Abstract
A new generation of silica encapsulated single quantum dots (QDs) was synthesized based on recent breakthroughs made in coating magnetic nanoparticles and their clusters. In comparison with the traditional Stöber sol-gel method, this new approach is significantly simpler, resulting in QDs with excellent luminescence, stability, size monodispersity, and tunable silica shell thickness. An important finding was that unlike previous reported magnetic and metallic nanoparticles, the QDs coated with only a layer of surfactant molecules were highly unstable and sensitive to the environment. As a consequence, the surfactant stabilized QDs must be prepared fresh and stored in dark before silica coating. The QDs become stable once silica shell formed on their surface and excess surfactants are removed. Further development of this technology particularly by incorporating drugs into the mesosized silica pores will open exciting opportunities in traceable delivery and controlled release of therapeutic agents.
Keywords: quantum dot, nanoparticle, mesoporous, fluorescence, silica, imaging, drug delivery
INTRODUCTION
Semiconductor QDs have captivated scientists and engineers over the past two decades due to their fascinating optical and electronic properties that are not available from either isolated molecules or bulk solids. Recent research has stimulated considerable interest in developing these quantum-confined nanocrystals as fluorescent probes for biomedical applications.1-3 In comparison with organic dyes and fluorescent proteins, QDs offer several unique advantages such as size- and composition-tunable emission from ultraviolet to infrared wavelengths, large absorption coefficients across a wide spectral range, and very high levels of brightness and photostability. Due to their broad excitation profiles and narrow/symmetric emission spectra, high-quality QDs are also well suited for combinatorial optical encoding, in which multiple colors and intensities are combined to encode thousands of genes, proteins, or small-molecule compounds.4-6
High-quality QDs are typically prepared at elevated temperatures in organic solvents, such as tri-n-octylphosphine oxide and hexadecylamine (TOPO and HDA, both of which are high boiling-point solvents containing long alkyl chains). These hydrophobic organic molecules not only serve as the reaction media, but also coordinate with unsaturated metal atoms on the QD surface to prevent formation of bulk semiconductors. As a result, the nanoparticles are capped with a monolayer of the organic ligands and are soluble only in organic solvents such as chloroform and toluene. For biological applications, these hydrophobic dots are made water-soluble generally by three approaches, ligand exchange, silica shell capping, and the recently developed amphiphilic polymer coating. The ligand exchange approach is easy to perform, but the resulting water-soluble QDs are only stable for a short period and its quantum yield decreases significantly,7 because the original hydrophobic surface ligands are replaced by hydrophilic ligands such as mercaptoacetic acid. The newly discovered amphiphilic polymer coating approach solved these problems by retaining the coordinating organic ligands on the QD surface.8 Typically, amphiphilic polymers contain both a hydrophobic segment or side-chain (mostly hydrocarbons) and a hydrophilic segment or group (such as polyethylene glycol or multiple carboxylate groups). A number of polymers have been reported including octylamine-modified low molecular weight polyacrylic acid, polyethylene glycol (PEG) derivatized phospholipids, block copolymers, and polyanhydrides.9-12 The hydrophobic domains strongly interact with TOPO on the QD surface, whereas the hydrophilic groups face outward and render QDs water soluble. Although the amphiphilic polymer coating represents the newest addition to the area of QD surface engineering and offers a number of advantages, silica shell capping remains as an attractive approach for QD solublization due to its stability, biocompatibility, and versatile surface chemistry. More importantly, the surface coating thickness can be precisely controlled in the range of 1-100s nm, which is difficult, if not impossible, to achieve based on the ligand exchange and amphiphilic polymer coating methods.
A number of papers have reported the successful encapsulation of QDs with silica, and the methods can be grouped into two general categories, Stöber sol-gel chemistry and microemulsion.13-22 For example, one of the earliest papers on the biological applications of silica capped QDs was reported by Alivisatos and co-workers.14 Although it demonstrates the potential of using QDs for multicolor cell labeling, the silica capping procedure itself is complicated and prone to formation of QD aggregates. Recently, a breakthrough procedure on coating magnetic nanoparticles (MNPs) and their clusters with mesoporous silica was developed by Hyeon et al.23, 24 In comparison with the Stöber and microemulsion methods, the surfactant templated mesoporous silica coating is simple, high-yield, and capable of tuning the silica shell thickness, yielding QDs with excellent optical properties and biocompatibility.
