Abstract
This report provides a perspective on metabolic glycoengineering methodology developed over the past two decades that allows natural sialic acids to be replaced with chemical variants in living cells and animals. Examples are given demonstrating how this technology provides the glycoscientist with chemical tools that are beginning to reproduce Mother Nature's control over complex biological systems – such as the human brain – through subtle modifications in sialic acid chemistry. Several metabolic substrates (e.g., ManNAc, Neu5Ac, and CMP-Neu5Ac analogs) can be used to feed flux into the sialic acid biosynthetic pathway resulting in numerous – and sometime quite unexpected – biological repercussions upon nonnatural sialoside display in cellular glycans. Once on the cell surface, ketone-, azide-, thiol-, or alkyne-modified glycans can be transformed with numerous ligands via bioorthogonal chemoselective ligation reactions, greatly increasing the versatility and potential application of this technology. Recently, sialic acid glycoengineering methodology has been extended to other pathways with analog incorporation now possible in surface-displayed GalNAc and fucose residues as well as nucleocytoplasmic O-GlcNAc-modified proteins. Finally, recent efforts to increase the “druggability” of sugar analogs used in metabolic glycoengineering, which have resulted in unanticipated “scaffold-dependent” activities, are summarized.
Keywords: carbohydrate-based drugs, glycosylation, metabolic glycoengineering, sialic acid
Overview and introduction
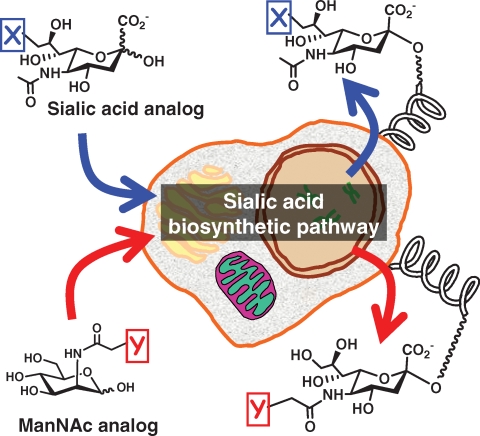
Metabolic glycoengineering has become the premier tactic for incorporating nonnatural “building-blocks” into the cellular architecture (Campbell et al. 2007; Aich and Yarema 2008). In this technique, a synthetic monosaccharide similar in structure to a natural precursor in a biosynthesis pathway for a cell-surface glycan, but bearing an unnatural chemical group, is incubated with cells or introduced into live animals (Kayser et al. 1992a). The modified monosaccharide enters a cell and is processed by the biosynthetic enzymes in a manner similar to the natural precursor, and the resulting cell-surface glycan bears the unnatural functional group (Figure 1). The sialic acid pathway pioneered – and subsequently has served as the workhorse – for metabolic glycoengineering because of its remarkable tolerance for both neuraminic acid (Oetke et al. 2001, 2002) and mannosamine (Kayser et al. 1992b; Keppler et al. 2001) precursors bearing nonnatural chemical substituents.
Fig. 1.
Metabolic sialic acid glycoengineering. A schematic view of chemically modified sialic acid (top) or ManNAc (bottom) analogs that intercept the sialic acid biosynthetic pathway of a mammalian cell and appear on the cell surface as the corresponding “X” or “Y” modified sialosides.
In theory, metabolic glycoengineering is a very straightforward technique. The sugar analogs can simply be added to the culture medium of a cell and the glycosylation machinery—exemplified by the sialic acid biosynthetic pathway (Figure 1)—functions as a “black box” that performs the synthetically challenging task of creating modified surface glycans. In practice, sialic acid glycoengineering gains incredible complexity by impinging on the underlying biology of this fascinating sugar. To introduce “sialobiology,” a brief overview of this topic is given in the next section of this article with an emphasis on complications (e.g., serious human disease) that arise from natural aberrations in sialic acid metabolism—clearly, the metabolic glycoengineer should keep these in mind to avoid doing harm. The chemical complexity of sialic acid, which has long been exploited by nature to modulate cellular functions in ways that glycoscientists are increasingly replicating and extending, is then described followed by a discussion of a significant advance in metabolic glycoengineering realized when chemical function groups appropriate for bioorthogonal chemistries were incorporated into monosaccharide analog design. This article concludes by describing how, in recent years, metabolic glycoengineering has been extended from sialic acid to additional glycosylation pathways thereby greatly increasing the versatility of this technology and in concert, analogs have been designed for greater efficiency, which has led to unexpected new activities.
An introduction to sialic acid
History
Terminology used by glycobiologists in general, and to describe sialic acids in particular, can be confusing to the nonspecialist; for example, these sugars are often referred to by a second name: neuraminic acids. The reason why there are two names in common – and often interchangeable – usage is historical in nature; the story begins in the mid 1930s when this sugar was isolated from submaxillary mucin by Gunnar Blix (Blix 1936) leading to the name “sialic acid” for this saliva-derived acidic compound. Shortly thereafter, by the early 1940s, Ernst Klenk independently isolated acidic glycosphingolipids (GSLs (Klenk 1941)), which were named gangliosides because they were abundant in brain gray matter and ganglial cells. Klenk identified sphingosine, fatty acid, hexoses – as well as a substance that gave a purple color with Bial's reagent that he called neuraminic acid – as constituents of the brain-derived glycolipids. Klenk's neuraminic acid was later found to be the same compound isolated from saliva by Blix and the nomenclature was clarified in 1957 (Blix et al. 1957) with the name “sialic acid” now collectively referring to a family of over 50 naturally occurring sugars (Angata and Varki 2002) as well as a growing number of synthetic sugars achieved through metabolic substrate-based sialic acid engineering methods (Keppler et al. 2001; Yarema 2001). Individual sugars, however, largely have retained neural-based nomenclature; for instance, the most common form of sialic acid in humans is N-acetylneuraminic acid (Neu5Ac) whose chemical structure was reported half a century ago by Saul Roseman's Laboratory (Comb and Roseman 1960).
Sialic acid bioprocessing – A complex, energetically expensive, and dangerous endeavor
Biosynthesis and Recycling
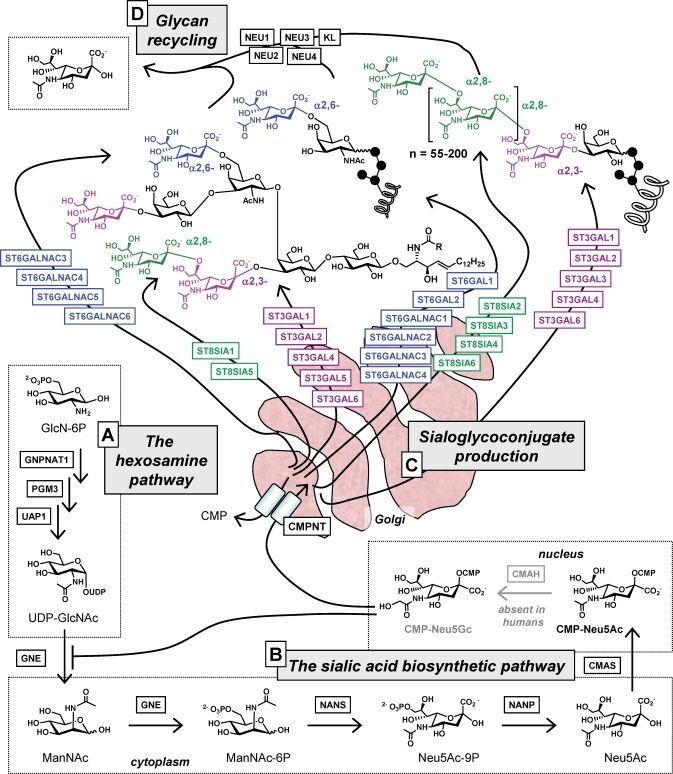
In the past two decades, the biosynthetic machinery for sialic acid in mammalian cells has been unraveled in considerable detail (Figure 2) and, in common with all facets of glycosylation, found to be complex and energetically expensive. To begin, sialic acid biosynthesis in mammalian cells is supported by flux through the hexosamine pathway, which produces UDP-GlcNAc (Figure 2A) (Hanover 2001). A relatively minor proportion of the millimolar levels of this cytosolic nucleotide sugar (Lau et al. 2007) is converted to ManNAc (Figure 2B), a dedicated precursor for sialic acid biosynthesis with no other roles in a cell (except in specialized situations when it can be epimerized back to GlcNAc (Luchansky et al. 2003)). Considering the other important metabolic fates for UDP-GlcNAc – this versatile intermediate is also used for O-GlcNAc protein modification, employed for glycoconjugate biosynthesis, or converted to UDP-GalNAc and used for the same purpose – it is not surprising that ManNAc production is kept under tight control. This regulation occurs through allosteric feedback inhibition of GNE, the bifunctional enzyme that controls flux into the sialic acid pathway (Keppler et al. 1999) through binding of the down-stream metabolite CMP-Neu5Ac (Seppala et al. 1991, 1999; Yarema et al. 2001). The pathway then moves from the cytosol into the nucleus where CMP-Neu5Ac is made and then into the lumen of the Golgi via CMP-Neu5Ac transporter (CMPNT, an anti-port transporter (Eckhardt et al. 1996)) where the activated nucleotide sugar is used to install α2,3-, α2,6-, or α2,8-linked sialosides into glycosphingolipids or glycoproteins by a suite of (in humans) 20 different sialyltransferases (Figure 2C). Finally, glycoconjugate-displayed sialic acids are hydrolyzed by one of several mammalian neuraminidases during glycan recycling (Figure 2D); the liberated sialic acids can be salvaged and reused by the cells.
Fig. 2.
Overview of mammalian sialic acid metabolism. (A) The hexosamine pathway converts glucosamine-6-phosphate (GlcN-6P) into the nucleotide sugar UDP-GlcNAc, which in turn is converted into ManNAc by GNE (pathway details are provided elsewhere (Hanover 2001)). (B) ManNAc is the dedicated metabolic precursor for the sialic acid biosynthetic pathway that produces Neu5Ac in the cytosol and then enters the nucleus where CMP-Neu5Ac is synthesized (in most nonhuman species, a proportion of CMP-Neu5Ac is converted to CMP-Neu5Gc). (C) These two nucleotide sugars are transported into lumen of the Golgi where they are used by several sialyltransferases (up to 20 in human cells, dependent on cell- and tissue-dependent expression patterns) to produce α2,3-, α2,6-, or α2,8-linked sialoglycoproteins or gangliosides. (D) Finally, sialosides are recycled by one of four neuraminidases, thereby regenerating sialic acid monomers that can be re-used by a cell. The full names of enzymes are given in Table S1 available online in the Supplementary Data.
Sialobiology
In addition to being energetically expensive (involving not the usual one, but rather two nucleotide sugars), sialoglycoconjugate biosynthesis is – as the disease-related abnormalities mentioned throughout this article testify – fraught with danger when biochemical events go awry. Moreover, even when nothing goes amiss with the host's metabolism, numerous pathogens exploit this sugar as an entry point to the human body. These pitfalls lead to the question of “Why does nature bother with sialylation?” The answer, of course, is that sialic acid plays immensely important roles in biological recognition by virtue of being situated at the outer periphery of the cell surface where it participates in numerous interactions that a cell makes with its microenvironment. Efforts to study and understand these functions have spawned the field of sialobiology, which seeks to uncover the role of this sugar in intimate relationships between a cell and its surroundings ranging from the nanometer scale (e.g., cell adhesion molecules) up to the whole organism level (e.g., the immune system and tissue-wide and system-level control through hormones).
Multiple Levels of Biological Control
Sialic acid exemplifies the several hierarchical levels through which sugars influence biological activity and engineer multi-layered regulation over complex systems. A brief discussion of these levels is now provided to introduce the idea that if similar control could be reproduced in the laboratory or clinically – perhaps through the metabolic glycoengineering methods described in this article – one could gain exquisite control over cells, tissues, and organisms in both health and disease. On one level, the simple presence or absence of sialic acid in a critical macromolecular context can profoundly influence biological activity. For example, the conversion of LacCer to GM3, or GM3 to GD3 by the sequential addition of sialic acid can alter the pro-apoptotic propensities of these glycoshingolipids (GSL) (Figure 3A). Showing similar influence over activity, the sialic acid residue of the sialyl Lewis X (sLeX) tetrasaccharide is required for mucins to support selectin-mediated tethering and rolling (Figure 3B) (McCarty et al. 2000). On a second level, the type of glycosidic linkage (e.g., α2,3-, α2,6-, or α2,8- as shown in Figure 2C) through which sialic acid is attached to the underlying glycoconjugate can profoundly influence activity; for example, the influenza virus discriminates between α2,3- and α-2,6-linked sialosides (Suzuki et al. 2000; Shinya et al. 2006).
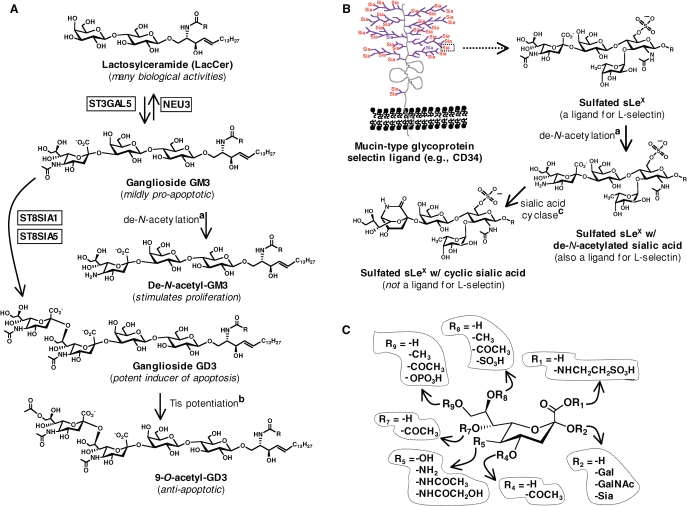
Fig. 3.
Sialic acid: a template for modulating biological activity. (A) Changes to sialic acid – either by the addition (or loss) of the entire monosaccharide or through submolecular chemical alterations – dramatically modulate the impact of gangliosides GM3 and GD3 on proliferation and apoptosis. (B) Sialic acid, as part of the sLeX tetrasaccharide that is multivalently displayed on mucin ligands for selectins, has a large impact on adhesion interactions exemplified here through a hypothesized cyclic form of sialic acid that abrogates binding interactions. (C) Sialic acid is a template for modulating biological activity with various “R” groups naturally found at virtually every position (Schauer 2000); to date, nonnatural substitutions (see Figure 4) have predominantly been situated at R5 and R9. The full names for enzymes are given in Table S1 (in the Supplementary Data available online) with more information for the conversion indicated by a sialic acid de-N-acetylation (Hanai et al. 1998), b Tis potentiation (Chen et al. 2006), and c sialic acid cyclase (Mitsuoka et al. 1999; Kannagi 2002) provided in the references given.
Zeroing in to the submolecular level, subtle (or not so subtle!) changes in the chemical structure of sialic acid can also have a dramatic impact on biological activity. For example, again illustrated in Figure 3A, de-acetylation of this sugar when found on GM3 converts this mildly pro-apoptotic GSL into a molecule that stimulates proliferation. Equally consequential is 9-O-acetylation of GD3, which transforms the potently pro-apoptotic parent molecule into an anti-apoptotic entity. Another example of the impact of submolecular changes to sialic acid on biological activity is provided by sLeX (Figure 3B) where the carboxylic acid group of this residue is sufficient to support tethering and rolling behavior under laboratory conditions (Yarema and Bertozzi 1998; Magnani 2004). Additional chemical features of sLeX, however – such as a hypothesized bicyclic form of sialic acid ((Mitsuoka et al. 1999) and sulfation of the 6-position of GlcNAc (Bistrup et al. 1999) or at the 6′-position of galactose (Bochner et al. 2005)) – determine the physiological relevance of sLeX-mediated recognition events (Varki 1997).
