Abstract
Rationale
Recent studies have investigated d-amphetamine as a potential agonist medication for cocaine dependence. In rats, a 14-day continuous infusion of d-amphetamine via osmotic mini-pump has been shown to decrease cocaine-reinforced responding under a progressive ratio (PR) schedule of reinforcement.
Objectives
This study was designed to assess the influences of the d-amphetamine treatment dose and self-administered cocaine dose on the magnitude of this effect.
Materials and methods
Experiment 1: Rats were trained to self-administer 1.5 mg/kg/inj cocaine under a PR schedule, then implanted with d-amphetamine mini-pumps for 14 days (days 1–7: 5 mg/kg/day; days 8–14: 7.5 mg/kg/day). Breakpoints were evaluated throughout the treatment period and 14 days post-treatment. Experiment 2: Rats were trained to self-administer cocaine under a PR schedule and initial dose-response curves were determined before implantation of d-amphetamine mini-pumps. During the 14-day d-amphetamine (5 mg/kg/day) treatment period, rats self-administered one of four cocaine doses (0.19, 0.38, 0.75 or 1.5 mg/kg/inj). A post-treatment PR dose-response curve and responding under a fixed ratio 1 (FR1) schedule were evaluated after mini-pump removal.
Results
Experiment 1: Breakpoints for 1.5 mg/kg/inj cocaine were unchanged by the increasing dose of d-amphetamine. Experiment 2: The PR dose-response curve was shifted downward after the treatment period in rats that had self-administered 0.19 and 0.38 mg/kg/inj cocaine. In contrast, rats in the 0.75 and 1.5 mg/kg/inj groups demonstrated increased rates of cocaine intake under an FR1 schedule after the treatment period.
Conclusions
These data suggest that continuous d-amphetamine treatment attenuates the reinforcing effects of cocaine.
Keywords: Addiction, Agonist therapy, d-Amphetamine, Cocaine, Osmotic mini-pump, Progressive ratio
Introduction
The search for a successful pharmacological treatment for cocaine dependence has been a considerable focus of the National Institute on Drug Abuse for several decades. Two distinct research strategies have been termed “bottom-up” and “top-down” (Vocci and Ling 2005). The bottom-up approach involves developing novel medications based on basic research findings on the neurochemistry and genetics of cocaine addiction. Although several promising compounds (Montoya and Vocci 2008; Rothman et al. 2008b, a; 2008b) and vaccines (Kosten 2005; Orson et al. 2008) are being developed and evaluated in this fashion, the Food and Drug Administration (FDA) has yet to approve a medication for the treatment of cocaine dependence. For this reason, recent research efforts have adopted a “top-down” approach by investigating the utility of medications that are currently marketed for the treatment of other disorders. d-Amphetamine is a stimulant medication that has been approved by the FDA for the treatment of both attention deficit hyperactivity disorder and narcolepsy. In recent years, its therapeutic potential for treating cocaine dependence has been evaluated in both clinical and preclinical populations.
Clinical studies using d-amphetamine in both immediate release (Shearer et al. 2003) and extended-release (Grabowski et al. 2001; 2004a; Rush et al. 2009) oral formulations have demonstrated beneficial effects in cocaine-dependent individuals, including decreases in subject ratings of cocaine effects and craving for the drug, objective measures of cocaine use, and use-related crime. These results have been attributed to the similarities between the effects of d-amphetamine and those of cocaine and the usefulness of d-amphetamine to serve as a replacement drug in cocaine-dependent individuals (Grabowski et al. 2004b; Shearer 2008). This idea of agonist replacement therapy was first proposed for the treatment of opiate dependence (Dole et al. 1966; Kreek 2000), and is currently a primary line of treatment for heroin and prescription opiate dependence (e.g., methadone maintenance and levo-alpha-acetyl-methadol treatment; Fiellin et al. 2006; Kreek and Vocci 2002) as well as tobacco smoking (i.e., nicotine replacement therapy; Fiore 2000; Stead et al. 2008).
In preclinical studies, whether d-amphetamine treatment produces an increase or decrease in the behavioral effects of cocaine has been shown to depend upon both the duration of treatment. Acute injections of d-amphetamine have been shown to augment the response to cocaine in studies of locomotor activity (Bonate et al. 1997; Ferrario and Robinson 2007; Schenk et al. 1991; Shuster et al. 1977) and conditioned place preference (Lett 1989; Shippenberg and Heidbreder 1995). In cocaine self-administration studies, acute pretreatment with d-amphetamine produces a similar leftward shift in the cocaine dose-response curve on measures of acquisition and maintenance of cocaine-reinforced responding (Barrett et al. 2004; Ferrario and Robinson 2007; Horger et al. 1992; Li et al. 2006; Mendrek et al. 1998), as well as responding previously reinforced by cocaine (Lynch et al. 1998; Schenk and Partridge 1999). By contrast, extended treatment with d-amphetamine appears to produce a reduction in the reinforcing effects. Treatment with d-amphetamine for at least 7 days either by twice-daily SC injections (Peltier et al. 1996), slow IV infusions three times per hour (Negus 2003; Negus and Mello 2003b, a; 2003b), or constant infusion from an osmotic mini-pump (Chiodo et al. 2008) has been shown to decrease cocaine self-administration in rats and monkeys. This effect is thought to be somewhat selective for cocaine-reinforced responding, as food-reinforced responding was either unaffected (Chiodo et al. 2008) or only transiently decreased (Negus and Mello 2003a) by extended d-amphetamine treatment.
Our laboratory has previously reported that breakpoints under a progressive ratio (PR) schedule were diminished following continuous d-amphetamine treatment (5 mg/kg/day) via SC osmotic mini-pump (Chiodo et al. 2008). The magnitude of this effect was dependent on the duration of treatment and the unit dose of cocaine. Seven days of continuous d-amphetamine infusion had the greatest effect on the lowest unit injection dose of cocaine (0.19 mg/kg/inj). Breakpoints associated with a dose in the middle of the curve (0.75 mg/kg/inj) were not significantly reduced by 7 days of d-amphetamine treatment; however a significant decrease was seen with 14 days of treatment. This longer duration of treatment did not affect the highest dose tested (1.5 mg/kg/inj).