MATERIALS AND METHODS
Reagents and instruments
Unless specified, chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification. TOPO coated CdSe/ZnS core/shell QDs were provided by Oceannanotech LLC as a gift. Methoxy poly(ethylene glycol) succinimidyl glutarate (MW 2000) was purchased from Laysan Bio, Inc. (Arab, AL). A UV-2450 spectrophotometer (Shimadzu, Columbia, MD) and a Fluoromax4 fluorometer (Horiba Jobin Yvon, Edison, NJ) were used to characterize the absorption and emission spectra of the original and modified QDs. The dry and hydrodynamic radii of QDs were measured on a CM100 transmission electron microscope (Philips EO, Netherlands) and a Zetasizer NanoZS size analyzer (Malvern, Worcestershire, UK). True-color fluorescence images were obtained with a Nikon digital camera.
Synthesis of mesoporous silica coated QDs
The synthesis of mesoporous silica coated QDs was developed based on the MNP encapsulation protocols described by Hyeon et al.23, 24 Briefly, for approximately 20 nm-thick silica coating, 3.0 μM CdSe/ZnS QDs (emission peak 622nm) in 0.5 ml chloroform was mixed with 5 ml cetyltrimethylammonium bromide (CTAB) aqueous solution (55 mM). A homogeneous microemulsion was obtained with vigorous stirring. The mixture was then heated at 50 °C for approximately 15 min to evaporate the chloroform, resulting in a clear aqueous solution of QDs stabilized with CTAB. The QD solution was diluted with 45 ml NaOH solution (13mM) preheated to 50 °C, followed by addition of 0.5 ml of tetraethylorthosilicate (TEOS) and 3 ml of ethylacetate. The reaction was kept on stirring for 3 hours and allowed to slowly cool down to room temperature. The silica encapsulated QDs were rinsed with ethanol repeatedly to remove the unreacted precursors and the surfactant porogen. For silica shell thickness of approximately 10 and 30 nm, the procedures were virtually the same except that the concentrations of the QD chloroform solution were 5.5 μM and 1.1 μM, respectively.
Pegylation of silica coated QDs
Mesoporous silica coated nanoparticles were first functionalized with primary amines. During the silica shell formation, 3-aminopropyltriethoxysilane (APTES) was added 10 minutes after TEOS addition. The aminated particles were purified with ethanol rinsing and suspended in 20 ml ethanol for reaction with PEG. 50 mg of methoxy poly(ethylene glycol) succinimidyl glutarate (MW 2000) was dissolved in 20 ml of ethanol and added to the QD-silica particle solution. The mixture was stirred for 3 h at 40°C, followed by washing with ethanol and water to remove any unreacted PEG. The pegylated particles were dispersed in phosphate buffer solution (PBS, 10 mM, pH 7.4).
QD quenching study in CTAB
The three QD samples used in the comparative stability study are QDs stabilized with CTAB, QD-mesoporous silica in the presence of CTAB (unpurified after silica formation), and QD-mesoporous silica in the absence of CTAB (purified after silica shell formation), and their concentrations were 30 nM. Because the CTAB used in the silica shell synthesis was 55 mM, the first two samples were also incubated in 55mM CTAB. For the last sample, CTAB was removed by repeated rinsing. To test the QD stability, the samples were either kept in dark or exposed to ambient room light. Periodically, their fluorescence emission was measured with the fluorometer (excitation wavelength 400 nm).
