Abstract
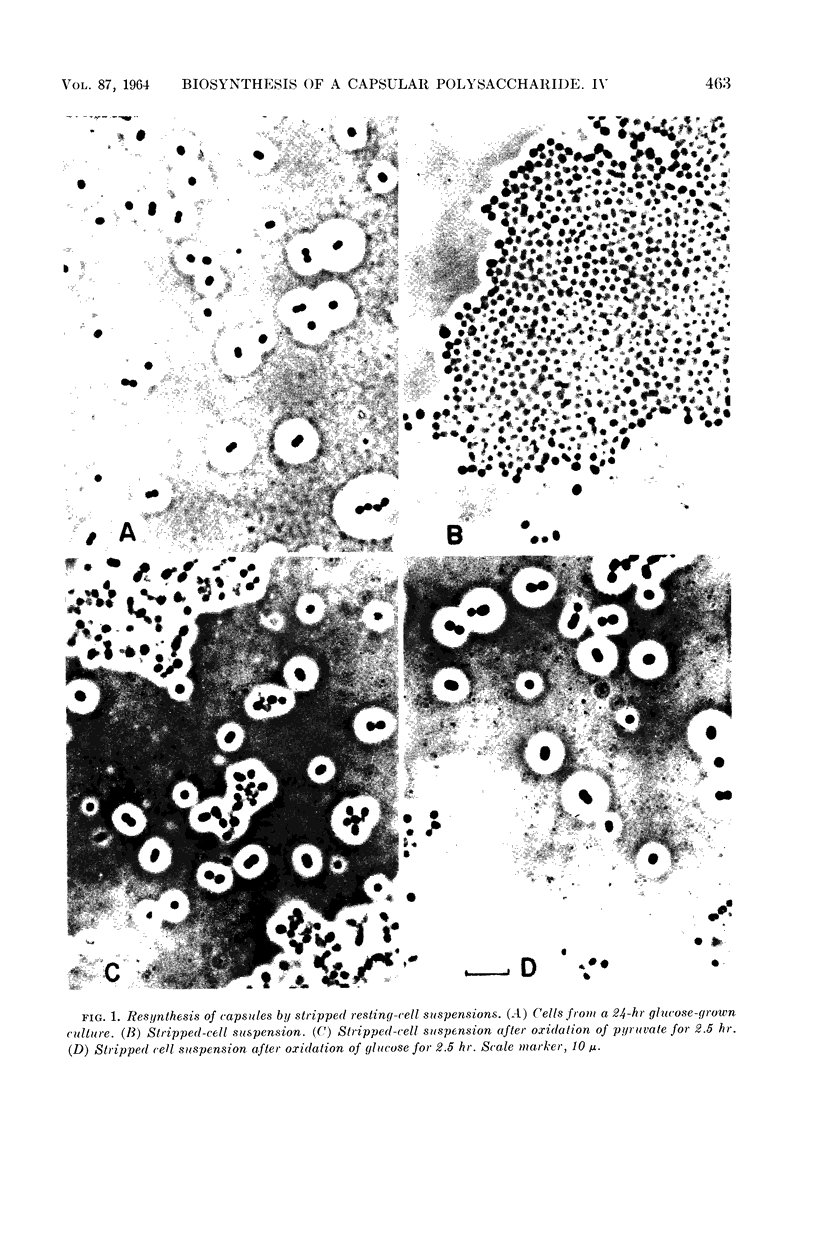
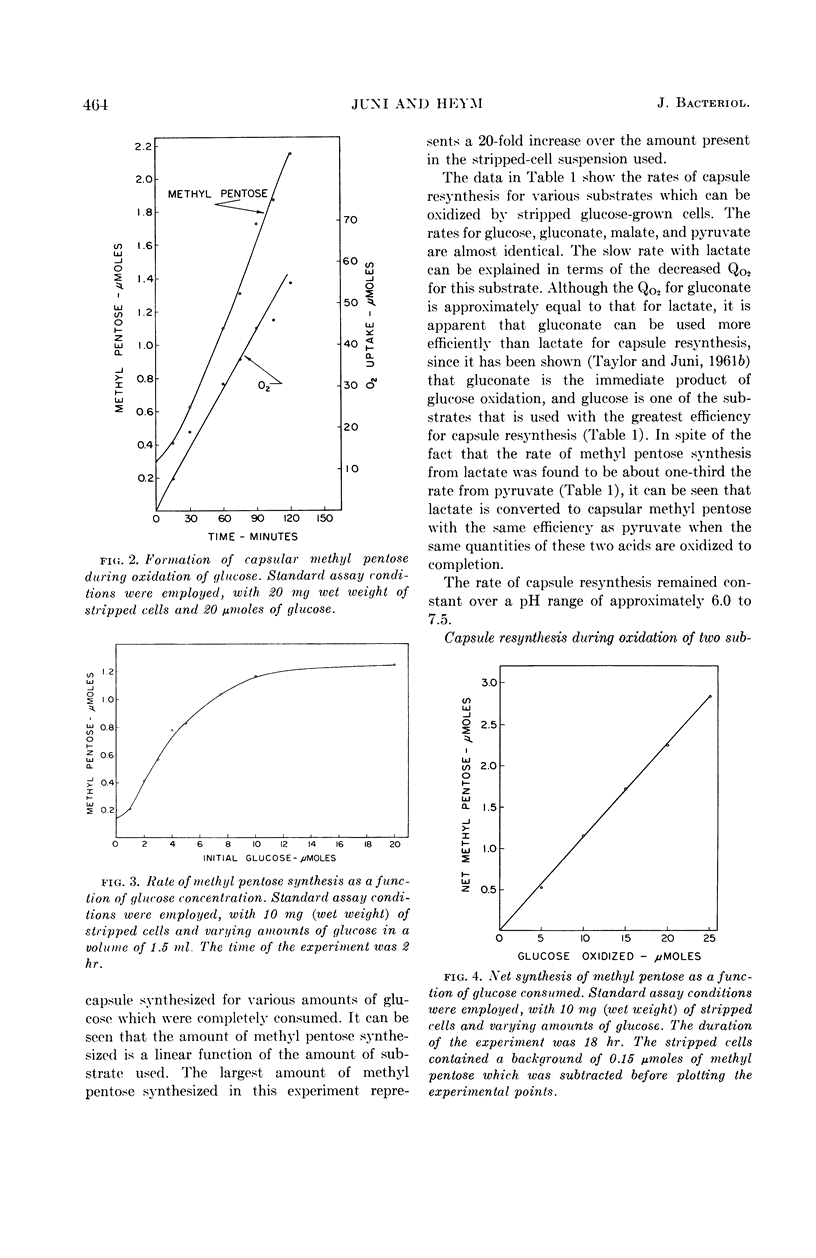
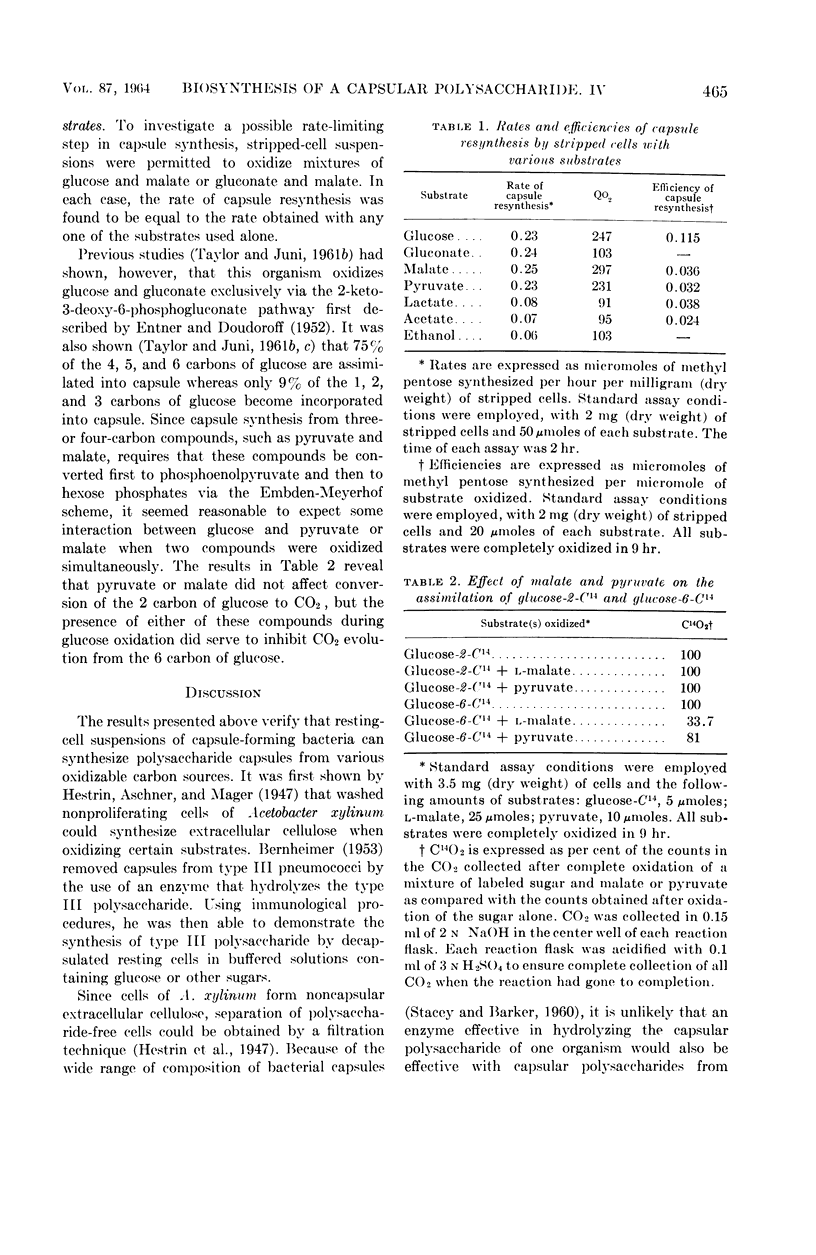
Juni, Elliot (Emory University, Atlanta, Ga.), and Gloria A. Heym. Pathways for biosynthesis of a bacterial capsular polysaccharide. IV. Capsule resynthesis by decapsulated resting-cell suspensions. J. Bacteriol. 87:461–467. 1964.—Methods were devised for stripping capsules from encapsulated bacteria. By use of stripped resting-cell suspensions of a gram-negative capsule-forming coccus, it was shown that polysaccharide capsule resynthesis depends upon the presence of air and an oxidizable substrate. Capsule resynthesis proceeds linearly with time. For a given quantity of stripped cells, the net amount of polysaccharide capsule synthesized is a linear function of the amount of substrate oxidized. The only factor that appears to limit the extent of capsule synthesis by resting cells is the amount of substrate utilized. A series of photomicrographs of wet mounts made in India ink show the appearance of stripped cells and resynthesized capsules formed during