With numerous other natural modifications to the core structure of sialic acid now documented (see Figure 3C) (Angata and Varki 2002), the just-discussed examples of biological activities influenced by chemical features of sialic acid are merely the tip of the iceberg. For instance, the “nonhuman” glycolyl-modified form of Neu5Ac (i.e., Neu5Gc where R5 = −NHCOCH2OH, Figure 3C) provides another window, which is focused on the brain in the discussion below, into the manifold biological roles of sialic acid and how the chemical structure of this sugar influences function. Interestingly, Neu5Gc can be produced both enzymatically (e.g., by CMAH, Figure 2 and Table S1 in the Supplementary Data) and artificially through incubation of cells with the appropriate ManNAc analog (e.g., Ac5ManNGc, (Collins et al. 2000) presaging the manipulation of sialic acid through the metabolic glycoengineering methods described later in this article.
Sialic acid: A sugar that engineers the evolution, function, and pathology of the brain
Sialic Acid and Human Brain Evolution
Compared to sialic acid research, which sprung to life about three-quarters of a century ago, the biological effects of this sugar reach much farther into the past. The Varki group has proposed that the functional loss of CMP-N-acetylneuraminate monooxygenase (CMAH, the enzyme that converts Neu5Ac to the Neu5Gc “non-human” form of this sugar, Figure 2B (Irie et al. 1998)) a few million years ago played a role in human brain evolution (Varki 2002). This premise is supported by evidence that the mutation that abrogated the activity of CMAH transcripts found in present-day humans occurred after the Homo-Pan divergence (Chou et al. 1998) but prior to brain expansion during human evolution (Chou et al. 2002). Unlike most primate brains that stop growing relatively soon after birth, human brains continue to grow postnatally at a rate similar to the whole body, presumably because they were freed from the constraints imposed by Neu5Gc in other mammals that limit brain expansion after birth (Chou et al. 2002).
Sialic Acid and Brain Development – Is It a Glyconeutracetical?
Ganglioside levels vary significantly among mammals; interestingly, humans – a life form self-regarded as being highly intelligent – have over twice the amount found in any other species (Wang et al. 1998). Moreover, cerebral gray matter contains ∼900 μg/(g wet wt) of GSL-bound Neu5Ac, which is strikingly higher than the ≤30 μg/(g wet wt) found in most tissues and organs (Wang and Brand-Miller 2003; Schnaar 2004). Sialylated molecules play many roles in the brain beginning with the simple gangliosides GM3 and GD3 (Figure 3A) that influence early development by impacting cell proliferation and apoptosis (Bieberich et al. 2001). After birth, the GSL composition of the CNS undergoes dramatic changes (Yu et al. 2004) with close to 90% of the dozens of different gangliosides found in the mature brain limited to only four structures (GM1, GD1a, GD1b, GT1b).
In addition to their GSL-mediated contributions, sialic acids attached to proteins in each of the three glycosidic linkages (i.e., α2,3-, α2,6-, and α2,8-) common in mammals play key roles during brain development and then facilitate neuronal plasticity in the adult (Breen and Georgopoulou 2003). To provide representative examples of each linkage, CD34 is a stem cell marker that proliferating resident microglia express in response to acute neural injury (Ladeby et al. 2005) that predominantly displays α2,3-linked sialic acid in the form of sLeX (Lanza et al. 2001). Integrin function is modulated by α2,6-sialic acids (Yamamoto et al. 2001) in turn influencing adhesion, migration, and differentiation of neural progenitor cells (Tate et al. 2004). Finally, the neural cell adhesion molecule (NCAM) is modified by polysialic acid (PSA), a highly hydrated, negatively charged homopolymer of α2,8-linked sialic acids 55 or more residues in length that facilitates cell–cell interactions during cell migration, axonal pathfinding, axon branching, fasciculation, and learning (Cremer et al. 1994; Brusés and Rutishauser 2001). PSA endows the normally pro-adhesive NCAM with anti-adhesive characteristics that provide neural plasticity in many tissues during fetal development and uniquely in the brain in the adult (Mühlenhoff et al. 1998; Angata and Fukuda 2003).
Based on the multiple roles of sialic acid in CNS development, it intuitively follows that nervous tissue requires a copious supply of this sugar during growth. Evidence linking sialic acid intake with optimal brain development and cognitive abilities was reported for rat pups over 25 years ago (Morgan and Winick 1980). Since then several corroborating studies – reviewed by Wang and Brand-Miller (2003) – showed that dietary supplementation or intraperitoneal administration of Neu5Ac in newborn rodents increased cerebral and cerebellar glycoprotein and ganglioside sialic acid content; beneficial effects persisted into adulthood and occurred without affecting brain weight, cell size, cell number, or DNA, RNA, and protein content, suggesting that a sugar-specific mechanism was responsible. The analysis of a small number (∼25) of human infants who died of sudden infant death syndrome provides evidence that dietary intake of sialic acid also benefits human brain development as breast-fed infants had 32% higher ganglioside-bound and 22% higher protein-bound sialic acids compared to formula-fed infants consistent with the higher levels of sialic acids in breast milk (Wang and Brand-Miller 2003; Wang et al. 2003). All though far from conclusive, and by no means meant to promote unfounded claims from glyconutrient sham artists (Schnaar and Freeze 2008), these studies do suggest that exogenous sialic acids (i) can be utilized by cellular systems and (ii) have an impact on their biology; these two features comprise a recurring theme in metabolic glycoengineering.
Sialic Acid and Brain Disease
Sialic acid has been implicated in many neurological disorders ranging from inborn errors of metabolism (such as sialuria, Salla Disease, or infantile sialic acid storage disease) to the late onset degenerative disorders of the brain including schizophrenia, Parkinson's and Alzheimer's diseases; these diseases have been reviewed extensively elsewhere (Sampathkumar et al. 2006a) and only a few highlights are presented here. Not surprisingly considering their overriding importance in brain development, congenitial defects in ganglioside biosynthesis are particularly prone to result in neurologic dysfunction. GM3 alone is implicated in several such diseases that include infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase (Simpson et al. 2004). In mice, a lack of GM3 synthase activity results in complete hearing loss due to degeneration of the organ of Corti (Yoshikawa et al. 2009).
Moving to GSL of greater complexity, Tay-Sachs disease is a classic example of an inborn lysosomal storage disease characterized by massive accumulation of ganglioside GM2 (Fernandes Filho and Shapiro 2004). Although not usually regarded as a primary factor in the etiology of Tay-Sachs, sialic acid has been implicated in disease progression when neural cells respond to massively elevated levels of GM2 as a quasi-xenobiotic and undertake detoxification efforts. To rid themselves of GM2, cells employ taurine conjugation (a well-known method for mammals to facilitate the clearance of xenobiotics by increasing their polarity and aqueous solubility) of the sialic acid moiety of this ganglioside. In effect, the taurine-conjugated form of GM2 becomes an activated xenobiotic with potent surfactant properties that contribute to the pathophysiological symptoms of Tay-Sachs disease (Li et al. 2003). This example is germane to metabolic glycoengineering as a cautionary note because some of the more flamboyant nonnatural sialosides discussed later in this paper have the potential to change the biophysical properties of the sugars dramatically and lead to pathologies. Conversely, in cases where sugar analogs alter the profile of biosynthetic enzymes responsible for sialylation (Wang et al. 2006; Sampathkumar et al. 2006b) in potentially beneficial manners, an enticing possibility is that these agents could be exploited to correct aberrant sialylation.
Sialic acid – A metabolic engineering opportunity exploited by nature
To evaluate the prospect of manipulating sialylation for therapeutic purposes, we note that specific patterns of sialylation associated with health and disease are determined by two factors: (i) the rate of flux through the sialic acid pathway and (ii) the expression of an appropriate complement of sialyltransferases in a particular cell or tissue. Unfortunately, neither flux nor sialyltranferase expression is readily manipulated with sufficient precision to achieve specific endpoints. Surface sialylation patterns, for example, typically are insensitive to changes in metabolic flux because the Golgi concentration of CMP-Neu5Ac exceeds the Km for sialyltransferases (Monica et al. 1997); as a consequence, enhanced CMP-Neu5Ac levels do not translate into increased sialylation of glycoconjugates (conversely, a similar buffer exists against moderate decreases in flux). Exceptions may exist in specialized cases, such as the “high demand” imposed by the production of polysialic acids (Bork et al. 2005), or in hereditary inclusion body myopathy (HIBM), a disease where flux is compromised due to mutations in GNE (Eisenberg et al. 2001); this latter situation can be remedied in mice by supplementation with exogenous ManNAc (Galeano et al. 2007; Malicdan et al. 2009).
Manipulating sialylation by modulating sialyltranferases is also problematic (once again, specialized exceptions may exist) because of redundancy in enzymatic activity, highly specific small molecule inhibitors are lacking, and genetic methods – even siRNA approaches – remain beset by technical issues (such as off-target effects and a lack of efficient in vivo delivery methods). Fortunately, a third level of control over sialic acid display exists that avoids many of the difficulties inherent in controlling the rate of flux or the expression of the biosynthetic (or recycling) enzymes. As introduced above, this ability lays in the “submolecular biochemical engineering” approach where a specific portion of the sialic acid molecule is subject to a chemical modification (Figure 3C) (Keppler et al. 2001). This age-old strategy is exemplified by the postulated impact of Neu5Gc on human brain evolution; this molecule, when compared to Neu5Ac, amply demonstrates the critical influence of a single atom – in this case an added oxygen – in specifying the activity of much larger sialoglycoconjugates.
Modified sialic acid – Building on nature's metabolic glycoengineering
Sialic acid – A molecular template for chemical diversity
Striking examples beyond Neu5Gc where nature modulates biological function through the submolecular biochemical engineering of sialic acid include the influence of acetylation over cell growth and death (Figure 3A) and sLeX on leukocyte recruitment (Panel B). Comparing the de-N-acetyl form of sialic acid in these two settings vividly illustrates the nuanced and context-dependent nature of sialic acid-dependent responses; the absence of the N-acetyl group from the sialic acid moiety of sLeX has no impact on the selectin binding activity of this tetrasaccharide while loss from GM3 converts a mildly pro-apoptotic GSL to a growth-stimulatory entity. These examples, and many more yet to be discovered (at present the known diversity in natural sialic acid structures – over 50 (Angata and Varki 2002) – greatly outpaces insight into their individual biological functions), make it clear that nature has long known that a monosaccharide is a versatile template for modifying biological activity. Based on a desire to mimic nature's ability to modulate biological activity by using sialic acid as a chemical template – a concept now embraced for many monosaccharides in drug discovery efforts (Meutermans et al. 2006) – metabolic sialic acid engineering was born almost two decades ago.
Considering how chemical engineering of sialic acid occurs naturally, through enzymatic methods, this approach may appear at first to be as intransient as the strategies based on modulating the rate of flux or manipulating biosynthetic enzymes considered above. Insight gained from the study of “nonhuman” Neu5Gc in tumors (Malykh et al. 2001), however, offered a possible solution. Specifically, Neu5Gc found in cancerous tissue in humans was initially puzzling because the inactivating mutation of CMAH, which was the loss of an entire exon, could not plausibly have been subject to a reciprocal activating mutation even in the mutagenic milieu of a tumor.
The mystery of Neu5Gc display in human tissues was solved by the discovery that dietary Neu5Gc – which is richly supplied by “red meat” and dairy products – could be taken up by cells, intercept the sialic acid pathway, and be incorporated into the host's glycans (Tangvoranuntakul et al. 2003). This observation decisively showed that the biosynthetic machinery for sialic acid was permissive to nonnatural metabolites, thus laying a conceptual foundation for sialic acid glycoengineering (in reality, the pioneers of this technology were not privy to these more recent insights provided by Neu5Gc, making the early experiments performed almost two decades ago all that much more remarkable). In the next sections of this report, a retrospective look at various supplementation strategies used to display nonnatural sialosides in living cells – organized by various classes of metabolites used to intercept the sialic acid pathway – is given.
The sialic acid pathway can be intercepted at multiple points
CMP-Sialic Acid Analogs
The first conclusive reports of the incorporation of nonnatural sialic acids into cellular glycoconjugates date from the late 1980s (Gross and Brossmer 1988) and were based on discovery that sialyltransferases were remarkably permissive for substrates bearing certain bulky functional groups (Figure 4). Remarkably, even bulky fluorophores appended to CMP-Neu5Ac (Gross and Brossmer 1988; Gross 1992; Brossmer and Gross 1994) could be biosynthetically incorporated into glycans (Gross et al. 1989; Gross and Brossmer 1995). The downside to this early nucleotide sugar-based approach was that the multiply charged analogs were not membrane permeable imposing a severe obstacle to their use in living cells where two sets of membranes had to be crossed for colocalization of the substrate with sialyltransferase inside the Golgi lumen. Consequently, a nucleotide sugar donor approach was only useful in semi-permeabilized cells or for cell-free chemoenzymatic synthesis.
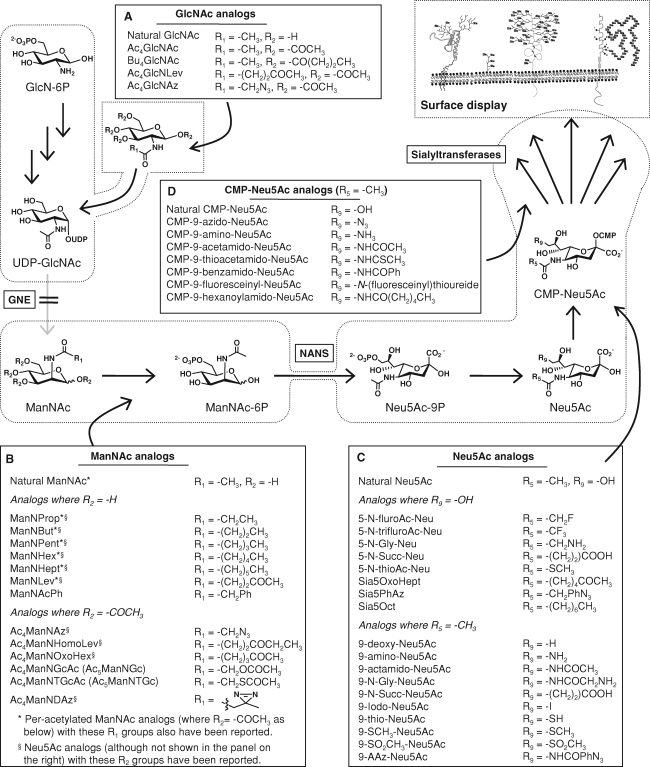
Fig. 4.
Pathway options for sialic acid glycoengineering. Analogs can be introduced into the sialic acid metabolic machinery (as shown in detail in Figure 2) at several points. (A) GlcNAc analogs can enter the hexosamine pathway by salvage mechanisms but subsequent entry into the sialic acid pathway is severely restricted by the strict substrate specificity of GNE. (B) ManNAc analogs can feed directly into the canonical sialic acid pathway but constraints on the size and position of nonnatural modifications to this metabolite are posed by the bottleneck enzyme NANS. (C) Sialic acid analogs bypass the NANS bottleneck and can be modified at both the C5 (N-acyl) and C9 (OH) positions. (D) CMP-Neu5Ac analogs enter the pathway at the final stage and need only to be processed by sialyltransferases; however, the membrane impermeability of these compounds severely restricts their intake into the Golgi lumen where they are required for biosynthetic incorporation. Additional analogs, and literature references for the analogs shown in this figure, are provided in Table S2 of the Supplementary Data available online.
