Aiello P, Zheng H. Successful eradication of sexually transmitted diseases in the People’s Republic of China: implications for the 21st century. J Infect Dis. 1996;174(Suppl 2):S223–9. doi: 10.1093/infdis/174.supplement_2.s223. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Wang N, Jia M, Wang G, Ding G, Jia M, et al. Sexually transmitted infections among female sex workers in Kaiyuan city, Yunnan province, China: potential for HIV transmission. Sex Transm Infect. 2009;85:290–5. doi: 10.1136/sti.2008.033100. [DOI] [PubMed] [Google Scholar]
- 30.Chitwarakorn A. HIV/AIDS and sexually-transmitted infections in Thailand: Lessons learned and challenges ahead. In: Narain JP, ed. AIDS in Asia: the challenge ahead Delhi: Sage Publications India; 2004. [Google Scholar]
- 31.Leng HB, Wantha SS, Saidel T, Sun LP, Sopheap S, Natpratan C, et al. Success of Cambodian HIV prevention efforts confirmed by low prevalence of sexually transmitted infections and declining HIV and risk behaviors. In: Abstracts, XIV International Conference on AIDS, Barcelona, Spain, 6-10July2002 [Google Scholar]
- 32.Meda N, Ndoye I, M’Boup S, Wade A, Ndiayee S, Niang C, et al. Low and stable HIV infection rates in Senegal: natural course of the epidemic or evidence for success of prevention? AIDS. 1999;13:1397–405. doi: 10.1097/00002030-199907300-00018. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Hawes SE, Gaye A, Sow PS, Ndoye I, Manhart LE, et al. HIV prevalence, previous HIV testing and condom use with clients and regular partners among Senegalese commercial sex workers. Sex Transm Infect. 2007;83:534–40. doi: 10.1136/sti.2007.027151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moses S, Ngugi EN, Costigan A, Kariuki C, Maclean I, Brunham RC, et al. Response of a sexually transmitted infection epidemic to a treatment and prevention programme in Nairobi, Kenya. Sex Transm Infect. 2002;78(suppl 1):i114–20. doi: 10.1136/sti.78.suppl_1.i114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steen R, Dallabetta G, Neilsen G. Antibiotic chemoprophylaxis and HIV infection in Kenyan sex workers. JAMA. 2004;292:921. doi: 10.1001/jama.292.8.921-a. [DOI] [PubMed] [Google Scholar]
- 36.Grosskurth H, Mosha F, Todd J, Mwijarubi E, Klokke A, Senkoro K, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346:530–6. doi: 10.1016/S0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 37.Wawer MJ, Sewankambo NK, Serwadda D, Quinn TC, Paxton LA, Kiwanuka N, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Lancet. 1999;353:525–35. doi: 10.1016/S0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 38.Kamali A, Quigley M, Nakiyingi J, Kinsman J, Kengeya-Kayondo J, Gopal R, et al. Syndromic management of sexually-transmitted infections and behaviour change interventions on transmission of HIV-1 in rural Uganda: a community randomised trial. Lancet. 2003;361:645–52. doi: 10.1016/S0140-6736(03)12598-6. [DOI] [PubMed] [Google Scholar]
- 39.Steen R, Mogasale V, Wi T, Singh AK, Das A, Daly C, et al. Pursuing scale and quality in STI interventions with sex workers: initial results from Avahan India AIDS Initiative. Sex Transm Infect. 2006;82:381–5. doi: 10.1136/sti.2006.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pisani E, Girault P, Gultom M, Sukartini N, Kumalawati J, Jazan S, et al. HIV, syphilis infection, and sexual practices among transgenders, male sex workers and other men who have sex with men in Jakarta, Indonesia. Sex Transm Infect. 2004;80:536–40. doi: 10.1136/sti.2003.007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilcher CD, Price MA, Hoffman IF, Galvin S, Martinson FE, Kazembe PN, et al. Frequent detection of acute primary HIV infection in men in Malawi. AIDS. 2004;18:517–24. doi: 10.1097/00002030-200402200-00019. [DOI] [PubMed] [Google Scholar]
- 42.Rothenberg RB, Wasserheit JN, St Louis ME, Douglas JM. The effect of treating sexually transmitted diseases on the transmission of HIV in dually infected persons: a clinic-based estimate. Sex Transm Dis. 2000;27:411–6. doi: 10.1097/00007435-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Korenromp EL, White RG, Orroth KK, Bakker R, Kamali A, Serwadda D, et al. Determinants of the impact of sexually transmitted infection treatment on prevention of HIV infection: a synthesis of evidence from the Mwanza, Rakai, and Masaka intervention trials. J Infect Dis. 2005;191(Suppl 1):S168–78. doi: 10.1086/425274. [DOI] [PubMed] [Google Scholar]
- 44.Orroth KK, White RG, Korenromp EL, Bakker R, Changalucha J, Habbema JDF, et al. Empirical observations underestimate the proportion of human immunodeficiency virus infections attributable to sexually transmitted diseases in the Mwanza and Rakai sexually transmitted disease treatment trials: simulation results. Sex Transm Dis. 2006;33:536–44. doi: 10.1097/01.olq.0000204667.11192.71. [DOI] [PubMed] [Google Scholar]
- 45.Steen R. Eradicating chancroid. Bull World Health Organ. 2001;79:818–26. [PMC free article] [PubMed] [Google Scholar]
- 46.Nagot N, Abdoulaye O, Foulongne V, Konaté I, Weiss HA, Vergne L, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 47.Celum CL, Robinson NJ, Cohen MS. Potential effect of HIV type 1 antiretroviral and herpes simplex virus type 2 antiviral therapy on transmission and acquisition of HIV type 1 infection. J Infect Dis. 2005;191(Suppl 1):S107–14. doi: 10.1086/425272. [DOI] [PubMed] [Google Scholar]
- 48.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–73. doi: 10.1016/S0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 49.Rojanapithayakorn W, Hanenberg R. The 100% condom program in Thailand. AIDS. 1996;10:1–7. doi: 10.1097/00002030-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Jessamine PG, Brunham RC. Rapid control of a chancroid outbreak: implications for Canada. Can Med Assoc J. 1990;142:1081–5. [PMC free article] [PubMed] [Google Scholar]