These data raise an important concern regarding the potential clinical usefulness of d-amphetamine treatment. Ideally a medication should produce a downward shift in the entire dose-response function (Grabowski et al. 2004b). A rightward shift would also demonstrate a therapeutic treatment effect, although this would be surmountable by higher unit doses. To date we have been unable find a dose and duration of d-amphetamine treatment which reduces responding at the peak of the dose-effect curve. Data from Chiodo et al. 2008 suggest that the slope of the curve had been affected but the position of the peak of the curve remained unchanged. A failure of any treatment to reduce the reinforcing efficacy of cocaine suggests that the putative therapeutic effects would be entirely surmountable.
Two approaches were used in the present study in order to document a possible reduction in the magnitude of cocaine’s reinforcing effects as measured under a PR schedule. Negus and Mello (2003a) demonstrated in monkeys that higher doses of d-amphetamine augmented the degree to which breakpoints maintained by cocaine were reduced. In addition, clinical studies using agonist therapies for treating both psychostimulant and opiate dependence have suggested that an increase in treatment dose is more effective based on several factors, including a patient’s drug history (Caplehorn et al. 1993; Fleming and Roberts 1994; Trafton et al. 2006). Therefore our first approach was to reassess whether a larger dose of d-amphetamine might have a significant effect on self-administration of higher doses of cocaine. In the present study we escalated the dose of d-amphetamine during the second week of treatment and assessed the effect on self-administration of the most effective dose of cocaine (1.5 mg/kg/inj). Our second approach was to examine the dose-response relationship in animals that demonstrate a decrease in responding at lower doses of cocaine, as this was not completely addressed in our previous study. Chiodo et al. (2008) used a between subjects design in which each group of animals was tested on a single dose of cocaine. In the present study, dose response curves under a PR schedule were evaluated in every subject before and after 14 days of continuous d-amphetamine treatment. As the PR schedule does not yield a complete explanation of drug intake behavior on its own, we also examined changes in the rate of cocaine intake under a fixed ratio 1 (FR1) schedule of reinforcement. Here we report a substantial downward shift in the cocaine dose-effect curve as measured under a PR schedule (demonstrating a reduction in reinforcing effects) after continuous d-amphetamine infusion. Additionally, we show an increased rate of cocaine intake under an FR1 schedule in animals that did not show a change in PR breakpoints, thus revealing dissociation between responding under these two schedules of reinforcement.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, Ind., USA) weighing approximately 350 g at the start of the experiments were used as subjects. Rats were maintained on a reverse 12 h light/dark cycle (lights on at 3 PM) with food and water available ad libitum. All rats were habituated to this schedule for a minimum of 3 days before entering the experiment. Throughout the experiments, rats were housed individually in stainless steel custom made experimental chambers (30 × 30 × 30 cm).
Surgery
Each rat was anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (8 mg/kg) and implanted with a chronically indwelling Silastic® jugular catheter (CamCaths, Cambridgeshire, UK) prior to the beginning of the study. The tubing of the catheter extended from the jugular vein to a subcutaneous plastic anchor which exited through the skin on the dorsal surface in the region of the scapulae (Roberts and Goeders 1989). Tygon® tubing (enclosed by a stainless steel protective tether), was connected to the plastic anchor to attach the catheter to a counterbalanced fluid swivel (Instech Laboratories, Inc., Plymouth Meeting, PA., USA) mounted above the experimental chamber. The swivel was then connected to an infusion pump (Razel Scientific Instruments, Inc., Stamford, CT) outside of the experimental chamber with Tygon tubing. Self-administration began after rats had 3–5 days to recover from surgery. During the course of the experiments, catheters were flushed daily with heparinized saline in order to maintain patency.
Rats were implanted SC with an osmotic mini-pump (Alzet Model 2001, Durect Corp. Cupertino, CA; see Theeuwes and Yum 1976) filled with d-amphetamine once their self-administration behavior was stable (as described in the experiments below). Briefly, animals were anesthetized with a mixture of oxygen, nitrogen and halothane (4%) and ventilated during surgery using halothane (1.5%). An incision in the skin was made between the scapulae (rostral to the plastic catheter anchor), the mini-pump was inserted with the flow moderator pointing rostrally, and the wound was closed using nylon sutures or surgical glue. The mini-pump was replaced after 7 days using the same procedure so that each rat received 14 continuous days of d-amphetamine treatment. The mini-pumps were filled with d-amphetamine at a concentration which would result in the delivery of either 5 or 7.5 mg/kg over 24 h, as determined by each subject’s body weight at the time of implantation.
Cocaine self-administration
For all experiments, self-administration occurred 7 days per week. A single active lever extended into the experimental chamber each day at 10 AM (i.e., during the dark phase of the light/dark cycle) to indicate the beginning of a self-administration session. The lever was linked to the infusion pump through a computer so that a 4–5 s (depending on body weight) injection of cocaine was delivered once an animal pressed the lever and completed the response requirement. Initially, rats were trained to self-administer 1.5 mg/kg/injection under an FR1 schedule of reinforcement. A 20-s time-out period, signalled by retraction of the lever and illumination of a light, occurred after each lever press. After an animal self-administered 40 injections within 6 h, the lever was retracted until the start of the next daily session. Self-administration training was termed complete following 5 consecutive daily sessions during which the animal self-administered all 40 injections while maintaining consistent post-infusion pauses of 5–8 min between each of the injections.
Following training, rats began to self-administer cocaine (1.5 mg/kg/inj in Experiment 1 or 0.19–1.5 mg/kg/inj in Experiment 2) in daily 6-h sessions under a PR schedule. On this schedule, response requirements were increased through the following ratio progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, etc. (Richardson and Roberts 1996). The breakpoint was defined as the number of completed ratios (i.e., the number of reinforcers delivered) before 1 h elapsed without completion of the next ratio. Each session lasted 6 h, but rats typically reached a breakpoint within 3 h and exhibited only minimal non-reinforced responding for the remainder of the session.