ManNAc Analogs
In a circuitous manner, an attempt at cancer therapy serendipitously provided an alternative strategy for introducing nonnatural monomer building blocks into surface glycans via a cell's biosynthetic machinery. Specifically, fluorinated ManNAc analogs intended to block sialylation in cancer cells were found – instead of inhibiting the biosynthetic pathway – to actually be incorporated into surface components, most plausibly sialic acid (Hadfield et al. 1983; Schwartz et al. 1983). Extending this tentative discovery in a deliberate manner, the Reutter group employed hexosamine analogs to install nonnatural monosaccharides into surface displayed glycans (Kayser et al. 1992a; Kayser et al. 1992b) and, for reasons explained below, ManNAc has remained the monosaccharide vehicle of choice for sialic acid glycoengineering. Dozens of analogs – mainly with the chemical modification intended for cell surface glycan display situated at the N-acyl position – have been synthesized and tested in biological assays; a sampling of these compounds is given in Figure 4 and more extensive listings are provided in the Supplementary Data to this article and elsewhere (Aich and Yarema 2008).
Sialic Acid Analogs
Sialic acid analogs, which are efficiently taken up by cells (Oetke et al. 2001; Bardor et al. 2005), provide a second viable alternative to CMP-Neu5Ac analogs for surface display of nonnatural sialosides. Many of these analogs are modified at the N-acyl position (see Figure 4 for representative structures) with the same functional groups found at the N-acyl position of ManNAc analogs (Oetke et al. 2002; Saxon et al. 2002), essentially mimicking cell surface sialoside modifications provided by the hexosamine derivatives. An important benefit of the sialic acid analogs, however, is that they bypass the “bottleneck” posed by N-actylneuraminic acid synthase (NANS, with the bottleneck indicated schematically in Figure 4) in two important ways. First, NANS is restrictive for long or branched N-acyl modifications to ManNAc (Jacobs et al. 2001; Viswanathan et al. 2003) limiting substitutions to three or less additional carbons (or equivalently sized groups such as an azide). Second, the pathway does not readily accommodate substitutions at the C6-position of ManNAc (Lawrence et al. 2000) that ultimately appear at the C9-position of sialic acid (Tanner 2005). While the C6-position can be modified in ManNAc analogs, this strategy leads to a large loss in efficiency (∼97% reduction, Lawrence et al. 2000)) because this hydroxyl is usually phosphorylated before ManNAc is converted to sialic acid. Consequently, the accessibility of the 9-position of Neu5Ac analogs without compromising metabolic efficiency positions sialic acid analogs as superior reagents (Oetke et al. 2002; Han et al. 2005) for modulating activities influenced the C9-OH group, such as Siglec binding. Additional scenarios where sialic acid analogs are either preferred or absolutely necessary include organisms such as certain bacteria (and possibly plants (Shah et al. 2003)) where sialic acid biosynthesis does not utilize ManNAc (Schilling et al. 2001; Goon et al. 2003) but rather depends on a direct source of sialic acids.
GlcNAc Analogs are a Poor Option for Sialic Acid Engineering
The previous sections describe three options (CMP-Neu5Ac, ManNAc, and Neu5Ac analogs) for cell surface sialic acid replacement. Of these, the Neu5Ac analogs are superior in several respects; in particular, they bypass the NANS bottleneck and provide options to install chemical variation at either the N-acyl or 9-OH positions. In reality, practical issues (such as the lower cost and synthetic tractability of ManNAc compared to sialic acid) come into play and the use of ManNAc analogs in sialic acid glycoengineering experiments continues to outpac e sialic acid. Glucosamine analogs continue the trend toward facile synthesis and economy, spurring efforts to evaluate the incorporation of GlcNAc analogs into the hexosamine pathway, which in turn feeds into the sialic acid pathway through GNE (Figure 2A and B). These efforts – exemplified by GlcNLev and GlcNDAz, which failed to be displayed on the cell surface as Sia5Lev (Yarema et al. 1998) or Sia5DAz (Tanaka and Kohler 2008), respectively – presumably were not successful because of the obstacle presented by GNE, an enzyme that appears to have strict specificity for natural UDP-GlcNAc. Similarly, as will be discussed later, GlcNAc analogs are poorly, if at all, incorporated into N-glycans, mucins, or GAGs, but are instead selectively partitioned into nucleocytoplasmic O-GlcNAc-modified proteins.
Summarizing analog choices for sialic acid glycoengineering, the first decade of work provided several options – coalescing around ManNAc analogs – for modifying sialoside display. By now the reader may be wondering if these efforts were paying off in the quest to complement – or even one-up – natural mechanisms by which cells modulate biology through glycochemistry. Therefore, even though additional exciting chemistry-based developments lay on the horizon (and have been reaching fruition of late), we next outline biological response elicited by early analogs and potential applications for the “first generation” of these compounds.
Applications: The impact of N-acyl-modified sialosides on biology
Enhancing the Immunogenicity of Carbohydrate Epitopes for Cancer Treatment
An initial opportunity – or apprehension, depending on one's perspective – of sialic acid engineering was that the modified sugars would constitute immunogenic epitopes when introduced into the body. These concerns were given credence by reports that 9-O-acetylation of GD3 (Figure 2A) produced an antigenic form of this ganglioside (Cheresh et al. 1984) as well as studies showing that Neu5Gc was not only immunogenic in humans but also an important factor in the evolution and functioning of the human immune system, especially in the context of Siglec biology (Brinkman-Van der Linden et al. 2000; Crocker and Varki 2001). The demonstration that Sia5Pr (Hayrinen et al. 1995; Liu et al. 2000) and Sia5Lev could be antigenic (Lemieux and Bertozzi 2001) extended the immunogenic precedent set by Neu5Gc and 9-O-Neu5Ac to nonnatural sialic acids created by metabolic glycoengineering.
Today, from a perspective compiled from almost two decades of animal testing with multiple analogs, nonnatural sialic acids – despite having antigenic potential in some situations – are generally too weakly immunogenic to raise a red flag for in vivo sialic acid glycoengineering experiments. Similarly, they are typically ineffective for applications where a strong immune response is desired. The concept of deliberately trying to evoke immunogenicity (which may seem counterintuitive on the surface) once again has a natural precedent from Neu5Gc where appearance in human tumors (Malykh et al. 2001) elicits a complex interplay with the host immunity (Hedlund et al. 2008) that may have therapeutic benefits that include “tagging” the cancerous tissue for eradication by the host immune system.
In one of the first experiments where connections between modified sialic acid, tumor growth, and the immune system were explored, the Jennings lab increased the immunogenicity of tumor cells by metabolic incorporation of N-propionyl derivatized residues into polysialic acid. By adjusting the dose and timing of administration of the nonnatural sugar, they were able to control the targeting of an antibody specific for the altered polysialic acid (Hayrinen et al. 1995) and selectively kill tumor cells through an innovative passive immunotherapy approach (Liu et al. 2000). The Guo group has methodically extended this strategy by systematically surveying N-acyl groups for immunogenicity and has found that most substituents are weakly, if at all, antigenic, consistent with a lack of an overt immune response in animal studies. However, N-phenylacetyl analogs – when incorporated into tumor-associated carbohydrate antigens (TACA) such as sTn (Wu and Guo 2006; Wang et al. 2008) or GM3 (Chefalo et al. 2006; Wang et al. 2007) – are sufficiently antigenic to raise antibody titers (Chefalo et al. 2004). In a two-pronged strategy, a tumor bearing animal can be treated with the analog, which selectively partitions into TACA. The animal is also treated with the antibody raised against the modified sialoside, which labels the glycoengineered tumor cells and helps target them for eradication by the host's immune response.
Modulating Viral Binding
Based on the immune system's ability to distinguish subtle structural differences between natural sialic acids such as Neu5Ac, Neu5Gc, and 9-O-AcNeu5Ac (Higa et al. 1985) and to recognize nonnatural forms such as Sia5Pr and Sia5PhAc, it was intriguing to postulate that similar discrimination extended to other biological recognition phenomenon. In this vein, the modulation of viral binding by metabolic glycoengineering was evaluated and used to weaken, rather than strengthen as had been demonstrated for the immunogenicity of TACA, responses. In particular, the binding and infectivity of influenza virus – where the N-acyl side chain of sialic acid is in close proximity to the edge of the hemagglutinin (HA) binding pocket – are dampened by sialosides with elongated N-acyl chains y (Herrler et al. 1990). As surveyed in a previous review (Keppler et al. 2001), attenuation of viral binding and infectivity is typical but occasionally the opposite response does occur, as shown by the Reutter group who incubated kidney epithelial and B lymphoma cells with ManNProp, ManNBu, and ManNPent. All three precursors were metabolized into the corresponding unnatural cell surface sialic acids by both cell types and influenced polyoma virus binding but infection was either inhibited by up to 95% or – depending on the N-acyl group – increased by as much as 7-fold (Keppler et al. 1995). These experiments are typical of metabolic glycoengineering endeavors to date, where the biological response can often be predicted in advance but unanticipated and sometimes difficult-to-explain outcomes are by no means a rarity.
Altering Cell Adhesion
Sialic acid is involved in cell–cell and cell–substrate adhesion in numerous ways; well-known examples include the impact of α2,8-linked polysialic acid on NCAM, the importance of the α2,3-linked sialic acid of sLeX in leukocyte tethering and rolling, and the role of α2,6-linked sialic acids in integrin function. This information, combined with the examples provided above where sialic acid engineering altered the binding of biological molecules (antibodies and viruses) to cells, suggests that ManNAc analogs – by installing nonnatural sialosides that interact differently with their cognate receptors – are fairly general tools for altering cell adhesion. Pursuing this objective by inhibiting adhesive interactions by installing “teflon” on a cell, glycoengineering has been employed to fluorinate mammalian cell surfaces resulting in modestly decreased adhesion of HL-60 cells to fibronectin (Dafik et al. 2008). By contrast, ManNProp promotes binding these cells to fibronectin (Villavicencio-Lorini et al. 2002); unexpectedly, however, this gain of binding was not directly correlated with cell surface sialic acid display but rather was linked to transcriptional regulation of integrins.
Modified Sialosides – Are they Signaling Molecules?
Corroborating evidence that gene expression is influenced by sialic acid glycoengineering has been gleaned from a decade of studies, beginning with experiments where ManNAc analogs altered the fate of embryonic neural cells (Schmidt et al. 1998) and culminating with recent evidence that ManNAc analogs can function as signaling molecules (Kontou et al. 2008), perhaps through the metabolic intermediate CMP-Neu5Ac (or an analog such as CMP-Sia5Pr) during the steps where the biosynthetic pathway transits the nucleus. In a more convoluted explanation, analog incorporation into surface glycans could alter the “sugar code” and subsequently perturb the decoding process by which lectins engage signaling pathways (Dam and Brewer 2002; Gabius et al. 2004; Gabius 2008). Mechanistically, lectin binding relies on the cluster glycoside effect (Lundquist and Toone 2002) and it is plausible that an analog such as ManNPr, which replaces up to 85% (Mantey et al. 2001) but more typically from 30 to 70% of Neu5Ac with Sia5Pr (Gagiannis et al. 2007), could affect the avidity of recognition molecules that employ multivalent sialoside recognition. Another way that nonnatural sialic acids, in particular those containing longer alkyl chains that impair carbohydrate–carbohydrate hydrogen bonding interactions, could indirectly alter gene expression is through their impact on the biophysical properties of lipid rafts (or, “the glycosynapse” (Hakomori 2002)) that “lubricate cell signaling” (Allende and Proia 2002). Clearly, resolving these complex issues will require a considerable future effort, which will be well repaid if the analogs become a new class of reagents for controlling signaling pathways.
As briefly surveyed in this section, two decades of sialic acid metabolic glycoengineering experiments, which have intentionally (or serendipitously) emulated natural mechanisms that employ structurally altered sialosides to modulate biology, have spawned interesting applications (e.g., novel cancer immunotherapy) as well as unsolved msyteries (e.g., the question “how do ManNAc analogs engage transcription?”). We will next shift gears and focus on a development that – depending on one's perspective – either exacerbates the complexity of these challenges or conveniently sidesteps them.
Introducing “chemical biology” and bioorthogonal chemistries
Chemoselective ligation and bioorthogonal chemistry – Exemplified by the ketone
An important conceptual advance in metabolic glycoengineering occurred when chemical functional groups not normally found in sugars were incorporated into analog design. The impetus behind efforts to endow sialic acids with bioorthogonal chemical functional groups was the enticing ability to perform chemoselective ligation reactions on the surfaces of living cells and even in vivo (Lemieux and Bertozzi 1998). In these reactions, the two participating functional groups – which ideally are abiotic – have finely tuned reactivity that avoids interference with coexisting functionalities, and they recognize only each other while ignoring their cellular surroundings ultimately forming stable covalent adducts under physiological conditions (Saxon and Bertozzi 2000).
The ketone group—incorporated into the sialic acid pathway using ManNLev (Figure 4B)—introduced the concept of bioorthogonal chemistry to sialic acid glycoengineering (Mahal et al. 1997); ketones were selected for these pilot experiments because they are not found in proteins, lipids, or carbohydrates and are thus absent from the cell surface. Moreover, ketones already had been successfully exploited for drug delivery via chemoselective ligation reactions (Rideout 1986; Rideout et al. 1990) demonstrating the concept of bioorthogonal ketone-based chemistry in living cells. Once a ketone is displayed on the cell surface (Figure 5A), chemical nuances of chemoselective ligation partners open interesting possibilities. For example, conjugation with hydrazide-derivatized ligands – which does not occur rapidly at physiological pH – can be driven forward by an excess of reagent. Once internalized into a low pH vesicle the reaction rate increases substantially but, in the absence of excess unligated ligand, the reverse reaction takes place liberating the ligand inside a cellular compartment. If the cell in question happens to be diseased and in need of detection or eradication, this strategy can be used to load a cell with an MRI contrast agent (Lemieux et al. 1999), a small molecule drug (Nauman and Bertozzi 2001), a gene vector (Lee et al. 1999), or a toxin such as ricin (Mahal et al. 1997). By contrast, aminooxy-derivatized ligands are not pH sensitive and ligands delivered by this route are recycled from internal vesicles back to the cell surface (Nauman and Bertozzi 2001); this strategy is preferred when prolonged surface display of a ligand is beneficial.
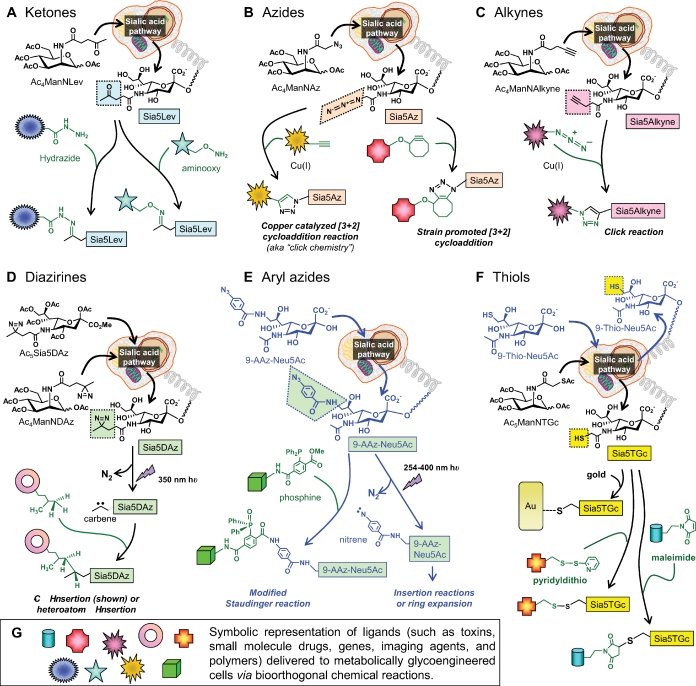
Fig. 5.