oxidation of pyruvate and glucose by stripped resting-cell suspensions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZIMAN M., BURGER-RACHAMIMOV H. Synthesis of cellulose from pyruvate by succinate-grown cells of Acetobacter xylinum. J Bacteriol. 1962 Oct;84:625–630. doi: 10.1128/jb.84.4.625-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHEIMER A. W. Synthesis of type III pneumococcal polysaccharide by suspensions of resting cells. J Exp Med. 1953 May;97(5):591–600. doi: 10.1084/jem.97.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGUID J. P. The demonstration of bacterial capsules and slime. J Pathol Bacteriol. 1951 Oct;63(4):673–685. doi: 10.1002/path.1700630413. [DOI] [PubMed] [Google Scholar]
- ENTNER N., DOUDOROFF M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952 May;196(2):853–862. [PubMed] [Google Scholar]
- GROMET-ELHANAN Z., HESTRIN S. Synthesis of cellulose by Acetobacter xylinum. VI. Growth on citric acid-cycle intermediates. J Bacteriol. 1963 Feb;85:284–292. doi: 10.1128/jb.85.2.284-292.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., KREBS H. A. Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle. Nature. 1957 May 18;179(4568):988–991. doi: 10.1038/179988a0. [DOI] [PubMed] [Google Scholar]
- SCHRAMM M., GROMET Z., HESTRIN S. Synthesis of cellulose by Acetobacter Xylinum. 3. Substrates and inhibitors. Biochem J. 1957 Dec;67(4):669–679. doi: 10.1042/bj0670669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., JUNI E. Pathways for biosynthesis of a bacterial capsular polysaccharide. I. Carbohydrate metabolism and terminal oxidation mechanisms of a capsuleproducing coccus. J Bacteriol. 1961 May;81:694–703. doi: 10.1128/jb.81.5.694-703.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., JUNI E. Pathways for biosynthesis of a bacterial capsular polysaccharide. I. Characterization of the organism and polysaccharide. J Bacteriol. 1961 May;81:688–693. doi: 10.1128/jb.81.5.688-693.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., JUNI E. Pathways for biosynthesis of a bacterial capsular polysaccharide. III. Syntheses from radioactive substrates. J Biol Chem. 1961 May;236:1231–1234. [PubMed] [Google Scholar]