Experiment 1: Effect of increasing the dose of continuous d-amphetamine on cocaine self-administration
This experiment investigated the effects of an increasing dose of d-amphetamine (5–7.5 mg/kg/day; SC) on self-administration of 1.5 mg/kg/inj cocaine under a PR schedule. After the completion of training under an FR1 schedule, animals (N = 8) self-administered 1.5 mg/kg/inj cocaine until they achieved a stable 3-day baseline period, wherein the 3-day range of breakpoints (i.e., number of injections) did not exceed three. At this point, animals were implanted with mini-pumps that delivered 5 mg/kg/day d-amphetamine. These mini-pumps were removed after 7 days and replaced with new mini-pumps that delivered 7.5 mg/kg/day d-amphetamine for 7 additional days. This dose of d-amphetamine was chosen because previous studies have shown that doses of 10 mg/kg/day and above (delivered via mini-pump) cause depressive-like symptoms, rapid weight loss, and neurotoxicity during and after the treatment period (Cryan et al. 2003; Eison et al. 1983; Martin-Iverson and Lodge 1991; Nielsen 1981; Robinson and Camp 1987; Ryan et al. 1990). Also, a gradual increase in d-amphetamine dose has been shown to protect against the neurotoxic effects associated with the higher dose (Robinson and Camp 1987). Self-administration of 1.5 mg/kg/inj cocaine occurred daily during this 14-day treatment period. After Day 14, mini-pumps were removed and post-treatment breakpoints were assessed for 14 additional days.
Experiment 2: Changes in the dose-response curve for self-administered cocaine after 14 days of continuous d-amphetamine treatment
The effect of 5 mg/kg/day SC infusion of d-amphetamine via osmotic mini-pump on cocaine-reinforced responding over a range of cocaine doses (0.19–1.5 mg/kg/inj) was investigated. After animals (N = 32) reached the training criterion, they self-administered 0.75 mg/kg/inj cocaine under a PR schedule of reinforcement until they reached 3 consecutive days of stable breakpoints. Next, animals were tested on each of 4 cocaine doses (0.19, 0.38, 0.75 and 1.5 mg/kg/inj) in a Latin square design during 4 consecutive testing days to determine an initial dose-response curve. At this point, animals were implanted with osmotic mini-pumps that delivered a continuous infusion of 5 mg/kg/day d-amphetamine over 14 days (see Surgery). During this 14-day treatment period, animals were divided into 4 groups (with equivalent baseline breakpoints; N = 8), and each group self-administered 1 of the 4 component cocaine doses of the initial dose-response curve (0.19, 0.38, 0.75 or 1.5 mg/kg/inj) in daily PR sessions. Mini-pumps were removed on Day 14 and the cocaine dose-response curve was reassessed in all animals by using the initial Latin square design. Following completion of the final dose-response curve, each animal self-administered 40 injections of 1.5 mg/kg/inj cocaine under an FR1 schedule.
Drugs
Cocaine HCl (National Institute on Drug Abuse, Rockville, MD) was dissolved in sterile 0.9% saline (containing heparin, 10 USP units/ml) in concentrations of 0.625, 1.25, 2.5 and 5 mg/ml (expressed as the salt) and passed through a microfilter. d-Amphetamine sulfate (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile 0.9% saline.
Data Analysis
Breakpoint was used as the main dependent measure for self-administration under the PR schedule. In Experiment 1, breakpoints from the final baseline testing day and the 14 days during d-amphetamine treatment were analyzed using a one-way analysis of variance (ANOVA) with repeated measures (SigmaPlot 11; Systat Software Inc.). In Experiment 2, the initial PR dose-response curves before mini-pump implantation were compared between all 4 groups using a two-way ANOVA with GROUP (i.e., based on which of the 4 cocaine doses was self-administered during the d-amphetamine treatment period) and DOSE (i.e., the 4 cocaine doses in the dose-response curve; 0.19, 0.38, 0.75 and 1.5 mg/kg/inj) as factors. Breakpoints during the d-amphetamine treatment period for all 4 groups were compared using a two-way ANOVA with repeated measures with GROUP and DAY as factors. Total cocaine intake during the d-amphetamine treatment period was compared between groups using a one-way ANOVA. Finally, a two-way repeated measures ANOVA which compared the post-treatment dose-response curve to the initial dose-response curve was done for each group individually, with DOSE (i.e., the 4 cocaine doses in the dose-response curve; 0.19, 0.38, 0.75 and 1.5 mg/kg/inj) and TREATMENT (i.e., before d-amphetamine vs. after d-amphetamine treatment) as factors. Changes in rate of intake under the FR1 schedule (i.e., mean number of injections per hr) were analyzed for each group of animals in Experiment 2. A two-way ANOVA with repeated measures was used to compare the rate of intake from Day 5 of training under the FR1 schedule to that of the FR1 session conducted after completion of the post-treatment dose-response curve. Values of p<0.05 were considered statistically significant and Holm-Sidak tests were used in post hoc analyses.