Bioorthogonal ligation reactions, exemplified by glycan-displayed ketones, azides, alkynes, diazirines, aryl azides, and thiols. (A) Ketones displayed on cells incubated with ManNLev or Ac4ManNLev (Jacobs et al. 2001) can be ligated with hydrazide in a pH-dependent reaction (Yarema et al. 1998) or with aminooxy in a pH-independent reaction (Nauman and Bertozzi 2001). (B) Azides can be displayed in sialosides using Ac4ManNAz and ligated with phosphines using the Bertozzi-modified Staudinger reaction (shown for aryl azides in (E) (Saxon and Bertozzi 2000) or via [3+2] cycloaddition with alkynes using copper-catalyzed click reactions or strain-promoted cyclootyne (Agard et al. 2004). (C) Conversely, alkynes can be displayed in glycans (e.g., by using Ac4ManNAlkyne) and ligated with azide-derivatized ligands or fluorophores (Hsu et al. 2007). (D) The diazirine-containing analog Ac4ManNDAz (or Ac5Sia5DAz) installs photoactivatable Sia5DAz onto the cell surface where irradiation produces carbenes that undergo insertion reactions with neighboring biological molecules (Tanaka and Kohler 2008), thus crosslinking in situ sialoside ligands. (E) Aryl azides can be incorporated into sialic acid via 9-AAZ-Neu5Ac, which can then be subject to the modified Staudinger reaction referenced in (B) or irradiated to provide crosslinking reactions similar to those resulting from diazirine in (D) (Han et al. 2005). (F) Thiols can be incorporated into sialosides using Ac5ManNTGc (Sampathkumar, Li, Jones, et al. 2006) or 9-thio-Neu5Ac (Oetke et al. 2002), which can then bind to gold or undergo the chemoselective reaction with pyridyldithio- or maleimide-derivatized ligands. (G) In this figure, ligands being delivered to a cell are represented symbolically with specific examples provided in the main text and references therein.
Despite its utility in proof-of-concept experiments and interesting chemical properties, the ketone was far from an ideal chemoselective ligation partner. Biologically, the ketone is only bioorthogonal on the cell surface; inside the cell, metabolites with ketones (and their more reactive aldehyde cousins) are common and compete for chemoselective ligation of Sia5Lev with hydrazide or aminooxy groups. From a chemical perspective, the ketone-hydrazide coupling is optimal at a lethal pH of ≤5; Sia5Lev labeling, therefore, is typically performed at an intermediate pH of 6.5 where both cell viability and labeling efficiency are compromised by about 75% (Yarema 2002). Although interest in ManNLev continues, with progress being made toward improved bioorthogonal reaction conditions (e.g., aniline-promoted aminooxy coupling (Zeng et al. 2009)) and recently reported applications (e.g., tracking trogocytosis (Daubeuf et al. 2007) and probing the mechanism of gangliogenesis in the enteric nervous system (Faure et al. 2007)), additional options for bioorthogonal metabolic glycoengineering chemistry have now been developed that provide the metabolic glycoengineer with attractive alternatives.
Additional bioorthogonal functional groups
Azides
Azides became the second (chronologically speaking) bioorthogonal functional group used in sialic acid glycoengineering when Ac4ManNAz was used to install Sia5Az into surface sialosides (Figure 5B). Azides have several advantages over ketones with perhaps the most striking being that they are completely abiotic allowing intracellular as well as cell surface chemoselective ligation strategies to be pursued. From a chemical perspective, the original azide reaction strategy employed an elegant modification of the Staudinger reaction (Saxon and Bertozzi 2000; Saxon et al. 2002). More recently, glycan-displayed azides have been exploited in the increasingly ubiquitous “click” reaction. One pitfall of click chemistry, however, is the copper catalysis, which can be cytotoxic for applications where ongoing cell viability is required; a solution to this problem may lie in a strain-promoted click reaction (Baskin et al. 2007) compatible for use in living cells and in vivo imaging (Laughlin et al. 2008).
Alkynes and Photoactivated Crosslinking Sugars
Click chemistry was first used in metabolic glycoengineering by installing the azide group into surface-displayed glycans; the reverse strategy where the alkyne is displayed in the glycan (Figure 5C) has also been demonstrated (Hsu et al. 2007). The alkyne exemplifies how the current modestly sized bevy of bioorthogonal chemical strategies now available has been constantly growing; for example photoactivatable crosslinking sugars have been recently reported. In one manifestation, the Kohler group developed the versatile diazirine-containing ManNAc analog Ac4ManNDAz (Tanaka and Kohler 2008) (Bond et al. 2009) (Figure 5D) and in another, the Paulson group installed the photo-activated aryl azide group into CD22-displayed glycans of CD22 (Han et al. 2005; Yarema and Sun 2005) (Figure 5E). As discussed earlier in the context of pathway bottlenecks, the C9-position of sialic acid was able to accommodate the bulky aryl azide group, while the more restrictive N-acyl position was permissive for the diazirine modification.
Thiols
The thiol group – installed into the glycocalyx via thioglycolyl analogs (e.g., Ac5ManNTGc, (Sampathkumar, Jones, Yarema 2006), Figure 5F) – possesses its own unique set of pros and cons. Unlike azides or diazirines, thiols are not abiotic but are common within a cell and do not even have the advantage of the ketone of being absent from the cell surface. Thiols, however, are not found in the glycocalyx, the intended destination for modified sialosides, positioning Sia5TGc with a degree of chemical uniqueness. Also, thiols are well tolerated and rather inert within the reducing environment of the nucleocytoplasm (if anything, they may be protective if they function analogously to glutathione, although there is not yet evidence for this premise). Once thiols reach the oxidizing conditions found in the secretory pathway, where protein disulfide bonds are generated (Frand et al. 2000), thiolated sialosides also form cis-disulfide linkages that are maintained in the slightly oxidizing cell surface milieu under tissue culture conditions (Sampathkumar, Jones, Yarema 2006). Rather than being an impediment, the “masking” of thiols in this manner provides additional chemical versatility insofar as certain ligation partners, such as gold, bind to both forms of sulfur whereas others, such as maleimide, only react with free thiols. Therefore, differential binding can be achieved depending on the probe used and modulation of oxidizing conditions (e.g., disulfides can be “unmasked” and reactivated with mild TCEP treatment (Sampathkumar, Li, Jones, et al. 2006) or controlling the cysteine/cystine ratio in the culture medium) thereby providing an additional layer of control, on a time scale of seconds to minutes, rather than the multi-day intervals needed for de novo biosynthetic display.
The second wave of – chemistry-based – applications
Tools for Analog Detection and Glycan Imaging
In tandem with the development of analogs endowed with chemical functionalities not naturally found in sugars, the metabolic glycoengineering community has sought to develop applications for this new technique. The ability to tag sialic acids with unique chemical functionality in living systems was first exploited for targeting with imaging agents, such as fluorophores, for analysis of surface expression by flow cytometry (Mahal et al. 1997) or confocal microscopy (Sampathkumar, Li, Jones, et al. 2006). These methods allowed estimates to be made of the complete transit of nonnatural analogs through the biosynthetic pathway (Yarema et al. 1998) and enabled recycling kinetics to be probed by using chemical ligation reactions that were (or were not) sensitive to the lower pH of the endosomal vesicles (Nauman and Bertozzi 2001). In the past few years, chemoselective labeling has progressed from cell culture experiments and rodent testing where samples are harvested before analysis (Kayser et al. 1992a; Gagiannis et al. 2007) to imaging in living animals such as zebrafish (Laughlin et al. 2008).
Emerging Glycomics Techniques
Empowered by the ability to label glycans with unique chemical functional groups that subsequently can be selectively captured and isolated from a complex biological milieu, metabolic glycoengineering is becoming a valued contributor to glycoproteomics. To date, variations of the “tagging-via-substrate” strategy (Sprung et al. 2005; Nandi et al. 2006), which have mostly been applied to sugars other than sialic acid (as discussed below), have been particularly fruitful for identifying O-GlcNAc modified proteins. In one approach, an analog with a chemical tag (such as a ketone or azide) is metabolically incorporated directly into the glycoconjugate, replacing a natural O-GlcNAc (for example) with a O-GlcNLev or O-GlcNAz residue (Khidekel et al. 2003, 2004; Vocadlo et al. 2003; Gurcel et al. 2008). In a variation, Qasba and colleagues have developed mutant glycosyltransferases that label glycoconjugates carrying specific sugar moieties, such as O-GlcNAc, with monosaccharides endowed with bioorthogonal functional groups (Qasba et al. 2008; Boeggeman et al. 2009). Moving forward, as commercialization of this methodology reaches fruition coupled with analogs that target a greater range of glycosylation pathways, metabolic glycoengineering will undoubtedly gain importance for glycomics efforts (Campbell and Yarema 2005).
Drug Delivery and Cancer Targeting
An increasing number of modern drugs consist of large molecules such as viruses, peptides, and nucleic acids. Delivery to a particular organ or cell type and subsequent uptake by the target can be severely hampered by the lack of specific receptors or transport mechanisms for these drug candidates leading to efforts to chemically engineer cell surfaces to enhance their interactions with drugs and drug delivery systems. In one example, synthetic peptide-based receptors that control the selective permeability of the cell membrane to large drug molecules have been inserted into cell surfaces to facilitate the uptake of exogenous proteins (Martin and Peterson 2003). Of relevance to sialic acid glycoengineering, metabolically engineered glycans, such as ketone-bearing Sia5Lev sialosides (Figure 5A), represent new synthetic receptors for drug delivery. Already, ManNLev has been used to install ketones for chemoselective-ligation-based killing of cancer cells via ricin (Mahal et al. 1997, 1999); small molecules, such as the cancer drug doxorubicin, have been delivered by this manner (Nauman and Bertozzi 2001), as they have much larger entities, such as the adenovirus used for gene therapy (Lee et al. 1999).
Moving to a larger size scale, Iwasaki and coworkers decorated 2-methacryloyloxyethyl phosphorylcholine polymer nanoparticles bearing hydrazide groups (PMBH) with anticancer drugs such as doxorubicin or paclitaxel. Exposure of these constructs, which react with the ketone groups of Sia5Lev, to ManLev-treated cells reduced viability while untreated cells remained unaffected indicating that the drugs were selectively delivered to the glycoengineered cells (Iwasaki et al. 2007). Work reported by Iwasaki and colleagues also raised the intriguing possibility that polymers capable of recognizing engineered glycans, such as those bearing Sia5Lev, could be used to adsorb cells and thus function as an adhesive filter to separate cancer cells from their normal counterparts (Iwasaki et al. 2005).
Polymer-based Adhesion Approaches – Toward Creating Artificial Cell Niches
An interesting observation from the experiments reported by Iwasaki and coworkers was that after metabolically glycoengineered cells were attached to a polymer, cell viability could be maintained (or diminished) through the chemistries present on the surfaces of both the cell and the material. This finding builds on over a decade of reports – beginning with the abolishment of normal contact-dependent inhibition of cell growth in cell incubated with ManNProp (Wieser et al. 1996) – that sialic acid influences adhesion-dependent cell–cell or cell–ECM interactions. The contributions of chemical functional groups installed into sialisodes via metabolic glycoengineering became apparent when Shakesheff's group demonstrated that aldehyde groups can be effectively introduced into the cell surface glycoconjugates of adherent cell monolayers by mild periodate oxidation and exploited to aggregate cells (De Bank et al. 2003). This technique, which can be mimicked by replacing aldehyde generated through chemical oxidation with ketones derived from ManNLev, demonstrated that metabolic glycoengineering can be valuable for engineering the surface properties of cells to manipulate their adhesion, behavior, and function.
To build on the growing evidence that adhesion plays a major role in cell fate during development, our laboratory used Ac5ManNTGc to install thiols onto the cell surface as the Neu5TGc form of sialic acid (Sampathkumar, Jones, Yarema 2006) (Figure 5F). In contrast to thiols situation in membrane proteins that have only limited accessibility to the microenvironment (due, for example, to formation of disulfide bridges), thiols displayed on sialic acid are at the outer periphery of the glycocalyx and are highly accessible. As a result, normally nonadhesive blood cells expressing Neu5TGc spontaneous undergo cell–cell clustering and readily adhere to a gold matrix (either to a 2D flat surface (Sampathkumar, Li, Jones, et al. 2006) or 3D nanofibers. Interestingly, Neu5TGc triggered the differentiation of a human embryoid body-derived germ cell line grown on gold toward a neuron lineage, and the growth of normally nonadherent Jurkat cells on the nanofibers led to adhesion and dramatic changes in morphology without compromising viability. These results, combined with earlier reports that metabolic glycoengineering alters the fate of embryonic neural cells (Schmidt et al. 1998) and the subsequent finding by Schnaar's group that Neu5Gc ablates MAG binding and spurs neurite outgrowth (Collins et al. 2000), position metabolic glycoengineering as a fascinating tool for future efforts in tissue engineering and regenerative medicine.
Extending metabolic glycoengineering to additional pathways
The connectness of glycosylation pathways poses potential problems
Isotope labeling studies have shown that exogenously supplied monosaccharides (such as glucose, which can enter the hexosamine pathway shown in Figure 2A) can broadly partition into many metabolites including other sugars (Jin et al. 2004). Extending this concept to metabolic glycoengineering, the ability of natural hexosamines to be interconverted through the connectedness of glycosylation pathways (as briefly outlined in Figure 6 and shown in more detail in other publications (Goon and Bertozzi 2002; Murrell et al. 2004) or in online resources such as the KEGG Pathway database (Hashimoto et al. 2006; Kanehisa et al. 2006)) raises the possibility that the nonnatural monosaccharide analogs could become broadly distributed throughout all glycans. While not hindering some uses of this technology, such as attaching cells to a tissue culture scaffold or directing therapeutic agents to cancer cells experiencing a global increase in glycosylation, this possibility does render certain applications less meaningful. For example, glycomics experiments where the objective is to metabolically label and isolate a specific class of glycans (such as sialic acids targeted by ManNAc analogs, mucins targeted by GalNAc analogs, or O-GlcNAcylated proteins targeted by GlcNAc analogs) would suffer.
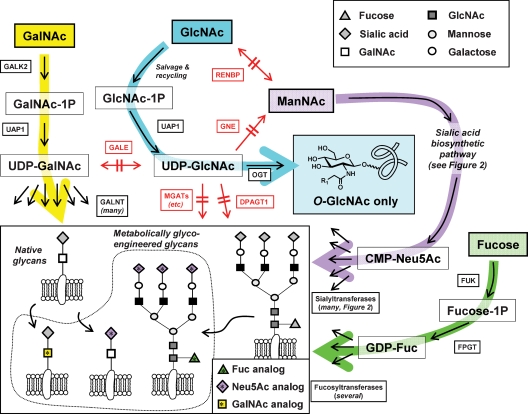
Fig. 6.