RESULTS AND DISCUSSION
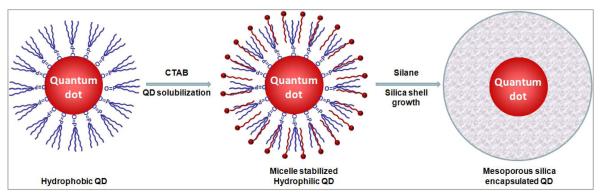
Figure 1 schematically illustrates the experiment procedure of making monodisperse mesoporous silica encapsulated QDs. The cationic surfactant molecule, CTAB, plays two important roles in this process, solublization of hydrophobic QDs into aqueous solutions and templating the nanometer-sized pore formation. In the first step, when QDs in chloroform are added into the CTAB aqueous solution, the CTAB molecules stabilize the oil droplets leading to formation of an oil-in-water microemulsion. Subsequent evaporation of the volatile organic solvent by heating drives CTAB molecules to directly interact with the QD surface ligands through hydrophobic interactions. The alkyl chains of the CTAB and the QD surface ligands intercalate into each other, rendering the CTAB cationic headgroup (quaternary amine) facing outward and the QD-CTAB complex water-soluble.25 In the second step, when silane compounds polymerize to form silica shells on QD surface, the CTAB acts as templates for mesopore formation (also known as porogen). Systematic investigation on mesoporous nanoparticles by Ostafin and coworkers has suggested a three-stage reaction: (i) hydrolysis of alkoxysilanes and formation of silica oligomers, (ii) formation of silica/CTAB primary particles, and (iii) mesopore growth via aggregation of the primary particles.26 Excess surfactant molecules can be removed by repeated rinsing with ethanol after the reaction.
Figure 1.
Schematic illustration of the procedure for mesoporous silica coating of QDs. Hydrophobic QDs initially coated with trioctylphosphine oxide are solubilized with CTAB. Subsequent addition of silane compounds leads to formation of mesoporous silica shell templated by the CTAB.
Using red QDs (emission 622 nm), we systematically investigated the conditions for QD-silica formation. In contrast to magnetic nanoparticles and metallic nanoparticles, the photophysical properties of QDs are highly sensitive to the environment and especially to the surface coating, which makes mesoporous silica coating more difficult. A surprising finding was that the fluorescence of CTAB-stabilized QDs was slowly quenched under ambient room light illumination in approximately 2 days, which was not observed previously.25 This effect is especially dramatic when the QD concentration is relatively low, such as 30 nM used in the current study. Figure 2a shows two CTAB stabilized QD samples. The sample to the left was prepared and aged for 2 days under normal room light illumination, whereas the sample to the right was prepared fresh. Clearly, the fluorescence of the aged sample was completely quenched, which was also confirmed by quantitative fluorescence spectroscopy measurements (inset). More interestingly, when the CTAB capped QDs were kept in dark, the fluorescence intensity decrease was minimal (data not shown), indicating that the quenching effect is likely due to photo-induced oxidation.27
Figure 2.
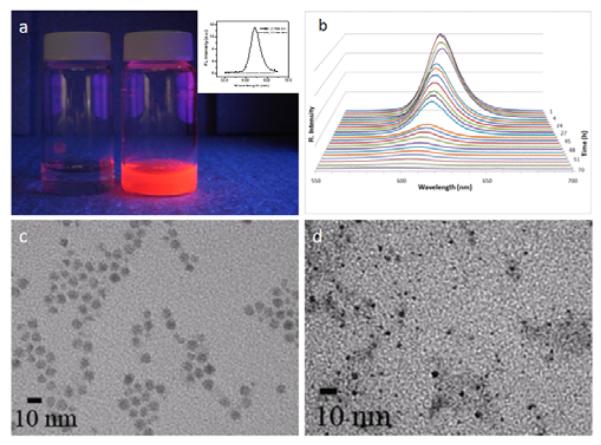
Photo-induced quenching of the CTAB-coated QDs. (a) 30 nM QD-CTAB solution made fresh (right) and aged for 48 hours (left). The fluorescence of the aged sample clearly diminished, which was also confirmed with spectroscopic studies (inset). (b) Time lapse spectroscopic measurements show that not only the fluorescence intensity decreased over time, the fluorescence emission peak also blue shifted from 622 nm to 600 nm, indicating QD decomposition. This result was also confirmed by TEM studies of fresh (c) and aged (d) samples. The particle size reduced significantly.
