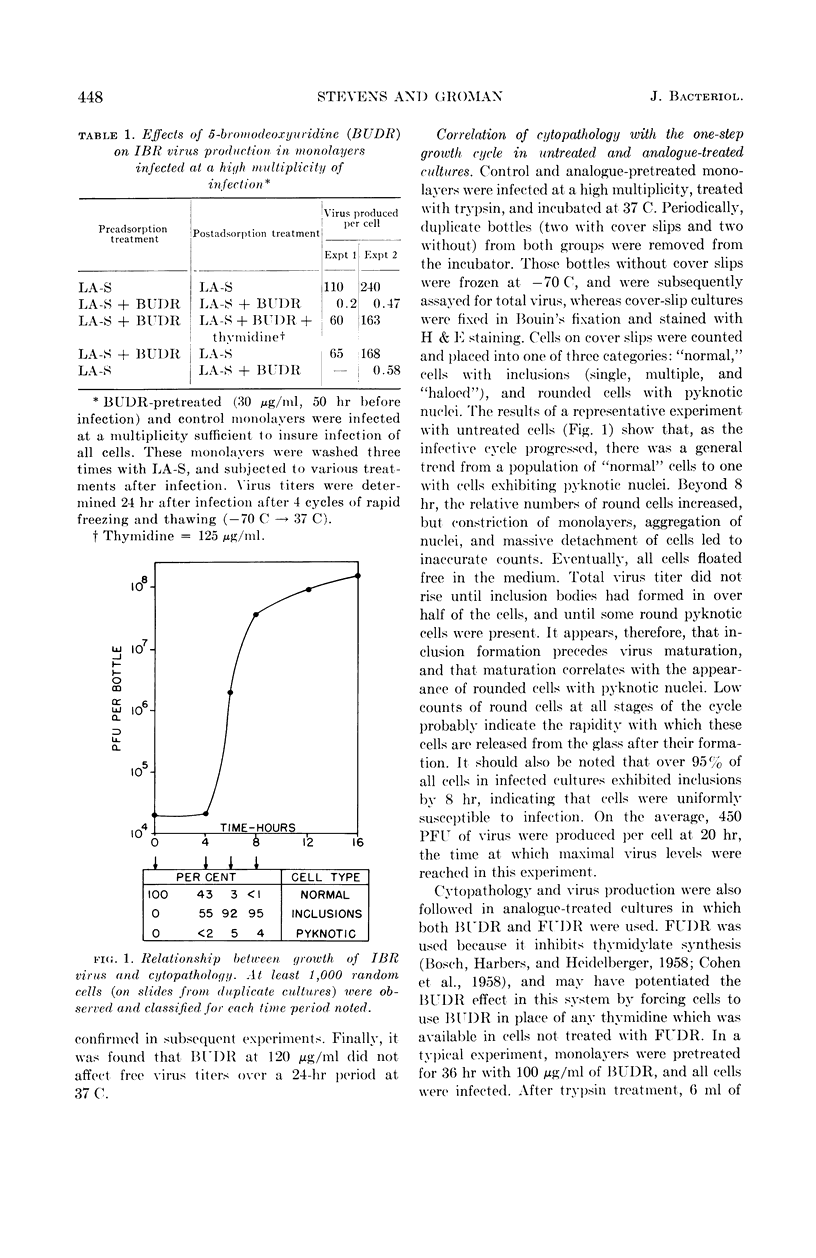
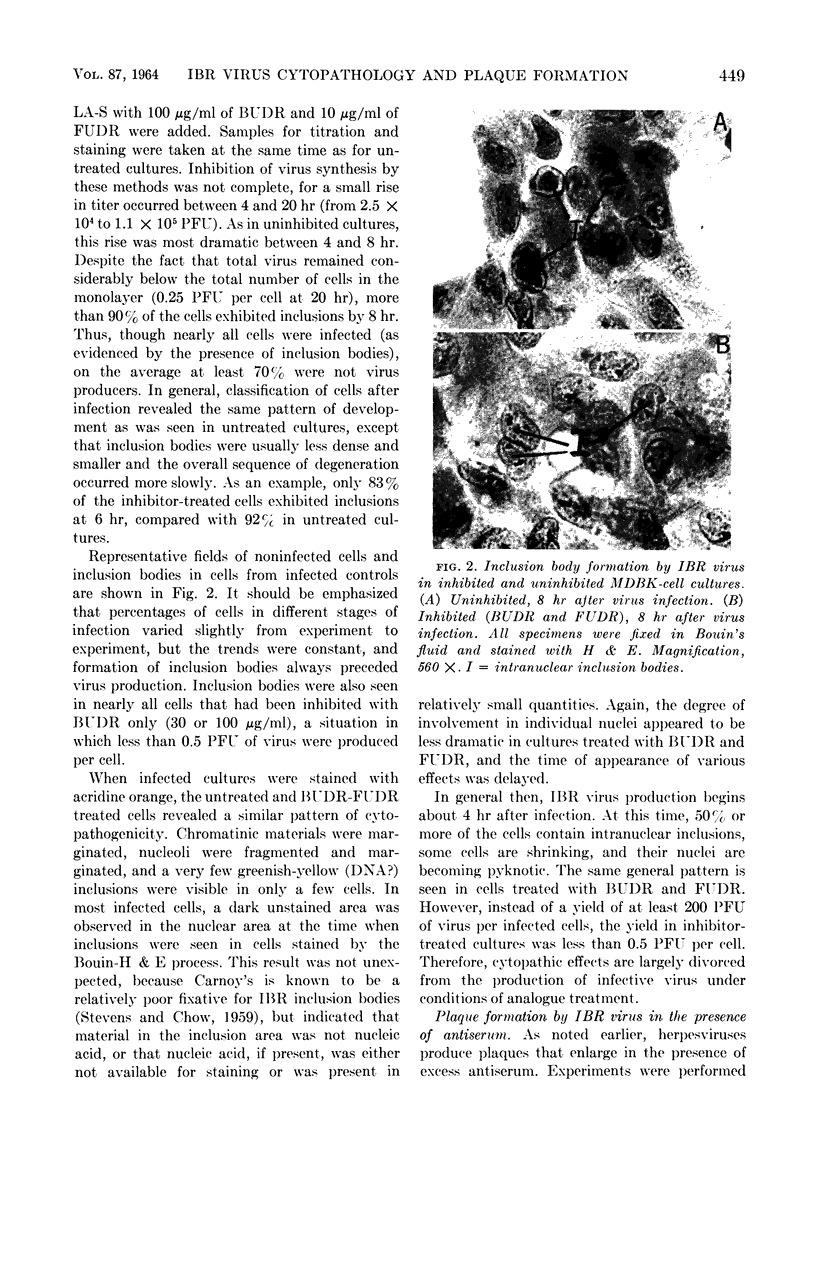
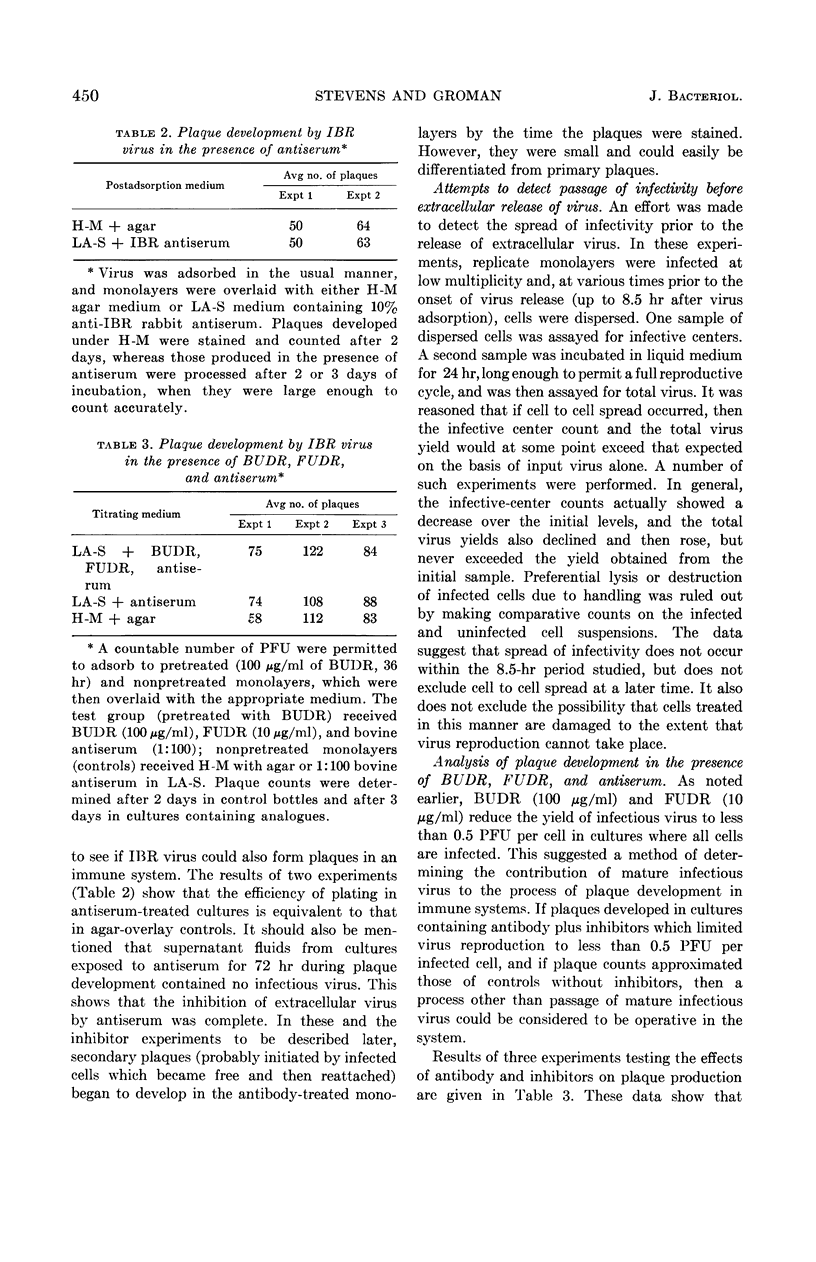
Abstract
Stevens, Jack G. (University of Washington, Seattle), and Neal B. Groman. Infectious bovine rhinotracheitis virus replication, cytopathology, and plaque formation in the presence and absence of nucleic acid analogues. J. Bacteriol. 87:446–453. 1964.—Cytopathology induced by infectious bovine rhinotracheitis (IBR) virus was correlated with the one-step growth cycle. Nuclear alterations, including the development of inclusion bodies, preceded the appearance of virus. It was found that similar effects occurred in the presence of 5-bromodeoxyuridine (BUDR) and 5-fluorodeoxyuridine (FUDR), compounds which depress the yield of “standard” virus from a range of 116 to 500 to less than 0.5 plaque-forming units per cell. As with known members of the herpesvirus group, IBR virus plaques developed and enlarged indefinitely in the presence of specific antibody. An analysis of the mechanism operative in this process was undertaken. The evidence suggested that neither viral nor subviral particles capable of replicating “standard” virus passed between cells during the first 8 hr of infection. This