RESULTS
Experiment 1
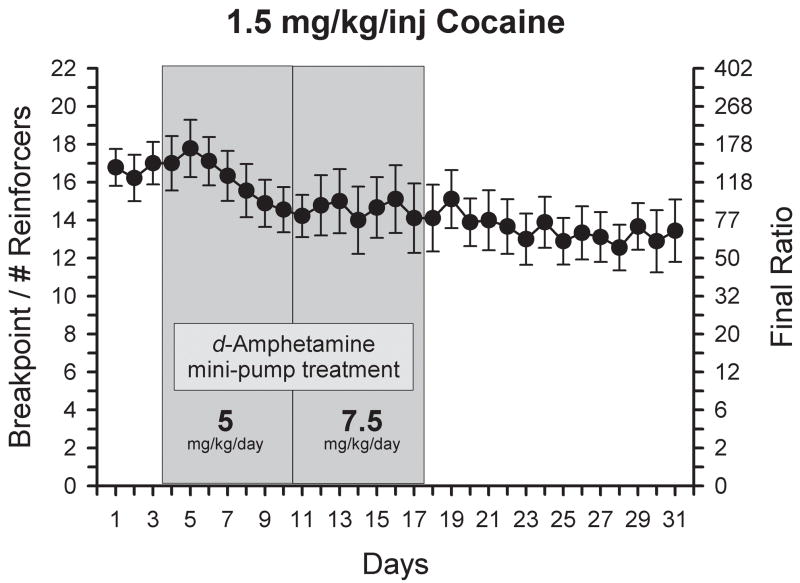
Figure 1 shows the effect of a constant infusion of d-amphetamine via an osmotic mini-pump for 14 days (7 days of 5 mg/kg/day and 7 days of 7.5 mg/kg/day) on breakpoints maintained by 1.5 mg/kg/inj cocaine. Comparison of the final baseline day and all 14 treatment days with a one-way ANOVA with repeated measures did not reveal a significant difference [F(14, 98)=0.92, ns] throughout the d-amphetamine treatment period.
Figure 1.
Effect of increasing the dose of continuous d-amphetamine on self-administration of 1.5 mg/kg/inj cocaine under a PR schedule. Points represent the mean (±SEM) breakpoints. Shaded portions represent the d-amphetamine treatment period (7 days of 5 mg/kg/day and 7 days of 7.5 mg/kg/day). The final ratio values corresponding to breakpoints are represented on the right y axis.
Experiment 2
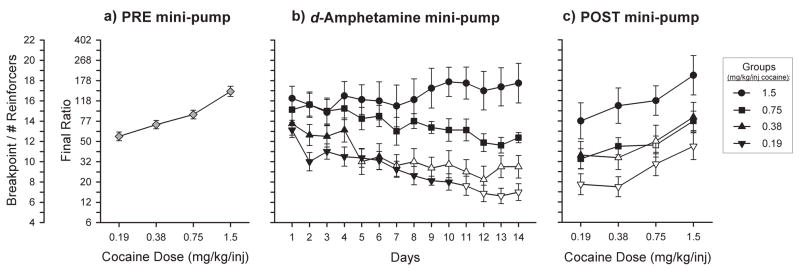
Figure 2a illustrates the initial breakpoint dose-response curve for all animals (N = 32) prior to d-amphetamine treatment. Two-way ANOVA revealed a significant effect of cocaine DOSE [F(3, 127)=17.16, p<0.001]. Animals were randomly assigned to 1 of 4 groups to be tested on 0.19, 0.38, 0.75 or 1.5 mg/kg/inj cocaine. Figure 2b illustrates the effect of 14 days of continuous d-amphetamine treatment on breakpoints associated with these 4 groups (N = 8). Repeated measures ANOVA revealed a significant effect of GROUP [F(3, 479)=10.83, p<0.001], DAY[F(13, 479)=10.28, p<0.001], and a significant GROUP x DAY interaction [F(42, 479)=12.89, p<0.001].
Figure 2.
Changes in the dose-response curve for self-administered cocaine after 14 days of continuous d-amphetamine (5 mg/kg/day) treatment. All points represent the mean (±SEM) breakpoints under a PR schedule. The final ratio values corresponding to breakpoints are represented on the additional y axis. a) Initial dose-response curve established in all animals (N = 32) after training sessions were completed. b) 14-day d-amphetamine treatment period during which animals were divided into four groups to self-administer one of four cocaine doses (▼: 0.19, ▲: 0.38, ■: 0.75, or ●: 1.5 mg/kg/inj). c) Final dose-response curves for each of the four groups after removal of d-amphetamine mini-pumps. Open symbols represent a significant difference from the same dose in the initial dose-response curve.
The post-treatment cocaine dose-response curves for all 4 groups are shown in Figure 2c. ANOVA revealed a significant effect of GROUP [F(3, 127)=18.17, p<0.001] and DOSE [F(3, 127)=6.84, p<0.001]. Repeated measures ANOVA comparing initial and post-treatment dose-response curves within each group revealed a significant difference for the 0.19 mg/kg/inj group [F(1, 63)=37.96, p<0.001] and post hoc Holm-Sidak analysis confirmed significant differences for all doses in the dose-response curve [0.19 dose: t=4.15, df=7, p<0.001; 0.38 dose: t=5.61, df=7, p<0.001; 0.75 dose: t=4.26, df=7, p<0.001; 1.5 dose: t=4.71, df=7, p<0.001]. For the 0.38 mg/kg/inj group, there was a significant effect of TREATMENT [F(1, 63)=5.92, p<0.05], and this is accounted for by significant differences when tested on the 0.38 dose [t=2.29, df=7, p<0.05] and the 0.75 dose [t=2.20, df=7, p<0.05]. There was not an effect of TREATMENT for the 0.75 mg/kg/inj group [F(1, 63)=3.80, ns] or the 1.5 mg/kg/inj group [F(1, 63)=0.54, ns].
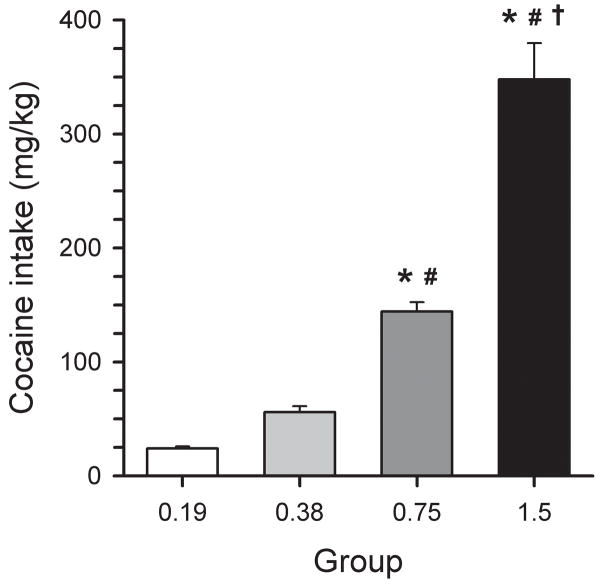
The total amount of cocaine self-administered during the 14-day d-amphetamine treatment period is illustrated in Figure 3. One-way ANOVA revealed a significant difference between groups [F(3, 31)=76.85, p<0.001]. Holm-Sidak post hoc analysis revealed significant difference for all comparisons except between the 0.19 mg/kg/inj and 0.39 mg/kg/inj groups [t=1.36, df=3, ns].