Selective pathway utilization of analogs is ensured by the nonpermissivity of enzymes at critical junctures. The interconnectedness of glycosylation pathways linking hexosamines (and fucose) used in metabolic glycoengineering is diagrammed indicating enzymes that are restrictive to analog utilization (the complete names of the enzymes are provided in Table S1 in the Supplementary Data available online). The restrictive nature of key enzymes results in the partitioning of GalNAc, GlcNAc, ManNAc, and fucose analogs into selectively targeted classes of mature glycans (inset, indicated with the starred symbols).
Empirical observations have largely discounted the possibility of unruly or universal partitioning of monosaccharide analogs into nontargeted glycans thus debunking the theoretical possibility that analog targeting is not feasible. Fortuitously, rigorous substrate discrimination of key enzymes situated at critical junctures between various nucleotide sugars facilitates precise targeting to various pathways, with ManNAc, GlcNAc, and GalNAc all experiencing distinctive metabolic fates and selective incorporation into a restricted set of surface-displayed glycans (Figure 6). The ability of pathways to discriminate between hexosamine isomers has spawned exciting developments in metabolic glycoengineering in recent years as the methods pioneered with sialic acid have been extended to additional glycosylation pathways, as summarized next.
Metabolic glycoengineering: Moving beyond sialic acid
GlcNAc
The selective utilization of nonnatural monosaccharide analogs by one (but not by a second or even third) pathway branch is epitomized by GlcNAc. The natural form of this monosaccharide can feed into sialic acid metabolism by one of two mechanisms. First, GlcNAc can be directly converted to ManNAc by GlcNAc 2-epimerase (Luchansky et al. 2003); alternately, it can be phosphorylated and intercept the hexosamine pathway via a salvage mechanism (Hinderlich et al. 2000; Ligos et al. 2002; Weihofen et al. 2006) and subsequently be converted to UDP-GlcNAc, which is the canonical feedstock for sialic acid biosynthesis (see Figure 2). Despite these possible routes, an early experiment showed that GlcNLev – the ketone-derivatized isomer of ManNLev (see Figure 4) – was not incorporated into cell surface elements (Yarema et al. 1998) thereby indicating that this GlcNAc analog was not converted to its ManNAc counterpart and thus was unable to gain access to the sialic acid pathway.
The lack of surface display of ketones in GlcNLev-incubated cells was consistent with a complete inability of these analogs to enter the hexosamine pathway. This possibility, however, was eliminated by a study that showed that GlcNAc analogs do partition into O-GlcNAcylated nucleocytosolic proteins (Khidekel et al. 2004); this result established that GlcNAc analogs do intercept the hexosamine pathway and are converted into the corresponding nonnatural forms of UDP-GlcNAc required for O-GlcNAcylation of proteins (as shown by the OGT-catalyzed reaction in Figure 6). As also outlined in Figure 6, other fates for UDP-GlcNAc besides conversion into ManNAc or use in O-GlcNAcylation include initiation of N-glycan biosynthesis by DPATG1, serving as a substrate for MGAT1/5 “branching” enzymes, or conversion to UDP-GalNAc by GALE. The lack of surface display of GlcNAc analogs indicates that modified forms of UDP-GlcNAc (such as UDP-GlcNLev or UDP-GlcNDAz) are not used by any of the GlcNAc transferase family members; neither are they converted to ManNAc by GNE, and finally, they are not epimerized to UDP-GalNAc. Instead, of the many enzymes that process natural UDP-GlcNAc, only OGT is highly permissive for nonnatural variants of this versatile metabolite.
GalNAc
Unlike GlcNAc analogs that do not gain biosynthetic access to surface glycans, GalNAc analogs are successfully incorporated into cell surface elements via biosynthetic routes. This feat presumably occurs through analog utilization by salvage pathways (e.g., N-acetylgalactosamine 1-kinase, GALK2 (Thoden and Holden 2005) and UDP-GalNAc pyrophophorylase (UAP1 or AGX1) (Wang-Gillam et al. 1998; Bourgeaux et al. 2005)). Once a GalNAc analog is converted to the nucleotide sugar (e.g., an UDP-GalNAc analog), it competes for replacement of natural GalNAc found in O-linked glycans (Hang et al. 2003) and (most likely) GAGs (Hang and Bertozzi 2001).
Fucose
The final monosaccharide that has (so far) shown facile analog incorporation into cell surface glycans in mammals is fucose. On one hand, this ability is not surprising insofar as oral supplementation corrects the metabolic defect found in Type 2 leukocyte adhesion disorder (Marquardt et al. 1999). This result established the ability of exogenous fucose, as well as analogs (Sawa et al. 2006; Hackenberger and Hinderlich 2007), to intercept the biosynthetic pathway and be utilized by the enzymes involved in the installation of fucose into surface glycans (i.e., FUK, FPGT, and a fucosyltransferase, Figure 6). On the other hand, biosynthetic processing of fucose analogs breaks the mold set by amino sugars as exclusive vehicles for metabolic glycoengineering. It is tempting to speculate that the increased biosynthetic permissivity for fucose compared to other hexoses is a consequence of its configuration as a rare l-sugar, compared to the more common d-configuration of other mammalian hexoses and hexosamines.
Precise targeting – Remaining ambiguities
As outlined above, a considerable amount of information describing the metabolic utilization of monosaccharide analogs and their selective partitioning into targeted classes of glycans has been compiled. Many unanswered questions remain, however; for example, do any of the 20 sialyltransferases shown in Figure 2 – or the similar suites of GalNAc- and fucosyltransferases alluded to in Figure 6 – have unique specificities for analogs? To date, evidence points in both directions. On one hand, when natural flux through the sialic acid pathway is restricted, thus biasing the biosynthetic incorporation of nonnatural sialosides such as Sia5Prop (Mantey et al. 2001), up to 85% of the native sialosides can be replaced with their engineered counterparts. Moreover, nonnatural sialic acids have been detected broadly in N-linked and O-linked glycans as well as in GSL; similarly, they occur in the entire range of α2,3-, α2,6-, and α2,8-linked sialosides under conditions of high flux. A more interesting, and so far untested, scenario involves what occurs at very low levels – e.g., 1% or 0.1% – of replacement and asking the question “would the nonnatural metabolites selectively partition into certain classes of glycans when the firehose supplying the pathway is replaced with an eyedropper?” To date, preliminary but intriguing evidence of such discrimination comes from studies that show α2,8-sialyltransferases respond selectively to ManNAc analogs (Mahal et al. 2001; Horstkorte et al. 2004), likely through the interaction with downstream CMP-Neu5Ac metabolites (Miyazaki et al. 2008). If family member-specific analog processing proves to be a general feature of glycosyltransferases, an additional layer of control may be possible that entails specifically directing nonnatural moieties to (or away from) α2,3-, α2,6- or α2,8-linked sialosides situated primarily on proteins or lipids.
Solving a practical problem leads to new challenges and opportunities
Designing analogs for increased efficiency leads to metabolic quirks
An issue faced by a practical-minded metabolic glycoengineer is the generally modest (or even abysmal) efficiency at which nonnatural analogs enter a cell; levels as high as 30–50 millimolar are often needed to maximize cellular responses. In some cases, the biosynthetic pathway cannot be saturated even at 75 or 100 millimolar, at which point cells cultured in vitro suffer nonspecific cytotoxicity due to osmotic imbalance. Moving to in vivo experiments, costs for medium-sized or large animal testing becomes prohibitive. Exploiting an age-old solution – exemplified by aspirin where cell-impermeable hydrophilic compounds are rendered more lipophilic through ester derivatization with short chain fatty acids (SCFA) such as acetate (Lavis 2008) – sugar analogs have been peracetylated to increase cellular uptake (Hadfield et al. 1983; Sarkar et al. 1995; Jones et al. 2004). In the case of ManNAc, evidence that increased lipophilicity enhances uptake was provided by an experiment where propionyl- (Pr4ManNAc) and n-butanoyl- (Bu4ManNAc) analogs were used with increased efficiency compared to peracetylated ManNAc (Ac4ManNAc, Figure 7A) (Kim, Sampathkumar, et al. 2004). Once inside a cell, the SCFA moieties (e.g., acetate, propionate, or n-butyrate, Figure 7B) of O-acylated ManNAc analogs are removed by nonspecific esterases generating four equivalents of SCFA and the core sugar, which can then intercept and transit the targeted biosynthetic pathway (Figure 7C).
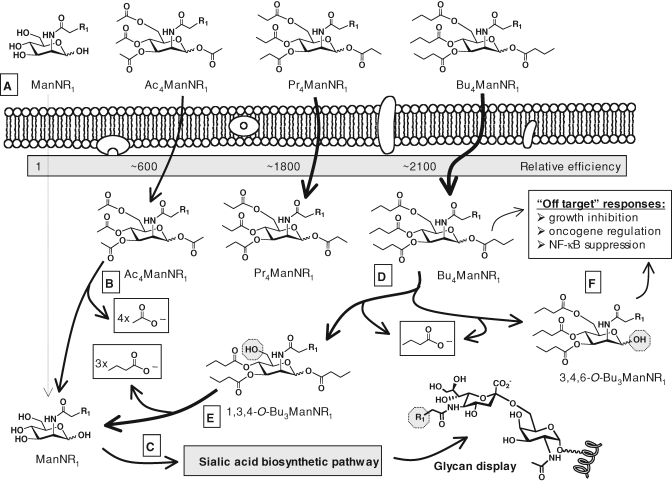
Fig. 7.
SCFA-analog hybrids enhance cellular uptake and endow analogs with novel activities. (A) Peracetylated, perpropionylated, and pern-butanoylated analogs are taken up by cells with progressively greater efficiency, presumably due to enhanced lipophilicity that facilitates transporter-independent cellular uptake (i.e., membrane diffusion). (B) Once inside a cell, the SCFA groups, such as acetate, are removed by nonspecific esterases regenerating the “core” sugar (C) that – in the case of ManNAc – enters the sialic acid pathway en route to surface glycan display (“R1”-modified examples of peracetylated analogs are shown in Figure 5). (D) Perbutanolylated hexosamines, represented by Bu4ManNR1, generate n-butyrate upon esterase processing that can modulate transcription through HDACi activity as well as the core sugar that can access the sialic acid pathway (C). Along the route to complete SCFA hydrolysis, tributanolyated ManNAc isomers elicit dramatically different cellular effects with (E) “1,3,4-O-Bu3” analogs supporting high flux with negligible cytotoxicity while (F) “3,4,6-O-Bu3” isomers trigger a bevy of NF-κB-associated “off-target” responses.
Although SCFA-monosaccharide hybrid molecules have proved to be valuable agents in dozens of studies, puzzling wrinkles arose that threatened the broad use of these compounds. Peracetylated ManNAc analogs (several examples are shown in Figure 5), for example, proved to be mildly toxic in cell culture whereas their fully unprotected counterparts were not (Kim, Sampathkumar, et al. 2004). While a nagging hindrance, especially when trying to achieve robust labeling with very high analog concentrations, analog cytotoxicity has generally been a manageable issue that can be worked around. As an illustration, fluorinated ManNAc analogs can be highly toxic (Hadfield et al. 1983) but their sialylated counterparts were not (Dafik et al. 2008). Moreover, cytotoxicity reported during in vitro experiments has not extended systematically to in vivo experiments.
Nonetheless, the mechanism behind analog toxicity intrigued our laboratory. Obvious explanations, such as detergent activity that disrupted membranes, have been discounted (this possibility was ruled out, in part, by the development of resistant cell lines (Kim, Jones, et al. 2004) and lack of an effect in liposome leakage assays (our laboratory, unpublished results). Similarly, HDACi activity emanating from hydrolyzed SCFA – while playing a minor role – could not explain the potency of various analogs (Sampathkumar, Jones, Meledeo, et al. 2006; Elmouelhi et al. 2009). For example, despite having equal equivalents of SCFA, Ac4ManNLev was considerably more toxic than the non-ketone containing counterpart Ac4ManNPent or Ac4ManNOxoHex where the ketone was moved down the chain one unit (Kim, Sampathkumar, et al. 2004). None of these analogs were toxic in the nonacetylated form or when equimolar concentrations of acetate were added to the culture to more accurate mimic the hydrolysis products of the prodrug. These results suggested that SCFA-ManNAc analogs have structure–activity relationships (SAR) that engage cellular receptors and elicit off-target effects through “scaffold-dependent” or “whole-molecule” mechanisms.
SCFA-monosaccharides exemplify “carbohydrates as a scaffold for drug discovery”
Over the past year, the “scaffold-depend” activity of SCFA-hexosamine hybrid molecules has come into focus through experiments that were initially inspired by the observation that Bu4ManNAc was more effective at killing cancer cells than perbutanoylated mannose (Bu5Man), a compound that only transiently inhibited the growth of the cells (Sampathkumar, Jones, Meledeo, et al. 2006). Similarly, Bu4ManNAc inhibited the invasive tendencies of metastatic breast cancer cells whereas Bu5Man did not (Campbell et al. 2008). Because the hexosamine analog was superior in both respects to Bu5Man, a clinically tested cancer drug candidate that held modest efficacy (Sampathkumar, Campbell, et al. 2006), we anticipated testing Bu4ManNAc in vivo and noted that a tributanoylated hexosamine would have a favorable Lipinski rule of five properties for oral availability and proceeded to evaluate two isomers: 1,3,4-O-Bu3ManNAc (Figure 7E) and 3,4,6-O-Bu3ManNAc (Figure 7F).
Based on long-held dogma that the biological activity of SCFA-hexosamines results from their hydrolysis products, we expected that 1,3,4-O-Bu3ManNAc and 3,4,6-O-Bu3ManNAc would elicit identical cellular responses because they both produce three equivalents of n-butyrate and one equivalent of ManNAc upon complete hydrolysis. Therefore, the dramatically different activities of these isomers, where 1,3,4-O-Bu3ManNAc lacked the off-target responses of 3,4,6-O-Bu3ManNAc but instead provided high flux with negligible toxicity (Aich et al. 2008), were surprising. In relation to metabolic glycoengineering, these SAR will allow researchers to carefully match analog design to an intended application. For example, 1,3,4-O-Bu3ManNLev lacks the toxicity of peracylated ManNLev analogs and supports highly efficient installation of Sia5Lev into surface glycans (Aich et al. 2008). In other situations, exemplified by 3,4,6-O-Bu3ManNAc, the “off-target” responses (Figure 7F) hold attractive anti-cancer properties that may be valuable of themselves or important complements to toxin- (Mahal et al. 1997) or immune-mediated (Liu et al. 2000), analog-based approaches to cancer treatment. More broadly, the SAR exhibited by 1,3,4-O-Bu3ManNAc and 3,4,6-O-Bu3ManNAc provide an outstanding example of the rise of carbohydrates as templates for drug discovery, a topic reviewed in detail elsewhere (Meutermans et al. 2006).
Summary and conclusions
The first two decades of metabolic sialic acid glycoengineering have largely been chemistry driven but with enough biology added to the mix to firmly establish this methodology as a potent new tool for the experimental glycobiologist and pique the interest of the clinician. Moving forward, advances in chemistry are sure to continue as the pace of discovery has accelerated in the synthesis of analogs that target new pathways, enable new bioorthogonal chemical strategies, and endow the analogs with increased efficiency in recent years. Efforts to further understand mechanism are also in full swing as fascinating – albeit vexing – new wrinkles are being thrown to the cell biologist, one of which is the ability of analogs to modulate cell signaling pathways and gene expression by unanticipated mechanisms. Finally, although translation of sialic acid glycoengineering methods to commercial products and the clinic therapies has been painstakingly slow (in part because of ongoing advances in the basic chemistry and the need to understand underlying mechanisms before rationale applications can be pursued) progress is now being made on both fronts and we foresee an optimistic future for this burgeoning technology.