is the time preceding the release of extracellular virus from initially infected cells. With BUDR and FUDR, it was shown that plaques also developed in this system in the virtual absence of production of “standard” infectious virus. However, a class of analogue-dependent virus was found which may have been at least partly responsible for plaque formation in the analogue-treated system. The relative contributions of subviral particles or of a self-sustaining molecular disorganization to the process have not been completely assessed as yet.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. A., PEREIRA H. G., ANDREWES C. H. Observations on the virus of infectious bovine rhinotracheitis, and its affinity with the Herpesvirus group. Virology. 1961 Jun;14:276–285. doi: 10.1016/0042-6822(61)90204-5. [DOI] [PubMed] [Google Scholar]
- BEN-PORAT T., KAPLAN A. S. The chemical composition of herpes simplex and pseudorabies viruses. Virology. 1962 Mar;16:261–266. doi: 10.1016/0042-6822(62)90246-5. [DOI] [PubMed] [Google Scholar]
- BOSCH L., HARBERS E., HEIDELBERGER C. Studies on fluorinated pyrimidines. V. Effects on nucleic acid metabolism in vitro. Cancer Res. 1958 Apr;18(3):335–343. [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRESSER I., ENDERS J. F. The effect of trypsin on representative myxoviruses. Virology. 1961 Apr;13:420–426. doi: 10.1016/0042-6822(61)90273-2. [DOI] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. Improved method for staining cell monolayers for virus plaque counts. J Bacteriol. 1959 Oct;78:596–597. doi: 10.1128/jb.78.4.596-597.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOH P. C. Effects of amethopterin on cell growth and viral synthesis. Proc Soc Exp Biol Med. 1960 Nov;105:296–300. doi: 10.3181/00379727-105-26089. [DOI] [PubMed] [Google Scholar]
- MADIN S. H., DARBY N. B., Jr Established kidney cell lines of normal adult bovine and ovine origin. Proc Soc Exp Biol Med. 1958 Jul;98(3):574–576. doi: 10.3181/00379727-98-24111. [DOI] [PubMed] [Google Scholar]
- MAYOR H. D., DIWAN A. R. Studies on the acridine orange staining of two purified RNA viruses: poliovirus and tobacco mosaic virus. Virology. 1961 May;14:74–82. doi: 10.1016/0042-6822(61)90134-9. [DOI] [PubMed] [Google Scholar]
- ORSI E. V., CABASSO V. J. Infectious bovine rhinotracheitis (IBR). IV. Cytological changes in infected bovine kidney and HeLa cultures. Proc Soc Exp Biol Med. 1958 Jul;98(3):637–639. doi: 10.3181/00379727-98-24133. [DOI] [PubMed] [Google Scholar]