Figure 3.
Total cocaine intake (mg/kg) during the 14-day d-amphetamine treatment period for groups of animals that self-administered various doses of cocaine. * significant difference from the 0.19 mg/kg/inj group (p<0.01). # significant difference from the 0.38 mg/kg/inj group (p<0.01). † significant difference from the 0.75 mg/kg/inj group (p<0.01).
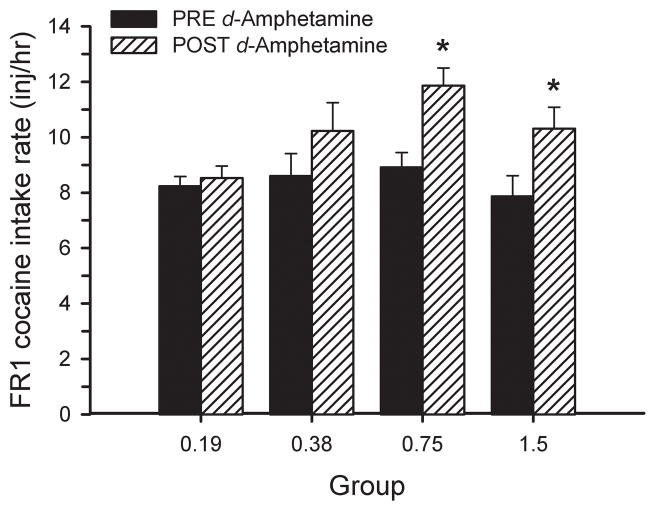
The change in cocaine intake rate under an FR1 schedule is shown in Figure 4. One animal from each group was excluded from the analyses due to catheter failure prior to the post-treatment FR1 testing session. There was no difference in intake rates for the final day of FR1 training (i.e., before d-amphetamine treatment) between the 4 groups [F(3, 27)=0.68, ns]. Two-way ANOVA with repeated measures revealed an effect of TIME [F(1, 55)=15.99, p<0.001] with significant differences for the 0.75 group [t=3.23, df=6, p<0.01] and the 1.5 mg/kg/inj group [t=2.67, df=6, p<0.01].
Figure 4.
Changes in the intake rate (mean inj/hr) for 1.5 mg/kg/inj cocaine under an FR1 schedule after 14 days of continuous d-amphetamine treatment. For each group, solid bars represent the baseline cocaine intake rate during the final day of FR1 training and hatched bars represent the cocaine intake rate after the d-amphetamine treatment period. * significant difference from baseline (p<0.01).
Figure 5 shows the cumulative records from a representative animal (from the 1.5 mg/kg/inj group) while self-administering 1.5 mg/kg/inj under a PR schedule before (i.e., PRE) and after (i.e., POST) the 14-day d-amphetamine treatment period. The breakpoint remained the same in both sessions, whereas increased rate of drug intake can be observed.
Figure 5.
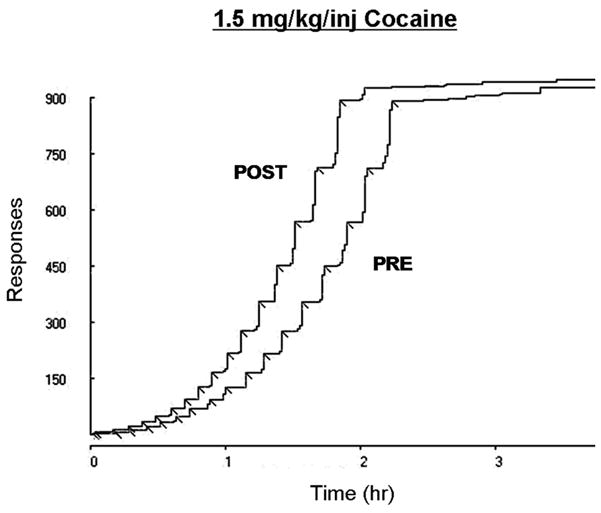
Cumulative records from a representative animal in the 1.5 mg/kg/inj group illustrating an increased rate of cocaine intake under a PR schedule after 14 days of continuous d-amphetamine treatment. Each drug infusion (i.e., breakpoint) is represented by a diagonal inflection. PRE: 1.5 mg/kg/inj test session from the initial dose-response curve. POST: 1.5 mg/kg/inj test session from the post-treatment dose-response curve.
DISCUSSION
In the present study we investigated the capacity of continuous d-amphetamine treatment, delivered via osmotic mini-pump, to decrease cocaine-reinforced responding and the extent to which cocaine dose, the level of cocaine exposure, and d-amphetamine treatment dose influenced the magnitude of this effect. The major finding of the present study is that continuous d-amphetamine treatment can produce a substantial downward shift in the cocaine dose-effect curve as measured under a PR schedule of reinforcement.