To further investigate the quenching effect, we set out to quantitatively characterize the fluorescence quenching over time. Figure 2b shows that for CTAB coated QDs, the fluorescence intensity decreased by 90% in 2 days and dropped to background level in approximately 3 days. In addition, the emission peak position also blue shifted for more than 20 nm before the fluorescence intensity reduced to background level, indicating decomposition of the QD nanocrystals. Indeed, high resolution TEM images of freshly prepared QD-CTAB and QD-CTAB aged for 48 hours revealed that the particle size had been reduced significantly (Figure 2c, d). Complete particle deformation (not detectable by TEM and UV absorption) was also observed upon further aging of the particles. These observations are completely different from the amphiphilic lipid and polymer encapsulated QDs9-12 although these compounds share the same mechanism as CTAB in solubilization of hydrophobic QDs. A potential solution to this QD instability problem is to use CdSe QDs with much thicker CdS or ZnS coating, which has been shown to not only better protect the CdSe core but also suppress the ‘blinking’ effect of QDs.28, 29
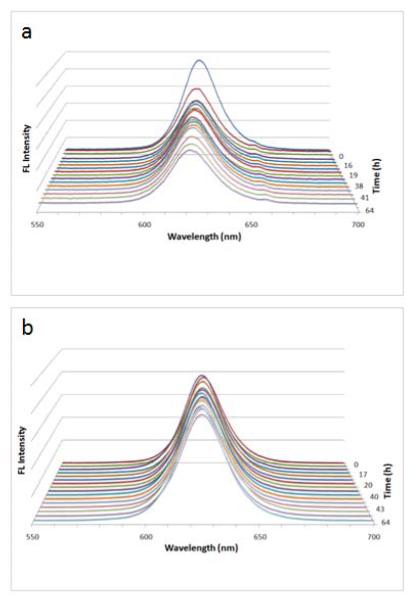
Essential to the success of QD encapsulation is to maintain its fluorescence emission, the signature of semiconductor QDs. Therefore, we used freshly prepared QD-CTAB and proceeded to study the stability of mesoporous silica encapsulated QDs in the presence and in the absence of CTAB. Figure 3a shows that under identical experiment conditions, the mesoporous silica coated QDs were also partially quenched by the CTAB, although at a significantly reduced rate compared with CTAB-stabilized QDs. Over the same time course, the fluorescence intensity decreased by 45% and the spectral blue shift was 3 nm. This reduced quenching rate could result from limited diffusion of CTAB through the mesoporous silica shell and stronger binding between QDs and silica than between QDs and CTAB. Using non-porous silica shells, Koole et al. have previously demonstrated that silane oligomers have stronger binding affinity to QDs than most surfactants (except thio compounds) and can replace the surfactant during silica deposition.30 In comparison, the optical properties of the final purified QD-silica particles (excess CTAB removed by centrifugation) remain unchanged in aqueous buffer as shown in Figure 3b.
Figure 3.
Photo induced quenching of the silica-coated QDs. (a) In the presence of excess CTAB, the QD fluorescence intensity was reduced by 45%, and peak position blue shifted by 3 nm. (b) When the CTAB was purified out, the QD fluorescence remained unchanged under identical experiment conditions.
For potential applications in biology and medicine, the nanoparticles should not only maintain their optical properties but also colloidal stability. With large excess of CTAB in solution (before the CTAB molecules were purified out), the QD-silica particles were highly positively charged with a zeta potential value of 50 mv, which made the nanoparticles highly soluble in aqueous solutions. When the excess CTAB was removed, however, the zeta potential reduced to −15 mv, which rendered the QD-silica to slowly form clusters over a few days. Further severe aggregation of such particles with proteins and other biomolecules was also observed, precluding them from being used in most biomedical applications. We solved this problem by functionalizing the silica surface with a layer of PEG molecules. For chemical conjugation, the innate hydroxyl groups on the silica shell have poor reactivity compared with primary amines. We, therefore, added a small amount of amine-containing APTES in the silica shell formation experiment. The primary amines are highly reactive to the monofunctional succinimidyl glutarate ester PEG. After PEG coating, QD-silica nanoparticles were highly dispersible in aqueous solution without forming aggregates over long storage time.
With these experimental parameters optimized, we successfully synthesized the QD-silica nanoparticles. TEM measurements showed that the nanoparticles were highly monodisperse with a ‘dry’ size of 49.3±1.3 nm and were separated from each other (Figure 4a). The TEM micrograph of higher magnification revealed that the mesoporous silica shell was approximately 20 nm thick and the QDs were located in the center of the silica particle (Figure 4b), indicating isotropic growth of silica shell on the surface of the QDs rather than directional growth.26 The size uniformity was also confirmed with dynamic light scattering (DLS), which measures the hydrodynamic size of the nanoparticles in solution. The DLS result showed a hydrodynamic diameter of 69.0±1.6 nm, much larger than the particle dry size. This is because the nanoparticles were pegylated and charged in solution, thus creating an electrical double layer on the nanoparticle surface, thereby increasing the colloidal hydrodynamic radius compared to the actual size.31
Figure 4.