- REISSIG M., KAPLAN A. S. The morphology of noninfective pseudorabies virus produced by cells treated with 5-fluorouracil. Virology. 1962 Jan;16:1–8. doi: 10.1016/0042-6822(62)90196-4. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B. Polykaryocytosis. Cold Spring Harb Symp Quant Biol. 1962;27:327–342. doi: 10.1101/sqb.1962.027.001.031. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P. The rate of formation of vaccinia deoxyribonucleic acid and vaccinia virus. Virology. 1960 Jan;10:150–152. doi: 10.1016/0042-6822(60)90015-5. [DOI] [PubMed] [Google Scholar]
- STEVENS J. G., CHOW T. L. Effects of some fixatives on inclusion bodies of infectious bovine rhinotracheitis. Proc Soc Exp Biol Med. 1959 Apr;100(4):856–859. doi: 10.3181/00379727-100-24803. [DOI] [PubMed] [Google Scholar]
- STEVENS J. G., GROMAN N. B. A nucleic acid analogue dependent animal virus. Biochem Biophys Res Commun. 1963 Jan 18;10:63–66. doi: 10.1016/0006-291x(63)90269-9. [DOI] [PubMed] [Google Scholar]
- STEVENS J. G., GROMAN N. B. PROPERTIES OF INFECTIOUS BOVINE RHINOTRACHEITIS VIRUS IN A QUANTITATED VIRUS-CELL CULTURE SYSTEM. Am J Vet Res. 1963 Nov;24:1158–1163. [PubMed] [Google Scholar]