Our initial aim of this study was to decrease breakpoints for a dose of cocaine at the peak of the curve by increasing the treatment dose of d-amphetamine. We had previously found that 14 days of 5 mg/kg/day d-amphetamine was not strong enough to affect the reinforcing magnitude of 1.5 mg/kg/inj cocaine in our previous study (Chiodo et al. 2008). Therefore in Experiment 1 of the present study, animals that self-administered 1.5 mg/kg/inj cocaine received a higher d-amphetamine dose during the second half of the 14-day treatment period. The treatment dose was increased from 5 mg/kg/day to 7.5 mg/kg/day only after the first week in order to avoid the toxic effects (i.e., stereotypy, excessive weight loss, depressive-like behaviors and neurochemical deficits) seen with extended mini-pump delivery of higher d-amphetamine doses (Cryan et al. 2003; Eison et al. 1983; Martin-Iverson and Lodge 1991; Nielsen 1981; Ryan et al. 1990). We found that breakpoints for the high dose of cocaine (1.5 mg/kg/inj) were unchanged during a week-long period of d-amphetamine infusion with 5 mg/kg/day (as expected) and remained at baseline levels throughout the subsequent week of 7.5 mg/kg/day d-amphetamine as well.
Using a second strategy to demonstrate a downward shift in the curve, we focused on our previous positive results in which 14 days of d-amphetamine treatment was shown to reduce breakpoints for low unit doses of cocaine. The present results replicated the principle findings of Chiodo et al. (2008), by showing that the decrease in breakpoints during the 14-day d-amphetamine treatment depends on the unit injection dose of cocaine. Again, breakpoints associated with lower unit doses of cocaine were reduced during the treatment period, whereas those associated with the higher doses were not. Our previous study used a between subjects design in which each group of animals was tested on a different dose of cocaine. In the present study, dose-response curves under a PR schedule were evaluated within all groups before and after 14 days of continuous d-amphetamine treatment. This design clearly shows that the effect of d-amphetamine is not restricted to a specific unit dose but instead translates into a shift of the entire dose-response curve. More specifically, a downward shift in the cocaine dose-response curve was seen after animals self-administered low to moderate doses of cocaine (0.19–0.75 mg/kg/inj) under a PR schedule during a 14-day d-amphetamine (5 mg/kg/day) treatment period, whereas the dose-response curve was unchanged for animals that self-administered a high dose of cocaine (1.5 mg/kg/inj) during the treatment period.
Extended d-amphetamine treatment has been shown to decrease responding under a PR schedule (in nonhuman primates and rats) using several paradigms (Chiodo et al. 2008; Negus and Mello 2003a; Peltier et al. 1996). Peltier et al. (1996) showed that 7 days of twice-daily IP d-amphetamine injections decreased breakpoints for cocaine in rats. Negus and Mello found similar decreases in the reinforcing efficacy of cocaine in monkeys when d-amphetamine was delivered every 20 minutes through an IV cannula (Negus and Mello 2003a) and this effect also applied to responding under a second-order schedule (Negus and Mello 2003b) and in a food-drug choice procedure (Negus 2003). Our present data and that from our previous study replicate this general finding, and also highlight differences that occur between these various methods of extended d-amphetamine treatment. It has been shown that the time between d-amphetamine injections can be very influential on subsequent behavioral responses to psychostimulants (Ellison and Morris 1981; Nelson and Ellison 1978). Moreover, when d-amphetamine is delivered via osmotic mini-pump, animals do not exhibit sensitized behavioral responses that are typically seen after repeated intermittent injections (i.e., acoustic startle; Kokkinidis 1984). This could account for the differences in post-treatment responding seen between the studies. For example, breakpoints returned to baseline very quickly after the d-amphetamine treatment had ended in the studies by Peltier et al. (1996) and Negus and Mello (2003a). However, we have shown that breakpoints reinforced by low cocaine doses not only decrease after continuous d-amphetamine treatment delivered via an osmotic mini-pump but also remain below baseline for up to two weeks after the d-amphetamine mini-pumps were removed (Chiodo et al. 2008).
Tolerance has conventionally been used to explain decreases in cocaine self-administration over time and extended d-amphetamine treatment has been thought to create cross-tolerance to the reinforcing effects of cocaine (Peltier et al. 1996). If tolerance were to account for our data, one would expect to see the greatest decrease in breakpoints in conjunction with a higher dose of the daily d-amphetamine infusion, a higher self-administered cocaine dose, or a greater amount of total cocaine exposure. However, the findings of the present study as well as those found previously (Chiodo et al. 2008) diminish the role of tolerance in decreasing cocaine self-administration under a PR schedule. First of all, breakpoints for 1.5 mg/kg/inj cocaine (i.e., the highest cocaine dose tested here) remained at the baseline level throughout the d-amphetamine treatment period regardless of the d-amphetamine dose. Next, the greatest decrease in breakpoints after either 7 or 14 days of continuous d-amphetamine treatment was seen for the lowest dose of cocaine (0.19 mg/kg/inj), while breakpoints for the highest dose (1.5 mg/kg/inj) remained at baseline throughout and well beyond the treatment period. Finally, the results from the present study suggest that although the reinforcing effects of cocaine can be decreased after continuous d-amphetamine treatment, the magnitude of this effect cannot be entirely predicted by the total amount of self-administered cocaine during the d-amphetamine treatment period. As shown in Figures 2b and 3, animals that self-administered 1.5 mg/kg/inj during the d-amphetamine treatment period not only received the greatest concentration of cocaine per each IV infusion, but they also self-administered considerably more injections of cocaine over 14 days than animals in the other groups. Despite earlier studies linking high cocaine exposure to a decrease in the reinforcing efficacy of the drug (Hammer, Jr. et al. 1997), the present findings from the PR dose-response curves in Experiment 2 suggest the opposite when continuous d-amphetamine treatment is incorporated. Although the PR dose-response curve was unchanged in the 1.5 mg/kg/inj group, a substantial downward shift can be seen in the 0.19 mg/kg/inj group after d-amphetamine treatment. This is somewhat unexpected considering that the average amount of cocaine taken by these animals over 14 days was almost 15 times lower than that of the 1.5 mg/kg/inj group. Additionally, the diminished breakpoints in the 0.19 mg/kg/inj group are not merely a demonstration of extinction responding over time, as we have previously shown that breakpoints for this dose of cocaine progressively increase for at least seven days after d-amphetamine treatment has been terminated (Chiodo et al. 2008).