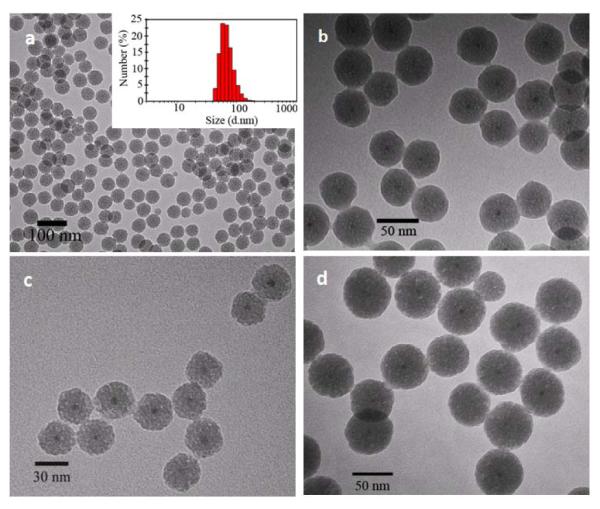
TEM micrographs of the QD-silica core/shell nanopartilcles with tunable shell thickness. (a) and (b) Low and high magnification images of QDs coated with approximately 20 nm silica shell. (Inset) DLS measurement shows that the hydrodynamic diameter of the QD-silica (20 nm shell) in aqueous buffer is 69.0±1.6 nm. (c) QDs coated with approximately 10 nm silica shell. (d) QDs coated with approximately 30 nm silica shell.
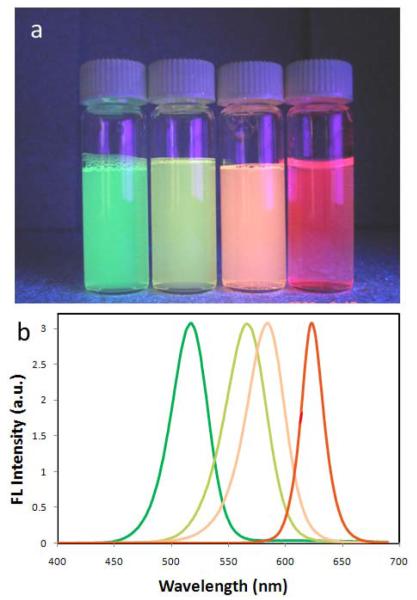
An important feature of this mesoporous silica coating procedure is that it allows precise control of the silica shell thickness simply by varying the QD/silane precursor ratios, which is extremely difficult for the aforementioned ligand exchange and amphiphilic polymer coating methods. Figure 4d&e show QDs coated with approximately 10 and 30 nm thick silica, and again the nanoparticles are highly monodisperse. To probe the possibility of making multicolor mesoporous silica encapsulated QDs, we further tested another three colors of QDs of smaller sizes. As shown in Figure 5, the mesoporous silica coated QDs were also highly soluble and fluorescent in aqueous solutions with emission wavelength nearly identical to the original organic soluble nanoparticles.
Figure 5.
Mesoporous silica encapsulation of multicolor QDs. (a) Fluorescent photographs of QDs encapsulated in mesoporous silica shell emitting light at 520, 570, 590, and 623 nm under UV illumination. (b) Corresponding emission spectra of the QD-silica samples shown in (a).
Conclusion
In conclusion, we have developed a new generation of silica encapsulated single quantum dots. A major finding is that in contrast to the previously reported amphiphilic macromolecules, CTAB based surface coating can solubilize QDs into water but significantly reduces their stability. Under room light illumination, the QD fluorescence can be quenched in 1-2 days due to particle decomposition, which was not observed in previous studies. As a result, the CTAB coated dots should be used fresh in making mesoporous silica coated QDs. We have also shown that this procedure can be applied for coating QDs of various sizes, and that the thickness of the silica coating layer can be varied by changing the QD-to-silica precursor molar ratio. We envision further development of this technology, particularly by incorporating drugs into the mesosized silica pores, will open exciting opportunities in traceable delivery and controlled release of therapeutic agents.