Supplementary Data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
The National Institutes of Health for financial support (EB005692-03) and (CA112314-04).
Conflict of interest statement
None declared.
Glossary
Abbreviation
- GSL
glycoshingolipids
- NCAM
neural cell adhesion molecule
- PSA
polysialic acid
- TACA
tumor-associated carbohydrate antigens
References
- Agard NJ, Prescher JA, Bertozzi CR. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J Am Chem Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- Aich U, Campbell CT, Elmouelhi N, Weier CA, Sampathkumar S-G, Choi SS, Yarema KJ. Regioisomeric SCFA attachment to hexosamines separates metabolic flux from cytotoxcity and MUC1 suppression. ACS Chem Biol. 2008;3:230–240. doi: 10.1021/cb7002708. [DOI] [PubMed] [Google Scholar]
- Aich U, Yarema KJ. Metabolic oligosaccharide engineering: Perspectives, applications, and future directions. In: Fraser-Reid B, Tatsuta K, Thiem J, editors. Glycosciences. Berlin Heidelberg: Springer-Verlag; 2008. pp. 2136–2190. [Google Scholar]
- Allende ML, Proia RL. Lubricating cell signaling pathways with gangliosides. Curr Opin Struct Biol. 2002;12:587–592. doi: 10.1016/s0959-440x(02)00376-7. [DOI] [PubMed] [Google Scholar]
- Angata K, Fukuda M. Polysialyltransferases: Major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie. 2003;85:195–206. doi: 10.1016/s0300-9084(03)00051-8. [DOI] [PubMed] [Google Scholar]
- Angata T, Varki A. Chemical diversity in the sialic acids and related α-keto acids: An evolutionary perspective. Chem Rev. 2002;102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci USA. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich E, MacKinnon S, Silva J, Yu RK. Regulation of apoptosis during neuronal differentiation by ceramide and b-series complex gangliosides. J Biol Chem. 2001;276:44396–44404. doi: 10.1074/jbc.M107239200. [DOI] [PubMed] [Google Scholar]
- Bistrup A, Bhakta S, Lee JK, Belov YY, Gunn MD, Zuo F-R, Chiao-Chain H, Kannagi R, Rosen SD, Hemmerich S. Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for l-selectin. J Cell Biol. 1999;145:899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blix G. Über die Kohlenhydratgruppen des Submaxillarismucins. Hoppe-Seyler's Z Physiol Chem. 1936;240:43–54. [Google Scholar]
- Blix G, Gottschalk A, Klenk E. Proposed nomenclature in the field of neuraminic and sialic acids. Nature. 1957;179:1088. doi: 10.1038/1791088b0. [DOI] [PubMed] [Google Scholar]
- Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280:4307–4312. doi: 10.1074/jbc.M412378200. [DOI] [PubMed] [Google Scholar]
- Boeggeman E, Ramakrishnan B, Pasek M, Manzoni M, Puri A, Loomis KH, Waybright TJ, Qasba PK. Site specific conjugation of fluoroprobes to the remodeled Fc N-glycans of monoclonal antibodies using mutant glycosyltransferases: Application for cell surface antigen detection. Bioconjug Chem. 2009;20:1228–1236. doi: 10.1021/bc900103p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond MR, Zhang H, Vu PD, Kohler JJ. Photocrosslinking of glycoconjugates using metabolically incorporated diazirine-containing sugars. Nat Protoc. 2009;4:1044–1063. doi: 10.1038/nprot.2009.85. [DOI] [PubMed] [Google Scholar]
- Bork K, Reutter W, Gerardy-Schahn R, Horstkorte R. The intracellular concentration of sialic acid regulates the polysialylation of neural cell adhesion molecule. FEBS Lett. 2005;579:5079–5083. doi: 10.1016/j.febslet.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Bourgeaux V, Piller F, Piller V. Two-step enzymatic synthesis of UDP-N-acetylgalactosamine. Bioorg Med Chem Lett. 2005;15:5459–5462. doi: 10.1016/j.bmcl.2005.08.088. [DOI] [PubMed] [Google Scholar]
- Breen KC, Georgopoulou N. The role of protein phosphorylation in α2,6(N)-sialyltransferase activity. Biochem Biophys Res Commun. 2003;309:32–35. doi: 10.1016/s0006-291x(03)01529-8. [DOI] [PubMed] [Google Scholar]
- Brinkman-Van der Linden EC, Sjoberg ER, Juneja LR, Crocker PR, Varki N, Varki A. Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs. J Biol Chem. 2000;275:8633–8640. doi: 10.1074/jbc.275.12.8633. [DOI] [PubMed] [Google Scholar]
- Brossmer R, Gross HJ. Fluorescent and photoactivatable sialic acids. Meth Enzymol. 1994;247:177–193. doi: 10.1016/s0076-6879(94)47014-6. [DOI] [PubMed] [Google Scholar]
- Brusés JL, Rutishauser U. Roles, regulation, and mechanism of polysialic acid function during neural development. Biochimie. 2001;83:635–643. doi: 10.1016/s0300-9084(01)01293-7. [DOI] [PubMed] [Google Scholar]
- Campbell CT, Aich U, Weier CA, Wang JJ, Choi SS, Wen MM, Maisel K, Sampathkumar S-G, Yarema KJ. Targeting pro-invasive oncogenes with short chain fatty acid-hexosamine analogs inhibits the mobility of metastatic MDA-MB-231 breast cancer cell. J Med Chem. 2008;51:8135–8147. doi: 10.1021/jm800873k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CT, Sampathkumar S-G, Weier C, Yarema KJ. Metabolic oligosaccharide engineering: Perspectives, applications, and future directions. Mol Biosyst. 2007;3:187–194. doi: 10.1039/b614939c. [DOI] [PubMed] [Google Scholar]
- Campbell CT, Yarema KJ. Large-scale approaches for glycobiology. Gen Biol. 2005;6:236. doi: 10.1186/gb-2005-6-11-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefalo P, Pan Y, Nagy N, Guo Z, Harding CV. Efficient metabolic engineering of GM3 on tumor cells by N-phenylacetyl-d-mannosamine. Biochemistry. 2006;45:3733–3739. doi: 10.1021/bi052161r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefalo P, Pan Y-B, Nagy N, Harding C, Guo Z-W. Preparation and immunological studies of protein conjugates of N-acylneuraminic acids. Glycoconj J. 2004;20:407–414. doi: 10.1023/B:GLYC.0000033997.01760.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Challa AK, Varki A. 9-O-Acetylation of exogenously added ganglioside GD3. The GD3 molecule induces its own O-acetylation machinery. J Biol Chem. 2006;281:7825–7833. doi: 10.1074/jbc.M512379200. [DOI] [PubMed] [Google Scholar]
- Cheresh DA, Reisfeld RA, Varki A. O-Acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science. 1984;225:844–846. doi: 10.1126/science.6206564. [DOI] [PubMed] [Google Scholar]
- Chou HH, Hayakawa T, Diaz S, Krings M, Indriati E, Leakey M, Paabo S, Satta Y, Takahata N, Varki A. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci USA. 2002;99:11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BE, Fralich TJ, Itonori S, Ichikawa Y, Schnaar RL. Conversion of cellular sialic acid expression from N-acetyl- to N-glycolylneuraminic acid using a synthetic precursor, N-glycolylmannosamine pentaacetate: Inhibition of myelin-associated glycoprotein binding to neural cells. Glycobiology. 2000;10:11–20. doi: 10.1093/glycob/10.1.11. [DOI] [PubMed] [Google Scholar]
- Comb DG, Roseman S. The sialic acids: I. The structure and enzymatic synthesis of N-acetylneuraminic acid. J Biol Chem. 1960;235:2529–2537. [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Varki A. Siglecs in the immune system. Immunology. 2001;103:137–145. doi: 10.1046/j.0019-2805.2001.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafik L, d’Alarcao M, Kumar K. Fluorination of mammalian cell surfaces via the sialic acid biosynthetic pathway. Bioorg Med Chem Lett. 2008;18:5945–5947. doi: 10.1016/j.bmcl.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam TK, Brewer CF. Thermodynamic studies of lectin–carbohydrate interactions by isothermal titration calorimetry. Chem Rev. 2002;102:387–429. doi: 10.1021/cr000401x. [DOI] [PubMed] [Google Scholar]
- Daubeuf S, Aucher A, Sampathkumar SG, Preville X, Yarema KJ, Hudrisier D. Chemical labels metabolically installed into the glycoconjugates of the target cell surface can be used to track lymphocyte/target cell interplay via trogocytosis: Comparisons with lipophilic dyes and biotin. Immunol Invest. 2007;36:687–712. doi: 10.1080/08820130701674596. [DOI] [PubMed] [Google Scholar]
- De Bank PA, Kellam B, Kendall DA, Shakesheff KM. Surface engineering of living myoblasts via selective periodate oxidation. Biotechnol Bioeng. 2003;81:800–808. doi: 10.1002/bit.10525. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Mühlenhoff M, Bethe A, Gerardy-Schahn R. Expression cloning of the Golgi CMP-sialic acid transporter. Proc Natl Acad Sci USA. 1996;93:7572–7576. doi: 10.1073/pnas.93.15.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, Barash M, Shemesh M, Sadeh M, Grabov-Nardin G, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–87. doi: 10.1038/ng718. [DOI] [PubMed] [Google Scholar]
- Elmouelhi N, Aich U, Paruchuri VDP, Meledeo MA, Campbell CT, Wang JJ, Srinivas R, Khanna HS, Yarema KJ. Hexosamine template. A platform for modulating gene expression and for sugar-based drug discovery. J Med Chem. 2009;52:2515–2530. doi: 10.1021/jm801661m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure C, Chalazonitis A, Rhéaume C, Bouchard G, Sampathkumar S-G, Yarema KJ, Gershon MD. Gangliogenesis in the enteric nervous system: Roles of the polysialylation of the neural cell adhesion molecule and its regulation by bone morphogenetic protein-4. Dev Dyn. 2007;236:44–59. doi: 10.1002/dvdy.20943. [DOI] [PubMed] [Google Scholar]
- Fernandes Filho JA, Shapiro BE. Tay-Sachs disease. Arch Neurol. 2004;61:1466–1468. doi: 10.1001/archneur.61.9.1466. [DOI] [PubMed] [Google Scholar]
- Frand AR, Cuozzo JW, Kaiser CA. Pathways for protein disulphide bond formation. Trends Cell Biol. 2000;10:203–210. doi: 10.1016/s0962-8924(00)01745-1. [DOI] [PubMed] [Google Scholar]
- Gabius HJ. Glycans: Bioactive signals decoded by lectins. Biochem Soc Trans. 2008;36:1491–1496. doi: 10.1042/BST0361491. [DOI] [PubMed] [Google Scholar]
- Gabius H-J, Siebert H-C, André S, Jiménez-Barbero J, Rüdiger H. Chemical biology of the sugar code. ChemBioChem. 2004;5:740–764. doi: 10.1002/cbic.200300753. [DOI] [PubMed] [Google Scholar]
- Gagiannis D, Gossrau R, Reutter W, Zimmermann-Kordmann M, Horstkorte R. Engineering the sialic acid in organs of mice using N-propanoylmannosamine. Biochim Biophys Acta. 2007;1770:297–306. doi: 10.1016/j.bbagen.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Galeano B, Klootwijk R, Manoli I, Sun M, Ciccone C, Darvish D, Starost MF, Zerfas PM, Hoffmann VJ, Hoogstraten-Miller S, et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest. 2007;117:1585–1594. doi: 10.1172/JCI30954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goon S, Bertozzi CR. Metabolic substrate engineering as a tool for glycobiology. J Carbohydr Chem. 2002;21:943–977. [Google Scholar]
- Goon S, Schilling B, Tullius MV, Gibson BW, Bertozzi CR. Metabolic incorporation of unnatural sialic acids into Haemophilus ducreyi lipooligosaccharides. Proc Natl Acad Sci USA. 2003;18:3089–3094. doi: 10.1073/pnas.0437851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross HJ, Brossmer R. Enzymatic introduction of a fluorescent sialic acid into oligosaccharide chains of glycoproteins. Eur J Biochem. 1988;177:583–589. doi: 10.1111/j.1432-1033.1988.tb14410.x. [DOI] [PubMed] [Google Scholar]
- Gross HJ, Brossmer R. Enzymatic transfer of sialic acids modified at C-5 employing four different sialyltransferases. Glycoconj J. 1995;12:739–746. doi: 10.1007/BF00731233. [DOI] [PubMed] [Google Scholar]
- Gross HJ, Rose U, Krause JM, Paulson JC, Schmid K, Feeny RE, Brossmer R. Transfer of synthetic sialic acid analogues to N- and O-linked glycoprotein glycans using four different mammalian sialyltransferases. Biochemistry. 1989;28:7386–7392. doi: 10.1021/bi00444a036. [DOI] [PubMed] [Google Scholar]
- Gross HJ. Fluorescent CMP-sialic acids as a tool to study the specificity of the CMP-sialic acid carrier and the glycoconjugate sialylation in permeabilized cells. Eur J Biochem. 1992;203:269–275. doi: 10.1111/j.1432-1033.1992.tb19856.x. [DOI] [PubMed] [Google Scholar]
- Gurcel C, Vercoutter-Edouart A-S, Fonbonne C, Mortuaire M, Salvador A, Michalski J-C, Lemoine J. Identification of new O-GlcNAc modified proteins using a click-chemistry-based tagging. Anal Bioanal Chem. 2008;390:2089–2097. doi: 10.1007/s00216-008-1950-y. [DOI] [PubMed] [Google Scholar]
- Hackenberger CPR, Hinderlich S. Chemoselective labeling of engineered fucosylated glycoproteins. ChemBioChem. 2007;8:1763–1765. doi: 10.1002/cbic.200700417. [DOI] [PubMed] [Google Scholar]
- Hadfield AF, Mella SL, Sartorelli AC. N-acetyl-d-mannosamine analogues as potential inhibitors of sialic acid biosynthesis. J Pharm Sci. 1983;72:748–751. doi: 10.1002/jps.2600720709. [DOI] [PubMed] [Google Scholar]
- Hakomori S-I. The glycosynapse. Proc Natl Acad Sci USA. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Collins BE, Bengtson P, Paulson JC. Homo-multimeric complexes of CD22 revealed by in situ photoaffinity protein-glycan crosslinking. Nat Chem Biol. 2005;1:93–97. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- Hanai N, Dohi T, Nores GA, Hakomori S. A novel ganglioside, de-N-acetyl-GM3 (II3NeuNH2LacCer), acting as a strong promoter for epidermal growth factor receptor kinase and as a stimulator for cell growth. J Biol Chem. 1998;263:6296–6301. [PubMed] [Google Scholar]
- Hang HC, Bertozzi CR. Ketone isoteres of 2-N-acetamidosugars as substrates for metabolic cell surface engineering. J Am Chem Soc. 2001;123:1242–1243. doi: 10.1021/ja002962b. [DOI] [PubMed] [Google Scholar]