It should be emphasized that high drug intake during the treatment period was associated with an increased rate of drug intake under an FR1 schedule; however this change in FR1 responding did not predict a change in responding under the PR schedule. In Experiment 2, the mean rate of cocaine self-administration under an FR1 schedule was substantially increased for animals in the 0.75 and 1.5 mg/kg/inj groups after the d-amphetamine treatment period whereas none of the doses on the breakpoint dose-response curve was changed in these animals (see Figures 2c and 4). An increase in the rate of cocaine intake might be interpreted as an indicator of behavioral tolerance (Emmett-Oglesby and Lane 1992); however this tolerance is seemingly limited to a specific aspect of reinforcement. For example, although the rate of cocaine intake under a PR schedule appears to be increased after the d-amphetamine treatment period for animals in the 1.5 mg/kg/inj group (see Figure 5 for a representative cumulative record) the final breakpoints did not show any evidence of tolerance. Conversely, animals in the 0.19 mg/kg/inj group demonstrated the largest downward shift in the PR dose-response curve but no change in the rate of intake under an FR1 schedule. This dissociation between breakpoints under a PR schedule and rate of intake under an FR schedule is not unique to the present study (see Brebner et al. 2000; Richardson and Roberts 1991; Roberts et al. 1989; 1996) thus indicating that the two measures reveal distinct information about the factors that control cocaine self-administration. According to behavioral economic theory, a PR schedule would appear to address the concept of “price” whereas responding under an FR1 schedule would appear to address the concept of “consumption” (see Hursh 1991; 2005 for review). In a recent study, price and consumption were found to be weak predictors of one another (Oleson and Roberts 2009).
Overall, our data suggest that continuous treatment with a low dose of d-amphetamine treatment might be of some benefit to human cocaine users. In clinical studies of d-amphetamine treatment, improvements in subjective ratings of cocaine use and craving as well as cocaine-related crime (Grabowski et al. 2001; 2004a; Shearer et al. 2003) can be viewed as measures of harm reduction for both the cocaine user and society in general (Des Jarlais 1995). In preclinical self-administration studies, changes in the pattern of drug intake reveal similar information about the value of a pharmacological treatment. The present study demonstrated changes in intake pattern on two different schedules of reinforcement as well as a substantial downward shift in the cocaine dose-response curve.
With regard to the specific clinical application of these findings, several factors remain to be determined. First, we found that the magnitude to which d-amphetamine infusion affects cocaine-reinforced responding is dependent on the self-administered cocaine dose. Since the greatest decrease in breakpoints occurred in animals that had the least amount of cocaine exposure (i.e., the 0.19 mg/kg/inj group), this might suggest that any therapeutic effect of d-amphetamine would be limited to humans who use cocaine casually instead of those who are the most dependent on cocaine. While this is not ideal in terms of treatment success, this would appear to translate well to the clinic situation wherein a patient’s drug history plays a considerable role in the therapeutic effect of any treatment medication (McLellan and Alterman 1991). However, the results from this study are not a complete evaluation of the effectiveness of a slow continuous infusion of d-amphetamine and therefore cocaine self-administration under other schedules of reinforcement must be tested in order to establish the parameters of this treatment method. For example, we previously found that continuous d-amphetamine treatment had no effect in animals that did not have access to cocaine during the treatment period (Chiodo et al. 2008), suggesting that the therapeutic effect requires a combination of d-amphetamine and cocaine. Further study is necessary to determine whether the cocaine must be self-administered to see this effect (indicating a psychological or associative explanation) or if a noncontingent delivery of cocaine in combination with d-amphetamine will also decrease subsequent cocaine self-administration (indicating a pharmacological explanation).
Acknowledgments
Funding provided by Grants R01DA14030 and P50DA06634-14.
References
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Bonate PL, Swann A, Silverman PB. Context-dependent cross-sensitization between cocaine and amphetamine. Life Sci. 1997;60:L1–L7. doi: 10.1016/s0024-3205(96)00591-7. [DOI] [PubMed] [Google Scholar]
- Brebner K, Phelan R, Roberts DC. Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology (Berl) 2000;148:314–321. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- Caplehorn JR, Bell J, Kleinbaum DG, Gebski VJ. Methadone dose and heroin use during maintenance treatment. Addiction. 1993;88:119–124. doi: 10.1111/j.1360-0443.1993.tb02769.x. [DOI] [PubMed] [Google Scholar]
- Chiodo KA, Lack CM, Roberts DC. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology (Berl) 2008;200:465–473. doi: 10.1007/s00213-008-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC. Harm Reduction - A Framework for Incorporating Science Into Drug Policy. American Journal of Public Health. 1995;85:10–12. doi: 10.2105/ajph.85.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade. Arch Intern Med. 1966;118:304–309. [PubMed] [Google Scholar]
- Eison MS, Eison AS, Iversen SD. Two routes of continuous amphetamine administration induce different behavioral and neurochemical effects in the rat. Neurosci Lett. 1983;39:313–319. doi: 10.1016/0304-3940(83)90319-1. [DOI] [PubMed] [Google Scholar]
- Ellison G, Morris W. Opposed stages of continuous amphetamine administration: parallel alterations in motor stereotypies and in vivo spiroperidol accumulation. Eur J Pharmacol. 1981;74:207–214. doi: 10.1016/0014-2999(81)90532-x. [DOI] [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Lane JD. Tolerance to the reinforcing effects of cocaine. Behav Pharmacol. 1992;3:193–200. [PubMed] [Google Scholar]
- Ferrario CR, Robinson TE. Amphetamine pretreatment accelerates the subsequent escalation of cocaine self-administration behavior. Eur Neuropsychopharmacol. 2007;17:352–357. doi: 10.1016/j.euroneuro.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Friedland GH, Gourevitch MN. Opioid dependence: rationale for and efficacy of existing and new treatments. Clin Infect Dis. 2006;43(Suppl 4):S173–S177. doi: 10.1086/508180. [DOI] [PubMed] [Google Scholar]
- Fiore MC. US public health service clinical practice guideline: treating tobacco use and dependence. Respir Care. 2000;45:1200–1262. [PubMed] [Google Scholar]