Acknowledgements
This work was supported in part by NIH, NSF, and the Department of Bioengineering at the University of Washington. X.G. thanks the NSF for a Faculty Early Career Development award (CAREER). P.Z. acknowledges the UW Center for Nanotechnology for a generous graduate student fellowship. We are also grateful to Prof. Terry Kavanagh for fruitful discussion on nanotoxicity, Dr. Y Andrew Wang at Oceannanotech for help with QD synthesis, and the Department of Biology and Center for Nanotechnology for their electron microscopes.
References
- 1.Alivisatos P. The use of nanocrystals in biological detection. Nature Biotechnology. 2004;22:47–52. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 2.Gao X, Yang L, Petros JA, Marshall FF, Simons JW, Nie S. In vivo molecular and cellular imaging with quantum dots. Current Opinion in Biotechnology. 2005;16:63–72. doi: 10.1016/j.copbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Materials. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 4.Gao XH, Nie SM. Doping mesoporous materials with multicolor quantum dots. Journal of Physical Chemistry B. 2003;107:11575–11578. [Google Scholar]
- 5.Gao XH, Nie SM. Quantum dot-encoded mesoporous beads with high brightness and uniformity: Rapid readout using flow cytometry. Analytical Chemistry. 2004;76:2406–2410. doi: 10.1021/ac0354600. [DOI] [PubMed] [Google Scholar]
- 6.Han MY, Gao XH, Su JZ, Nie S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nature Biotechnology. 2001;19:631–635. doi: 10.1038/90228. [DOI] [PubMed] [Google Scholar]
- 7.Chan WCW, Nie SM. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 8.Gao XH, Nie SM. Molecular profiling of single cells and tissue specimens with quantum dots. Trends in Biotechnology. 2003;21:371–373. doi: 10.1016/S0167-7799(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 9.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 10.Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnology. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 11.Pellegrino T, Manna L, Kudera S, Liedl T, Koktysh D, Rogach AL, Keller S, Radler J, Natile G, Parak WJ. Hydrophobic nanocrystals coated with an amphiphilic polymer shell: A general route to water soluble nanocrystals. Nano Letters. 2004;4:703–707. [Google Scholar]
- 12.Wu XY, Liu HJ, Liu JQ, Haley KN, Treadway JA, Larson JP, Ge NF, Peale F, Bruchez MP. Immunofluorescent labeling of cancer marker her2 and other cellular targets with semiconductor quantum dots. Nature Biotechnology. 2003;21:41–46. doi: 10.1038/nbt764. [DOI] [PubMed] [Google Scholar]
- 13.Gerion D, Pinaud F, Williams SC, Parak WJ, Zanchet D, Weiss S, Alivisatos AP. Synthesis and properties of biocompatible water-soluble silica-coated cdse/zns semiconductor quantum dots. Journal of Physical Chemistry B. 2001;105:8861–8871. [Google Scholar]
- 14.Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 15.Darbandi M, Thomann R, Nann T. Single quantum dots in silica spheres by microemulsion synthesis. Chemistry of Materials. 2005;17:5720–5725. [Google Scholar]
- 16.Wolcott A, Gerion D, Visconte M, Sun J, Schwartzberg A, Chen SW, Zhang JZ. Silica-coated cdte quantum dots functionalized with thiols for bioconjugation to igg proteins. Journal of Physical Chemistry B. 2006;110:5779–5789. doi: 10.1021/jp057435z. [DOI] [PubMed] [Google Scholar]
- 17.Selvan ST, Patra PK, Ang CY, Ying JY. Synthesis of silica-coated semiconductor and magnetic quantum dots and their use in the imaging of live cells. Angewandte Chemie-International Edition. 2007;46:2448–2452. doi: 10.1002/anie.200604245. [DOI] [PubMed] [Google Scholar]