- Hang HC, Yu C, Kato DL, Bertozzi CR. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc Natl Acad Sci USA. 2003;100:14846–14851. doi: 10.1073/pnas.2335201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JA. Glycan-dependent signaling: O-Linked N-acetylglucosamine. FASEB J. 2001;15:1865–1876. doi: 10.1096/fj.01-0094rev. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Goto S, Kawano S, Aoki-Kinoshita KF, Ueda N, Hamajima M, Kawasaki T, Kanehisa M. KEGG as a glycome informatics resource. Glycobiology. 2006;16:63R–70R. doi: 10.1093/glycob/cwj010. [DOI] [PubMed] [Google Scholar]
- Hayrinen J, Jennings H, Raff HV, Rougon G, Hanai N, Gerardy-Schahn R, Finne J. Antibodies to polysialic acid and its N-propyl derivative: Binding properties and interaction with human embryonal brain glycopeptides. J Infect Dis. 1995;171:1481–1490. doi: 10.1093/infdis/171.6.1481. [DOI] [PubMed] [Google Scholar]
- Hedlund M, Padler-Karavani V, Varki NM, Varki A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci USA. 2008;105:18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrler G, Gross HJ, Milks G, Paulson JC, Klenk HD, Brossmer R. Use of a sialic acid analogue to analyze the importance of the receptor-destroying enzyme for the interaction of influenza C virus with cells. Acta Histochem Suppl. 1990;40:39–41. [PubMed] [Google Scholar]
- Higa HH, Rogers GN, Paulson JC. Influenza virus hemagglutinins differentiate between receptor determinants bearing N-acetyl-, N-glycolyl-, and N,O-diacetylneuraminic acids. Virology. 1985;144:279–282. doi: 10.1016/0042-6822(85)90325-3. [DOI] [PubMed] [Google Scholar]
- Hinderlich S, Berger M, Schwarzkopf M, Effertz K, Reutter W. Molecular cloning and characterization of murine and human N-acetylglucosamine kinase. Eur J Biochem. 2000;267:3301–3308. doi: 10.1046/j.1432-1327.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- Horstkorte R, Mühlenhoff M, Reutter W, Nöhring S, Zimmermann-Kordmann M, Gerardy-Schahn R. Selective inhibition of polysialyltransferase ST8SiaII by unnatural sialic acids. Exp Cell Res. 2004;298:268–274. doi: 10.1016/j.yexcr.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Hsu T-L, Hanson SR, Kishikawa K, Wang S-K, Sawa M, Wong C-H. Alkynyl sugar analogs for the labeling and visualization of glycoconjugates in cells. Proc Natl Acad Sci USA. 2007;104:2614–2619. doi: 10.1073/pnas.0611307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273:15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Tabata E, Kurita K, Akiyoshi K. Selective cell attachment to a biomimetic polymer surface through the recognition of cell-surface tags. Bioconjug Chem. 2005;16:567–575. doi: 10.1021/bc049707r. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Maie H, Akiyoshi K. Cell-specific delivery of polymeric nanoparticles to carbohydrate-tagging cells. Biomacromolecules. 2007;8:3162–3168. doi: 10.1021/bm700606z. [DOI] [PubMed] [Google Scholar]
- Jacobs CL, Goon S, Yarema KJ, Hinderlich S, Hang HC, Chai DH, Bertozzi CR. Substrate specificity of the sialic acid biosynthetic pathway. Biochemistry. 2001;40:12864–12874. doi: 10.1021/bi010862s. [DOI] [PubMed] [Google Scholar]
- Jin ES, Jones JG, Merritt M, Burgess SC, Malloy CR, Sherry AD. Glucose production, gluconeogenesis, and hepatic tricarboxylic acid cycle fluxes measured by nuclear magnetic resonance analysis of a single glucose derivative. Anal Biochem. 2004;327:149–155. doi: 10.1016/j.ab.2003.12.036. [DOI] [PubMed] [Google Scholar]
- Jones MB, Teng H, Rhee JK, Baskaran G, Lahar N, Yarema KJ. Characterization of the cellular uptake and metabolic conversion of acetylated N-acetylmannosamine (ManNAc) analogues to sialic acids. Biotechnol Bioeng. 2004;85:394–405. doi: 10.1002/bit.10901. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, Katayama T, Araki M, Hirakawa M. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 2006;34:D354–D357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R. Regulatory roles of carbohydrate ligands for selectins in the homing of lymphocytes. Curr Opin Struct Biol. 2002;12:599–608. doi: 10.1016/s0959-440x(02)00365-2. [DOI] [PubMed] [Google Scholar]
- Kayser H, Zeitler R, Kannicht C, Grunow D, Nuck R, Reutter W. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-d-hexosamines as precursors. J Biol Chem. 1992a;267:16934–16938. [PubMed] [Google Scholar]
- Kayser H, Geilen CC, Paul C, Zeitler R, Reutter W. Incorporation of N-acyl-2-amino-2-deoxy-hexoses into glycosphingolipids of the pheochromocytoma cell line PC 12. FEBS Lett. 1992b;301:137–140. doi: 10.1016/0014-5793(92)81233-c. [DOI] [PubMed] [Google Scholar]
- Keppler OT, Hinderlich S, Langner J, Schwartz-Albiez R, Reutter W, Pawlita M. UDP-GlcNAc 2-epimerase: A regulator of cell surface sialylation. Science. 1999;284:1372–1376. doi: 10.1126/science.284.5418.1372. [DOI] [PubMed] [Google Scholar]
- Keppler OT, Horstkorte R, Pawlita M, Schmidt C, Reutter W. Biochemical engineering of the N-acyl side chain of sialic acid: Biological implications. Glycobiology. 2001;11:11R–18R. doi: 10.1093/glycob/11.2.11r. [DOI] [PubMed] [Google Scholar]
- Keppler OT, Stehling P, Herrmann M, Kayser H, Grunow D, Reutter W, Pawlita M. Biosynthetic modulation of sialic acid-dependent virus-receptor interactions of two primate polyoma viruses. J Biol Chem. 1995;270:1308–1314. doi: 10.1074/jbc.270.3.1308. [DOI] [PubMed] [Google Scholar]
- Khidekel N, Arndt S, Lamarre-Vincent N, Lippert A, Poulin-Kerstien KG, Ramakrishnan B, Qasba PK, Hsieh-Wilson LC. A chemoenzymatic approach toward the rapid and sensitive detection of O-GlcNAc posttranslational modifications. J Am Chem Soc. 2003;125(52):16162–16163. doi: 10.1021/ja038545r. [DOI] [PubMed] [Google Scholar]
- Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Exploring the O-GlcNAc proteome: Direct identification of O-GlcNAc-modified proteins from the brain. Proc Natl Acad Sci USA. 2004;101:13132–13137. doi: 10.1073/pnas.0403471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Jones MB, Rhee JK, Sampathkumar S-G, Yarema KJ. Establishment of N-acetylmannosamine (ManNAc) analogue-resistant cell lines as improved hosts for sialic acid engineering applications. Biotechnol Prog. 2004;20:1674–1682. doi: 10.1021/bp049841q. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Sampathkumar S-G, Jones MB, Rhee JK, Baskaran G, Yarema KJ. Characterization of the metabolic flux and apoptotic effects of O-hydroxyl- and N-acetylmannosamine (ManNAc) analogs in Jurkat (human T-lymphoma-derived) cells. J Biol Chem. 2004;279:18342–18352. doi: 10.1074/jbc.M400205200. [DOI] [PubMed] [Google Scholar]
- Klenk E. Neuraminsäure, das Spaltprodukt eines neuen Gehirnlipoids. Hoppe-Seyler's Z Physiol Chem. 1941;268:50–58. [Google Scholar]
- Kontou M, Bauer C, Reutter W, Horstkorte R. Sialic acid metabolism is involved in the regulation of gene expression during neuronal differentiation of PC12 cells. Glycoconj J. 2008;25:237–244. doi: 10.1007/s10719-008-9104-1. [DOI] [PubMed] [Google Scholar]
- Ladeby R, Wirenfeldt M, Dalmau I, Gregersen R, García-Ovejero D, Babcock A, Owens T, Finsen B. Proliferating resident microglia express the stem cell antigen CD34 in response to acute neural injury. Glia. 2005;50:121–131. doi: 10.1002/glia.20159. [DOI] [PubMed] [Google Scholar]
- Lanza F, Healy L, Sutherland DR. Structural and functional features of the CD34 antigen: An update. J Biol Regul Homeost Agents. 2001;15:1–13. [PubMed] [Google Scholar]
- Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavis LD. Ester bonds in prodrugs. ACS Chem Biol. 2008;3:203–206. doi: 10.1021/cb800065s. [DOI] [PubMed] [Google Scholar]
- Lawrence SM, Huddleston KA, Pitts LR, Nguyen N, Lee YC, Vann WF, Coleman TA, Betenbaugh MJ. Cloning and expression of the human N-acetylneuraminic acid phosphate synthase gene with 2-keto-3-deoxy-d-glycero-d-galacto-nononic acid biosynthetic ability. J Biol Chem. 2000;275:17869–17877. doi: 10.1074/jbc.M000217200. [DOI] [PubMed] [Google Scholar]
- Lee JH, Baker TJ, Mahal LK, Zabner J, Bertozzi CR, Wiemar DF, Welsh MJ. Engineering novel cell surface receptors for virus-mediated gene transfer. J Biol Chem. 1999;274:21878–21884. doi: 10.1074/jbc.274.31.21878. [DOI] [PubMed] [Google Scholar]
- Lemieux GA, Bertozzi CR. Chemoselective ligation reactions with proteins, oligosaccharides and cells. Trends Biotechnol. 1998;16:506–513. doi: 10.1016/s0167-7799(98)01230-x. [DOI] [PubMed] [Google Scholar]
- Lemieux GA, Bertozzi CR. Modulating cell surface immunoreactivity by metabolic induction of unnatural carbohydrate antigens. Chem Biol. 2001;8:265–275. doi: 10.1016/s1074-5521(01)00008-4. [DOI] [PubMed] [Google Scholar]
- Lemieux GA, Yarema KJ, Jacobs CL, Bertozzi CR. Exploiting differences in sialoside expression for selective targeting of MRI contrast reagents. J Am Chem Soc. 1999;121:4278–4279. [Google Scholar]
- Li Y-T, Maskos K, Chou C-W, Cole RB, Li S-C. Presence of an unusual GM2 derivative, taurine-conjugated GM2, in Tay–Sachs brain. J Biol Chem. 2003;278:35286–35291. doi: 10.1074/jbc.M306126200. [DOI] [PubMed] [Google Scholar]
- Ligos JM, de Lera TL, Hinderlich S, Guinea B, Sánchez L, Roca R, Valencia A, Bernad A. Functional interaction between the Ser/Thr kinase PKL12 and N-acetylglucosamine kinase, a prominent enzyme implicated in the salvage pathway for GlcNAc recycling. J Biol Chem. 2002;277:6333–6343. doi: 10.1074/jbc.M105766200. [DOI] [PubMed] [Google Scholar]
- Liu T, Guo Z, Yang Q, Sad S, Jennings HJ. Biochemical engineering of surface α2,8 polysialic acid for immunotargeting tumor cells. J Biol Chem. 2000;275:32832–32836. doi: 10.1074/jbc.C000573200. [DOI] [PubMed] [Google Scholar]
- Luchansky SJ, Yarema KJ, Takahashi S, Bertozzi CR. GlcNAc 2-epimerase can serve a catabolic role in sialic acid metabolism. J Biol Chem. 2003;278:8036–8042. doi: 10.1074/jbc.M212127200. [DOI] [PubMed] [Google Scholar]
- Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev. 2002;102:555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- Magnani JL. The discovery, biology, and drug development of sialyl Lea and sialyl Lex. Arch Biochem Biophys. 2004;426:122–131. doi: 10.1016/j.abb.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Mahal LK, Charter NW, Angata K, Fukuda M, Koshland DE, Jr, Bertozzi CR. A small-molecule modulator of poly-α2,8-sialic acid expression on cultured neurons and tumor cells. Science. 2001;294:380–382. doi: 10.1126/science.1062192. [DOI] [PubMed] [Google Scholar]
- Mahal LK, Yarema KJ, Bertozzi CR. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- Mahal LK, Yarema KJ, Lemieux GA, Bertozzi CR. Chemical approaches to glycobiology: Engineering cell surface sialic acids for tumor targeting. In: Inoue Y, Lee YC, Troy FA II, editors. Sialobiology and Other Novel Forms of Glycosylation. Osaka (Japan): Gakushin Publishing Company; 1999. pp. 273–280. [Google Scholar]
- Malicdan MCV, Noguchi S, Hayashi YK, Nonaka I, Nishino I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med. 2009;15:690–695. doi: 10.1038/nm.1956. [DOI] [PubMed] [Google Scholar]
- Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001;83:623–634. doi: 10.1016/s0300-9084(01)01303-7. [DOI] [PubMed] [Google Scholar]
- Mantey LR, Keppler OT, Pawlita M, Reutter W, Hinderlich S. Efficient biochemical engineering of cellular sialic acids using an unphysiological sialic acid precursor in cells lacking UDP-N-acetylglucosamine 2-epimerase. FEBS Lett. 2001;503:80–84. doi: 10.1016/s0014-5793(01)02701-6. [DOI] [PubMed] [Google Scholar]
- Marquardt T, John K, Freeze HH, Harns E, Vesteweber D. Correction of leukocyte adhesion deficiency type II with oral fucose. Blood. 1999;94:3976–3985. [PubMed] [Google Scholar]
- Martin SE, Peterson BR. Non-natural cell surface receptors: Synthetic peptides capped with N-cholesterylglycine efficiently deliver proteins into mammalian cells. Bioconjug Chem. 2003;14:67–74. doi: 10.1021/bc025601p. [DOI] [PubMed] [Google Scholar]
- McCarty OJT, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood. 2000;96:1789–1797. [PubMed] [Google Scholar]
- Meutermans W, Le GT, Becker B. Carbohydrates as scaffolds in drug discovery. ChemMedChem. 2006;1:1164–1194. doi: 10.1002/cmdc.200600150. [DOI] [PubMed] [Google Scholar]
- Mitsuoka C, Ohmori K, Kimura N, Kanamori A, Komba S, Ishida H, Kiso M, Kannagi R. Regulation of selectin binding activity by cyclization of sialic acid moiety of carbohydrate ligands on human leukocytes. Proc Natl Acad Sci USA. 1999;96:1597–1602. doi: 10.1073/pnas.96.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Angata K, Seeberger PH, Hindsgaul O, Fukuda M. CMP substitutions preferentially inhibit polysialic acid synthesis. Glycobiology. 2008;18:187–194. doi: 10.1093/glycob/cwm132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monica TJ, Andersen DC, Goochee CF. A mathematical model of sialylation of N-linked oligosaccharides in the trans-Golgi network. Glycobiology. 1997;7:515–521. doi: 10.1093/glycob/7.4.515. [DOI] [PubMed] [Google Scholar]
- Morgan BL, Winick M. Effects of administration of N-acetylneuraminic acid (NANA) on brain NANA content and behavior. J Nutr. 1980;110:416–424. doi: 10.1093/jn/110.3.416. [DOI] [PubMed] [Google Scholar]
- Mühlenhoff M, Eckhardt M, Gerardy-Schahn R. Polysialic acid: Three-dimensional structure, biosynthesis, and function. Curr Opin Struct Biol. 1998;8:558–564. doi: 10.1016/s0959-440x(98)80144-9. [DOI] [PubMed] [Google Scholar]
- Murrell MP, Yarema KJ, Levchenko A. The systems biology of glycosylation. ChemBioChem. 2004;5:1334–1447. doi: 10.1002/cbic.200400143. [DOI] [PubMed] [Google Scholar]
- Nandi A, Sprung R, Barma DK, Zhao Y, Kim SC, Falck JR, Zhao Y. Global identification of O-GlcNAc-modified proteins. Anal Chem. 2006;78:452–458. doi: 10.1021/ac051207j. [DOI] [PubMed] [Google Scholar]