- Fleming PM, Roberts D. Is the prescription of amphetamine justified as a harm reduction measure? J R Soc Health. 1994;114:127–131. doi: 10.1177/146642409411400303. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004a;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004b;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Hammer RP, Jr, Egilmez Y, Emmett-Oglesby MW. Neural mechanisms of tolerance to the effects of cocaine. Behav Brain Res. 1997;84:225–239. doi: 10.1016/s0166-4328(97)83332-3. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology (Berl) 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration and drug abuse policy. J Exp Anal Behav. 1991;56:377–393. doi: 10.1901/jeab.1991.56-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Galuska CM, Winger G, Woods JH. The economics of drug abuse: a quantitative assessment of drug demand. Mol Interv. 2005;5:20–28. doi: 10.1124/mi.5.1.6. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L. Effects of chronic intermittent and continuous amphetamine administration on acoustic startle. Pharmacol Biochem Behav. 1984;20:367–371. doi: 10.1016/0091-3057(84)90272-7. [DOI] [PubMed] [Google Scholar]
- Kosten T, Owens SM. Immunotherapy for the treatment of drug abuse. Pharmacol Ther. 2005;108:76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Methadone-related opioid agonist pharmacotherapy for heroin addiction. History, recent molecular and neurochemical research and future in mainstream medicine. Ann N Y Acad Sci. 2000;909:186–216. doi: 10.1111/j.1749-6632.2000.tb06683.x. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Vocci FJ. History and current status of opioid maintenance treatments: blending conference session. J Subst Abuse Treat. 2002;23:93–105. doi: 10.1016/s0740-5472(02)00259-3. [DOI] [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology (Berl) 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Li SM, Campbell BL, Katz JL. Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J Pharmacol Exp Ther. 2006;317:1088–1096. doi: 10.1124/jpet.105.100594. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Heaser WA, Carroll ME. Effects of amphetamine, butorphanol, and morphine pretreatment on the maintenance and reinstatement of cocaine-reinforced responding. Exp Clin Psychopharmacol. 1998;6:255–263. doi: 10.1037//1064-1297.6.3.255. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Lodge BA. Effects of chronic treatment of rats with "designer" amphetamines on brain regional monoamines. Can J Physiol Pharmacol. 1991;69:1825–1832. doi: 10.1139/y91-270. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI. Patient treatment matching: a conceptual and methodological review with suggestions for future research. NIDA Res Monogr. 1991;106:114–135. [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl) 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Montoya ID, Vocci F. Novel medications to treat addictive disorders. Curr Psychiatry Rep. 2008;10:392–398. doi: 10.1007/s11920-008-0063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine-and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 2003a;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine-and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Nelson LR, Ellison G. Enhanced stereotypies after repeated injections but not continuous amphetamines. Neuropharmacology. 1978;17:1081–1084. doi: 10.1016/0028-3908(78)90045-x. [DOI] [PubMed] [Google Scholar]
- Nielsen EB. Rapid decline of stereotyped behavior in rats during constant one week administration of amphetamine via implanted ALZET osmotic minipumps. Pharmacol Biochem Behav. 1981;15:161–165. doi: 10.1016/0091-3057(81)90171-4. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology. 2009;34:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orson FM, Kinsey BM, Singh RA, Wu Y, Gardner T, Kosten TR. Substance abuse vaccines. Ann N Y Acad Sci. 2008;1141:257–269. doi: 10.1196/annals.1441.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier RL, Li DH, Lytle D, Taylor CM, Emmett-Oglesby MW. Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther. 1996;277:212–218. [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat. Life Sci. 1991;49:833–840. doi: 10.1016/0024-3205(91)90248-a. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996;15:417–423. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Goeders NE. Drug self-administration: experimental methods and determinants. In: Boulton AA, Baker GB, Greenshaw AJ, editors. Neuromethods: Psychopharmacology. Vol. 13. Humana Press, Inc; Clifton, NJ: 1989. pp. 349–398. [Google Scholar]
- Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol. 2008a;75:2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dopamine/serotonin releasers as medications for stimulant addictions. Prog Brain Res. 2008b;172:385–406. doi: 10.1016/S0079-6123(08)00919-9. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR. Cocaine effects during D-amphetamine maintenance: a human laboratory analysis of safety, tolerability and efficacy. Drug Alcohol Depend. 2009;99:261–271. doi: 10.1016/j.drugalcdep.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan LJ, Linder JC, Martone ME, Groves PM. Histological and ultrastructural evidence that D-amphetamine causes degeneration in neostriatum and frontal cortex of rats. Brain Res. 1990;518:67–77. doi: 10.1016/0006-8993(90)90955-b. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Cocaine-seeking produced by experimenter-administered drug injections: dose-effect relationships in rats. Psychopharmacology (Berl) 1999;147:285–290. doi: 10.1007/s002130051169. [DOI] [PubMed] [Google Scholar]
- Schenk S, Snow S, Horger BA. Pre-exposure to amphetamine but not nicotine sensitizes rats to the motor activating effect of cocaine. Psychopharmacology (Berl) 1991;103:62–66. doi: 10.1007/BF02244075. [DOI] [PubMed] [Google Scholar]
- Shearer J. The principles of agonist pharmacotherapy for psychostimulant dependence. Drug Alcohol Rev. 2008;27:301–308. doi: 10.1080/09595230801927372. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van BI, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. J Pharmacol Exp Ther. 1995;273:808–815. [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology (Berl) 1977;52:185–190. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- Theeuwes F, Yum SI. Principles of the design and operation of generic osmotic pumps for the delivery of semisolid or liquid drug formulations. Ann Biomed Eng. 1976;4:343–353. doi: 10.1007/BF02584524. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Minkel J, Humphreys K. Determining effective methadone doses for individual opioid-dependent patients. PLoS Med. 2006;3:e80. doi: 10.1371/journal.pmed.0030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci F, Ling W. Medications development: successes and challenges. Pharmacol Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]