- 18.Tan TT, Selvan ST, Zhao L, Gao SJ, Ying JY. Size control, shape evolution, and silica coating of near-infrared-emitting pbse quantum dots. Chemistry of Materials. 2007;19:3112–3117. [Google Scholar]
- 19.Yang YH, Jing LH, Yu XL, Yan DD, Gao MY. Coating aqueous quantum dots with silica via reverse microemulsion method: Toward size-controllable and robust fluorescent nanoparticles. Chemistry of Materials. 2007;19:4123–4128. [Google Scholar]
- 20.Bakalova R, Zhelev Z, Aoki I, Ohba H, Imai Y, Kanno I. Silica-shelled single quantum dot micelles as imaging probes with dual or multimodality. Analytical Chemistry. 2006;78:5925–5932. doi: 10.1021/ac060412b. [DOI] [PubMed] [Google Scholar]
- 21.Zhelev Z, Ohba H, Bakalova R. Single quantum dot-micelles coated with silica shell as potentially non-cytotoxic fluorescent cell tracers. Journal of the American Chemical Society. 2006;128:6324–6325. doi: 10.1021/ja061137d. [DOI] [PubMed] [Google Scholar]
- 22.Selvan ST, Tan TT, Ying JY. Robust, non-cytotoxic, silica-coated cdse quantum dots with efficient photoluminescence. Advanced Materials. 2005;17:1620. + [Google Scholar]
- 23.Kim H. S. K. Jaeyun, Lee Nohyun, Kim Taeho, Kim Hyoungsu, Yu Taekyung, Song In Chan, Moon Woo Kyung, Hyeon Taeghwan. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angewandte Chemie International Edition. 2008;9999 doi: 10.1002/anie.200802469. NA. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Lee JE, Lee J, Yu JH, Kim BC, An K, Hwang Y, Shin C-H, Park J-G, Hyeon T. Magnetic fluorescent delivery vehicle using uniform mesoporous silica spheres embedded with monodisperse magnetic and semiconductor nanocrystals. J. Am. Chem. Soc. 2006;128:688–689. doi: 10.1021/ja0565875. [DOI] [PubMed] [Google Scholar]
- 25.Fan H, Leve EW, Scullin C, Gabaldon J, Tallant D, Bunge S, Boyle T, Wilson MC, Brinker CJ. Surfactant-assisted synthesis of water-soluble and biocompatible semiconductor quantum dot micelles. Nano Lett. 2005;5:645–648. doi: 10.1021/nl050017l. [DOI] [PubMed] [Google Scholar]
- 26.Nooney RI, Thirunavukkarasu D, Chen Y, Josephs R, Ostafin AE. Self-assembly of mesoporous nanoscale silica/gold composites. Langmuir. 2003;19:7628–7637. [Google Scholar]
- 27.van Sark WGJHM, Frederix PLTM, Van den Heuvel DJ, Gerritsen HC, Bol AA, van Lingen JNJ, de MelloDonega C, Meijerink A. Photooxidation and photobleaching of single cdse/zns quantum dots probed by room-temperature time-resolved spectroscopy. J. Phys. Chem. B. 2001;105:8281–8284. [Google Scholar]
- 28.Chen Y, Vela J, Htoon H, Casson JL, Werder DJ, Bussian DA, Klimov VI, Hollingsworth JA. “giant” multishell cdse nanocrystal quantum dots with suppressed blinking. J. Am. Chem. Soc. 2008;130:5026–5027. doi: 10.1021/ja711379k. [DOI] [PubMed] [Google Scholar]
- 29.Mahler B, Spinicelli P, Buil S, Quelin X, Hermier J-P, Dubertret B. Towards non-blinking colloidal quantum dots. Nat Mater. 2008;7:659–664. doi: 10.1038/nmat2222. [DOI] [PubMed] [Google Scholar]
- 30.Koole R, van Schooneveld MM, Hilhorst J, Doneg d. M., #xe1 C, Hart DC, #x2bc, van Blaaderen A, Vanmaekelbergh D, Meijerink A. On the incorporation mechanism of hydrophobic quantum dots in silica spheres by a reverse microemulsion method. Chem. Mater. 2008;20:2503–2512. [Google Scholar]
- 31.Larson DR, Zipfel WR, Williams RM, Clark SW, Bruchez MP, Wise FW, Webb WW. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science. 2003;300:1434–1436. doi: 10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]