- Nauman DA, Bertozzi CR. Kinetic parameters for small-molecule drug delivery by covalent cell surface targeting. Biochim Biophys Acta. 2001;1568:147–154. doi: 10.1016/s0304-4165(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Oetke C, Brossmer R, Mantey LR, Hinderlich S, Isecke R, Reutter W, Keppler OT, Pawlita M. Versatile biosynthetic engineering of sialic acid in living cells using synthetic sialic acid analogues. J Biol Chem. 2002;277:6688–6695. doi: 10.1074/jbc.M109973200. [DOI] [PubMed] [Google Scholar]
- Oetke C, Hinderlich S, Brossmer R, Reutter W, Pawlita M, Keppler OT. Evidence for efficient uptake and incorporation of sialic acid by eukaryotic cells. Eur J Biochem. 2001;268:4553–4561. doi: 10.1046/j.1432-1327.2001.02379.x. [DOI] [PubMed] [Google Scholar]
- Qasba PK, Boeggeman E, Ramakrishnan B. Site-specific linking of biomolecules via glycan residues using glycosyltransferases. Biotechnol Prog. 2008;24:520–526. doi: 10.1021/bp0704034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout D. Self-assembling cytotoxins. Science. 1986;233:561–563. doi: 10.1126/science.3523757. [DOI] [PubMed] [Google Scholar]
- Rideout D, Calogeropoulou T, Jaworski J, McCarthy M. Synergism through direct covalent bonding between agents: A strategy for rational design of chemotherapeutic combinations. Biopolymers. 1990;29:247–262. doi: 10.1002/bip.360290129. [DOI] [PubMed] [Google Scholar]
- Sampathkumar S-G, Campbell CT, Weier C, Yarema KJ. Short-chain fatty acid-hexosamine cancer prodrugs: The sugar matters! Drug Future. 2006;31:1099–1116. [Google Scholar]
- Sampathkumar S-G, Jones MB, Meledeo MA, Campbell CT, Choi SS, Hida K, Gomutputra P, Sheh A, Gilmartin T, Head SR, et al. Targeting glycosylation pathways and the cell cycle: Sugar-dependent activity of butyrate-carbohydrate cancer prodrugs. Chem Biol. 2006;13:1265–1275. doi: 10.1016/j.chembiol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Sampathkumar S-G, Jones MB, Yarema KJ. Metabolic expression of thiol-derivatized sialic acids on the cell surface and their quantitative estimation by flow cytometry. Nat Protoc. 2006;1:1840–1851. doi: 10.1038/nprot.2006.252. [DOI] [PubMed] [Google Scholar]
- Sampathkumar S-G, Li AV, Jones MB, Sun Z, Yarema KJ. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat Chem Biol. 2006;2:149–152. doi: 10.1038/nchembio770. [DOI] [PubMed] [Google Scholar]
- Sampathkumar S-G, Li A, Yarema KJ. Sialic acid and the central nervous system: Perspectives on biological functions, detection, imaging methods and manipulation. CNS Neurol Disord – Drug Targets. 2006a;5:425–440. doi: 10.2174/187152706777950729. [DOI] [PubMed] [Google Scholar]
- Sampathkumar S-G, Li AV, Yarema KJ. Synthesis of non-natural ManNAc analogs for the expression of thiols on cell surface sialic acids. Nat Protoc. 2006b;1:2377–2385. doi: 10.1038/nprot.2006.319. [DOI] [PubMed] [Google Scholar]
- Sarkar AK, Fritz TA, Taylor WH, Esko JD. Disaccharide uptake and priming in animal cells: Inhibition of sialyl Lewis X by acetylated Gal β1,4GlcNAc β-O-naphthalenemethanol. Proc Natl Acad Sci USA. 1995;92:3323–3327. doi: 10.1073/pnas.92.8.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa M, Hsu T-L, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong C-H. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Natl Acad Sci USA. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- Saxon E, Luchansky SJ, Hang HC, Yu C, Lee SC, Bertozzi CR. Investigating cellular metabolism of synthetic azidosugars with the Staudinger ligation. J Am Chem Soc. 2002;124:14893–14902. doi: 10.1021/ja027748x. [DOI] [PubMed] [Google Scholar]
- Schauer R. Achievements and challenges of sialic acid research. Glycoconj J. 2000;17:485–499. doi: 10.1023/A:1011062223612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling B, Goon S, Samuels NM, Gaucher SP, Leary JA, Bertozzi CR, Gibson BW. Biosynthesis of sialylated lipooligosaccharides in Haemophilus ducreyi is dependent on exogenous sialic acid and not mannosamine. Incorporation studies using N-acylmannosamine analogues, N-glycolylneuraminic acid, and 13C-labeled N-acetylneuraminic acid. Biochemistry. 2001;40:12666–12677. doi: 10.1021/bi0107849. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Stehling P, Schnitzer J, Reutter W, Horstkorte R. Biochemical engineering of neural cell surfaces by the synthetic N-propanoyl-substituted neuraminic acid precursor. J Biol Chem. 1998;273:19146–19152. doi: 10.1074/jbc.273.30.19146. [DOI] [PubMed] [Google Scholar]
- Schnaar RL. Glycolipid-mediated cell–cell recognition in inflammation and nerve regeneration. Arch Biochem Biophys. 2004;426:163–172. doi: 10.1016/j.abb.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Schnaar RL, Freeze HH. A “glyconutrient sham”. Glycobiology. 2008;18:652–657. doi: 10.1093/glycob/cwm098. [DOI] [PubMed] [Google Scholar]
- Schwartz EL, Hadfield AF, Brown AE, Sartorelli AC. Modification of sialic acid metabolism of murine erythroleukemia cells by analogs of N-acetylmannosamine. Biochim Biophys Acta. 1983;762:489–497. doi: 10.1016/0167-4889(83)90051-4. [DOI] [PubMed] [Google Scholar]
- Seppala R, Lehto VP, Gahl WA. Mutations in the human UDP-N-acetylglucosamine 2-epimerase gene define the disease sialuria and the allosteric site of the enzyme. Am J Hum Genet. 1999;64:1563–1569. doi: 10.1086/302411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala R, Tietze F, Krasnewich D, Weiss P, Ashwell G, Barsh G, Thomas GH, Packman S, Gahl WA. Sialic acid metabolism in sialuria fibroblasts. J Biol Chem. 1991;266:7456–7461. [PubMed] [Google Scholar]
- Shah MM, Fujiyama K, Flynn CR, Joshi L. Sialylated endogenous glycoconjugates in plant cells. Nat Biotechnol. 2003;21:1470–1471. doi: 10.1038/nbt912. [DOI] [PubMed] [Google Scholar]
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: Influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Simpson MA, Cross H, Proukakis C, Priestman DA, Neville DCA, Reinkensmeier G, Wang H, Wiznitzer M, Gurtz K, Verganelaki A, et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat Genet. 2004;36:1225–1229. doi: 10.1038/ng1460. [DOI] [PubMed] [Google Scholar]
- Sprung R, Nandi A, Chen Y, Chan Kim S, Barma D, Falck JR, Zhao Y. Tagging-via-substrate strategy for probing O-GlcNAc modified proteins. J Proteome Res. 2005;4:950–957. doi: 10.1021/pr050033j. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ito T, Suzuki T, Holland J, Robert E, Chambers TM, Kiso M, Ishida H, Kawaoka Y. Sialic acid species as a determinant of the host range of influenza A viruses. J Virol. 2000;74:11825–11831. doi: 10.1128/jvi.74.24.11825-11831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Kohler JJ. Photoactivatable crosslinking sugars for capturing glycoprotein interactions. J Am Chem Soc. 2008;130:3278–3279. doi: 10.1021/ja7109772. [DOI] [PubMed] [Google Scholar]
- Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner ME. The enzymes of sialic acid biosynthesis. Bioorg Chem. 2005;33:216–228. doi: 10.1016/j.bioorg.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Tate MC, García AJ, Keselowsky BG, Schumm MA, Archer DR, LaPlac MC. Specific β1 integrins mediate adhesion, migration, and differentiation of neural progenitors derived from the embryonic striatum. Mol Cell Neurosci. 2004;27:22–31. doi: 10.1016/j.mcn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Thoden JB, Holden HM. The molecular architecture of human N-acetylgalactosamine kinase. J Biol Chem. 2005;280:32784–32791. doi: 10.1074/jbc.M505730200. [DOI] [PubMed] [Google Scholar]
- Varki A. Selectin ligands: Will the real ones please stand up? J Clin Invest. 1997;99:158–162. doi: 10.1172/JCI119142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution. Am J Phys Anthropol. 2002;116:54–69. doi: 10.1002/ajpa.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villavicencio-Lorini P, Laabs S, Danker K, Reutter W, Horstkorte R. Biochemical engineering of the acyl side chain of sialic acids stimulates integrin-dependent adhesion of HL60 cells to fibronectin. J Mol Med. 2002;80:671–677. doi: 10.1007/s00109-002-0382-y. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Lawrence S, Hinderlich S, Yarema KJ, Lee YC, Betenbaugh M. Engineering sialic acid synthetic ability into insect cells: Identifying metabolic bottlenecks and devising strategies to overcome them. Biochemistry. 2003;42:15215–15225. doi: 10.1021/bi034994s. [DOI] [PubMed] [Google Scholar]
- Vocadlo DJ, Hang HC, Kim E-J, Hanover JA, Bertozzi CR. A chemical approach for identifying O-GlcNAc-modified proteins in cells. Proc Natl Acad Sci USA. 2003;100:9116–9121. doi: 10.1073/pnas.1632821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Brand-Miller J. The role and potential of sialic acid in human nutrition. Eur J Clin Nutr. 2003;57:1351–1369. doi: 10.1038/sj.ejcn.1601704. [DOI] [PubMed] [Google Scholar]
- Wang B, Brand Miller J, McNeil Y, McVeagh P. Sialic acid concentration of brain gangliosides: Variation among eight mammalian species. Comp Biochem Physiol A Mol Integr Physiol. 1998;119A:435–439. doi: 10.1016/s1095-6433(97)00445-5. [DOI] [PubMed] [Google Scholar]
- Wang B, McVeagh P, Petocz P, Brand-Miller J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am J Clin Nutr. 2003;78:1024–1029. doi: 10.1093/ajcn/78.5.1024. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ekanayaka SA, Wu J, Zhang J, Guo Z. Synthetic and immunological studies of 5′-N-phenylacetyl sTn to develop carbohydrate-based cancer vaccines and to explore the impacts of linkage between carbohydrate antigens and carrier proteins. Bioconjug Chem. 2008;19:2060–2067. doi: 10.1021/bc800243f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang J, Guo Z. Efficient glycoengineering of GM3 on melanoma cell and monoclonal antibody-mediated selective killing of the glycoengineered cancer cell. Bioorg Med Chem. 2007;15:7561–7567. doi: 10.1016/j.bmc.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Sun Z, Li AV, Yarema KJ. Roles for GNE outside of sialic acid biosynthesis: Modulation of sialyltransferase and BiP expression, GM3 and GD3 biosynthesis, proliferation and apoptosis, and ERK1/2 phosphorylation. J Biol Chem. 2006;281:27016–27028. doi: 10.1074/jbc.M604903200. [DOI] [PubMed] [Google Scholar]
- Wang-Gillam A, Pastuszak I, Elbein AD. A 17-amino acid insert changes UDP-N-acetylhexosamine pyrophosphorylase specificity from UDP-GalNAc to UDP-GlcNAc. J Biol Chem. 1998;273:27055–27057. doi: 10.1074/jbc.273.42.27055. [DOI] [PubMed] [Google Scholar]
- Weihofen WA, Berger M, Chen H, Saenger W, Hinderlich S. Structures of human N-acetylglucosamine kinase in two complexes with N-acetylglucosamine and with ADP/glucose: insights into substrate specificity and regulation. J Mol Biol. 2006;364:388–399. doi: 10.1016/j.jmb.2006.08.085. [DOI] [PubMed] [Google Scholar]
- Wieser JR, Heisner A, Stehling P, Oesch R, Reutter W. In vivo modulated N-acyl side chain of N-acetylneuraminic acid modulates the cell-dependent inhibition of growth. FEBS Lett. 1996;395:170–173. doi: 10.1016/0014-5793(96)01029-0. [DOI] [PubMed] [Google Scholar]
- Wu J, Guo Z. Improving the antigenicity of sTn antigen by modification of its sialic acid residue for development of glycoconjugate cancer vaccines. Bioconjug Chem. 2006;17:1537–1544. doi: 10.1021/bc060103s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Oviedo A, Sweeley C, Saito T, Moskal JR. α2,6-Sialylation of cell-surface N-glycans inhibits glioma formation in vivo. Cancer Res. 2001;61:6822–6829. [PubMed] [Google Scholar]
- Yarema KJ. New directions in carbohydrate engineering: A metabolic substrate-based approach to modify the cell surface display of sialic acids. BioTechniques. 2001;31:384–393. doi: 10.2144/01312rv02. [DOI] [PubMed] [Google Scholar]
- Yarema KJ. A metabolic substrate-based approach to engineering new chemical reactivity into cellular sialoglycoconjugates. In: Al-Rubeai M, editor. Cell Engineering Vol. 3. Glycosylation. Dordrecht (The Netherlands): Kluwer Academic Publishers; 2002. pp. 171–196. [Google Scholar]
- Yarema KJ, Bertozzi CR. Chemical approaches to glycobiology and emerging carbohydrate-based therapeutic agents. Curr Opin Chem Biol. 1998;2:49–61. doi: 10.1016/s1367-5931(98)80035-5. [DOI] [PubMed] [Google Scholar]
- Yarema KJ, Goon S, Bertozzi CR. Metabolic selection of glycosylation defects in human cells. Nat Biotechnol. 2001;19:553–558. doi: 10.1038/89305. [DOI] [PubMed] [Google Scholar]
- Yarema KJ, Mahal LK, Bruehl RE, Rodriguez EC, Bertozzi CR. Metabolic delivery of ketone groups to sialic acid residues. Application to cell surface glycoform engineering. J Biol Chem. 1998;273:31168–31179. doi: 10.1074/jbc.273.47.31168. [DOI] [PubMed] [Google Scholar]
- Yarema KJ, Sun Z. A photochemical snapshot of CD22 binding. Nat Chem Biol. 2005;1:69–70. doi: 10.1038/nchembio0705-69. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Go S, Takasaki K, Kakazu Y, Ohashi M, Nagafuku M, Kabayama K, Sekimoto J, Suzuki S, Takaiwa K, et al. Mice lacking ganglioside GM3 synthase exhibit complete hearing loss due to selective degeneration of the organ of Corti. Proc Natl Acad Sci USA. 2009;106:9483–9488. doi: 10.1073/pnas.0903279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RK, Bieberich E, Xia T, Zeng G. Regulation of ganglioside biosynthesis in the nervous system. J Lipid Res. 2004;45:783–793. doi: 10.1194/jlr.R300020-JLR200. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Ramya TNC, Dirksen A, Dawson PE, Paulson JC. High-efficiency labeling of sialylated glycoproteins on living cells. Nat Meth. 2009;6:207–299. doi: 10.1038/nmeth.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.